Abstract
Calcinosis cutis (CC) is characterized by deposit of calcium salts in the skin and subcutaneous tissue; its clinical presentation consists of indurated painful nodules, which can ulcerate and become superinfected. CC treatment remains a challenge, yet successful treatment with intralesional (IL) sodium thiosulfate (STS) has been reported in several CC subtypes. Herein we are reporting on a case series of 5 patients with CC successfully treated with IL-STS. We describe the 18–22 MHz ultrasound characteristics of the lesions and on follow-up after treatment. Ultrasound imaging was useful in guiding IL-STS injections and confirming response to treatment.
Keywords: Calcinosis cutis, Ultrasound imaging, Echography, Intralesional sodium thiosulfate, Treatment, Follow-up
Introduction
Calcinosis cutis (CC) is characterized by deposit of insoluble calcium salts in the skin and subcutaneous tissue [1]. CC can be painful, limit joint mobility, become ulcerated and superinfected [2–4]. Five main subtypes of CC have been described: dystrophic, metastatic, idiopathic, iatrogenic, and calciphylaxis [1, 5]. Dystrophic CC is the most common type of cutaneous calcification; it can be found in 25–40% of patients with limited systemic sclerosis, and 30% of adults and 70% of children and adolescents with dermatomyositis [1]. There are several therapeutic options for CC, including drugs (warfarin, diltiazem, bisphosphonates, ceftriaxone, probenecid, minocycline, intravenous immunoglobulins, aluminium hydroxide, intralesional corticosteroids, colchicine, rituximab, and cyclophosphamide), physical treatments (CO2 laser, hyperbaric oxygen, and extracorporeal shock wave lithotripsy), and surgical approaches (curettage or excision) [6]. Nevertheless, their efficacy and supporting evidence are limited; thus, treatment of CCs is not standardized and remains a therapeutic challenge. In 2004, Cicone et al. published the first report of calciphylaxis treated with intravenous (IV) sodium thiosulfate (STS) [7]. Several cases of CC with good response to topical [8, 9], or intralesional (IL) STS have been subsequently reported [2–4, 10–14]. However, outcome evaluations are usually only clinical, with its intrinsic limitations. To our knowledge, treatment of CC with IL-STS with ultrasound follow-up has been reported just in one case [15]. We report on a case series of five patients with CC who presented a good response to IL-STS therapy clinically and ultrasonographically, and we describe the ultrasound features of CC prior to and during IL-STS therapy.
Materials and methods
Cutaneous ultrasound examinations were performed in all patients with CC who were referred to or followed up at our department and had been treated with IL-STS. Ultrasound examination was performed at the baseline visit and at each follow-up visit. The findings were retrospectively reviewed. All the dermatologic ultrasound examinations were performed by the same physician, a dermatologist accredited by the Spanish (SEEC) and European (EFSUMB) societies of ultrasound, which has 8 years of experience in cutaneous ultrasound. Images were obtained using an Esaote MyLab™ Seven ultrasound system, with either 18 MHz or 22 MHz linear probes, because a very important technical requirement for an ultrasound diagnostic system is the maximum depth of ultrasound signal visualization [16]. Frequencies of 20 MHz reach a maximum depth of between 8 and 10 mm, optimum for skin and subcutaneous tissue evaluation [17]. All patients were treated with ultrasonographically-guided intralesional injections of 250 mg/mL STS solution, injected using a 30G needle at a dosage of maximum 1–1.2 mL per lesion. The frequency of injections was monthly, except in two cases because of patients’ availability: case 5 was treated every 3–4 months, and in case 4 the last dose was administered after a 2 months’ interval. Informed consent was obtained for all participants before IL-STS treatment.
Results
Five patients were included. Patients’ characteristics, their CC subtype, previous CC treatments, IL-STS regimen and adverse events are summarized in Table 1. No systemic adverse event was reported in any patient.
Table 1.
Main features of patients, calcinosis cutis, and treatment
| Patient | Age (years) | Gender | Underlying disease | CC location | CC subtype | Previous CC treatments | IL-STS (250 mg/mL) regimen: months when it was administrated | Treatment response (no/partial/complete) | Adverse events | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | Female | Goodpasture’s syndrome | Fingers | Metastatic | Sevelamer (a non-calcium phosphate binder) | 0, 1, 2, 3, 4 | Complete | Pain with injection | |
| 2 | 58 | Female | Lupus panniculitis | Left buttock | Dystrophic | Topical clobetasol, Tacrolimus cream, Methotrexate, Methyl mycophenolate, Prednisone, and Hydroxychloroquine | 0, 1, 2. She is waiting for a new IL-STS injection because of clinical worsening | Partial | Pain with injection | |
| 3 | 68 | Female | Limited systemic sclerosis | Left elbow | Dystrophic | Antibiotic | 0, 1, 2, 3 | Partial |
Ulceration on the injection site, 24 h after the fourth injection Pain with injection |
|
| 4 | 74 | Female | Skin burn during childhood | Left thigh | Dystrophic | Surgical excision (two times) | 0, 1, 2, 3, 5 | Partial | Pain with injection | |
| 5 | 54 | Female | Undifferentiated connective tissue disease | Left forearm | Dystrophic | Surgical excision (one time) | 0, 3, 7 | Partial | Pain with injection | |
CC calcinosis cutis, IL intralesional, STS sodium thiosulfate
Case 1
A 67-year-old woman with renal insufficiency secondary to Goodpasture’s syndrome, with altered calcium-phosphorus product (6.9 mmol2/L2), developed painful (8/10 in pain visual analogue scale (VAS)) nodules on the volar surfaces of the third finger of her left hand (Fig. 1a) and first finger of her right hand (Fig. 1c). Ultrasound examination showed hyperechoic deposits in the hypodermis with posterior acoustic shadow and positive Doppler signal (Fig. 1e). A punch-biopsy specimen revealed calcium deposits in the superficial and deep dermal interstitium, surrounded by foreign-body granulomatous reaction, consistent with metastatic CC. Lesions did not improve after 3 months of sevelamer—a non-calcium phosphate binding agent—, so we started treatment with IL-STS. Significant pain improvement was achieved since the first administration. After four monthly injections, ultrasound images showed complete clearance of calcium deposits and disappearance of Doppler activity (Fig. 1f). Complete clinical resolution (0/10 in pain VAS) was achieved 7 months after the initiation of treatment (Fig. 1b, d).
Fig. 1.
Clinical image before starting treatment with IL-STS, showing a reddish, spontaneously painful, hard nodule on the left middle finger pad (a). Monthly IL-STS administrations were started with complete resolution of the CC 7 months later, 3 months after the last injection (b). Clinical image before starting treatment with IL-STS, showing a reddish, spontaneously painful, hard nodule on the right thumb pad (c). Monthly IL-STS administrations were started with complete resolution of the lesions 7 months later, 3 months after the last injection (d). Cutaneous ultrasound image—using the 22 MHz probe—of the patient’s left 3rd finger pad before starting IL-STS showed hyperechoic deposits (yellow arrows) in the hypodermis with intense posterior acoustic shadow and positive Doppler signal (e). Cutaneous ultrasound image—with the 22 MHz probe—of her left 3rd finger pad after four monthly doses of IL-STS, showed complete resolution of the calcium deposit and complete disappearance of Doppler activity (f)
Case 2
A 58-year-old woman with lupus panniculitis on her left buttock developed pain (6/10 in pain VAS), induration, and central ulceration of the lesion. Ultrasound examination showed hyperechoic structures with posterior acoustic shadow and positive Doppler signal (Fig. 2a, b); the lesions were clinically and ultrasonographically compatible with dystrophic CC. The lesion presented a torpid evolution after multiple medical treatments such as topical clobetasol, tacrolimus cream, methotrexate, methyl mycophenolate, prednisone, and hydroxychloroquine. Because of that, treatment with IL-STS was started. After 1 month, the ulcers had healed, and the patient did not develop new lesions. Two months after the initiation of IL-STS therapy, the patient reported important pain relief (2/10 in pain VAS). Under ultrasound examination, dermal and subcutaneous tissue calcifications looked more fragmented. After three monthly administrations of IL-STS, treatment was stopped because of improvement. Ultrasonographic images 4 months after the first IL-STS treatment showed smaller and more fragmented calcifications with minimally positive Doppler signal (Fig. 2c, d).
Fig. 2.
Cutaneous ultrasound image (18 MHz probe) of the lesion on her left buttock, before starting treatment with IL-STS (a). Several hyperechogenic images (yellow arrows) with posterior acoustic shadow can be observed, which tally with CC (a). Cutaneous ultrasound image (18 MHz probe) of the lesion on her left buttock, before starting treatment with IL-STS, which shows positive Doppler signal (b). Cutaneous ultrasound image (18 MHz probe) of the lesion on her left buttock, 4 months after the initiation of IL-STS therapy (c). Smaller and more fragmented calcifications (yellow arrows) can be observed (c). Cutaneous ultrasound image (18 MHz probe) of the lesion on her left buttock, 4 months after IL-STS therapy was initiated, showing lower intensity of colour Doppler signal (d)
Case 3
A 68-year-old woman was under follow-up by our department for a limited systemic sclerosis. She presented with redness, heat, swelling, pain (7/10 in pain VAS), and a whitish papule draining a serous liquid in her left elbow (Fig. 3a). Ultrasonographic exam showed hyperechogenic images in the subcutaneous tissue and positive grade 2 Doppler signal (Fig. 3c). Clinic and ultrasonographic examinations where suggestive of superinfected dystrophic CC. Antibiotic treatment was started with partial improvement of elbow pain and inflammation. Due to persisting pain and inflammation, treatment with monthly injections of IL-STS was started. After 1 month of therapy, the patient reported remarkable improvement in pain (3/10 in pain VAS). After 2 months of treatment, inflammatory signs had disappeared, but induration was still palpable and ultrasound imaging showed small calcifications and positive Doppler signal. After four monthly injections of IL-STS, pain had remitted completely (0/10 in pain VAS). Ultrasonographically, only some small calcifications were apparent, with negative Doppler signal (Fig. 3d). An asymptomatic self-healing ulceration developed on the injection site 24 h after the fourth injection (Fig. 3b).
Fig. 3.
Front-view clinical image before starting treatment with IL-STS, showing erythema and increased volume of the patient’s left elbow (a). Front-view clinical image 4 months after IL-STS therapy was initiated—after four injections of IL-STS, where a remarkable improvement of the inflammatory signs can be noticed (b). A central crust is observed, which was an adverse event of the IL-STS injection (b). Cutaneous ultrasound image (22 MHz probe) of her left elbow, before starting treatment with IL-STS, showing multiple hyperechoic images (yellow arrows) and positive Doppler signal (c). Cutaneous ultrasound image (22 MHz probe) of her left elbow, 4 months after IL-STS therapy was initiated, where only some small calcifications (yellow arrows) with negative Doppler signal were objectivated (d)
Case 4
A 74-year-old woman, who suffered from a skin burn on the anterior aspect of her left thigh during childhood and developed secondary dystrophic CC, was referred to our department because of lesion recurrence after two surgical resections. Physical examination revealed an indurated, erythematous, painful (7/10 in pain VAS), ulcerated plaque (Fig. 4a). Ultrasound images showed multiple linear hyperechoic images with posterior acoustic shadow and negative Doppler signal (Fig. 4c, d). Histopathologic examination of the surgical specimens confirmed the diagnostic of dystrophic CC. Treatment with IL-STS was initiated, with injections at months 0, 1, 2, 3, and 5. The patient reported improvement of the pain (3/10 in pain VAS) and inflammation after 1 month of therapy, and complete pain relief (0/10 in pain VAS) 2 months after the start of IL-STS. Five months after the start of therapy the patient remained asymptomatic, and only two points of calcification persisted clinically and ultrasonographically (Fig. 4b, e), enabling a new surgical resection.
Fig. 4.
Clinical image before therapy with IL-STS was initiated, when multiple calcifications where palpable in the anterior aspect of her left thigh (a). Clinical image 5 months after starting treatment with IL-STS, when only two palpable calcification points remained (b). Cutaneous ultrasound image (22 MHz probe) before starting treatment with IL-STS, where a linear hyperechoic image (yellow arrows) in the subcutaneous tissue with posterior acoustic shadow, and slightly positive Doppler signal could be objectivated (c, d). This image corresponds to one of the multiple calcifications she had (c, d). Cutaneous ultrasound image (18 MHz probe) after four administrations of IL-STS—5 months after IL-STS was started (e). At that moment, only the two calcifications (yellow arrows) that can be observed on this image remained (e). In addition, Doppler signal had become negative (e)
Case 5
A 54-year-old woman with undifferentiated connective tissue disease was being followed-up at our department. She presented a painful (8/10 in pain VAS) tender indurated subcutaneous lesion in her left forearm, where five years ago a dystrophic CC was excised. Ultrasound exam showed two linear hyperechoic lesions with posterior acoustic shadow, consistent with dystrophic CC (Fig. 5a). IL-STS therapy was started. Three months later the patient reported significant pain improvement (2/10 in pain VAS). A significant reduction in the size of calcifications was confirmed ultrasonographically (Fig. 5b). Due to the persistence of calcifications, an additional injection of IL-STS was administered 4 months later.
Fig. 5.
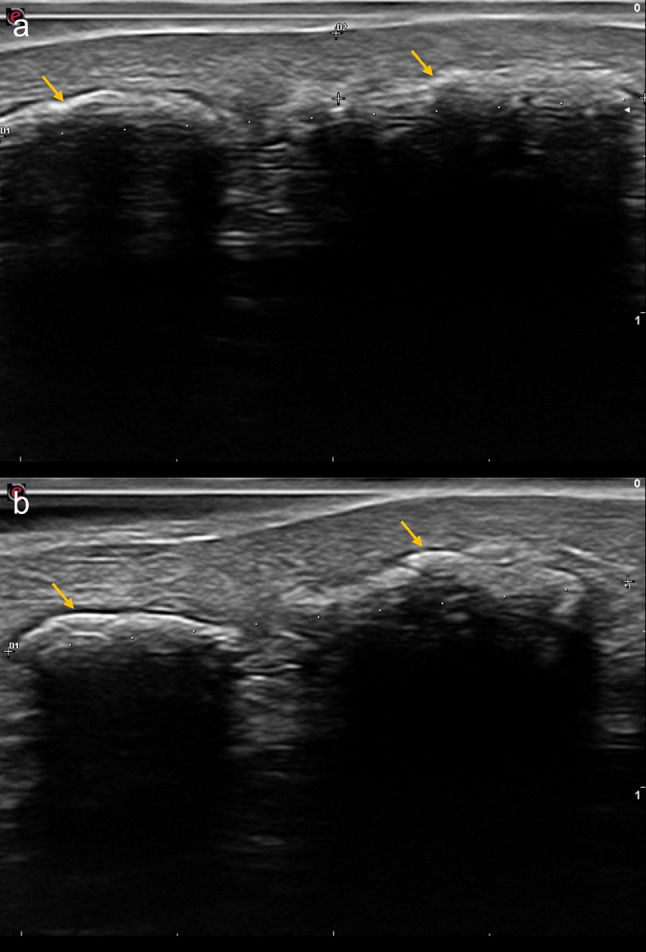
Cutaneous ultrasound image (18 MHz probe) before starting treatment with IL-STS showed two hyperechoic linear lesions (yellow arrows) with posterior acoustic shadow that measured 20.7 mm from the left side of fragment 1 to the contralateral side of fragment 2 (a). Cutaneous ultrasound image (18 MHz probe) on the day of the second IL-STS injection, 3 months after the first one (b). The size of the initial lesion had decreased, measuring 20 mm from the left side of fragment 1 (yellow arrow) to the contralateral side of fragment 2 (yellow arrow) (b)
Discussion
Ultrasound is a very useful technique for assessing the musculoskeletal system [18]. Cutaneous ultrasound examination is helpful for diagnosis of CC. These lesions are ultrasonographically characterized by hyperechogenic structures accompanied by a posterior acoustic shadow [5, 12]. In addition, ultrasound images help to determine CC thickness, length, and location or depth. However, to date, ultrasound morphologic characteristics of CC and its ultrasound treatment follow-up have been scarcely reported [5, 12, 19, 20].
Despite these promising capacities of cutaneous ultrasonography, histopathologic study remains the gold standard for diagnosis of CC. However, ultrasound can provide the key to diagnosis if histopathologic examination is inconclusive or cannot be performed [20, 21]. Remarkably, the efficiency of biopsy in highly necrotic CC lesions is low, and in locations such as the scrotum and penis biopsy is not recommended because of the risk of necrosis progression [20, 22]. Already in 2003, Popken et al. [21], and some years later Lorente-Luna et al. [20], stated that ultrasound is the imaging technique of choice for early diagnosis and monitoring of calcium deposits, since it has higher sensibility and specificity than X-ray studies. Furthermore, as in the presented cases, CC ultrasound can be used to guide the injection of STS, as in IL treatments for other disorders [23]. In addition, ultrasound may be helpful in evaluating treatment response as it assists to differentiate fibrosis from the persistence of calcifications [15]. Regarding treatment of CC with IL-STS, in a recently published retrospective analysis including 80 patients with CC treated with topical/intradermal/intravenous STS [24], all patients with CC 2 cm or less in size treated with IL- STS showed complete response to treatment and dissolution of calcium deposits. As regards our patients, one of them (case 1) had complete resolution of the CC, while all the others reported notable pain improvement as early as one to 2 months after the first IL-STS administration. Ultrasonographically, all of them showed a decrease in the size of calcifications that started 2–5 months after the beginning of treatment. The therapeutic mechanism of action of STS in CC is not fully understood, but is probably related to its calcium chelating, antioxidant, and vasodilator properties [4, 12, 13]. The main limitations of IL route of administration are injection pain and risk of local infection [2–4, 12, 13]. Case 3 in our series developed a self-healing ulceration 24 h after the fourth injection. Until now, there are no reports of systemic adverse events with IL-STS. In addition, this route can reach locations deeper than topical administration, with a lower risk of systemic adverse events than IV administration [2, 11, 13]. Some of the AEs associated with the IV administration are hypotension, nausea, vomiting, metabolic acidosis, QT interval prolongation, hypernatremia, volume overload, headache, and decreased bone density [2, 4, 9, 14]. Intralesional route of administration may be especially useful in patients who are not on dialysis and therefore do not have a venous access or require frequent medical visits, as well as in those with intolerance to the AEs (especially severe gastrointestinal symptoms) of IV-STS [2, 4, 14]. There is no standardized protocol for IL-STS treatment. The most frequently reported regimen is injection of variable volumes (0.1–15 mL) of STS at a concentration of 150–250 mg/mL, administered at intervals ranging from monthly to twice weekly [4, 10, 11, 13]. We used IL-STS 250 mg/mL—the most widely described concentration in the literature—and monthly intervals. In order to minimise pain at the time of injection some authors have mixed sodium thiosulfate with lidocaine [4, 14], and some others have administered lidocaine for local anaesthesia prior to the STS injection [2, 11, 13]. In our case series, IL-STS diminished pain and inflammation, split up calcium deposits, and decreased the number of calcifications. Furthermore, IL-STS may simplify subsequent surgical excision—as in our case 4—if required.
In conclusion, this case series highlights the usefulness of dermatologic ultrasound examination as a contribution to diagnosis and follow-up in patients with CC, as well as a guide for IL-STS administration. Moreover, it shows the therapeutic efficacy of IL-STS for different CC subtypes, with quick resolution of its associated disabling pain. Prospective, placebo-controlled trials with adequate numbers of patients are required to confirm the therapeutic benefit of IL-STS in CC and define the ideal dosing concentrations and injection intervals.
Declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Author contributions
All authors contributed to the study conception and design. Data collection and analysis was performed by Carla Tubau and Esther Roé-Crespo. The first draft of the manuscript was written by Carla Tubau. All authors revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Ethics approval
Institutional Review Board submission is waived at our Institution for retrospective studies based on anonimized clinical records.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1, 2, 3, 4, 5.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reiter N, El-Shabrawi L, Leinweber B, Berghold A, Aberer E. Calcinosis cutis: part I. Diagnostic pathway. J Am Acad Dermatol. 2011;65:1–12. doi: 10.1016/j.jaad.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 2.Goossens J, Courbebaisse M, Caudron E, et al. Efficacy of intralesional sodium thiosulfate injections for disabling tumoral calcinosis: two cases. Semin Arthritis Rheum. 2017;47:451–455. doi: 10.1016/j.semarthrit.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner-Nielsen J, Olesen AB. Treatment of skin calcifications with intra-lesional injection of sodium thiosulphate: a case series. Acta Derm Venereol. 2016;96:257–258. doi: 10.2340/00015555-2206. [DOI] [PubMed] [Google Scholar]
- 4.Strazzula L, Nigwekar SU, Steele D, Tsiaras W, Sise M, Bis S, Smith GP, Kroshinsky D. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946–949. doi: 10.1001/jamadermatol.2013.4565. [DOI] [PubMed] [Google Scholar]
- 5.Jiménez-Gallo D, Ossorio-García L, Linares-Barrios M. Calcinosis cutis and calciphylaxis. Actas Dermosifiliogr. 2015;106:785–794. doi: 10.1016/j.ad.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Reiter N, El-Shabrawi L, Leinweber B, Berghold A, Aberer E. Calcinosis cutis: part II. Treatment options. J Am Acad Dermatol. 2011;65:15–22. doi: 10.1016/j.jaad.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 7.Cicone JS, Petronis JB, Embert CD, Spector DA. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis Off J Natl Kidney Found. 2004;43:1104–1108. doi: 10.1053/j.ajkd.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Wolf EK, Smidt AC, Laumann AE. Topical sodium thiosulfate therapy for leg ulcers with dystrophic calcification. Arch Dermatol. 2008;144:1560–1562. doi: 10.1001/archderm.144.12.1560. [DOI] [PubMed] [Google Scholar]
- 9.García-García E, López-López R, Álvarez-Del-Vayo C, Bernabeu-Wittel J. Iatrogenic calcinosis cutis successfully treated with topical sodium thiosulfate. Pediatr Dermatol. 2017;34:356–358. doi: 10.1111/pde.13116. [DOI] [PubMed] [Google Scholar]
- 10.Smith GP. Intradermal sodium thiosulfate for exophytic calcinosis cutis of connective tissue disease. J Am Acad Dermatol. 2013;69:e146–147. doi: 10.1016/j.jaad.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Gunasekera NS, Maniar LEG, Lezcano C, Laga AC, Merola JF. Intralesional sodium thiosulfate treatment for calcinosis cutis in the setting of lupus panniculitis. JAMA Dermatol. 2017;153:944–945. doi: 10.1001/jamadermatol.2017.0966. [DOI] [PubMed] [Google Scholar]
- 12.Ossorio-García L, Jiménez-Gallo D, Arjona-Aguilera C, Linares-Barrios M. Calcifilaxis tratada con tiosulfato sódico intralesional. Actas Dermosifiliogr. 2016;107:359–362. doi: 10.1016/j.ad.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Isoherranen K, Bouchard L, Kluger N. Benefits of intralesional injections of sodium thiosulfate in the treatment of calciphylaxis. Int Wound J. 2017;14:955–959. doi: 10.1111/iwj.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuhaili B, Al-Talib K. Successful treatment of single infected calciphylaxis lesion with intralesional injection of sodium thiosulfate at high concentration. Wounds Compend Clin Res Pract. 2019;31:E54–E57. [PubMed] [Google Scholar]
- 15.López-Sundh AE, Quintana-Sancho A, Durán-Vian C, Reguero-DelCura L, Corrales-Martínez AF, Gómez-Fernández C, González-López MA. Clinical and ultrasound response to intralesional sodium thiosulfate for the treatment of calcinosis cutis in the setting of systemic sclerosis. A case-based review. Clin Rheumatol. 2020 doi: 10.1007/s10067-020-05523-4. [DOI] [PubMed] [Google Scholar]
- 16.Scorza A, Lupi G, Sciuto SA, Bini F, Marinozzi F (2015) A novel approach to a phantom based method for maximum depth of penetration measurement in diagnostic ultrasound: a preliminary study. In: 2015 IEEE Int. Symp. Med. Meas. Appl. MeMeA Proc., pp 369–74
- 17.Hernández C, del Boz J, de Troya M. ¿Es la ecografía cutánea de alta frecuencia una alternativa en el diagnóstico y manejo del carcinoma basocelular? Actas Dermosifiliogr. 2014;105:107–111. doi: 10.1016/j.ad.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Han D-S, Wu W-T, Hsu P-C, Chang H-C, Huang K-C, Chang K-V. Sarcopenia is associated with increased risks of rotator cuff tendon diseases among community-dwelling elders: a cross-sectional quantitative ultrasound study. Front Med. 2021;8:630009. doi: 10.3389/fmed.2021.630009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konno K, Ishida H, Morikawa P, Uno A, Niizawa M, Naganuma H, Masamune O, Niizawa M. Sonographic appearance of extensive subcutaneous calcification. J Clin Ultrasound JCU. 1992;20:415–418. doi: 10.1002/jcu.1870200612. [DOI] [PubMed] [Google Scholar]
- 20.Lorente-Luna M, Alfageme Roldán F, González Lois C. Ultrasound diagnosis of calcified skin deposits. Actas Dermosifiliogr. 2015;106:586–588. doi: 10.1016/j.ad.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Popken F, König DP, Tantow M, Rütt J, Kausch T, Peters KM. Possibility of sonographic early diagnosis of heterotopic ossifications after total hip-replacement. Unfallchirurg. 2003;106:28–31. doi: 10.1007/s00113-002-0461-0. [DOI] [PubMed] [Google Scholar]
- 22.Olaoye OA, Koratala A. Patient with ESRD with vascular calcifications and ischaemic complications. BMJ Case Rep. 2017 doi: 10.1136/bcr-2017-222674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu P-C, Chang K-V, Wu W-T, Wang J-C, Özçakar L. Effects of ultrasound-guided peritendinous and intrabursal corticosteroid injections on shoulder tendon elasticity: a post hoc analysis of a randomized controlled trial. Arch Phys Med Rehabil. 2021;102:905–913. doi: 10.1016/j.apmr.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Howard RM, Smith GP. Treatment of calcinosis cutis with sodium thiosulfate therapy. J Am Acad Dermatol. 2020;83:1518–1520. doi: 10.1016/j.jaad.2020.06.996. [DOI] [PubMed] [Google Scholar]






