Abstract
Degradation of nitriles by mixed biofilms of nitrile-hydrolyzing bacteria Alcaligenes faecalis 2 and Rhodococcus ruber gt 1 grown on basalt and carbon carriers, in a submerged packed-bed reactor was studied. It was shown the formation of a massive mixed biofilm of Al. faecalis 2 and R. ruber gt 1 and the effective removal of nitriles and products of their degradation from the reaction medium. After the accumulation of carboxylic acid and some of the unprocessed substrate, the system adapts to 600–1000 h of biofilter operation, which is expressed in a decrease in the content of substrate and reaction products in the medium. The rate of acetonitrile and acrylonitrile utilization was 0.072–0.086 and 0.039–0.215 g/h, respectively, and acrylonitrile utilization with maximum rate was realized by a mixed biofilm on carbon fibers. Biofilms grown on mixed fibers in a "sandwich"-type reactor had the best characteristics for the transformation of aceto- and acrylonitrile (removal capacity of 99.6–99.9%, nitrile utilization rate of 0.080–0.095 g/h). Biofilms grown on basalt fiber with a diameter of 4–12 μm are also well suited for the degradation of acetonitrile (removal capacity of 100%, nitrile utilization rate of 0.086 g/h). The results of metagenomic analysis showed the resistance of Al. faecalis 2 and R. ruber gt 1 mixed biofilms against leaching from a biofilter and to competitive growth in an open system, indicating the advantages of biofilms over homogeneous biomass for wastewater treatment from nitrile compounds. Biofilms of two species of nitrile hydrolyzing bacteria on basalt and carbon fibers effectively purify water from nitriles in a submerged packed-bed reactor.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-022-01030-z.
Keywords: Biofilter, Organocyanides, Acrylonitrile, Acetonitrile, Wastewater treatment
Introduction
Nitriles or organocyanides, are organic compounds, containing one or more cyano groups bound to an organic radical. They are natural widespread compounds of wildlife that are synthesized by plants as the precursors of hormones, reserve substances, etc. [1]. Typical examples of synthetic nitriles include aceto- and acrylonitrile that are widely used as solvents and extracts, or used as the components in pharmaceuticals, plastics, synthetic rubbers, herbicides, and pesticides [2–8]. Large production volumes of aceto- and acrylonitrile contribute to the presence of these compounds in wastewater. It is widely known that aceto- and acrylonitrile are highly toxic and can cause nausea, vomiting, headache, lethargy, respiratory depression, impaired judgment, tachycardia, coma, convulsions and possibly death [2, 3, 5]. Moreover, acrylonitrile has been classified as a Group B1 probable human carcinogen. To date, therefore, the issue of wastewater treatment from aceto- and acrylonitrile is highly topical.
Currently, a number of microbial strains are known to carry out one-stage nitrile hydrolysis by nitrilase (EC 3.5.5.1) to carboxylic acids or two-stage nitrile hydrolysis by nitrile hydratase (EC 4.2.1.84) to amides and by amidase (EC 3.5.1.4) to carboxylic acids. Genes encoding nitrile hydratase and amidase are usually expressed within the same operon [9, 10]. For biotechnological purposes nitrile-hydrolyzing bacteria are usually obtained by screening and selection of bacteria for the elevated nitrile hydratase or amidase activity. Therefore, mixed suspension cultures of such bacteria are used to convert nitrile to the carboxylic acid without contaminating the system with residual amide [11, 12]. Nitrile-hydrolyzing bacteria have the ability to form the biofilms, which are potentially suitable for the use as biocatalysts in the processes of local treatment of effluents, liquid and solid industrial wastes.
It has been shown that microbial cell-based biofilters can be used to degrade nitrile compounds into harmless intermediates, or ultimately, carbon dioxide and water. Chen et al. showed the efficiency of degradation of up to 150 mM propionitrile in batch and continuous-flow bioreactor based on immobilized Klebsiella oxytoca cells [13]. The recombinant bacterium B. subtilis N4/pHTnha-ami with organonitrile-degrading and biofilm-forming capability for acetonitrile, acrylonitrile, cis- and trans-crotononitrile removal was used in a moving bed biofilm reactor [14]. The process of organic cyanides degradation by biofilm of Rhodococcus rhodochrous BX2 with nitrile-degrading activity and Bacillus mojavensis M1 with biofilm-forming capacity in the lab-scale fluidized bed reactors was also developed [15]. A membrane-aerated biofilm reactor based on an adapted activated sludge consortium was developed. It was capable to completely removing acetonitrile at the removal capacity of up to 21.1 g/ m2 day and generated negligible amount of suspended sludge in the effluent [16]. However, increasingly stringent requirements for the treatment of industrial effluents obligate the development of new, more efficient decisions in the field of treatment. In this connection, it is relevant to study the process of nitrile degradation in biofiltration systems based on mixed biofilms of nitrile hydrolyzing bacteria grown on different carriers.
The objects of the study were strains of Rhodococcus ruber gt 1 and Alcaligenes faecalis 2 with high nitrile hydratase and amidase activity, respectively. We have proposed biofilms of nitrile hydrolyzing bacteria as an alternative method of biological treatment of nitrile wastewater. In this research the process of transformation of aceto- and acrylonitrile and utilization of transformation’s products (acrylamide, acetamide, acrylic acid, and acetic acid), as well as the time of continuous operation of the biofilter, depending on the different carriers have been studied.
Materials and Methods
Bacterial Strains and Cultivation
R. ruber gt1 (IEGM 612, Regional profiled collection of alkanotrophic microorganisms, acronym IEGM, www.iegm.ru/iegmcol/index.html), which possesses efficient nitrile-degrading capacity, and Al. faecalis 2, which has efficient amide-degrading capacity were obtained from anthropogenic contaminated soil of the Perm region and active sludge of municipal sewage treatment plant, respectively [17, 18]. Bacterial cultures were incubated at 30 °C in 50-mL flasks with shaking at 120 rpm on mineral salt medium (MSM) with the following composition (g/L): КH2PO4 1.0, К2HPO4∙ 3H2O 3.7, NaCl 0.5, MgSO4∙ 7H2O 0.5, FeSO4∙ 7H2O 0.005, CoCl2∙6H2O 0.01; pH 7.2–7.4. Glucose at a concentration of 0.1% was used as the carbon source for R. ruber gt 1; 0.01 М NH4Cl was used as the nitrogen source. Acetamide (0.1 M) served as the carbon and nitrogen source for Al. faecalis 2. To cultivate the mixed culture and the binary biofilm of Al. faecalis 2 and R. ruber gt 1, the mineral medium was supplemented with 0.1% glucose, 0.1 М acetamide, and 0.01 М NH4Cl.
Biofilm Growth
Biofilms of nitrile-hydrolyzing bacteria were incubated in 300 mL flasks containing 1 g of carbon fiber Carbopon activated ("SvetlogorskKhimvolokno", Belarus) or 7 g of basalt fiber ("Basalt Fiber", Russia) on mineral salt medium with the additives prescribed in paragraph 2.1 for 7 days at 30 °C. After that, the support with the biofilms grown on it was washed with potassium phosphate buffer and reactor column was packed with it.
Submerged Packed-Bed Reactor Experiments
Each experiment on the degradation of acrylonitrile and acetonitrile by biofilms of nitrile-hydrolyzing bacteria grown on carbon and mineral carriers was implemented in a laboratory submerged packed-bed reactor at a flow rate of 60 mL/min. Reactor is composed of polypropylene column with total volume of 40.84 mL and a 250-mL flask containing 200 mL of potassium phosphate buffer (Fig. 1). A total volume of circulating liquid was supplied through a reactor with a peristaltic pump LS 301 (Laboratory Equipment and Devices CJSC, St. Petersburg, Russia). Biofilms of nitrile-hydrolyzing bacteria on carriers were packed into the continuous-flow biofilter. Mineral fibers with a diameter of 4–12 μm and 22 μm in an amount of 7 g and carbon fiber in an amount of 1 g were used as carriers. In the case of using a biofilter based on two different carriers, mineral fibers with a diameter of 4–12 μm in an amount of 6.5 g and carbon fiber in an amount of 0.5 g laid in layers were used ("sandwich" type). Aceto- and acrylonitrile (95%) was daily added in the amount of 4 ml/day (to 15.7 g/L and 16.2 g/L respectively), and samples were taken (daily one hour after the substrate was added for 6 h), 1 ml each for analysis of residual amounts in the medium of the substrate (aceto- and acrylonitrile) and reaction products (acetamide and acetic acid or acrylamide and acrylic acid) throughout the experiment.
Fig. 1.
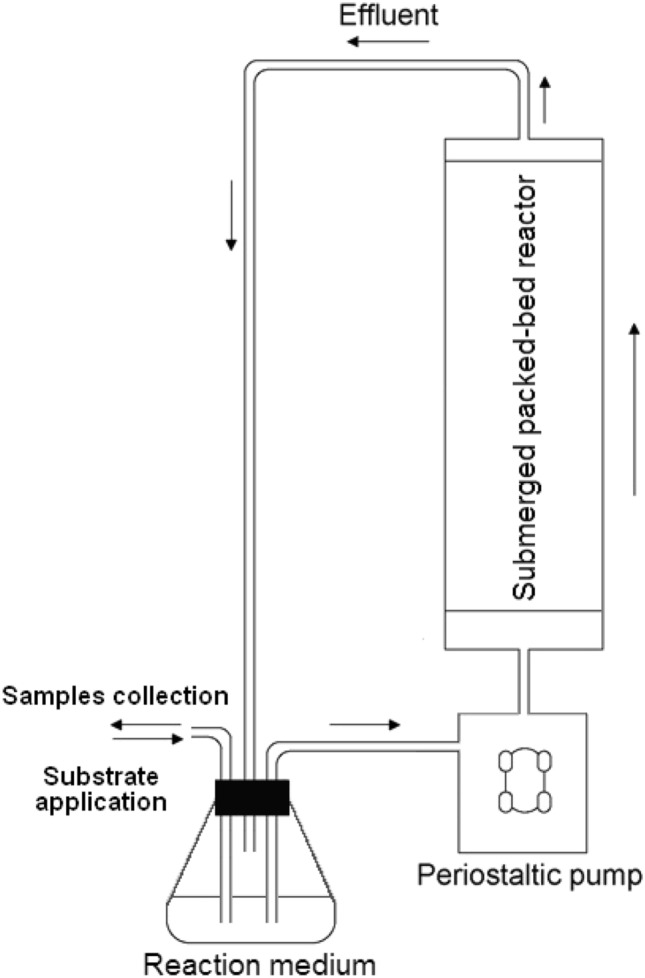
Schematic diagram of the submerged packed-bed reactor
Analysis of Residual Nitriles and Degradation Products
The concentrations of acetic acid and acetamide that resulted from the transformation of acetonitrile by cells, as well as acetonitrile per se were determined by gas chromatography on a GC-2014 (Shimadzu, Japan) chromatograph with a 2-m Polysorb-1 column. Nitrogen was used as the carrier gas; the flow rate was 35 ml/min at 180 °C. Analysis of acrylic acid, acrylamide and acrylonitrile was carried out at 190 °C.
Results and Discussion
The degradation of aceto- and acrylonitrile was studied separately for biofilms of nitrile-hydrolyzing bacteria grown on basalt fibers with a diameter of 22 μm, basalt fibers with a diameter of 4–12 μm, and carbon fibers of Carbopon activated. The experiments also included the use of a mixed biocatalyst ("sandwich" type biofilter): basalt fibers with a diameter of 4–12 μm was used as a carrier for Al. faecalis 2 biofilm, carbon fiber of Carbopon activated was used as a carrier for R. ruber gt 1. At the beginning of the work, the biofilter was filled with potassium phosphate buffer, and then 4 ml of aceto- or acrylonitrile were added. Samples for determining the content of the substrate and reaction products were taken during the day and through 24 h after the introduction of aceto- or acrylonitrile. The next day, the procedure was repeated: 4 ml of the substrate were added again and the samples were taken.
Biofilms on Basalt Fibers with a Diameter of 22 μm
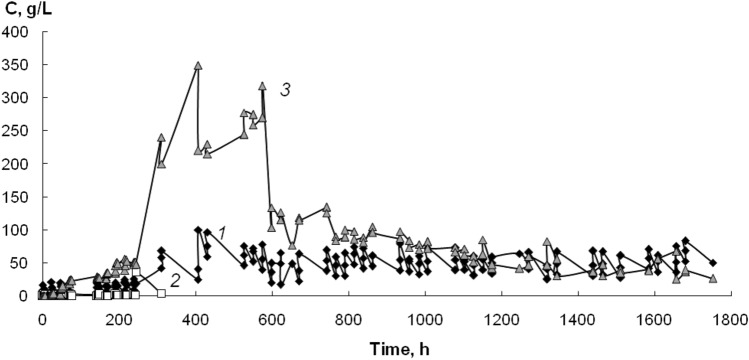
Throughout the experiment, R. ruber gt 1 cells transformed most of acetonitrile into acetamide. The accumulation of acetonitrile in the reaction medium was averaged to 25 g/L after 300 h of biofilter operation (Fig. 2). The accumulation of acetamide in small amounts was observed up to 250 h of operation, after which the concentration of amide in the medium decreased to a level below the detection limit, since Al. faecalis 2 cells began to utilize the acetamide. Along with this, acetic acid was accumulated in the medium to the highest concentration of 345 g/L. By 600 h of operation of the biofilter, an adaptation of the system took place, which was expressed in a decrease by about 3 times in the content of acetic acid in the reaction medium. Cells in the biofilm grew and multiplied, and by 600 h of operation, a rather massive biofilm was grown, which began to actively consume not only the substrate, but also the reaction products and stabilized the entire system.
Fig. 2.
Degradation of acetonitrile by mixed biofilm on basalt fibers with a diameter of 22 μm in a submerged packed-bed reactor. C—concentration of acetonitrile (1), acetamide (2) and acetic acid (3)
Thus, it was shown that high degradation rate of acetonitrile is maintained for a long time. This is due both to the high catalytic activity of R. ruber gt 1 and to the strength of the grown biofilm, which is formed mainly by cells of Al. faecalis 2.
A micrograph of a basalt fiber taken after working off a biofilter shows a massive biofilm up to 5 µm in diameter, which covers most of the surface of the fiber (Fig. S1). Previously, it was shown that Al. faecalis 2 has a high biofilm-forming ability due to the formation of a massive polymer matrix [19].
In a similar experiment for the transformation of acrylonitrile by biofilms grown on a 22 μm diameter basalt fiber, no reaction products (acrylamide and acrylic acid) were detected in the biofilter system (Fig. S2). This is due to the rapid consumption of the resulting acrylamide by Al. faecalis 2 cells, which predominate in the biofilm.
Al. faecalis 2 forms a massive biofilm on almost any carriers because it has an intermediate value of hydrophobicity / hydrophilicity of the cell wall surface. In the photograph of the biofilm of Al. faecalis 2 on a basalt fibers with a diameter of 22 μm, one can observe a massive polymer matrix, which forms a biofilm between the carrier fibers (Fig. S3). Metagenomic analysis of the biocatalyst sample used in the biofilter showed the presence of 81.69% (46,502 reads) Al. faecalis 2 and of 0.36% (206 reads) R. ruber gt 1 (Fig. S4). However, this did not significantly affect the overall work of the biofilter, since R. ruber gt 1 is a superproducer strain, and even a small numbers of cells of this strain were enough to degrade the introduced amount of nitrile.
Biofilms on Basalt Fibers with a Diameter of 4–12 μm
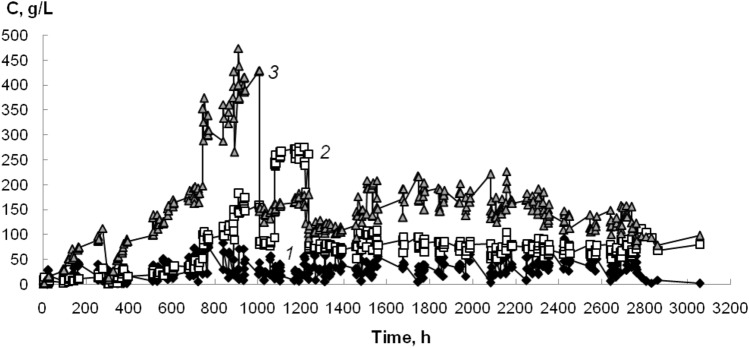
The degradation of acetonitrile with biofilms of nitrile-hydrolyzing bacteria on basalt fibers with a diameter of 4–12 μm was carried out (Fig. 3). Throughout the experiment (2527 h), a high rate of transformation of daily acetonitrile into acetamide was maintained. Moreover, the acetamide was not accumulated in the medium (the concentration of acetamide was 3 g/L on average throughout the experiment), but was immediately transformed into acetic acid.
Fig. 3.
Degradation of acetonitrile by mixed biofilm on basalt fibers with a diameter of 4–12 μm in a submerged packed-bed reactor. C—concentration of acetonitrile (1), acetamide (2) and acetic acid (3)
Also, during the experiment, a hasty growth was observed not only in the content of acetic acid in the reaction medium, as in the previous experiment, but also in acetonitrile to the concentrations of 651 g/L and 639 g/L, respectively. For 200 h of biofilter operation, the biocatalyst coped with a hasty growth in nitrile and acid concentrations, as in the previous experiment. These examples of adaptation prove that mixed biofilms are a self-sustaining system.
Degradation of acrylonitrile by biofilms on this carrier was different from the transformation of acetonitrile (Fig. S5). The biofilter worked for 968 h and all this time kept the concentration of acrylonitrile at a fairly low level (from 0 to 35 g/L, the average value of 13.7 g/L).
The transformation of acrylonitrile by a mixed biofilm on a 4–12 µm fibers was more efficient than on a 22 µm fibers, which can be associated with a larger surface area of the fibers with an equal volume of the bioreactor, therefore, with a larger biomass of biofilm (Fig. S6).
Biofilms on Carbon Fibers of Carbopon Activated
In the next series of experiments, a fundamentally different carrier was used, namely, the carbon fibers of Carbopon activated. It can be seen from microphotographs that R. ruber gt 1 forms a fairly good monolayer biofilm on a carbon fibers, in which individual cells are clearly observable (Fig. S7).
Metagenomic analysis of mixed biofilm samples taken after the operation of the biofilter showed the presence of 81.69% Al. faecalis 2 and 0.36% R. ruber gt 1 on basalt fibers with a diameter of 22 μm, 40.90% R. ruber gt 1 and 43.64% Al. faecalis 2 on basalt fibers with a diameter 4–12 µm, and 73.71% R. ruber gt 1 and 25.67% Al. faecalis 2 on carbon fibers of Carbopon activated. Carbon fibers has a lager degree of surface hydrophobicity than basalt fibers, and is therefore more preferable for the formation of biofilms by R. ruber gt 1 having a hydrophobic cell wall. R. ruber gt 1 synthesizes a less massive polymer matrix than Al. faecalis 2.
Despite the fact that in relation to Al. faecalis 2, the amount of R. ruber gt 1 cells on basalt fiber with a diameter of 22 μm was very small, this did not significantly affect the overall performance of the biofilter. Even a small amount of R. ruber gt 1 cells was sufficient to degrade the injected amount of nitrile, since R. ruber gt 1 has an average enzymatic activity of 240 µmol/mg cells per min compared to Al. faecalis 2, which has an average enzymatic activity of 1.5 µmol/mg cells per min. The micrographs show the presence of a R. ruber gt1 biofilm on a basalt fibers with a diameter of 22 μm (Fig. S8).
Data analysis showed the high efficiency of using a biocatalyst based on biofilms grown on carbon fibers in the process of acetonitrile transformation. The concentration of both nitrile and amide throughout the experiment does not exceed the amount of 60 g/L (Fig. S9). Acetic acid accumulates in the medium up to a maximum concentration of 326 g/L by 500–600 h of biofilter operation, as well as when using basalt fiber with a diameter of 22 μm as a carrier for biofilms.
The biocatalyst based on a mixed biofilm grown on a carbon support also degraded acrylonitrile at a fairly high rate. Acrylonitrile for a long time (1010 h) was almost completely transformed by bacterial cells into amide (Fig. S10). The average substrate content in the reaction medium was 11.4 g/L, which is by 17% and 71% lesser than in the experiments using basalt fibers with a diameter of 4–12 μm and 22 μm, respectively, as a carrier. This may be due to the high adsorption capacity of the carbon fibers. A certain amount of acrylonitrile is adsorbed on the biofilm-free surface of the carrier and is gradually degraded by bacteria, without accumulating in the reaction medium to high concentrations. The content of reaction products is also maintained at a fairly low level.
Biofilms on Basalt and Carbon Fibers in "Sandwich" Type Biofilter
The "sandwich" type packing of the biofilter consisted of layers of 4–12 µm basalt fibers with Al. faecalis 2 biofilms and carbon fibers layers with R. ruber gt 1 biofilms. Degradation of aceto- and acrylonitrile was carried out in such a biofilter. Cells of Al. faecalis 2 and R. ruber gt1 have different values of hydrophobicity of the surface and therefore varying adhesive ability to different carriers. Al. faecalis 2 better forms a biofilm on mineral carriers, whereas R. ruber gt 1 – on carbon carriers.
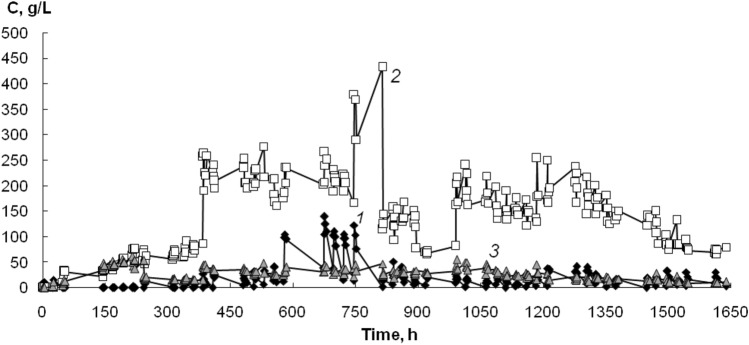
It has been shown that the concentration of non-utilized acetamide in the medium was 78 g/L on average, which significantly exceeded that in the biofilter with basalt fiber as a biofilm carrier (Fig. 4). In the experiment using a mixed carrier, the acetic acid being accumulated to an amount of 325 g/L. Then concentration of acetic acid decreased gradually, but remained at a high level. However, a relatively high concentration of the acetic acid at the exit of the biofilter will not pose a strong threat for environment, since the acetic acid is a substrate for microorganisms and it will be utilized by the native microbiota of the water reservoir.
Fig. 4.
Degradation of acetonitrile (1) by mixed biofilm on basalt and carbon fibers in "sandwich" type biofilter. C—concentration of acetonitrile (1), acetamide (2) and acetic acid (3)
The degradation of acrylonitrile in a biofilter based on mixed carrier has also been effective throughout the life of the biofilter (Fig. 5). In a "sandwich" type biofilter, acrylamide was accumulated in the medium during the transformation of acrylonitrile. This fact could be due to two reasons. First, the biofilm biomass of Al. faecalis 2 decreased compared to the experiments where other carriers were used, and this number of cells is not enough to transform the entire volume of acrylamide produced by R. ruber gt 1. Secondly, R. ruber gt 1 degrades acrylonitrile no worse than acetonitrile but acrylamide is a more difficult to consume substrate for Al. faecalis 2 than acetamide [20]. Therefore, Al. faecalis 2 cells immediately use the acrylic acid, just transformed from acrylamide, for further growth, and do not continue to transform the amide remaining in the medium.
Fig. 5.
Degradation of acrylonitrile by mixed biofilm on basalt and carbon fibers in "sandwich" type biofilter. C—concentration of acrylonitrile (1), acrylamide (2) and acrylic acid (3)
Removal capacities of nitriles by biofilms in a reactor with different type of packed bed was evaluated. All of the reactors studied were effective in removing aceto- and acrylonitrile from water. A common feature of the functioning of these reactors was the presence of an adaptation period, which was expressed in the accumulation of nitrile, amide or acid in the medium. After such a period, the system went into a state of balanced consumption of a toxic substrate applied daily. It was shown that acrylonitrile was most efficiently utilized in a reactor with an activated Carbopone with a mixed biofilm (Table 1). This is due to the fact that the biomass of R. ruber gt1 predominated in this type of biofilm due to the better adhesion of hydrophobic rhodococcal cells with high nitrile hydratase activity to a hydrophobic carrier. In turn, the least potency of utilization of acrylonitrile was observed in a reactor with basalt fibers with a diameter of 22 μm, which is associated with poorer adhesion of rhodococcal cells to hydrophilic basalt fibers. However, the longest operating time was noted for a "sandwich" type reactor, which indicates that this type of packed bed is promising for the treatment of toxic nitrile effluents.
Table 1.
Removal capacity of nitriles in submerged packed bed biofilm reactor
| Packed bed | Substrate | Time of work, h | Total amount of utilized nitrile, g | Nitrile utilization rate, g/h | Removal capacity, % |
|---|---|---|---|---|---|
| Basalt fibers with a diameter of 22 μm | Acetonitrile | 1752 | 138 | 0.079 | 92.8 |
| Acrylonitrile | 1234 | 48 | 0.039 | 54.0 | |
| Basalt fibers with a diameter of 4–12 μm | Acetonitrile | 2527 | 217 | 0.086 | 100 |
| Acrylonitrile | 968 | 66 | 0.068 | 94.6 | |
| Carbon fibers of Carbopon activated | Acetonitrile | 652 | 47 | 0.072 | 89.9 |
| Acrylonitrile | 1010 | 81 | 0.080 | 99.8 | |
| Basalt and carbon fibers in "sandwich" type | Acetonitrile | 2955 | 236 | 0.080 | 99.9 |
| Acrylonitrile | 1639 | 156 | 0.095 | 99.6 |
On average, a biofilter based on biofilms of a mixed culture of nitrile-hydrolyzing bacteria can operate in a normal mode without reducing the catalytic activity for 2000–3000 h. Metagenomic analysis data also revealed the resistance of mixed Al. faecalis 2 and R. ruber gt 1 biofilms against leaching from a biofilter and to competitive growth in an open system. In the submerged packed-bed biofilm reactor developed by us, high concentrations of aceto- and acrylonitrile (up to 15.7 g/L/day and 16.2 g/L/day) were utilized by nitrile hydrolyzing bacteria. A much lower removal capacity of packed-bed reactor was reported by Manolov et al. The removal capacity of the reactor filled with foamed glass beads with biofilm dropped when the acetonitrile load was increased to 3.0 g/L/day [21]. Li et al. reported biodegradation of acetonitrile by adapted biofilm in a membrane-aerated biofilm reactor. Above 550 mg/l in influent acetonitrile, biodegradation rates showed decline [16]. Biofilms formed by Bacillus subtilis N4-pHT01-nit almost completely degraded the initial concentration of acetonitrile (800 mg/L) within 24 h in a moving bed biofilm reactor [22]. To date, several biological methods have been developed to treat the wastewater containing nitriles and cyanides, mainly via using the activated sludge and suspended biomass [23–25]. The proposed solution differs from the existing ones in that the wastewater is purified from nitriles in a submerged packed-bed reactor with basalt or activated carbon fibers whereon a biofilm of nitrile-hydrolyzing bacteria is grown. We used a mixed culture of two nitrile-hydrolyzing strains of Al. faecalis 2 and R. ruber gt 1. Both of these strains are capable to biofilm formation. The fibrous structure of the carriers we use is favorable for the formation of a biofilm. R. ruber gt 1 with high nitrile hydratase activity transformed nitriles into the appropriate amides, while Al. faecalis 2 with pronounced amidase activity transformed amides into the corresponding carboxylic acids and utilized them during the metabolism. This leads to the complete mineralization of high nitrile concentration, and the biofilter can be at work for a long time without replacing the packed-bed.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The work was carried out within the framework of the state assignment on the topic " Search and selection of new promising microorganisms for the purposes of biotechnology. Creation of immunochemical diagnostic systems ", registration number R&D 122010800029-1.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Debabov VG, Yanenko AS. Biocatalytic hydrolysis of nitriles. Obz Zh Khim. 2011;1(4):376–394. doi: 10.1134/S2079978011030010. [DOI] [Google Scholar]
- 2.Achanzar WE, Mangipudy RS. Acrylonitrile. In: Wexler P, editor. Encyclopedia of toxicology. 3. London: Academic Press; 2014. pp. 76–78. [Google Scholar]
- 3.Caito S, Costa LG, Rongzhu L, Aschner M. Acrylonitrile. In: Aminoff MJ, Daroff RB, editors. Encyclopedia of the neurological sciences. 2. London: Academic Press; 2014. pp. 33–35. [Google Scholar]
- 4.Liu Q, Fang B, Bai X, Liu Y, Wu Y, Xu G, Guo C. Direct synthesis of nitriles from cleavage of C=C double bond with nitrite as the nitrogen source and oxidant. Tetrahedron lett. 2016;57(24):2620–2623. doi: 10.1016/j.tetlet.2016.04.108. [DOI] [Google Scholar]
- 5.Robles H. Acetonitrile. In: Wexler P, editor. Encyclopedia of toxicology. 3. London: Academic Press; 2014. pp. 40–42. [Google Scholar]
- 6.Zhai Y, St-Pierre J. Acetonitrile contamination in the cathode of proton exchange membrane fuel cells and cell performance recovery. Appl Energy. 2019;242:239–247. doi: 10.1016/j.apenergy.2019.03.086. [DOI] [Google Scholar]
- 7.Zhan W, Tong M, Ji L, Zhang H, Ge Z, Wang X, Li R. Continuous-flow synthesis of nitriles from aldehydes via Schmidt reaction. Chinese Chem Lett. 2019;30(5):973–976. doi: 10.1016/j.cclet.2019.01.006. [DOI] [Google Scholar]
- 8.Zheng L, Pan L, Miao J, Lin Y, Wu J. Application of a series of biomarkers in Scallop Chlamys farreri to assess the toxic effects after exposure to a priority hazardous and noxious substance (HNS) – Acrylonitrile. Environ Toxicol Pharm. 2018;64:122–130. doi: 10.1016/j.etap.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Balomajumber Ch, Agarwal VK. Enzymatic mechanism and biochemistry for cyanide degradation: a review. J Hazard Mater. 2010;176:1–13. doi: 10.1016/j.jhazmat.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 10.Prasad S, Bhalla TC. Nitrile hydratases (NHases): At the interface of academia and industry. Biotechnol Adv. 2010;28:725–741. doi: 10.1016/j.biotechadv.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Kohyama E, Yoshimura A, Aoshima D, Yoshida T, Kawamoto H, Nagasawa T. Convenient treatment of acetonitrile-containing wastes using the tandem combination of nitrile hydratase and amidase-producing microorganisms. Appl Microbiol Biotechnol. 2006;72:600–606. doi: 10.1007/s00253-005-0298-x. [DOI] [PubMed] [Google Scholar]
- 12.Kohyama E, Dohi M, Yoshimura A, Yoshida T, Nagasawa T. Remaining acetamide in acetonitrile degradation using nitrile hydratase- and amidase-producing microorganisms. Appl Microbiol Biotechnol. 2007;74:829–835. doi: 10.1007/s00253-006-0738-2. [DOI] [PubMed] [Google Scholar]
- 13.Chen CY, Chen SC, Fingas M, Kao CM. Biodegradation of propionitrile by Klebsiella oxytoca immobilized in alginate and cellulose triacetate gel. J Hazard Mater. 2010;177:856–863. doi: 10.1016/j.jhazmat.2009.12.112. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Sun Y, Yue Z, Huang M, Wang J, Chen X, An X, Zang H, Li D, Hou N. Combination of a recombinant bacterium with organonitrile-degrading and biofilm-forming capability and a positively charged carrier for organonitriles removal. J Hazard Mater. 2018;353:372–380. doi: 10.1016/j.jhazmat.2018.03.058. [DOI] [PubMed] [Google Scholar]
- 15.An X, Cheng Y, Huang M, Sun Y, Wang H, Chen X, Wang J, Li D, Li C. Treating organic cyanide-containing groundwater by immobilization of a nitrile-degrading bacterium with a biofilm-forming bacterium using fluidized bed reactors. Environ Pollut. 2018;237:908–916. doi: 10.1016/j.envpol.2018.01.087. [DOI] [PubMed] [Google Scholar]
- 16.Li T, Bai R, Ohandja D-G, Liu J. Biodegradation of acetonitrile by adapted biofilm in a membrane-aerated biofilm reactor. Biodegradation. 2009;20(4):569–580. doi: 10.1007/s10532-008-9246-7. [DOI] [PubMed] [Google Scholar]
- 17.Demakov VA, Vasil’ev DM, Maksimova YG, Pavlova YA, Ovechkina GV, Maksimov AY. Activated sludge bacteria transforming cyanopyridines and amides of pyridinecarboxylic acids. Microbiol. 2015;84:433–441. doi: 10.1134/S0026261715030030. [DOI] [PubMed] [Google Scholar]
- 18.Maksimov AY, Kuznetsova MV, Ovechkina GV, Kozlov SV, Maksimova YG, Demakov VA. Effects of nitriles and amides on the growth and nitrile hydratase activity of the Rhodococcus sp. strain gt1. Appl Biochem Microbiol. 2003;39(1):55–59. doi: 10.1023/A:1021798010261. [DOI] [PubMed] [Google Scholar]
- 19.Zorina AS, Maksimova YG, Demakov VA. Biofilm formation by monocultures and mixed cultures of Alcaligenes faecalis 2 and Rhodococcus ruber gt 1. Microbiol. 2019;88(2):164–171. doi: 10.1134/S0026261719020140. [DOI] [Google Scholar]
- 20.Maksimova YG, Vasil’ev DM, Zorina AS, Ovechkina GV, Maksimov AY. Acrylamide and acrylic acid biodegradation by Alcaligenes faecalis 2 planktonic cells and biofilms. Appl Biochem Microbiol. 2018;54(2):173–178. doi: 10.1134/S0003683818020084. [DOI] [Google Scholar]
- 21.Manolov T, Håkansson K, Benoit G. Continuous acetonitrile degradation in a packed-bed bioreactor. Appl Microbiol Biotechnol. 2005;66:567–574. doi: 10.1007/s00253-004-1744-x. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Yue Z, Feng F, Xi C, Zang H, An X, Liu K. A novel strategy for acetonitrile wastewater treatment by using a recombinant bacterium with biofilm-forming and nitrile-degrading capability. Chemosphere. 2016;161:224–232. doi: 10.1016/j.chemosphere.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Dash RR, Gaur A, Balomajumder C. Cyanide in industrial wastewaters and its removal: a review on biotreatment. J Hazard Mater. 2009;163:1–11. doi: 10.1016/j.jhazmat.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 24.An X, Cheng Y, Miao L, Chen X, Zang H, Li C. Characterization and genome functional analysis of an efficient nitrile-degrading bacterium, Rhodococcus rhodochrous BX2, to lay the foundation for potential bioaugmentation for remediation of nitrile-contaminated environments. J Hazard Mater. 2020;389:121906. doi: 10.1016/j.jhazmat.2019.121906. [DOI] [PubMed] [Google Scholar]
- 25.Alvillo-Rivera A, Garrido-Hoyos S, Buitrón G, Thangarasu-Sarasvathi P, Rosano-Ortega G. Biological treatment for the degradation of cyanide: a review. J Mater Res Technol. 2021;12:1418–1433. doi: 10.1016/j.jmrt.2021.03.030. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.