Abstract
Introduction
Riluzole, a benzothiazole sodium channel blocker is acknowledged as a neuroprotective agent in spinal cord injury (SCI). Most of this evidence is based on pre-clinical studies and its effectiveness in clinical setting is undetermined, heretofore.
Methods
A prospective, randomised-controlled study was conducted between April 2019 and March 2020 at a tertiary-level centre. Patients aged 18–65 years with sub-axial cervical spine injury, who presented within 72 h of injury with incomplete neuro-deficit, were included. They were randomised into groups A (riluzole was administered) and B (no adjuvants). All patients were followed up at 6 weeks/3/6/12 months, and clinical [ASIA motor/sensory scores/grade, SCIM3, and NRS (neuropathic pain)] and radiological evaluation was performed.
Results
Twenty-three and 20 patients were included in groups A and B. Two in group A were females, while others were males (p = 0.49). Mean age in groups A and B was 47.7 ± 14.8 and 51.2 ± 14.1 years (p = 0.44). Five patients died prior to 6th-week follow-up. Among the others, there was significant improvement in all neurological parameters in both groups (post-injury vs 1-year; motor score: p < 0.001, sensory score: p < 0.001, SCIM3: p < 0.001, NRS: p < 0.001). In both groups, initial significant improvement was noticed even at the 6th-week follow-up, which further continued until the end of 1 year. There was no statistically significant difference between groups A and B with respect to these neurological parameters (motor: p = 0.15, sensory: p = 0.39, SCIM3: p = 0.68, NRS: p = 0.06).
Conclusion
Administration of riluzole did not significantly improve neurological outcome/neuropathic pain in our cohort. Nevertheless, both our groups demonstrated an overall improvement in neurological outcome at 1 year, as compared with immediate post-injury status.
Keywords: Traumatic cervical spine injury, Riluzole, Neurological deficit, Neurological outcome, Benzothiazole molecule
Introduction
Over the past decade, in the context of acute spinal cord injury (SCI) management, the phrase “time is spine” has been gaining immense popularity [1]. In other words, with the background of the far-reaching and devastating aftermaths of traumatic SCIs; the significance of prompt interventions during the “early hours” in mitigating the eventual neurological impairments has been increasingly acknowledged [1–4]. In this context, the administration of supplemental neuro-protective drugs to curtail the secondary injury cascade has been gradually gaining importance [3–6].
Riluzole is a benzothiazole molecule, which inhibits the voltage-gated sodium channels, and thereby prevents glutamate release and mitigates cellular excito-toxicity [5, 7]. It is a US Food and Drug Administration (US-FDA)-approved drug for amyotrophic lateral sclerosis (ALS), and has also been demonstrated to have beneficial roles in Parkinson’s disease, Huntington’s disease, Alzheimer’s disease and other conditions presenting with spasticity [8–11]. Its role in ameliorating the outcome in patients with cervical myelopathy has also been discussed in a recent large, multi-centered clinical trial [12].
Considering these neuro-protective benefits, there has been a rising interest in evaluating its role in traumatic SCI situations [1, 5, 7, 12, 13]. Certain recent studies (mostly animal-based) have already shown sufficient support to its usefulness in such patients [1, 5, 7, 14, 15]. Nevertheless, its true benefits, ideal timing or dosage of administration and complications are still largely unknown [12–14]. The current study was thus planned to evaluate the implications and benefits of administering riluzole in patients presenting with acute cervical spine injury (CSI) with incomplete neurological deficit.
Materials and Methods
A prospective, randomised-controlled study was conducted between April 2018 and March 2019 at a single tertiary-level spine centre, after obtaining approval from our Institutional Review Board (IRB: Application No. 2019/03/01). Patients with cervical spine injury, who presented to our Emergency Department (ED) with incomplete neurological deficit, were considered for the study. All patients were initially managed in accordance with Advanced Trauma Life Support (ATLS) protocol and their cervical spines were appropriately immobilised. Detailed elicitation of clinical history and clinical assessment (including complete neurological evaluation) were carried out. After appropriate resuscitation, patients underwent routine blood investigations (complete blood counts, renal and liver function tests, blood grouping), and plain cervical radiographs (antero-posterior and lateral views—with adequate exposure to cervico-thoracic junction). In patients with evidence of or high suspicion for cervical injuries, advanced imaging including computed tomography (CT) and magnetic resonance imaging (MRI) were obtained.
Only patients aged between 18 and 65 years, who presented within 72 h of injury, involving sub-axial levels of injury (C3 to C7 levels) and incomplete neurological deficiencies (ISNCSCI grades B, C, D) were included. Patients who did not consent to participate, those with penetrating or open injuries, those with significant associated [head (GCS < 14), chest or abdominal injuries] injuries or poly-trauma, pre-existing renal or liver disease and pregnant women were excluded.
The included patients were randomised into two groups: groups A (in whom riluzole was administered) and B (in whom riluzole was not additionally administered). All patients in group A received a loading dose of riluzole (100 mg twice daily) within 72 h of injury (which was within 1 h of presenting to our Emergency Department). This was followed by a maintenance dose of 50 mg twice daily for the next 13 days. Patients in control group did not receive any additional medical therapy. The decisions regarding patient management in both the groups [conservative or surgical (anterior versus posterior approaches) intervention] were made by one of the senior consultants (SR, APS, RMK), based on the imaging findings, injury pattern and patient’s health status (after detailed discussions with patients and their families). Based on the standard practice at our Institution, all patients (in both groups) underwent surgical management within 12–24 h of presentation or at the earliest opportunity after they were deemed surgically and anaesthetically fit, after due consideration for all relevant, additional factors such as general systemic condition of the patient, age, associated comorbidities, nature of associated injuries, surgical procedure planned (anterior versus posterior or combined approaches), and the time of the day at presentation (late hours of the night versus morning hours, etc.). The patients were managed in an Intensive Care/high dependency Unit (ICU/HDU) during the initial days of admission, peri-operatively or until completely weaned off any respiratory support. Based on their general condition and neurological status, standardised post-operative rehabilitative regimens were followed. Patients, who required prolonged ventilator supports, were tracheostomised on 3rd to 5th post-operative days. All relevant details including the duration of hospital stay, adverse events and complications during their stay and in the initial 30 days following discharge, need for additional interventions and neurological status were documented.
The patients were followed up at 6 weeks, 3 months, 6 months, 1 year and 2 years’ intervals following their discharge. At each post-operative visit, detailed elicitation of clinical history (including complications), thorough clinical examination (including neurological evaluation), and radiological assessment (status of fracture healing, implant position or related complications) with plain radiographs were carried out. The neurological evaluation (performed pre-operatively and at each follow-up visit) consisted of assessment of American Spinal Injury Assessment (ASIA) motor, sensory scores and grades, spinal cord independence measure (SCIM3), and numerical rating scale (NRS) for neuropathic pain. In patients with any evidence of implant loosening or failure of fracture healing, additional advanced imaging (CT or MRI) were considered. A detailed comparison and analysis of the general outcome and these aforementioned neurological measures between the pre- and post-operative periods was performed (for both groups A and B). In accordance with our Institutional protocol, all patients were transferred to a physiotherapy/rehabilitation unit after discharge, if deemed medically fit. Those who are not medically fit, are initially transferred to the care of a step-down medical unit or discharged home with the necessary medical care, and then considered for rehabilitative services later.
Statistical Analysis
Chi-square and t-tests were used for analysing qualitative and quantitative variables, respectively. The data analysis was performed using SPSSV-27 (IBM Solutions, New York, United States) software. P value < 0.05 was considered as significant.
Results
Overall, there were 23 and 20 patients included in the groups A and B, respectively. Two patients in group A were females, while the remaining were male patients (p = 0.49). The mean age of patients in groups A and B was 47.7 ± 14.8 and 51.2 ± 14.1 years, respectively (p = 0.44). Six and three patients, respectively, in groups A and B, were smokers (no statistical difference between groups A and B; p = 0.47). In the group A, six patients were known diabetics, four were hypertensives, and one each had chronic obstructive pulmonary disease (COPD) and ankylosing spondylitis (AS), respectively. In group B, five were known diabetics, and one each had AS, chronic renal disease (CRD), ischaemic heart disease (IHD), hypertension and diffuse idiopathic skeletal hyperostosis (DISH). There was no statistically insignificant difference in the distribution of medical comorbidities between the groups (p = 0.93).
In group A, the mechanisms of injury were road traffic accident (RTA), fall from height and assault by an animal in 12, 10 and 1 patients, respectively. In group B, the mechanisms included road traffic accident (RTA), fall from height and assault by an animal in 9, 8 and 3 patients, respectively. Twelve and 9 patients in groups A and B, respectively, had other major associated skeletal or visceral injuries, respectively (p = 0.58). In group A, 17, 2 and 4 patients had single, two and three-level injuries, respectively. In group B, 16, 1 and 3 patients sustained single, two and three-level injuries, respectively (p = 0.72). Among the patients with single-level injuries in group A, 7, 3, 4 and 3 patients had C3-4, C4-5, C5-6 and C6-7 involvement, respectively. Among the group B patients with single-level involvement, 4, 9 and 3 patients had C4-5, C5-6 and C6-7 involvement, respectively. There was a statistically higher incidence of proximal level injuries (especially C3-4) in group A, as compared with group B (p = 0.02). There was no statistically significant difference in the post-injury neurological status between the two groups [motor (ASIA) score: p = 0.38, sensory (ASIA) score: p = 0.80, SCIM score: p = 0.60, ASIA grade: p = 0.12, numerical pain rating scale (NPRS): p = 0.38] (Tables 1, 2, 3).
Table 1.
Neurological parameters in groups A and B at admission and each follow-up visit (6 weeks, 3 months, 6 months and 1 year)
| At admission | 6 weeks | 3 months | 6 months | 1 year | |
|---|---|---|---|---|---|
| Motor score | |||||
| Group A | 43.0 ± 24.4 | 58.9 ± 22.8 | 68.7 ± 20.9 | 79.7 ± 19.1 | 91.2 ± 8.7 |
| Group B | 36.1 ± 26.5 | 51.8 ± 23.3 | 67.2 ± 20.6 | 76.0 ± 18.9 | 85.1 ± 14.2 |
| p | 0.38 | 0.36 | 0.83 | 0.57 | 0.15 |
| Sensory score | |||||
| Group A | 137.0 ± 48.8 | 160.3 ± 41.5 | 183.2 ± 33.1 | 198.8 ± 28.1 | 208.8 ± 11.2 |
| Group B | 133.7 ± 33.9 | 149.5 ± 34.1 | 168.6 ± 32.9 | 188.9 ± 29.0 | 202.4 ± 28.2 |
| p | 0.80 | 0.41 | 0.21 | 0.32 | 0.39 |
| Spinal cord independence measure (SCIM) version III | |||||
| Group A | 11.8 ± 6.9 | 41.7 ± 20.7 | 60.4 ± 23.0 | 77.3 ± 21.9 | 90.6 ± 8.7 |
| Group B | 12.9 ± 6.9 | 38.8 ± 13.9 | 60.4 ± 18.9 | 77.8 ± 19.9 | 88.5 ± 18.8 |
| p | 0.60 | 0.63 | 0.99 | 0.95 | 0.68 |
Table 2.
Neuropathic pain severity of patients in groups A and B at admission and each follow-up visit (6 weeks, 3 months, 6 months and 1 year)
| Numerical pain rating scale (NPRS) | |||||
|---|---|---|---|---|---|
| Group A | 8.5 ± 1.54 | 5.4 ± 1.54 | 3.4 ± 1.95 | 1.8 ± 1.54 | 0.6 ± 0.70 |
| Group B | 8.9 ± 1.17 | 5.0 ± 1.41 | 3.3 ± 1.73 | 2.2 ± 0.98 | 1.13 ± 0.28 |
| p | 0.38 | 0.45 | 0.76 | 0.43 | 0.06 |
Table 3.
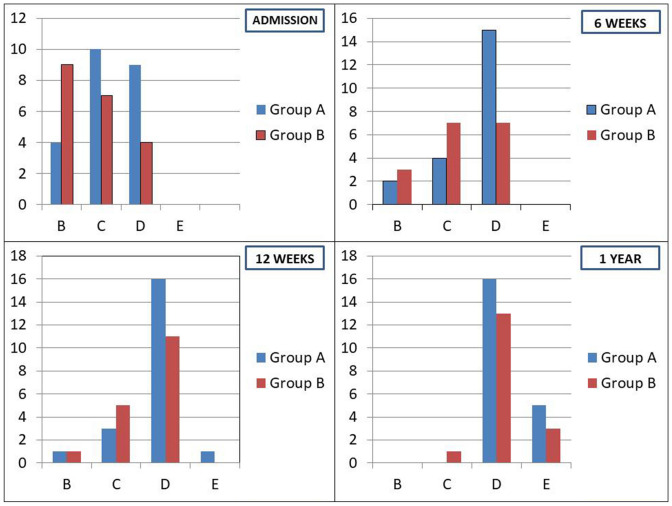
American Spinal Injury Association (ASIA) Grades in patients in groups A and B at admission and each follow-up visit (6 weeks, 3 months, 6 months and 1 year)
| ASIA grades | A | B | C | D | E | p | |
|---|---|---|---|---|---|---|---|
| At admission | Group A | 0 | 4 | 10 | 9 | 0 | 0.12 |
| Group B | 0 | 9 | 7 | 4 | 0 | ||
| 6 weeks | Group A | 0 | 2 | 4 | 15 | 0 | 0.05 |
| Group B | 0 | 3 | 7 | 7 | 0 | ||
| 3 months | Group A | 0 | 1 | 3 | 16 | 1 | 0.34 |
| Group B | 0 | 1 | 5 | 11 | 0 | ||
| 6 months | Group A | 0 | 1 | 1 | 14 | 5 | 0.73 |
| Group B | 0 | 0 | 1 | 14 | 2 | ||
| 1 year | Group A | 0 | 0 | 0 | 16 | 5 | 0.66 |
| Group B | 0 | 0 | 1 | 13 | 3 |
In both groups A and B, 19 patients were surgically managed. Among the surgically managed patients in group A, 13 underwent anterior surgery, 5 posterior-only surgeries, and 1 underwent combined antero-posterior surgery (Figs. 1 and 2). The distribution of surgical intervention in group B too was similar (12, 5 and 2 anterior, posterior-only and combined antero-posterior surgeries, respectively) (p = 0.99). The mean time interval between injury and administration of riluzole in group A patients was 41.6 ± 26.4 h (5, 12, and 6 patients had riluzole administered within 24 h, between 24 and 48 h, and between 48 and 72 h, respectively). In group A, the mean time interval between the injury and surgery was 72.6 ± 70.3; while in group B, the mean interval between the injury and surgical interval was 49.1 ± 23.5. In group A, three patients underwent surgery within 24 h following injury, 16 patients between 24 and 48 h, and the remaining underwent surgery beyond 48 h of injury. On the other hand, in group B, 2, 13 and 5 underwent surgery within 24 h, between 24 and 48 h and beyond 48 h of injury, respectively. These differences were not statistically significant (p = 0.17, 0.25). The mean number of days of hospitalisation in groups A and B were 8.6 ± 3.8 and 14.8 ± 24.7 days, respectively (p = 0.29). Three patients in group A and four in group B required undergoing tracheostomy and needed prolonged ventilator support. Overall, three patients in each group had an early, major complication (group A: one wound infection, one dural tear with early CSF leak, one atrial fibrillation; group B: one each with aspiration, respiratory distress and hematoma) (p = 0.50). There were no riluzole-related complications in any of our patients.
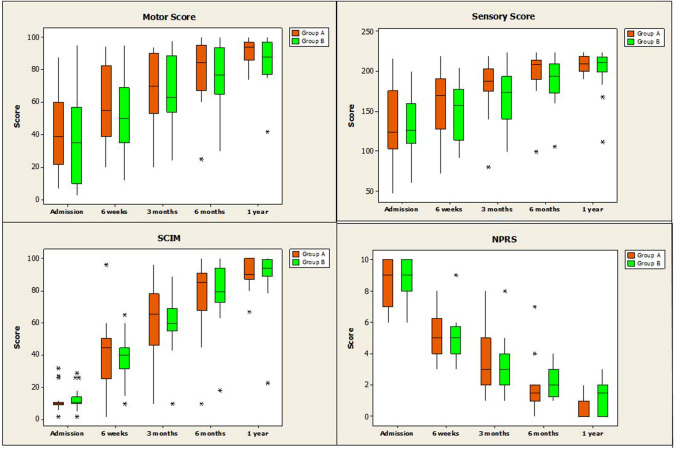
Fig. 1.
Box-plot representation for the neurological assessment parameters at different time points (sensory and motor components of ASIA score, SCIM and NPRS)
Fig. 2.
Bar diagrams showing the distribution of ASIA grades of patients at different time points
Long-Term Outcome in Groups A and B
In group A, two patients died during the follow-up period. The cause of death in both the patients was acute respiratory distress (at home during the 3rd and 4th weeks post-injury, respectively), possibly secondary to aspiration. Among the patients in group B, three died during the follow-up [one due to acute respiratory distress during the 2nd post-operative week, one following sepsis due to urinary infection (during 4th post-operative week) and another due to pneumonia (during the 6th week)]. The neurological recovery and neuropathic pain severity were assessed and statistically analysed only for the remaining patients during the follow-up (21 and 17 patients in groups A and B, respectively).
Neurological Status
A detailed evaluation of the neurological status of all patients was carried out at each visit. Overall, there was a significant neurological improvement in patients belonging to both groups, with regard to all the individual components considered.
Motor Score
In group A, the motor (ASIA) score improved from 47.5 ± 24.8 (immediate post-injury) to 58.9 ± 22.8 (6 weeks; p = 0.001), 79.7 ± 19.1 (6 months; p < 0.001) and 91.2 ± 8.7 (1 year; p < 0.001). In group B, the motor score (ASIA) improved from 40.3 ± 26.2 (immediate post-injury) to 51.8 ± 23.3 (6 weeks; p < 0.001), 76.0 ± 18.9 (6 months; p < 0.001) and 85.1 ± 14.2 (1 year; p < 0.001).
Sensory Score
In group A, the sensory (ASIA) score improved from 149.2 ± 44.4 (immediate post-injury) to 160.3 ± 41.5 (6 weeks; p = 0.001), 183.2 ± 33.1 (3 months; p < 0.001), 198.8 ± 28.1 (6 months; p < 0.001) and 208.9 ± 11.2 (1 year; p < 0.001). In group B, the sensory (ASIA) score improved from 135.1 ± 36.9 (immediate post-injury) to 149.5 ± 34.1 (6 weeks; p = 0.006), 168.6 ± 32.9 (3 months; p < 0.001), 188.9 ± 29.1 (6 months; p < 0.001) and 202.4 ± 28.2 (1 year; p < 0.001).
ASIA Grade
In group A, there was statistically significant improvement in ASIA grades at 6 weeks (p = 0.006), 3 months (p = 0.02), 6 months (p = 0.01), as compared to pre-operative neurological status. At the end of 1 year, although there was a trend towards improved ASIA grades in comparison with pre-operative status, there was no statistically significant difference (p = 0.11). In group B, there was a statistically significant improvement in ASIA grades at 6 weeks (p = 0.003), as compared to pre-operative neurological status. In group B patients, there was a trend towards improvement in ASIA grades at 3, 6 and 12 months. Nevertheless, there was no statistically significant difference (At 3 months: p = 0.18; 6 months: p = 0.05 and 1 year: p = 0.05).
All the patients in the current patient cohort (in both groups A and B), who died during the follow-up, had ASIA grade B neurology at presentation. In both groups, the eventual neurological improvement at the final follow-up time point primarily ranged between ASIA grades 1 and 2 [Group A: ASIA grade B (4 patients) at presentation—2 mortality, 2 patients with ASIA D (at final follow-up); ASIA grade C (10 patients) at presentation—10 ASIA D patients (at final follow-up); and ASIA grade D (9 patients) at presentation—4 ASIA D and 5 ASIA E (at final follow-up); Group B: ASIA grade B (9 patients) at presentation—3 mortality, 1 ASIA C and 5 patients with ASIA D (at final follow-up); ASIA grade C (7 patients) at presentation—7 ASIA D patients (at final follow-up); and ASIA grade D (4 patients) at presentation—1 ASIA D and 3 ASIA E (at final follow-up) (p = 0.36).
SCIM3 Score
Ingroup A, SCIM score improved significantly from 12.4 ± 7.7 (immediate post-injury) to 41.7 ± 20.7 (6 weeks; p < 0.001), 60.4 ± 23.0 (3 months; p < 0.001), 77.3 ± 21.9 (6 months; p < 0.001) and 90.6 ± 8.7 (1 year: p < 0.001). Ingroup B, SCIM score improved significantly from 14.2 ± 6.7 (immediate post-injury) to 38.8 ± 13.9 (6 weeks; p < 0.001), 60.4 ± 18.9 (3 months; p < 0.001), 77.8 ± 19.9 (6 months; p < 0.001) and 88.5 ± 18.8 (1 year: p < 0.001).
Neuropathic Pain
In group A, NPRS significantly improved from 8.5 ± 1.5 (immediate post-injury) to 5.4 ± 1.5 (6 weeks; p < 0.001), 3.4 ± 1.9 (3 months; p < 0.001), 1.8 ± 1.5 (6 months; p < 0.001) and 0.60 ± 0.70 (1 year: p < 0.001). In group B, NPRS significantly improved from 8.9 ± 1.2 (immediate post-injury) to 5.0 ± 1.4 (6 weeks; p < 0.001), 3.3 ± 1.7 (3 months; p < 0.001), 2.2 ± 0.9 (6 months; p < 0.001) and 1.250 ± 1.1 (1 year: p < 0.001).
The Effect of Riluzole on Neurological Recovery
Although there was a statistically significant improvement in a majority of the neurological parameters in both the groups during the course of follow-up, there was no statistically significant difference between groups A and B. In other words, administration of riluzole did not offer any significant additional benefit in our cohort of patients. The comparison of analysis between groups A and B have been tabulated below (Tables 1, 2 and 3). A sub-group analysis was also performed for analysing the relationship between the timing of riluzole administration (< 24 h, 24–48 h and > 48 h) and neurological recovery; which revealed no significant correlation between recovery and the time of riluzole administration [motor score (p = 0.44), sensory score (p = 0.21), ASIA grade (p = 0.99), SCIM3 score (p = 0.46) and neuropathic pain (p = 0.39].
Discussion
Traumatic SCIs are devastating injuries and early surgical decompression is the best available treatment option to ameliorate the overall outcome [16–19]. Although widely discussed, there is currently no neuro-protective therapeutic agent which has emerged hitherto as the standard of care in such patients [7, 20, 21]. Following traumatic SCIs, spinal cord undergoes a series of biological processes of responses and repair. The general goals of neuro-protective strategies include limiting the spinal cord damage and enhancing repair at every stage of healing [1, 7, 11, 12, 16, 18, 19, 22–25]. This includes modulation of inflammatory reactions, modification of microglial responses, mitigation of fibroblastic scar formation, stimulation of axonal re-growth and providing substrates to bridge the axonal gaps [6, 15, 18–20, 25, 26]. Therapeutic strategies to enhance SCI repair will, therefore, necessitate a series of approaches, each directed towards specific phases of reactionary and reparative processes [15].
During the early stages of secondary injury cascade, traumatic forces in combination with ischaemia lead to disruption of neuronal membranes and result in continuous activation of voltage-gated sodium ionic channels. This incessant, pathological activation leads to substantial increase in intracellular sodium levels, which in turn causes persistent influx of calcium ions through sodium-calcium exchange channels. Such large increases in intracellular calcium lead to extracellular releases of toxic amounts of glutamate, which is an excitatory neurotransmitter (NT). These reactive processes cause progressive ionic imbalance, formation of reactive oxidative molecules, failure of intracellular energy mechanism, cytotoxic oedema and severe tissue excito-toxicity, which eventually result in regional cellular death [1, 7, 12–14].
Riluzole is a sodium channel blocker, which is currently approved by USFDA for the management of ALS [8, 9]. In view of its mechanism of action directly targeting the sodium channels, which are fundamental to secondary cascade in SCI, it has been increasingly recommended as a potential neuroprotective agent [8, 23–25]. Its potential role in meliorating neuronal recovery in patients with brachial plexus avulsion injuries [27] and cervical root injuries [28] have already been reported on the basis of previous animal studies. Based on the available pre-clinical evidence, other clinical trials are underway to ascertain the role of this drug in patients with SCIs. The exact role of riluzole in clinical settings of traumatic SCIs is still largely unclear [1, 7, 14–16, 22, 26]. The current prospective, randomised study was thus planned evaluate the safety and efficacy of riluzole in patients undergoing surgical decompression and stabilisation following acute traumatic SCIs.
In the current study, riluzole was administered orally or via nasogastric (NG) tube in patients at a dose previous recommended in similar studies [1, 7, 11, 12, 14, 15, 19]. The dosage effective in animal studies was extrapolated to humans, based on the formula, human equivalent dose = Animal Dose (mg/kg) * (Animal wt/Human wt in kg). Only patients who could be administered the initial dose within 72 h of injury were included.
The groups A and B were similar with respect to a majority of baseline clinico-radiological characteristics. We could not observe any major riluzole-associated adverse reactions in our patients with the current dose of administration. Overall, we could observe a significant improvement in the neurological status of patients at every stage of follow-up in both groups A and B. The motor/sensory assessment, SCIM score and ASIA grades significantly improved in a majority of patients. Thus, surgical intervention within 72 h post-injury was a significant determinant of the overall outcome in both our groups.
However, when a comparison of the neurological outcome was made, there was no statistically significant difference observed between the two groups (A and B). Thus, at the current dose of administration, riluzole did not significantly alter the course of improvement significantly. On a detailed evaluation, nevertheless, we could observe that all the individual parameters of neurological evaluation (motor/sensory ASIA scores, SCIM score and ASIA grades) were numerically better in the cohort of patients, who were administered riluzole (group A). The small sample size in our study could have contributed to low statistical significance and a larger scale; multi-centered trial can help us understand the true benefits of adjuvant riluzole administration in traumatic SCIs more clearly.
Previous studies have reported reduced tissue cavitation, enhanced preservation of white matter and motor neurons, improved somatosensory evoked potentials (SSEPs) and locomotor functional scores in riluzole-treated animals with traumatic SCIs, as compared with control animals [15]. A rodent-based study by Wu et al. [22, 25] evaluated the functional, histo-pathological and molecular benefits of riluzole in SCI. They could observe that riluzole demonstrated a substantial penetration across the blood brain barrier (BBB) at 15 min after administration, showed a relatively long duration of availability (longer t1/2) within the spinal cord and plasma, effected a significant reduction in apoptosis and inflammation; as well as improved the axonal integrity, cytoskeletal structure and functional preservation of neural tissues. They reported that a post-injury administration of riluzole within 12 h in humans could offer similar benefits. In a recent systematic review discussing 16 pre-clinical studies of traumatic and non-traumatic SCI [29, 30], it was concluded that riluzole significantly improved locomotor scores, gait function, and measures of neuropathic pain. Based on this evidence, they recommended the need for large-scale clinical studies to analyse its impact in SCI situations.
Recently, riluzole has also been trialled as a potential agent in the management of cervical spondylomyelopathy (CSM). Karadimas et al. [23, 24], based on animal studies, reported that administration of riluzole reduced the incidence of ischaemia–reperfusion injuries after decompression surgery in CSM. In experimental settings, riluzole has been effective in reducing oxidative damage to DNA in ischaemic and post-traumatic situations [23]. In a recently-concluded multicentre, double-blind, placebo-controlled, randomised, phase 3 trial evaluating 290 patients with non-traumatic spinal cord injury (mostly, degenerative cervical myelopathy), it was concluded that adjuvant treatment with riluzole (for a duration of 6 weeks peri-operatively) did not improve functional outcome beyond surgical decompression (especially in moderate to severe myelopathy) [12]. In this trial, there was no statistically significant adverse event associated with riluzole administration. Rajasekaran et al. [26] also evaluated the effectiveness of riluzole as pharmacotherapeutic agent in early CSM using clinical assessment and diffusion tensor imaging (DTI) analysis. They observed no significant benefit of the drug as a stand-alone treatment for early CSM.
Apart from neuronal recovery, another potential benefit of riluzole in SCIs has been the alleviation of neuropathic pain severity. Neuropathic pain is characterised by allodynia and hyperalgesia, which may be initially localised to the primary zone of injury and later gradually spread to other regions that are not directly damaged (secondary hyperalgesia). Glutamate N-methyl-d-aspartate (NMDA) receptors are located within the dorsal horns of spinal cords, which have been identified to be critically involved in the transmission of nociceptive stimuli. Riluzole decreases phosphorylation of these NR1 and NR2B receptors in spinal dorsal horns, which in turn leads to attenuation of glutamate excitotoxicity, and subsequent decreases the neuronal sensitisation. Riluzole also leads to reduced microglial recruitment and activation in the dorsal horns, which also contributes to mitigation of neuropathic pain [22]. Previous studies by Fehlings et al. [7, 12] had suggested a possible role of riluzole in the management of severe neuropathic pain related to traumatic and non-traumatic SCIs. We also evaluated the neuropathic pain in all our patients. Overall, there was a significant improvement in the pain severity in both the groups with each follow-up. However, similar to the other outcome, we did not observe any major additional benefit associated with the adjuvant dose of riluzole.
Limitations
A major limitation of the current study was the small sample size (since this was a pilot study, we conformed to a smaller patient sample). The ongoing pandemic situation also significantly restricted our ability to recruit patients for the study. There was also some attrition of the patient cohort during the follow-up, owing to death of some patients. There was a significant difference in the distribution of upper level injuries (C3-4) between the two groups. This could contribute to imprecise observations on SCIM scores. In view of the delayed presentation of patients to the hospitals (as usually observed in developing nations), there was a certain delay in the administration of riluzole to the SCI patients in our series. This delay could have also influenced the overall outcome. Since multiple pre-, intra- and post-operative (early and late) factors could concomitantly impact the final outcome, the individual role of each of these parameters in determining the final outcome might not be entirely ruled out (although the distribution of many of the parameters were similar between groups A and B).
Conclusion
Overall, we could observe a statistically significant improvement in the neurological outcome of our cohort of patients with acute traumatic cervical SCI at 2 years’ follow-up time point, as compared with immediate post-injury neurological status. The administration of adjuvant riluzole therapy did not significantly improve the neurological outcome or neuropathic pain severity beyond surgical decompression in our cohort. Larger scale, prospective, multi-centered studies on this issue can help us clearly define the role of riluzole in traumatic SCIs.
Funding
IASA-ASSI (Indo American Spine Alliance—Association of Spine Surgeons of India) grant.
Declarations
Conflict of Interest
The authors have no conflicts of interest to disclose.
Ethical Standard Statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed Consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson JR, Fehlings MG. Riluzole for acute traumatic spinal cord injury: A promising neuroprotective treatment strategy. World Neurosurgery. 2014;81:825–829. doi: 10.1016/j.wneu.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. New England Journal of Medicine. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na(+)-K(+)-ATPase, the Na(+)-H+ exchanger, and the Na(+)-Ca2+ exchanger. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 1996;16:545–552. doi: 10.1523/JNEUROSCI.16-02-00545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz G, Fehlings MG. Secondary injury mechanisms of spinal cord trauma: a novel therapeutic approach for the management of secondary pathophysiology with the sodium channel blocker Riluzole. Progress Brain Research. 2002;137:177–190. doi: 10.1016/s0079-6123(02)37016-x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Al Mamun A, Yuan Y, et al. Acute spinal cord injury: Pathophysiology and pharmacological intervention (Review) Molecular Medicine Reports. 2021 doi: 10.3892/mmr.2021.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehlings MG, Wilson JR, Frankowski RF, et al. Riluzole for the treatment of acute traumatic spinal cord injury: rationale for and design of the NACTN Phase I clinical trial. Journal of Neurosurgery. Spine. 2012;17:151–156. doi: 10.3171/2012.4.AOSPINE1259. [DOI] [PubMed] [Google Scholar]
- 8.Bensimon G, Lacomblez L, Delumeau JC, et al. A study of riluzole in the treatment of advanced stage or elderly patients with amyotrophic lateral sclerosis. Journal of Neurology. 2002;249:609–615. doi: 10.1007/s004150200071. [DOI] [PubMed] [Google Scholar]
- 9.Bensimon G, Ludolph A, Agid Y, et al. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain: a Journal of Neurology. 2009;132:156–171. doi: 10.1093/brain/awn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallée A, Vallée J-N, Guillevin R, Lecarpentier Y. Riluzole: A therapeutic strategy in Alzheimer’s disease by targeting the WNT/β-catenin pathway. Aging. 2020;12:3095–3113. doi: 10.18632/aging.102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesuis SL, Kaplick PM, Lucassen PJ, Krugers HJ. Treatment with the glutamate modulator riluzole prevents early life stress-induced cognitive deficits and impairments in synaptic plasticity in APPswe/PS1dE9 mice. Neuropharmacology. 2019;150:175–183. doi: 10.1016/j.neuropharm.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Fehlings MG, Badhiwala JH, Ahn H, et al. Safety and efficacy of riluzole in patients undergoing decompressive surgery for degenerative cervical myelopathy (CSM-Protect): A multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. Lancet Neurology. 2021;20:98–106. doi: 10.1016/S1474-4422(20)30407-5. [DOI] [PubMed] [Google Scholar]
- 13.Meshkini A, Salehpour F, Aghazadeh J, et al. Riluzole can improve sensory and motor function in patients with acute spinal cord injury. Asian Journal of Neurosurgery. 2018;13:656–659. doi: 10.4103/ajns.AJNS_259_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehlings MG, Nakashima H, Nagoshi N, et al. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): A randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016;54:8–15. doi: 10.1038/sc.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman RG, Fehlings MG, Frankowski RF, et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. Journal of Neurotrauma. 2014;31:239–255. doi: 10.1089/neu.2013.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouanet C, Reges D, Rocha E, et al. Traumatic spinal cord injury: Current concepts and treatment update. Arquivos de Neuro-Psiquiatria. 2017;75:387–393. doi: 10.1590/0004-282X20170048. [DOI] [PubMed] [Google Scholar]
- 17.Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: Results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J-M, Long X-H, Zhou Y, et al. Is urgent decompression superior to delayed surgery for traumatic spinal cord injury? A meta-analysis. World Neurosurgery. 2016;87:124–131. doi: 10.1016/j.wneu.2015.11.098. [DOI] [PubMed] [Google Scholar]
- 19.Jug M, Kejžar N, Vesel M, et al. Neurological recovery after traumatic cervical spinal cord injury is superior if surgical decompression and instrumented fusion are performed within 8 hours versus 8 to 24 hours after injury: A single center experience. Journal of Neurotrauma. 2015;32:1385–1392. doi: 10.1089/neu.2014.3767. [DOI] [PubMed] [Google Scholar]
- 20.Ahuja CS, Fehlings M. Concise review: bridging the gap: Novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Translational Medicine. 2016;5:914–924. doi: 10.5966/sctm.2015-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahuja CS, Nori S, Tetreault L, et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Zhang Y, Zhang Y, et al. Riluzole improves functional recovery after acute spinal cord injury in rats and may be associated with changes in spinal microglia/macrophages polarization. Neuroscience Letters. 2020;723:134829. doi: 10.1016/j.neulet.2020.134829. [DOI] [PubMed] [Google Scholar]
- 23.Karadimas SK, Laliberte AM, Tetreault L, et al. Riluzole blocks perioperative ischemia-reperfusion injury and enhances postdecompression outcomes in cervical spondylotic myelopathy. Science Translational Medicine. 2015;7:316ra194. doi: 10.1126/scitranslmed.aac6524. [DOI] [PubMed] [Google Scholar]
- 24.Moon ES, Karadimas SK, Yu W-R, et al. Riluzole attenuates neuropathic pain and enhances functional recovery in a rodent model of cervical spondylotic myelopathy. Neurobiology of Diseases. 2014;62:394–406. doi: 10.1016/j.nbd.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Satkunendrarajah K, Teng Y, et al. Delayed post-injury administration of riluzole is neuroprotective in a preclinical rodent model of cervical spinal cord injury. Journal of Neurotrauma. 2013;30:441–452. doi: 10.1089/neu.2012.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajasekaran S, Aiyer SN, Shetty AP, et al. Effectiveness of Riluzole as a pharmacotherapeutic treatment option for early cervical myelopathy: A double-blinded, placebo-controlled randomised controlled trial. European Spine Journal: Official Publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2016;25:1830–1835. doi: 10.1007/s00586-015-4323-1. [DOI] [PubMed] [Google Scholar]
- 27.Gloviczki B, Török DG, Márton G, et al. Delayed spinal cord-brachial plexus reconnection after C7 ventral root avulsion: The effect of reinnervating motoneurons rescued by Riluzole treatment. Journal of Neurotrauma. 2017;34:2364–2374. doi: 10.1089/neu.2016.4754. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson KJ, Zhang S, Gilliland TM, Winkelstein BA. Riluzole effects on behavioral sensitivity and the development of axonal damage and spinal modifications that occur after painful nerve root compression. Journal of Neurosurgery. Spine. 2014;20:751–762. doi: 10.3171/2014.2.SPINE13672. [DOI] [PubMed] [Google Scholar]
- 29.Tetreault LA, Zhu MP, Wilson JR, et al. The impact of Riluzole on neurobehavioral outcomes in preclinical models of traumatic and nontraumatic spinal cord injury: Results from a systematic review of the literature. Global Spine Journal. 2020;10:216–229. doi: 10.1177/2192568219835516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L-Y, Tian Z-R, Yao M, et al. Riluzole promotes neurological function recovery and inhibits damage extension in rats following spinal cord injury: A meta-analysis and systematic review. Journal of Neurochemistry. 2019;150:6–27. doi: 10.1111/jnc.14686. [DOI] [PubMed] [Google Scholar]