Abstract
Purpose
The present study was stimulated by the continuous growth of commercially available high intensity focused ultrasound (HIFU) systems for fat reduction. Herein, HIFU was utilised for fat ablation using a single-element ultrasonic transducer operating in thermal mode.
Methods
The custom-made concave transducer that operates at 1.1 MHz was assessed on excised porcine adipose tissue for its ability to reduce fat. Ultrasonic sonications were executed on the adipose tissue utilising acoustical power between 14 and 75 W and sonication time in the range of 1–10 min. The mass of the adipose tissue sample was weighed afore and after ultrasonic sonications and the effect of the sonication on the mass change was recorded.
Results
Mass change was linearly dependent with either increasing acoustical power or sonication time and was in the range of 0.46–1.9 g. High acoustical power of 62.5 W for a sonication time of 1 min and a power of 75 W for a sonication time of 5 min, respectively resulted in the formation of a lesion or possible cavitation on the piece of excised adipose tissue.
Conclusion
The study demonstrated the efficacy of the proposed transducer in achieving a reduction of excised fat tissue. The present findings indicate the potential use of the transducer in a HIFU system indicated for the reduction of subcutaneous adipose tissue where increased values of acoustical power can result in increased amounts of fat reduction.
Keywords: HIFU, Adipose, Porcine, Fat, Reduction, Lipolysis
Introduction
Global societal changes occurring over the last 45 years such as the increase of desk-sitting jobs and hectic work schedules have led to decreases in physical activity and balanced diets, thus resulting in a threefold increase in the proportion of people who are characterised as overweight or obese [1]. As a result of overweight and obesity, excess body fat seems to accumulate in certain areas, mostly in the abdomen. Although some studies have shown that a combination of diet and exercise might reduce abdominal fat [2, 3], others have failed to do so [4]. Since people often require immediate and long-term results, they frequently choose radical treatments such as liposuction, where fat is permanently infiltrated by cannulae inserted in the skin through small incisions [5].
Liposuction is the second most common cosmetic surgical procedure performed after breast enlargement, with a total of 1,704,786 treatments completed globally in 2019 [6]. Liposuction involves the introduction of blunt-tipped cannulae and subcutaneous fat infiltration thus leaving blood vessels intact [7]. The cannulae are inserted through 1–1.5 cm skin incisions and fat cells are suctioned during the 2–4 h long procedure with optimal results seen 6–12 weeks post-treatment [8]. However, the surgical nature of the treatment has many side effects and can even cause mortality, especially with the use of general anaesthesia [8], resulting in 1 death per 5000 treatments [9]. In order to reduce the occurrence of adverse effects, several techniques have been introduced over time including power-assisted, ultrasound-assisted, laser-assisted and radiofrequency-assisted liposuctions [5].
Power-assisted liposuction uses pneumatic or electrical sources for the mechanical motion of the cannula thus resulting in reduced physician fatigue [10]. One such system commercially available is the PAL-650 (MicroAire, Charlottesville, Virginia, USA), which utilises a 3 mm cannula with mechanical motion speeds of up to 4000 cycles/min thus reducing the operation times, besides being a better suit for fibrous areas [11]. Ultrasound-assisted liposuction uses a transdermal probe that emits ultrasonic energy on the fat cells for their fragmentation while being equipped with a cannula for their suction. The VASER (Solta Medical, Pleasanton, California, USA) is a commercially available system utilising this technology at 36 kHz [12] and featuring 2.9–3.7 mm probes [13], considered to be as efficient as traditional liposuction, but with higher skin retraction, decreased amounts of blood loss [14] and increased operation times [12]. However, with this technique, side effects such as skin burns and seromas might occur [15]. Laser-assisted liposuction similarly utilises laser sources of either 980 nm, 1064 nm, or dual 1064/1320 nm wavelength for fat lysis, whereupon cells are suctioned with a cannula [16]. Energy is applied through a micrometre-sized probe and thus it requires smaller incisions than traditional or ultrasound-assisted liposuction [17]. The ProLipo Plus (Sciton, Palo Alto, CA, USA) [18] and the SmartLipo Triplex system (Cynosure, Westford, Massachusetts, USA) [19] are two commercially available devices transmitting energy at varied wavelengths that can either be utilised individually or in combination, depending on the therapy requirements. Radiofrequency-assisted liposuction utilises radiofrequency energy with simultaneous aspiration for fat cell destruction and suction. The BodyTite system (InMode, Yokneam Illit, Israel) has been FDA-approved in 2016 [20] and is equipped with an external electrode placed on the skin surface and an internal electrode that is inserted in the abdomen [21] through a 14G needle [22]. The technique has been proven efficient and requires a smaller operation time than laser-assisted liposuction [21]; however, it might lead to larger operation times compared to traditional liposuction [22] and is sometimes associated with skin burns [20].
With the significant risks reported for invasive liposuction techniques, non-invasive fat reduction techniques emerged with increased use, since better post-operative recovery with fewer complications is provided along with a decreased physician fatigue and no requirement for anaesthesia. Such techniques include cryolipolysis, radiofrequency (RF), low-level laser therapy (LLLT), high intensity focused ultrasound (HIFU) and high intensity focused electromagnetic field (HIFEM) where these accounted for 4.6 thousand treatments performed in 2019, nearly a 10% increase from 2015 [6].
Cryolipolysis for fat reduction was introduced in 2007 [23] and gained FDA approval for abdominal fat reduction in 2012 [24]. Fat cells are exposed to − 5 °C temperatures which induce adipocyte apoptosis, thus triggering inflammatory response 3-days post-treatment leading to lipolysis, with ultimate results seen 1–3 months post-treatment [25]. The CoolSculpting (Zeltiq Aesthetics, Pleasanton, California, USA) is a commercially available system for cryolipolysis that utilises an applicator, which is placed on the treatment area, whereupon the selected tissue is suctioned with vacuum so that subcutaneous fat can be selectively targeted with cold temperatures that are controlled through temperature sensors, with the treatment lasting 45–60 min per treatment area [25]. Shockwave therapy has been used post-treatment to increase patient comfort [26] and has led to the development of the CE marked ProShock system (PromoItalia, Milan, Italy) that combines the two modalities [27]. Cryolipolysis is performed without anaesthesia since it induces only temporary mild adverse effects (e.g. erythema, bruising and oedema) [28].
RF is the most popular non-invasive technique for fat reduction and can selectively target fat tissue due to its higher impedance [29]. Lipolysis is achieved by directing energy from electrodes to the area of interest and heating fat cells between 43 and 45 °C, with intervening tissue (i.e. skin) heated to the same effect; therefore a means of skin cooling is utilised, with treatments usually lasting between 0.5 and 2 h [30]. Commercially available systems include the Vanquish (BTL Aesthetics, Newcastle, UK) system [31], the Thermage (Solta Medical, Pleasanton, California, USA) [32], the TriPollar (Pollogen, Tel Aviv-Yafo, Israel) [30], the Trusculpt (Cutera, Brisbane, California, USA) [33] and the BodyFX (InMode, Yokneam Illit, Israel) [34]. Notably, the commercial Accent Prime (Alma Lasers, Caesarea, Israel) combines radiofrequency with ultrasound waves for a more efficient fat reduction [35]. The novel Vellashape (Candela Medical, Marlborough, Massachusetts, USA) system combines infrared energy, radiofrequency, and suction to enhance penetration of radiofrequency energy, achieving results 1-month post-treatment [36]. Some adverse effects include pain and bruising which subside a few hours post-treatment [34].
LLLT was FDA approved as a therapy for abdominal fat reduction in 2010 [37], and since then, several systems have emerged. Contrary to other techniques, LLLT does not induce adipocyte cell destruction, but induces pores in the cell membranes from which lipids are released [38] and then degraded through the lymphatic and circulatory systems [39]. Multiple treatments lasting approximately 30 min are administered for optimal results [40]. The American company Erchonia manufactured the first FDA approved LLLT system, the Zerona system which uses five diode heads each emitting a 635 nm laser [41]. The same company has also developed the Verju system (Erchonia, Melbourne, Florida, USA) which has been more recently FDA approved, and uses a lower laser wavelength (532 nm), thereby achieving better results and treatment times [42]. The SculpSure system (CynoSure, Westford, Massachusetts, USA) is different from the aforementioned systems since it emits energy through contact applicators and increases fat tissue temperature to 47 °C, thereby resulting in cell apoptosis [43]. The EON (Dominion Aesthetic Technologies, San Antonio, Texas, USA) is a novel, recently FDA-approved system utilising a laser wavelength of 1064 nm. The system is the first system utilising a robotic arm for movement of the energy source, thereby completely sparing the fatigue from the physician. The Venus Bliss (Venus Concept, Toronto, Canada) is another novel system with four diodes operating at 1060 nm [44]. LLLT has consistently reported the fewest side effects among all non-invasive techniques [37, 40, 41].
HIFU utilises ultrasound waves which can be focused on a single point in fat tissue locally increasing its temperature to 50–60 °C, thereby resulting in local cell apoptosis without damaging the intervening dermal tissues [45]. Contrary to the aforementioned techniques, the focus of the extracorporeal transducer can be adjusted relative to the patient’s needs, thus resulting in a safer treatment [45], with histopathology studies confirming that fat lysis is achieved only on the targeted area [46]. Treatments last 1–1.5 h and the apoptotic fat cells are degraded through the lymphatic and circulatory systems with initial results seen just 2 weeks post-treatment and optimal results 3-months after treatment [47]. While results can be seen after a single treatment, follow-up treatments are usually recommended since they can lead to further fat reduction [48].
LipoSonix (Medicis Technologies Corporation, Bothell, Washington, USA) is an FDA approved system intended for abdominal fat reduction using thermal ultrasound. Ultrasonic waves of 2 MHz can be focused from 1.1 to 1.8 cm in tissue to heat fat cells depending on the treatment needs [46]. Its efficacy has been extensively reported in the literature, achieving a mean reduction in waist circumference of 4.7 cm 3-months post-single-treatment [47, 49]. The LIPOcel system (Jeisys Medical, Seoul, South Korea) is another FDA approved system operating at 2 MHz, with the added benefit of a cooling system that protects from any skin side effects [50]. The system exploits the thermal effects of therapeutic ultrasound to destroy fat cells, with a reported reduction in waist circumference of 2–5.5 cm a month post-treatment [50]. The more novel Scizer system (Classys Inc., Seoul, Korea) uses HIFU pulses of 2 MHz that heat the targeted area to ablative temperatures. This system also uses a contact cooler and is equipped with two transducers that allow focusing at either 0.9 or 1.3 cm and has been shown to induce up to 3.4 cm waist circumference reduction [51].
On the contrary, the commercial Ultrashape system (Candela Medical, Marlborough, Massachusetts, USA) offers abdominal lipolysis based on the principle of ultrasonic cavitation. Specifically, the system utilises pulsed ultrasonic energy at 0.2 MHz that focuses at 1.5 cm, while treatment is guided by a real-time video feedback [48]. The system has been shown to result in a mean reduction in waist circumference of up to 2.5 cm after a single session [52] and up to 3.5 cm after three sessions [48].
HIFEM is the most recent FDA approved technique for abdominal fat reduction, having been approved in 2018. It differs from the abovementioned techniques since it also enhances muscle mass [53] and is mostly suited for non-obese, non-overweight people (Body Mass Index (BMI) < 25) [54], whereas most of the aforementioned techniques are suitable for people with 25 < BMI < 30 [55]. Moreover, for people to be candidates for either RF, cryolipolysis, LLLT or HIFU their abdominal thickness must exceed 2 cm, while for HIFEM an abdominal thickness of less than 2.5 cm is recommended [54]. There are two systems commercially available; the Emsculpt (BTL Aesthetics, Newcastle, UK) [54] and the StimSure (Cynosure, Westford, Massachusetts, USA) [56]. Both systems require multiple treatments not exceeding 30 min [57], and while there are published reports on the efficacy of Emsculpt (BTL Aesthetics) [54, 57] no such data exist for the StimSure (Cynosure). The Emsculpt system (BTL Aesthetics) has been found to induce up to 18% fat reduction along with an increase in muscle mass 3 months post-treatment [54], with the only reported side effect being muscle soreness which subsided 24 h post-treatment [54, 57].
Overall, non-invasive fat reduction techniques are increasingly being utilised due to their combination of proven efficacy and fewer side effects, allowing patients to perform the treatment as outpatients. However, since the area of non-invasive fat reduction techniques is fairly novel, it would highly benefit from innovative systems that could provide added advantages to the patients. In this respect, in the current study, we assess a HIFU system using a single-element HIFU transducer for evaluating its efficacy in achieving fat reduction on excised porcine adipose tissue.
Materials and methods
HIFU system
The HIFU system entailed an RF amplifier (AG1012, T&C Power Conversion Inc., Rochester, USA) and a custom-made single-element concave transducer that operates at 1.1 MHz, has a diameter of 64 mm and focuses ultrasonic beam at 63 mm.
Adipose tissue
Freshly excised porcine adipose tissue was obtained from a local slaughterhouse (Cypra Ltd, Nicosia, Cyprus). Adipose tissue was acquired by slaughterhouse personnel from the backfat of three pig carcasses. Three large pieces of backfat adipose tissue were acquired and supplied intact, with the subcutaneous fat layers having thicknesses in the 2–6 cm range. The adipose tissue pieces were supplied to the laboratory premises within several hours post-excision and were stored in a refrigerator. Preceding the conduction of any experiments, the fat tissue pieces were removed from the refrigerator and allowed to reach thermal equilibrium with the laboratory environment. Small samples were cut to the required length and width dimensions to facilitate accommodation of the adipose tissue samples in the mechanical design of the experimental set-up. The height of the adipose tissue samples was predetermined from the thicknesses of the supplied pieces.
Mechanical design of the experimental set-up
The focused transducer and sample of adipose tissue were accommodated in a 3D structure that had been printed utilising an industrial 3D printer (F270, Stratasys, Minnesota, USA) that prints parts from Acrylonitrile Butadiene Styrene (ABS) material. The transducer and adipose tissue were accommodated in the structure in an approach that allowed direct propagation of the ultrasound beam on the adipose tissue sample. The structure was designed and printed in a manner that allowed manual adjustment of the transducer-adipose tissue distance in prearranged 5 mm or 10 mm steps, thereby easily modifying the focal point of the transducer within the adipose tissue. The supplied pieces of adipose tissue had intact dermis and epidermis layers thus these were configured in a manner that they were not in the propagation path of the ultrasonic beam. In this regard, the ultrasound beam directly sonicated the subcutaneous fat layer, completely avoiding the dermis and epidermis layers. The 3D structure, transducer and adipose tissue were placed in a water tank that was filled with degassed water to a level that exceeded the top surface of the adipose tissue sample, so as to minimise ultrasonic reflections. A 3D printed (F270, Stratasys) ABS frame was configured on the 3D structure and was allocated on the top surface of the adipose tissue, securing the tissue sample so that its displacement during sonications would be avoided. The configuration of all individual parts as aforementioned is shown in Fig. 1.
Fig. 1.
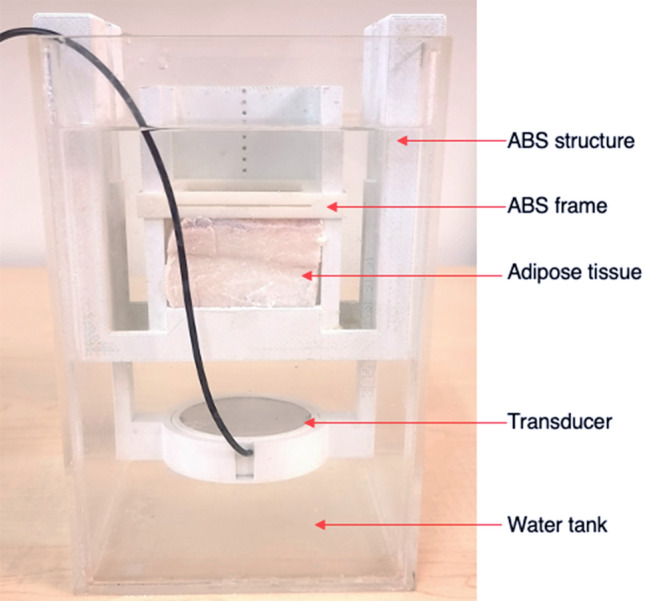
Photo of the experimental set-up
Evaluation of HIFU transducer on adipose tissue
Primarily, the adipose tissue sample was weighed utilising a high-precision balance (JSR-100, JScale, Erkelenz, Germany) and its mass was recorded. The sample was then introduced in the 3D printed ABS structure and was immersed in the water tank of the experimental set-up, whereupon ultrasonic sonications were performed. Post-sonication, the sample of adipose tissue was removed from the acrylic water tank of the experimental set-up and 15 min thereafter its mass was remeasured (JSR-100, JScale), and any difference from its initial mass (prior to sonication) was recorded. The 15-min timeframe was required to account for any amount of water absorbed during immersion of the adipose tissue sample in the water tank. Thereby, any changes arising between the initial mass of the adipose tissue sample and its mass as measured 15-min after ultrasonic sonications were attributed to the effect of the executed ultrasonic sonications and thus the observed mass change was documented as the amount of fat mass lost.
Originally, the distance between the transducer and adipose tissue was set at 40 mm, thus adjusting the focal point of the transducer to 23 mm within the adipose tissue layer. Ultrasonic sonications were implemented on 6 cm thick adipose tissue samples at either a constant acoustical power of 14 W for varied sonication times of 1, 2.5, 5, 7.5 or 10 min or different values of acoustical power of either 14, 25, 37.5, 50 or 75 W for a constant sonication time of 5 min in order to assess the effect of varying sonication parameters on the amount of adipose tissue mass lost. Regarding sonications performed at the acoustical power of 75 W for a sonication time of 5 min, these were executed at 4 different locations on a single piece of adipose tissue, and the average mass lost as a result of a single sonication pass was recorded. Contrary, all sonications performed at other abovementioned ultrasonic parameters were only executed once on pieces of adipose tissue and the amount of mass lost was recorded a single time.
The distance between the transducer-adipose tissue was then adjusted at 50 mm resulting in the focal point of the transducer being 13 mm within the adipose tissue layer. Ultrasonic sonication was executed once on a 2 cm thick piece of adipose tissue at an acoustical power of 62.5 W for a shorter sonication time of 1 min. Additionally, a single sonication was also performed utilising the abovementioned ultrasonic parameters on the same piece of adipose tissue but with the epidermis layer configured in the propagation path of the ultrasonic beam in order to assess the effect of the epidermis presence on the amount of fat mass lost as a result of the sonication.
Results
Sonications performed on a 6 cm thick piece of adipose tissue utilising an acoustical power of 14 W for varied sonication times of 1, 2.5, 5, 7.5 or 10 min resulted in distinctive mass changes recorded for each sonication time as shown in Fig. 2. Following linear regression analysis, mass change as a result of each sonication was proportionally linearly dependent with increasing sonication time (R2 = 0.9734). For sonication times between 1 and 10 min, mass changes in the range of 0.46–1.9 g were correspondingly recorded, with data showing a sharp increase. Sonications executed thereafter for a constant sonication time of 5 min and varied values of acoustical power of either 14, 25, 37.5, 50 or 75 W, similarly resulted in a proportional relationship (R2 = 0.9381) of mass change with increasing acoustical power as shown in Fig. 3. Mass changes in the range of 0.85–1.69 g were respectively recorded for acoustical power values between 14 and 75 W. The amounts of mass change for sonications performed at acoustical power values of 14–50 W were recorded for a single sonication, while the amount of mass change (1.69 g) recorded for the acoustical power of 75 W was the average mass change resulting from the four sonication passes executed at different locations. Furthermore, the four sonication passes executed at different locations on the tissue at the acoustical power of 75 W for a sonication time of 5 min resulted in the creation of cavitation in the form of vapour-filled cavities on the piece of adipose tissue as shown in Fig. 4.
Fig. 2.
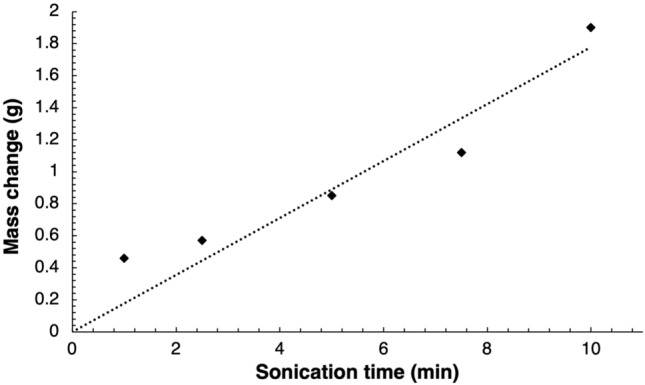
Mass change resulting sonications of the fat layer at acoustical power of 14 W for varied sonication times at 23 mm focal depth
Fig. 3.
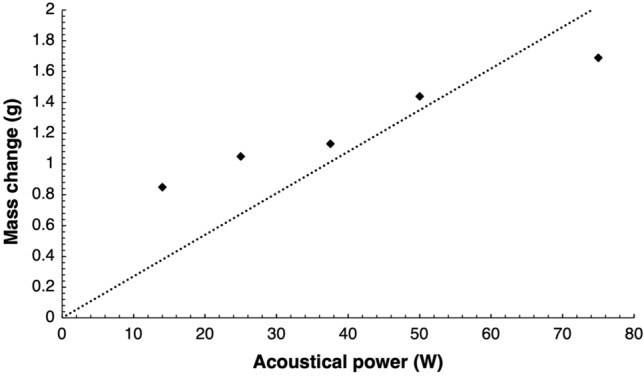
Mass change resulting sonications of the fat layer at varied acoustical power for a constant sonication time of 5 min at 23 mm focal depth
Fig. 4.
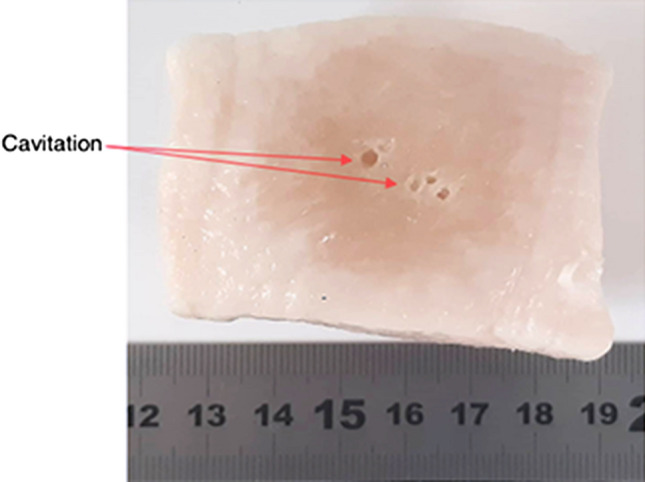
Cavitation formed on excised porcine adipose tissue resulting from four sonications performed at different locations using an acoustical power of 75 W for a sonication time of 5 min at 23 mm focal depth
Figure 5 shows the relative percentage change in fat mass as a result of the sonication executed at the 13 mm focal depth utilising the acoustical power of 62.5 W for a sonication time of 1 min on the adipose tissue layer. Figure 5 additionally shows the relative percentage change in fat mass attributed to the ultrasonic sonication that was executed at the same ultrasonic parameters but with the epidermis layer configured in the propagation path of the ultrasonic beam. A reduced by 0.18 relative percentage change in the fat mass was observed when the epidermal layer was configured in the propagation path of the ultrasonic beam. Furthermore, sonicating the adipose tissue at the abovementioned ultrasonic parameters resulted in the formation of a lesion on the piece of adipose tissue as shown in Fig. 6. The lesion was formed having a diameter of 14.05 mm and a length of 17.3 mm.
Fig. 5.

Percent mass change resulting sonication of either fat or epidermis layer at acoustical power of 62.5 W for a sonication time of 1 min at 13 mm focal depth
Fig. 6.
Lesion formed on excised porcine adipose tissue resulting from sonication of the fat layer at acoustical power of 62.5 W for a sonication time of 1 min at 13 mm focal depth. a Plane perpendicular to the beam, b plane parallel to the beam
Discussion
The primary objective of the study presented herein was the experimental evaluation of a 1.1 MHz concave ultrasonic transducer that focuses beam at 63 mm, for its ability to stimulate fat reduction of excised porcine adipose tissue. The proposed operating frequency of the transducer was precisely chosen to ensure the achievement of an adequate penetration depth within the adipose tissue since a lower frequency transducer is known to achieve a larger penetration depth. Remarkably, the chosen frequency was also agreeably found to be within the frequency range for transducers utilised in clinically approved systems that exploit either non-thermal [48] or thermal effects [46, 50, 51] of ultrasound in order to achieve a reduction of adipose tissue. The rationality for utilising porcine adipose tissue was that it showcases similar physiological characteristics with human adipose tissue [58] while being more readily accessible.
Reduction in the amount of excised adipose tissue was attained after ultrasonic sonications performed at varying values of either acoustical power or sonication time. The effect of the application of varied ultrasonic energy (sonication time × acoustical power) on the amount of fat reduction was examined through measurements performed on the mass of the adipose tissue sample prior to as well as after execution of ultrasonic sonications and calculation of any mass difference. Excised porcine dermis tissue has been previously shown to be susceptible to the rapid absorption of water during the first 20 min of its immersion in an aqueous environment [59]; thereby this resulted in significant amounts of water absorbed by the adipose tissue samples during the execution of the experimental procedure presented herein. In this regard, to account for the amount of absorbed water, the mass of the adipose tissue sample post-sonication was measured at a 15-min timeframe.
Conspicuously, the change in the mass of adipose tissue as recorded 15-min after execution of ultrasonic sonications was observed to be linearly dependent either on the use of increasing sonication time at a constant acoustical power of 14 W or increasing acoustical power upon the exploitation of a constant exposure time of 5 min. At the constant acoustical power of 14 W, increasing the exposure time by a tenfold, resulted in an increase of 1.44 g in the amount of mass change of fat tissue while an increase of 0.84 g was attained when increasing the acoustical power by approximately 19% at the constant sonication time of 5 min. Furthermore, notably, the four individual sonications executed at a high acoustical power of 75 W for a sonication time of 5 min were amply to result in the formation of small vapour-filled cavities on the piece of adipose tissue indicating the possible presence of cavitation mechanisms, contrary to sonications performed at lower values of acoustical power. Although in the present study no means of monitoring cavitation were introduced, it has been reported that acoustic cavitation thresholds are dependent on the properties of the tissue that is utilised as a propagation medium [60], with fat tissue having a greater possibility of showcasing such phenomena [61]. It has been previously shown that for sonications performed with a low-frequency transducer, the cavitation threshold does not change for excised or in vivo porcine adipose tissue, with the authors of the study [62] concluding that this relation might always apply notwithstanding patient-specific characteristics. In this regard, we form the assumption that similar results (vapour-filled cavities) with what is presented in the current study would be expected if the ultrasonic sonications executed at an acoustical power of 75 W presented herein, were performed on in vivo porcine adipose tissue.
In the present study, ultrasonic sonications were executed on the adipose tissue in a reverse direction compared to the proposed application, with the ultrasonic beam propagating directly to the fat layer, completely sparing the dermis and epidermis layers. The single sonication performed at a total ultrasonic energy of 3750 J in the normal direction, with the ultrasonic beam propagating from the epidermis to the fat layer, resulted in a decreased relative percentage change in the mass of the adipose tissue compared with the sonication performed under the same ultrasonic parameters and in the reverse direction (propagation directly to fat layer). The decreased mass change was attributed to the increased attenuation value of the epidermal layer relative to the corresponding attenuation value of fat layer [63], that considerably attenuated the beam and delivered less ultrasonic energy to the focal point. This result substantiates the findings of a study performed by Park et al. [64], where the coagulation point of a HIFU transducer was found at a smaller depth upon sonications performed on excised porcine skin with an intact epidermis layer compared to sonications executed on the skin without presence of the layer. Furthermore, herein it was observed, that the utilisation of a relatively high value of acoustical power (greater than 60 W) for short sonication time (1 min), formed a lesion on the sonicated 2 cm thick piece of adipose tissue. Notably, the formed lesion had a wide diameter and a length that was approximately extending from the fat layer to the epidermis, indicating deprived absorption of the acoustic field by the 2 cm thick piece of adipose tissue.
Although several studies [50, 65–67] have been executed on in vivo porcine adipose tissue for evaluating the effects of HIFU transducers on achieving a reduction of subcutaneous fat tissue, to the best of our knowledge, no such study has been performed on excised porcine adipose tissue. The results presented in this study showcase the potential utilisation of the proposed transducer in achieving a reduction of subcutaneous adipose tissue. Utilising an acoustical power of 25 W for 5 min resulted in a 1.05 g change in the mass of adipose tissue without any presence of cavitation effects in the form of vapour-filled cavities.
Taking into account the aforementioned results, upon future utilisation of the proposed transducer on in vivo porcine or human adipose tissue, it is assumed that a future system integrating 12 such transducers would result in a loss of 12.6 g (12 × 1.05 g) after a single session lasting 5 min. Considering that most HIFU treatments for fat reduction last approximately 60 min [47], a single session of that length would result in a loss of 151.2 g (12 × 12.6 g). Therefore, for loss of 1 kg of fat, 7 sessions would be required, each lasting 60 min. Furthermore, the acoustical power of 50 W for a sonication time of 5 min resulted in a 1.44 g change in the mass of fat tissue without the presence of vapour-filled cavities. Thereby, if an acoustical power of 50 W was used for the system integrating 12 transducers, a mass change of 207.36 g (12 × 12 × 1.44 g) would be respectively recorded for a 60-min-long session. As a result, utilising an acoustical power of 50 W, 5 sessions, each lasting 60 min, would now be required for the loss of 1 kg of fat mass. Thereby, it is assumed that a future system integrating 12 of the proposed transducers and utilising an acoustical power of 25 W or 50 W could respectively result in the loss of 1 kg of adipose tissue after 5 or 7 sessions each lasting 60 min.
Nonetheless, with the proposed transducer, the number of sessions required for loss of 1 kg of fat mass seems relatively large irrespective of the sonication parameters. Contrary to the current study where the mass of ex vivo adipose tissue was measured in order to assess the amount of fat loss, studies performed in vivo with commercially available systems, measured the amount of fat loss as a reduction in waist circumference [47, 49–52]. These clinical studies performed with commercially available systems have reported changes in waist circumference in the range of 2–5.5 cm [47, 49–52] after a single session, while an increased waist circumference reduction was observed with an increased number of sessions [48]. Taking into account that a change of 3 kg in body weight results in approximately a 3 cm reduction in waist circumference, mostly as a consequence of a reduction of the fat percentage [68], we can consider that loss of 1 kg of fat mass correspondingly results to 1 cm reduction in waist circumference. Thereby, loss of 1 kg of fat using the proposed transducer after the required number of 5–7 sessions, would result in approximately a 1 cm reduction in waist circumference. Comparing the aforementioned result with the 2–5.5 cm reduction in waist circumference resulting from a single session in previously performed trials [47, 49–52], the amount of reduction in waist circumference with the proposed transducer seems to be decreased, thus showcasing limitations of the study or the transducer. Ultimately, the number of sessions required with the proposed transducer for loss of 1 kg of fat could be greatly decreased by utilising a transducer that operates at the same frequency and has the same diameter as the proposed transducer but has a decreased radius of curvature. Utilising such a transducer would result in greater intensity gains [69], thus resulting in greater amounts of fat mass lost as a result of a single sonication, ultimately leading to a decreased number of sessions required for the loss of 1 kg of fat mass.
Nonetheless, limitations of the current study also exist when comparing the sessions required using the proposed transducer with the number of sessions implemented in studies previously performed using commercially available systems [47, 49–52]. The number of sessions required with the proposed transducer has been extracted from the amounts of fat mass lost as a result of sonications presented herein on the ex vivo adipose tissue. Contrary, the amount of waist circumference reduction as a result of the number of sessions applied in studies previously performed with commercially available systems was a result of sonications executed in vivo [47, 49–52]. Since in HIFU ablation for fat reduction apoptotic fat cells are slowly degraded through the circulatory and lymphatic systems [67], we assume that different and probably larger amounts of fat mass loss would be observed upon utilisation of the proposed transducer on in vivo porcine or human adipose tissue, thereby resulting in a reduced number of sessions required for the loss of 1 kg of fat mass.
Furthermore, commercially available systems utilising thermal effects of ultrasound, primarily operate at a frequency of 2 MHz [46, 50, 51]. The lower frequency proposed herein results in lower absorption and attenuation of the ultrasonic beam thereby the transducer can achieve improved delivery of thermal energy at the focal point [60]. Moreover, the ultrasonic beam of a lower frequency transducer is broader, thus it can achieve a larger ablative area in a single pass compared to higher frequency transducers. Furthermore, the focal length of the proposed transducer (63 mm) is considered greater than the range of focal lengths utilised in the commercially available HIFU systems. These systems can focus the ultrasonic beam from 8 mm for the LIPOcel system (Jeisys Medical, South Korea) [50] to 18 mm for the LipoSonix system (Medicis Technologies Corporation, USA) [46], however, the aforementioned focal points indicate distances taken below the skin surface. The average skin thickness is approximately 2 mm [70] and considering that commercial HIFU systems for fat reduction are appropriate for people with BMI in the range of 25–30, the average thickness of abdominal subcutaneous adipose tissue for that specific group has been reported in a study [70] as 11.10 mm and 17.62 mm for males and females respectively. Considering this information, in a potential clinical use of the transducer that is presented in the herein study, if a direct application with the skin is selected, its focal point would exceed the adipose tissue layer and thus focus in deeper areas of the abdominal cavity. Thereby, upon potential employment of the transducer in human subjects, the use of an ultrasound gel pad is strongly recommended, in order to achieve adequate focusing of the transducer within the layer of subcutaneous adipose tissue. Nevertheless, considering that the thickness of adipose tissue layer is strongly correlated with increasing BMI [71], and thicknesses up to 49 mm have been reported [72], the thickness of the potentially utilised ultrasound gel pad would be dependent on the individual measurements of each patient.
The promising results of the present study showcase the future use of the proposed transducer for achieving a reduction of subcutaneous tissue, thus expanding the range of available HIFU systems. The utilisation of the proposed transducer in humans can be achieved after further future preclinical experiments performed on in vivo porcine adipose tissue. Such experiments will provide detailed information regarding the appearance of any adverse effects, the amount of fat mass lost in vivo as well as provide the ability of histopathological examinations, thereby ensuring the efficacy and safety of the proposed transducer for its integration in a commercially available device for reduction of subcutaneous adipose tissue.
Acknowledgements
The project has been funded by the Research and Innovation Foundation of Cyprus under the project ABLABREAST (EXPLOITATION-A/0918/0006).
Declarations
Conflict of interest
All authors declare no conflicts of interest.
Availability of data and material
All data generated or analysed in the present study are available from the authors upon request.
Code availability
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (2021) Obesity and Overweight Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 25 Oct 2021
- 2.Okura T, Nakata Y, Lee DJ, Ohkawara K, Tanaka K. Effects of aerobic exercise and obesity phenotype on abdominal fat reduction in response to weight loss. Int J Obes. 2005;29(10):1259–1266. doi: 10.1038/sj.ijo.0803013. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RS, Shuman WP, Larson V, et al. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism. 1991;40(5):545–551. doi: 10.1016/0026-0495(91)90239-S. [DOI] [PubMed] [Google Scholar]
- 4.Despres JP, Pouliot MC, Moorjani S, et al. Loss of abdominal fat and metabolic response to exercise training in obese women. Am J Physiol. 1991;261(2 Pt 1):E159–E167. doi: 10.1152/ajpendo.1991.261.2.E159. [DOI] [PubMed] [Google Scholar]
- 5.Berry MG, Davies D. Liposuction: a review of principles and techniques. J Plast Reconstr Aesthetic Surg. 2011;64(8):985–992. doi: 10.1016/j.bjps.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 6.International Society of Aesthetic Plastic Surgery (2019) ISAPS Global Survey Results 2019. https://www.isaps.org/wp-content/uploads/2020/12/Global-Survey-2019.pdf. Accessed 25 Oct 2021
- 7.Illouz YG. Body contouring by lipolysis: a 5-year experience with over 3000 cases. Plast Reconstr Surg. 1983;72(5):591–597. doi: 10.1097/00006534-198311000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Venkataram J. Tumescent liposuction: a review. J Cutan Aesthet Surg. 2008;1(2):49–57. doi: 10.4103/0974-2077.44159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cárdenas-Camarena L, Gerardo LPA, Durán H, Bayter-Marin JE. Strategies for reducing fatal complications in liposuction. Plast Reconstr Surg Glob Open. 2017;5(10):1–5. doi: 10.1097/GOX.0000000000001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scuderi N, Tenna S, Spalvieri C, De Gado F. Power-assisted lipoplasty versus traditional suction-assisted lipoplasty: comparative evaluation and analysis of output. Aesthet Plast Surg. 2005;29(1):49–52. doi: 10.1007/s00266-004-0003-y. [DOI] [PubMed] [Google Scholar]
- 11.Fodor PB, Vogt PA. Power-assisted lipoplasty (PAL): a clinical pilot study comparing PAL to traditional lipoplasty (TL) Aesthet Plast Surg. 1999;23(6):379–385. doi: 10.1007/s002669900305. [DOI] [PubMed] [Google Scholar]
- 12.Hoyos AE, Prendergast PM. High definition body sculpting: art and advanced lipoplasty techniques. Berlin: Springer; 2014. [Google Scholar]
- 13.Jewell ML, Fodor PB, De Souza Pinto EB, Al Shammari MA. Clinical application of VASER-assisted lipoplasty: a pilot clinical study. Aesthet Surg J. 2002;22(2):131–146. doi: 10.1067/maj.2002.123377. [DOI] [PubMed] [Google Scholar]
- 14.Nagy MW, Vanek PF. A multicenter, prospective, randomized, single-blind, controlled clinical trial comparing vaser-assisted lipoplasty and suction-assisted lipoplasty. Plast Reconstr Surg. 2012;129(4):681–689. doi: 10.1097/PRS.0b013e3182442274. [DOI] [PubMed] [Google Scholar]
- 15.Roustaei N, Masoumi Lari SJ, Chalian M, Chalian H, Bakhshandeh H. Safety of ultrasound-assisted liposuction: a survey of 660 operations. Aesthet Plast Surg. 2009;33(2):213–218. doi: 10.1007/s00266-008-9293-9. [DOI] [PubMed] [Google Scholar]
- 16.Zelickson BD, Dressel TD. Discussion of laser-assisted liposuction. Lasers Surg Med. 2009;41(10):709–713. doi: 10.1002/lsm.20842. [DOI] [PubMed] [Google Scholar]
- 17.Kim K, Geronemus R. Laser lipolysis using a novel 1,064 nm Nd:YAG laser. Dermatol Surg. 2006;32(2):241–247. [PubMed] [Google Scholar]
- 18.Salzman MJ. My experience with the sciton ProLipo PLUS for laser lipolysis. Am J Cosmet Surg. 2010;27(2):89–91. doi: 10.1177/074880681002700209. [DOI] [Google Scholar]
- 19.Regula CG, Lawrence N. Update on liposuction: laser-assisted liposuction versus tumescent liposuction. Curr Dermatol Rep. 2014;3(2):127–134. doi: 10.1007/s13671-014-0074-1. [DOI] [Google Scholar]
- 20.Theodorou SJ, Del Vecchio D, Chia CT. Soft tissue contraction in body contouring with radiofrequency-assisted liposuction: a treatment gap solution. Aesthet Surg J. 2018;38:S74–S83. doi: 10.1093/asj/sjy037. [DOI] [PubMed] [Google Scholar]
- 21.Paul M, Mulholland RS. A new approach for adipose tissue treatment and body contouring using radiofrequency-assisted liposuction. Aesthet Plast Surg. 2009;33(5):687–694. doi: 10.1007/s00266-009-9342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theodorou SJ, Paresi RJ, Chia CT. Radiofrequency-assisted liposuction device for body contouring: 97 patients under local anesthesia. Aesthet Plast Surg. 2012;36(4):767–779. doi: 10.1007/s00266-011-9846-1. [DOI] [PubMed] [Google Scholar]
- 23.Manstein D, Laubach H, Watanabe K, Farinelli W, Zurakowski D, Anderson RR. Selective cryolysis: a novel method of non-invasive fat removal. Lasers Surg Med. 2008;40(9):595–604. doi: 10.1002/lsm.20719. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein EF, Bloom JD, Basilavecchio LD, Plugis JM. Non-invasive fat reduction of the flanks using a new cryolipolysis applicator and overlapping, two-cycle treatments. Lasers Surg Med. 2014;46(10):731–735. doi: 10.1002/lsm.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger N, Mai SV, Luebberding S, Sadick NS. Cryolipolysis for noninvasive body contouring: clinical efficacy and patient satisfaction. Clin Cosmet Investig Dermatol. 2014;7:201–205. doi: 10.2147/CCID.S44371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michon A. A prospective study determining patient satisfaction with combined cryolipolysis and shockwave therapy treatment for noninvasive body contouring. Aesthet Plast Surg. 2021;45(5):2317–2325. doi: 10.1007/s00266-021-02139-0. [DOI] [PubMed] [Google Scholar]
- 27.Ferraro GA, De Francesco F, Cataldo C, Rossano F, Nicoletti G, D’Andrea F. Synergistic effects of cryolipolysis and shock waves for noninvasive body contouring. Aesthet Plast Surg. 2012;36(3):666–679. doi: 10.1007/s00266-011-9832-7. [DOI] [PubMed] [Google Scholar]
- 28.Oh CH, Shim JS, Il Bae K, Chang JH. Clinical application of cryolipolysis in Asian patients for subcutaneous fat reduction and body contouring. Arch Plast Surg. 2020;47(1):62–69. doi: 10.5999/aps.2019.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alhaddad M, Boen M, Fabi S, Goldman MP. Noninvasive body contouring by dermal fillers, radiofrequency, and focused ultrasound: a review. Dermatol Rev. 2020;1(3):84–90. doi: 10.1002/der2.38. [DOI] [Google Scholar]
- 30.Manuskiatti W, Wachirakaphan C, Lektrakul N, Varothai S. Circumference reduction and cellulite treatment with a TriPollar radiofrequency device: a pilot study. J Eur Acad Dermatol Venereol. 2009;23(7):820–827. doi: 10.1111/j.1468-3083.2009.03254.x. [DOI] [PubMed] [Google Scholar]
- 31.Weiss R, Weiss M, Beasley K, Vrba J, Bernardy J. Operator independent focused high frequency ISM band for fat reduction: porcine model. Lasers Surg Med. 2013;45(4):235–239. doi: 10.1002/lsm.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anolik R, Chapas AM, Brightman LA, Geronemus R. Radiofrequency devices for body shaping: a review and study of 12 patients. Semin Cutan Med Surg. 2009;28(4):236–243. doi: 10.1016/j.sder.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Somenek MT, Ronan SJ, Pittman TA. A multi-site, single-blinded, prospective pilot clinical trial for non-invasive fat reduction of the abdomen and flanks using a monopolar 2 MHz radiofrequency device. Lasers Surg Med. 2020;53(3):337–343. doi: 10.1002/lsm.23295. [DOI] [PubMed] [Google Scholar]
- 34.Boisnic S, Divaris M, Nelson AA, Gharavi NM, Lask GP. A clinical and biological evaluation of a novel, noninvasive radiofrequency device for the long-term reduction of adipose tissue. Lasers Surg Med. 2014;46(2):94–103. doi: 10.1002/lsm.22223. [DOI] [PubMed] [Google Scholar]
- 35.Kapoor R, Shome D, Ranjan A. Use of a novel combined radiofrequency and ultrasound device for lipolysis, skin tightening and cellulite treatment. J Cosmet Laser Ther. 2017;19(5):266–274. doi: 10.1080/14764172.2017.1303169. [DOI] [PubMed] [Google Scholar]
- 36.Brightman L, Weiss E, Chapas AM, Karen J, Hale E, Bernstein L, Geronemus RG. Improvement in arm and post-partum abdominal and flank subcutaneous fat deposits and skin laxity using a bipolar radiofrequency, infrared, vacuum and mechanical massage device. Lasers Surg Med. 2009;41(10):791–798. doi: 10.1002/lsm.20872. [DOI] [PubMed] [Google Scholar]
- 37.Nestor MS, Newburger J, Zarraga MB. Body contouring using 635-nm low level laser therapy. Semin Cutan Med Surg. 2013;32(1):35–40. [PubMed] [Google Scholar]
- 38.Neira R, Arroyave J, Ramirez H, et al. Fat liquefaction: effect of low-level laser energy on adipose tissue. Plast Reconstr Surg. 2002;110(3):912–922. doi: 10.1097/00006534-200209010-00030. [DOI] [PubMed] [Google Scholar]
- 39.Jackson RF, Dedo DD, Roche GC, Turok DI, Maloney RJ. Low-level laser therapy as a non-invasive approach for body contouring: a randomized, controlled study. Lasers Surg Med. 2009;41(10):799–809. doi: 10.1002/lsm.20855. [DOI] [PubMed] [Google Scholar]
- 40.McRae E, Boris J. Independent evaluation of low-level laser therapy at 635 nm for non-invasive body contouring of the waist, hips, and thighs. Lasers Surg Med. 2013;45(1):1–7. doi: 10.1002/lsm.22113. [DOI] [PubMed] [Google Scholar]
- 41.Jackson RF, Stern FA, Neira R, Ortiz-Neira CL, Maloney J. Application of low-level laser therapy for noninvasive body contouring. Lasers Surg Med. 2012;44(3):211–217. doi: 10.1002/lsm.22007. [DOI] [PubMed] [Google Scholar]
- 42.Roche GC, Shanks S, Jackson RF, Holsey LJ. Low-level laser therapy for reducing the hip, waist, and upper abdomen circumference of individuals with obesity. Photomed Laser Surg. 2017;35(3):142–149. doi: 10.1089/pho.2016.4172. [DOI] [PubMed] [Google Scholar]
- 43.Bass LS, Doherty ST. Safety and efficacy of a non-invasive 1060 nm diode laser for fat reduction of the abdomen. J Drugs Dermatol. 2018;17(1):106–112. [PubMed] [Google Scholar]
- 44.Kislevitz M, Wamsley C, Kang A, Kilmer S, Hoopman J, Barillas J, Kenkel JM. Clinical evaluation of the safety and efficacy of a 1060-nm diode laser for non-invasive fat reduction of the abdomen. Aesthet Surg J. 2021;41(10):1–11. doi: 10.1093/asj/sjaa418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jewell ML, Solish NJ, Desilets CS. Noninvasive body sculpting technologies with an emphasis on high-intensity focused ultrasound. Aesthet Plast Surg. 2011;35(5):901–912. doi: 10.1007/s00266-011-9700-5. [DOI] [PubMed] [Google Scholar]
- 46.Gadsden E, Aguilar MT, Smoller BR, Jewell ML. Evaluation of a novel high-intensity focused ultrasound device for ablating subcutaneous adipose tissue for noninvasive body contouring: safety studies in human volunteers. Aesthet Surg J. 2011;31(4):401–410. doi: 10.1177/1090820X11405027. [DOI] [PubMed] [Google Scholar]
- 47.Fatemi A, Kane MAC. High-intensity focused ultrasound effectively reduces waist circumference by ablating adipose tissue from the abdomen and flanks: a retrospective case series. Aesthet Plast Surg. 2010;34(5):577–582. doi: 10.1007/s00266-010-9503-0. [DOI] [PubMed] [Google Scholar]
- 48.Moreno-Moraga J, Valero-Altés T, Martínez Riquelme A, Isarria-Marcosy MI, Royo De La Torre J. Body contouring by non-invasive transdermal focused ultrasound. Lasers Surg Med. 2007;39(4):315–323. doi: 10.1002/lsm.20478. [DOI] [PubMed] [Google Scholar]
- 49.Fatemi A. High-intensity focused ultrasound effectively reduces adipose tissue. Semin Cutan Med Surg. 2009;28(4):257–262. doi: 10.1016/j.sder.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Lee HJ, Lee MH, Lee SG, Yeo UC, Chang SE. Evaluation of a novel device, high-intensity focused ultrasound with a contact cooling for subcutaneous fat reduction. Lasers Surg Med. 2016;48(9):878–886. doi: 10.1002/lsm.22576. [DOI] [PubMed] [Google Scholar]
- 51.Hong JY, Ko JE, Choi SY, Kwon TR, Kim JH, Kim SY, Kim BJ. Efficacy and safety of high-intensity focused ultrasound for noninvasive abdominal subcutaneous fat reduction. Dermatol Surg. 2020;46(2):213–219. doi: 10.1097/DSS.0000000000002016. [DOI] [PubMed] [Google Scholar]
- 52.Ascher B. Safety and efficacy of ultrashape contour I treatments to improve the appearance of body contours: multiple treatments in shorter intervals. Aesthet Surg J. 2010;30(2):217–224. doi: 10.1177/1090820X09360692. [DOI] [PubMed] [Google Scholar]
- 53.Hoffmann K, Soemantri S, Hoffmann K, Hoffmann KKP. Body shaping with high-intensity focused electromagnetic technology. J Asthet Chir. 2020;13(2):64–69. doi: 10.1007/s12631-020-00220-2. [DOI] [Google Scholar]
- 54.Kinney BM, Lozanova P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: Safety and efficacy study of a dual tissue effect based non-invasive abdominal body shaping. Lasers Surg Med. 2019;51(1):40–46. doi: 10.1002/lsm.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guth F, Bitencourt S, Bedinot C, Sinigaglia G, Tassinary JAF. Immediate effect and safety of HIFU single treatment for male subcutaneous fat reduction. J Cosmet Dermatol. 2018;17(3):385–389. doi: 10.1111/jocd.12466. [DOI] [PubMed] [Google Scholar]
- 56.Cynosure StimSure. https://www.cynosureuk.com/product/stimsure/. Accessed 26 Oct 2021
- 57.Jacob CI, Paskova K. Safety and efficacy of a novel high-intensity focused electromagnetic technology device for noninvasive abdominal body shaping. J Cosmet Dermatol. 2018;17(5):783–787. doi: 10.1111/jocd.12779. [DOI] [PubMed] [Google Scholar]
- 58.Houpt KA, Houpt TR, Pond WG. The pig as a model for the study of obesity and of control of food intake: a review. Yale J Biol Med. 1979;52(3):307–329. [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Iwata T, Nam K, Kimura T, Wu P, Nakamura N, Hashimoto Y, Kishida A. Water absorption by decellularized dermis. Heliyon. 2018 doi: 10.1016/j.heliyon.2018.e00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperth. 2007;23(2):89–104. doi: 10.1080/02656730601186138. [DOI] [PubMed] [Google Scholar]
- 61.Li C, Chen S, Wang Q, Li H, Xiao S, Li F. Effects of thermal relaxation on temperature elevation in ex vivo tissues during high intensity focused ultrasound. IEEE Access. 2020;8:212013–212021. doi: 10.1109/ACCESS.2020.3040102. [DOI] [Google Scholar]
- 62.Kyriakou Z (2010) Use of High Intensity Focused Ultrasound to Destroy Subcutaneous Fat Tissue. Dissertation, University of Oxford
- 63.Baldeweck T, Laugier P, Berger G. In vitro study on porcine skin: attenuation profile estimation using auto-regressive modeling. Proc IEEE Ultrason Symp. 1995;2:1141–1144. doi: 10.1109/ultsym.1995.495762. [DOI] [Google Scholar]
- 64.Park JH, Lim SD, Oh SH, Lee JH, Yeo UC. High-intensity focused ultrasound treatment for skin: ex vivo evaluation. Ski Res Technol. 2017;23(3):384–391. doi: 10.1111/srt.12347. [DOI] [PubMed] [Google Scholar]
- 65.Jewell ML, Desilets C, Smoller BR. Evaluation of a novel high-intensity focused ultrasound device: preclinical studies in a porcine model. Aesthet Surg J. 2011;31(4):429–434. doi: 10.1177/1090820X11405026. [DOI] [PubMed] [Google Scholar]
- 66.Brown SA, Greenbaum L, Shtukmaster S, Zadok Y, Ben-Ezra S, Kushkuley L. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (Lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124(1):92–101. doi: 10.1097/PRS.0b013e31819c59c7. [DOI] [PubMed] [Google Scholar]
- 67.Kwon TR, Im S, Jang YJ, Oh CT, Choi EJ, Jung SJ, Hong H, Choi YS, Choi SY, Kim YS, Kim BJ. Improved methods for evaluating pre-clinical and histological effects of subcutaneous fat reduction using high-intensity focused ultrasound in a porcine model. Ski Res Technol. 2017;23(2):194–201. doi: 10.1111/srt.12319. [DOI] [PubMed] [Google Scholar]
- 68.Miyatake N, Matsumoto S, Miyachi M, Fujii M, Numata T. Relationship between changes in body weight and waist circumference in Japanese. Environ Health Prev Med. 2007;12(5):220–223. doi: 10.1265/ehpm.12.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hynynen K, Jones RM. Image-guided ultrasound phased arrays are a disruptive technology for non-invasive therapy. Phys Med Biol. 2016;61(17):R206–248. doi: 10.1088/0031-9155/61/17/R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akkus O, Oguz A, Uzunlulu M, Kizilgul M. Evaluation of skin and subcutaneous adipose tissue thickness for optimal insulin injection. J Diabetes Metab. 2012 doi: 10.4172/2155-6156.1000216. [DOI] [Google Scholar]
- 71.Ludescher B, Rommel M, Willmer T, Fritsche A, Schick F, MacHann J. Subcutaneous adipose tissue thickness in adults—correlation with BMI and recommendations for pen needle lengths for subcutaneous self-injection. Clin Endocrinol (Oxf) 2011;75(6):786–790. doi: 10.1111/j.1365-2265.2011.04132.x. [DOI] [PubMed] [Google Scholar]
- 72.De Lucia RE, Sleigh A, Finucane FM, Brage S, Stolk RP, Cooper C, Sharp SJ, Wareham SJ, Ong KK. Ultrasound measurements of visceral and subcutaneous abdominal thickness to predict abdominal adiposity among older men and women. Obesity. 2010;18(3):625–631. doi: 10.1038/oby.2009.309. [DOI] [PubMed] [Google Scholar]



