Abstract
Neurological conditions such as neurodegenerative diseases and mental health disorders are a result of multifactorial underpinnings, leading to individual-based complex phenotypes. Demystification of these multifactorial connections will promote disease diagnosis and treatment. Personalized treatment rather than a one-size-fits-all approach would enable us to cater to the unmet healthcare needs based on protein–protein and gene-environment interactions. Gut–brain axis, as the name suggests, is a two-way biochemical communication pathway between the central nervous system (CNS) and enteric nervous system (ENS), enabling a mutual influence between brain and peripheral intestinal functions. The gut microbiota is a major component of this bidirectional communication, the composition of which is varied depending on the age, and disease conditions, among other factors. Gut microbiota profile is typically unique and personalized therapeutic intervention can aid in treating or delaying neurodegeneration and mental health conditions. Besides, research on the gut microbial influence on these conditions is gaining attention, and a better understanding of this concept can lead to identification of novel targeted therapies.
Graphical Abstract
Keywords: Gut–brain axis, Neurodegeneration, Gut microbiota, Personalized medicine, Mental health
Introduction
Physical and mental health, social activity, sleep, and food are standard key drivers that promote good quality of life. For instance, a commonly neglected factor is sleep. Chronic sleep deprivation can aggravate declining brain function in turn leading to other diseases [1]. Better dietary patterns can lead to improved cognitive abilities, as shown by the Global Council on Brain Health (GCBH) [1]. Deteriorating brain conditions include dementia, neurodegeneration, unstable mental health patterns, behavioral and neuropsychiatric disorders. Neurodegenerative diseases (ND), represent a major part of the neurological disorders with heterogeneous conditions that are caused by multiple factors including progressive neurodegeneration ultimately impairing neuronal function and leading to the death of nerve cells [2]. Alzheimer’s disease (AD), and Parkinson’s disease (PD) are highly prevalent and common ND having multifactorial and complex etiology [3].
Mental illness is a broad term for disruption observed in regular mental health patterns leading to behavioral abnormalities and influenced by a combination of genetics [4], environment [5], psychological and social factors hence causing different presentations of the same disease. Diagnosis of a mental health condition can be complicated because of the complex underpinnings and varying inter-individual phenotypes [6]. Diagnosis and treatment of the disorders belonging to these categories are complicated. For example, targeting different underlying causes of Alzheimer’s disease like neuroinflammation, which can be reduced with the usage of drug Minocycline, primarily treats neuropsychiatric symptoms of AD [7]. Despite having the same disease, patients’ exhibit varied clinical phenotypes and genotypes. Health care could be compromised when a “one-drug-fits-all” approach is used for treatment. Therefore, treatments specifically tailored for patients’ needs are essential for producing better therapeutics and guide patient management. This has led to personalized way of medicine that has gained increasing importance in recent times [8]. Given the varied phenotypes of neurodegenerative and mental disorders, it would be essential to consider both protein–protein and gene-environment interactions (GxE) in the development of personalized medicine [9]. In this article, we discuss the gut–brain axis and how its disruption is interconnected to neurodegeneration, elaborating its mechanisms in AD and PD, and mental health disorders (depression, anxiety) and conditions (stress). We also emphasize the role of gut microbiota and how it can be utilized as a personalized treatment strategy for the conditions we chose to address.
Contributions of Gut–Brain Axis to Neurodegeneration and Mental Health: Disease Etiology and Treatment
Introduction to Gut–Brain Axis (GBA)
Early brain-gut interactions were shown by Pavlov in 1904, well known for his work in classic conditioning, in the cephalic phase of digestion where there was stimulation of gastric and pancreatic secretions in response to sensory signals [10]. Gut–Brain axis is bidirectional biochemical signaling or communication between the central nervous system and the enteric nervous system facilitated through neuro-immuno-endocrine pathways [11, 12]. This crosstalk between Central Nervous System (CNS) and Enteric Nervous System (ENS) is facilitated and interlinked through an autonomic nervous system (ANS) where the ANS with sympathetic (SNS) and parasympathetic (PSNS) limbs communicate the afferent signals from intestinal lumen to CNS through enteric, vagal and spinal nerves and efferent signals from CNS to intestinal wall. Communication from the brain to the intestinal cells is through neural, hormonal, and immune pathways. Immune pathways are one of the pathways of two-way communication [11].
Hypothalamic Pituitary Adrenal (HPA) Axis
HPA axis is an important part of the GBA’s hormonal influence. Dysregulation of this HPA axis can affect gut microbiota and intestinal permeability [13]. It is the core stress-efferent axis and any fluctuations within the stress levels will make the hypothalamus release corticotropin-releasing factor (CRF). CRF triggers the pituitary gland to release adrenal corticotropin hormone (ACTH) which stimulates the adrenal gland to release cortisol which is a major stress hormone. Cortisol negatively affects many body systems. Studies have shown that an upsurge in psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans, with mast cells involved in the mechanism [14]. Increasing intestinal permeability would lead to an unbalanced and unintegrated passage of microorganisms and their metabolites from the lumen to the bloodstream and rest of the body causing immune system responses and inflammation. As a result of dysbiosis, leaky gut syndrome can occur [15]. Together with the environmental stressors, increased systemic proinflammatory cytokines also activate the HPA axis [13]. This is the impact of dysregulation of the HPA axis or the hormonal influence of the brain on the intestinal functional effector cells (including epithelial cells, immune cells, smooth muscle cells, interstitial cells of cajal) [11]. The intestinal function effector cells are under the effect of gut microbiota, the colonization of which begins immediately after birth.
Vagus Nerve
Vagus nerve is a chief component of the gut–brain axis, and a segment of the parasympathetic nervous system whose function is to transmit information from the inner organs, including gut, heart, and lungs to the brain, hence involved in a wide range of functions. Parasympathetic nervous system in general has a complex influence on gastrointestinal (GI) activity [16]. Vagal efferent nerves send signals from brain to gut and the afferent ones send signals from gut to the brain. Afferent vagus nerves are associated with activation or regulation of the HPA axis, electrical stimulation of afferent vagus fibers induces the production of IL-1 beta in the brain which in turn is involved in activation or regulation of HPA axis. Vagus nerve is the longest cranial nerve arising from the brainstem and extending till the gut, leading to GI innervation. An ability to modulate gut microbiota, intestinal permeability and peripheral inflammation is observed in the vagus nerve, which is partly linked to a cholinergic pathway. Drugs targeting the cholinergic system have gained interest in treatment of neurological disorders like AD [26].
An abnormal or decreased vagal tone, a clinical measure of vagus nerve, is found in conditions including anxiety, and conditions resulting from dysbiosis like Inflammatory Bowel Diseases (IBD) and irritable bowel syndrome (IBS). Studies have shown that vagus nerve stimulation will aid in restoring homeostasis in the microbiota–gut–brain axis [17]. Treatments targeting the vagus nerve are found to be effective in increasing the vagal tone and inhibiting cytokine production therefore modulating the inflammation. The vagus nerve possesses anti-inflammatory properties that can also be utilized for developing therapeutic strategies for IBS [17], depression [18], and epilepsy [19].
Gut Microbiota
Escherichia and Enterococcus are two of the earliest colonizing bacteria. Every human has a unique profile of gut microbial composition [20]. Various processes ranging from digestion of food to immunological responses and psychological states are a function of the gut microbiota’s composition and hence, an alteration of the gut microbiota or dysbiosis can have various consequences. Gut microbiota are an important part of the gut–brain axis; they regionally interact with the intestinal walls and the ENS while also communicating with the CNS through the neuroendocrine and metabolic (metabolites released in the gut) pathways relayed through GBA. ENS development is partly modulated and mediated by gut microbiota [11, 20]. The gut microbiota research is also being extended to health benefits of other species such as poultry [21].
Modulation and protection of intestinal barrier and restoration of function of tight junctions and their integrity provenly through treatment with specific probiotics, for example, the suppression of chronic stress-mediated abnormalities of brain functions synergistically by Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 probiotic formulation which also restores tight junction barrier integrity [22]. The modulation of ENS sensory afferents takes place where the gut microbiota interacts with ENS through production of various neurotransmitters such as histamine, acetylcholine, serotonin, GABA, melatonin, and a biologically active form of catecholamine at the local level [11].
How Microbial Metabolites Modulate Brain Functions?
The major metabolites produced by gut microbiota include short-chain fatty acids (SCFAs) like acetic acid, butyric acid, and propionic acid [23]. The SCFAs also impact memory and learning processes and can even stimulate the mucosal serotonin release [23]. Other examples of GABA producing bacteria are Lactobacillus and Bifidobacterium, and serotonin producing bacteria are Candida and Enterococcus [24]. Tryptophan metabolism is an essential modulator of GBA at various levels. Metabolism of dietary substrates like amino acids by gut microbiota is an important modulatory and regulatory mechanism in tryptophan metabolism which helps in maintaining internal homeostasis. Tryptophan is a substrate for serotonin production in the brain and gut, and melatonin production in the pineal gland [25]. However, the serotonin produced in the CNS is small compared to the serotonin production in the gut which is about 90% of total body serotonin, mainly produced from enterochromaffin cells. Depression and low mood conditions have been linked with the downregulation of tryptophan metabolism [25]. Another mechanism is the effect of gut microbiota on mucosal immune activation. A balanced interaction between intestinal microbiota and mucosal immunity enables intestinal homeostasis, an imbalance of which can trigger prolonged conditions like IBD, Crohn’s disease (CD) and ulcerative colitis (UC). IBD is a heterogeneous group of chronic inflammation with a multifactorial etiology influenced by microbial, genetic, and environmental interactions. Intestinal microflora manipulation and fecal microbiota transplantation have been playing an important role in effective prevention and treatment strategy respectively [26].
Neurodegenerative Disorders
Gut microbiota Dysbiosis as a Contributing Factor in Neurodegenerative Diseases
Recent research has highlighted the role of gut microbiota in healthy brain development, function, and ageing. Healthy gut microbiota can improve overall health markers in the elderly, reduce levels of systemic inflammation, maintain healthy body weight, reduce weakness, and improve life expectancy [28]. These revelations in regard to studies done on animal models provide certain helpful insights on the role of a diverse and healthy gut microbial colony that can improve brain health thereby reducing the offshoot chances of development of ND. A study suggested that mice peripherally administered with Lactobacillus, demonstrated anti-inflammatory and antioxidant effects on the brain [28]. The examination resulted in a severe reduction of gut microbiota and twofold reduction in neurogenesis, in mice brains when administered with several broad-spectrum antibiotics, such as ampicillin or metronidazole [27]. Due to the action of these antibiotics, a decline in neurogenesis capability was observed, which consequently was restored in mice that received the probiotic mixture VSL#3, containing Bifidobacterium breve, Bifidobacterium longum among others. Administration of VSL#3 probiotics showed beneficial effects on GI health, such as decreased intestinal permeability after intestinal injury. Therefore, these studies show that the gut microbiota can have a significant impact on brain neurogenesis, which is crucial in preventing a decline in age-related memory and cognitive functions [27].
The deregulation of microbiota has been recognized as one of the factors that lead to a variety of disorders and diseases, including cardiovascular disease, liver disease, autism, AD, and PD (aspects of which are mainly focused in this review). Although each of these diseases has its unique defining characteristics, they are characterized, at least in part, by chronic inflammation, which may be the result of associated microbial dysbiosis. The effects of microbial diseases on human health are not limited to peripheral diseases but can also extend to various brain diseases [27]. The mechanism of gut microbiota in the progression of AD and PD have also been shown in Table 1.
Table 1.
Mechanism of Gut Microbiota in the Progression of AD and PD
| Alteration pathway | Mechanism | Modulation for treatment | |
|---|---|---|---|
| AD | PD | ||
| Phosphorylation | Deposition of Aβ oligomers and fibrils due to the misfolding of proteins leads to pathological changes AD characteristics. The inability to process toxic and proinflammatory inclusions is thought the basis of oxidative stress, abnormal immune activation, and chronic diseases [33] | Phosphorylation of α-syn leads to mitochondrial morphology damage and mitochondrial dysfunction, synaptic vesicle reduction, and motor impairment, while Tau phosphorylation shows a decrease in the number of microtubules and destruction of axonal myelin sheath contributing to neuronal death and neurodegeneration conjointly [36] | Probiotics, Prebiotics and FMT |
| Inflammation | LPS-induced neuroinflammation induce Aβ aggregation and memory impairment, with a significant reduction of brain-derived neurotrophic factor (BDNF) levels, while enhancing the expression of apoptosis markers like cytochrome C, Bax, caspase-9, and caspase-3 [34] | Decreased SCFA concentrations within the intestine reduce anti-inflammatory activities, supporting local and systemic inflammation. Study done mice models showed that injection of LPS resulted in proinflammation and loss of dopaminergic neurons [31] | |
| Oxidative Stress | Lactobacillus, E. coli, and Bifidobacterium have shown the metabolic activity to convert nitrate and nitrite into nitric oxide, higher levels of which increase permeability of BBB and form peroxynitrite, contributing to neurotoxicity in AD [35] | LPS triggered oxidative stress in CNS promotes the release of NO and excess NO promotes the nitration and polymerization of α-syn which is important for the progression dopaminergic neurodegeneration [23] | |
Alzheimer’s Disease (AD)
AD is a progressive neurodegenerative disease caused due to the formation of senile plaques consisting of misfolded β-amyloid (Aβ) fibrils and oligomers, along with hyperphosphorylated tau protein in regions of the brain like the cerebral cortex and hippocampus [30]. Despite the fact that the first case of AD was diagnosed almost 100 years ago, there isn’t any concrete therapy to cure the disease except drugs to temporarily relieve the symptoms and extend lifespan. Recent studies hint at the involvement of GBA in the pathophysiology of ND via microbial dysbiosis caused by the effects of changes in daily diet, exposure to antibiotics, and response to probiotics [29]. Research in this aspect shows the direct association between the gut microbiome dysbiosis and Aβ peptide aggregates in epithelial cells of the intestine. The excretion of various immunogenic components, lipopolysaccharides (LPS) and amyloid species by the gut microbiome into the local intestinal environment led to their polymerization and formation of insoluble fibrous protein aggregates which stimulates oxidative stress and further protein aggregation [31]. Further, parallel studies suggest the molecular mimicry by bacterial amyloid proteins of human Aβ owing to their structural overlap. This has the potential to induce an immune response by a foreign antigen against the self-antigens, sharing structural similarities, ultimately resulting in inflammatory responses towards cerebral Aβ. Bacterial species like Lactobacillus spp. and Bifidobacterium spp. inherently possess the ability to metabolize glutamate, an excitatory neurotransmitter helpful in the production of GABA, an inhibitory neurotransmitter [29]. These observations direct at thinking that altering the gut microbiota can compromise the endogenous production of GABA. This alteration of GABA results in altered signals in the brain which is known to be linked to AD thereby cognitive impairment and mood disorders. In addition, gut bacteria may also influence peripheral neuronal function through the production of short-chain fatty acids, neuromodulators developed as a result of bacterial fermentation of fiber supplemented by diet in the colon [31]. Generally, SCFAs stimulate the nervous system to release hormones like serotonin, which can affect the central nervous system’s cognitive operations and processes related to memory and learning capabilities. Also, catabolism of SCFAs to ketones suggestively provides an alternative/substitute source of ATP to the brain, which can counter the disrupted glucose metabolism, progressively as reported in AD patients [31].
Parkinson’s Disease (PD)
PD is the second most prevalent age-related ND, after AD. PD is characterized by progressive degeneration of dopaminergic lesions in the substantia nigra (SN) and a gradual reduction in dopamine levels, which affect both motor and non-motor functions. Patients suffering from PD show motor symptoms, such as resting tremors. While the cause of PD is yet unknown, it is believed to involve both genetic and environmental (severe head trauma) factors. Evidence suggests that the pro-inflammatory signaling molecules, like cytokines or enzymes and oxidative stress, are considered major etiological factors to neurodegeneration and dopaminergic cell death in PD. Even though there are dopamine supplementation drugs (Levodopa) for the therapy at present, it is only for temporary relief from the symptoms but does not help in the reduction of progressive degeneration [31].
Alpha-synuclein is a dynamic protein that is present in cells of the body and particularly in PD patients, higher expression of αSYN at the terminals of neurons is observed. This protein is soluble in nature and helps in regulating the dopaminergic neurons which produce the neurotransmitter dopamine. The imbalanced deposition of αSYN, followed by a β-sheet structure formation leads to compromised physiologic membrane-binding capacity, resulting in misfolded protein aggregates and further fibrils which make up the Lewy bodies dopaminergic neurons of SN (basal ganglia located in midbrain). These Lewy bodies eventually take up large space in the neurons resulting in the death and disappearance of dopaminergic cells. Similar to the pathophysiology of AD, the relation between gut microbiota and PD pathophysiology has been researched extensively. Various studies done on specific strains of probiotics like Bacillus subtilis have shown to inhibit the accumulation of alpha-synuclein when introduced in the gut of Caenorhabditis elegans model [32]. It triggered the formation of B. subtilis biofilm and production of nitric oxide, which protected an aging Caenorhabditis elegans from αSYN aggregation and also presented its reversion [32].
Gut–Brain Axis Role in Behavior, Mental Health, Psychiatry
Gut microbiota are involved in secretion of major neurotransmitters like oxytocin, dopamine, GABA, acetylcholine, noradrenaline, and histamine or in providing precursors for biosynthetic pathways [24, 37]. Lactobacillus subspecies are involved in many secretions. Feeding of Lactobacillus reuteri from a human to mice rendered an increase in oxytocin levels and improvement in wound healing capacity, and a subsequent benefit in social behavior [38]. Bacillus subspecies B. cereus, B. mycoides, B. subtilis are involved in dopamine production. Lactobacillus and Bifidobacterium microbes are producers of GABA [37]. Gut microbes are involved in signaling the enterochromaffin cells to produce serotonin through tryptophan metabolism, which has been discussed in the previous section. These neurotransmitters and neuroactive molecules are involved in behavioral and neurological processes. Alterations of Brain Derived Neurotrophic Factor (BDNF) are involved in pathogenesis of various conditions like schizophrenia, depression, epilepsy [39], aging and AD [40]. BDNF levels are crucial for normal mood levels. Administration of Lactobacillus, Bifidobacterium, or probiotics such as fructo-oligosaccharide help in increasing the hippocampal and peripheral levels of BDN [41]. Chronic stress dysregulates BDNF expression both of which are part of the etiology of depression. Acute stress leads to modified cortisol levels, whereas chronic stress causes an imbalanced and dysregulated HPA axis activity [42]. The stress also alters gut microbiota composition which in turn leads to changes in HPA axis and hence mood disorders [42]. Good gut microbiota has the ability to reduce anxiety and depression levels through inhibition of cortisol increase. Probiotic-induced corticosterone level reduction is observed in normal mice, which is an indicator of the HPA axis activity [43]. Vagus nerve plays a pivotal role as the interface of GBA and gut microbiota [17]. Stress inhibits vagus nerve leading to GI conditions and gut microbial compositions. Ingestion of Lactobacillus rhamnosus (JB-1) by mouse model showed brain region dependent modifications of GABA expression, reduced anxiety, stress and depression. Vagus nerve stimulation (VNS) has been effectively alleviating depression, epilepsy and PTSD [44].
Inflammatory signal pathway is vital for the control of neurological functions by the intestinal microbiota. An important immune component is LPS. LPS is involved in modulating amygdala’s activity, a major part of the limbic system involved in emotions [45]. Administering LPS to healthy individuals induced depression-like behaviors; and neuroinflammation in mice. LPS production happens as a result of GI imbalances and is also correlated with sickness behavior [46]. Abnormal anxiety and depression-like negative behaviors are expressed in germ-free, pathogen infected, stress or antibiotics exposed animals. Studies show the differences in emotional behavior with respect to varying gut microbiota compositions. Reversal of behavioral deficits and abnormal immune responses is seen post administration of Bifidobacterium infantis in the MS (maternal separation) rat, which is usually used to study early life stress. Similarly, a probiotic containing both Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reversed increased levels of intestinal permeability [22] and depressive behavior in rat models of myocardial infarction [47]. Improved social interaction, speech and language skills are also being observed after probiotic administration in these models [48].
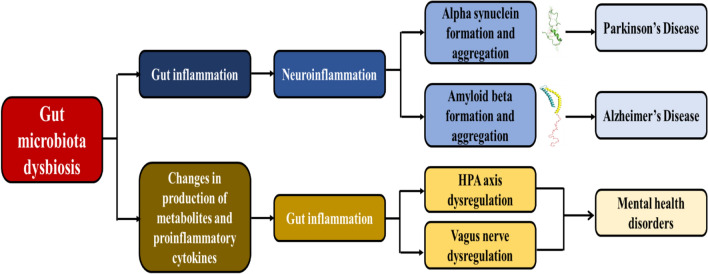
The relation between depression and microbiota–gut–brain axis is increasingly studied for exploring the possibility of new clinical interventions. Monoamine imbalances and intestinal permeability defects in gut dysbiosis is a condition common to clinical depression, interconnecting gut microbiota and mood [43]. In stress-related psychiatric conditions and depression, there is an increased expression and production of proinflammatory cytokines. Transcription of these cytokines is influenced by gut microbiota, and the SCFA metabolites are involved in reducing or inhibiting production of proinflammatory cytokines [49]. Therefore, inflammation is a major contributor to the pathophysiology of depression. This inflammation is also involved in activating the HPA axis and reducing the neurotransmitter precursor availability [49]. In fact, COX-2 inhibitor, an anti-inflammatory drug has been shown to be favorable in treating major depression and schizophrenia [50]. Fecal microbiota transplantation (FMT) is performed with the objective to achieve a health benefit through administration or transfer of fecal matter solution from a healthy donor into the patient or recipient’s intestinal tract which causes a change in intestinal microbiota composition [51]. FMT is used successfully in the treatment of Clostridium difficile infection. FMT enables replication of behavioral phenotypes and gut conditions of the host by the recipient. FMT from IBS-D patients to germ-free mice led to altered gut and immune function, and behavior [52]. Microbiota transfer from a high anxiety mouse model to low anxiety mouse model increased anxiety levels in the low anxiety mouse model [53]. Also, when this was performed in the opposite direction, where a low anxiety mouse model’s gut microbiota is transferred into a high anxiety mouse mode; there is a reduction in anxiety levels [53]. This phenomenon has also been observed in depression [54]. FMT from depression patients to GF rats resulted in implication of conditions including depression, anxiety, anhedonia, and tryptophan alterations similar to the host [55]. A brief representation of how gut microbiota dysbiosis leads to neurodegeneration and mental health disorders is shown in Fig. 1.
Fig. 1.
Gut microbiota dysbiosis leading to ND and mental health disorders
Potential of This Concept as a Personalized Treatment Strategy
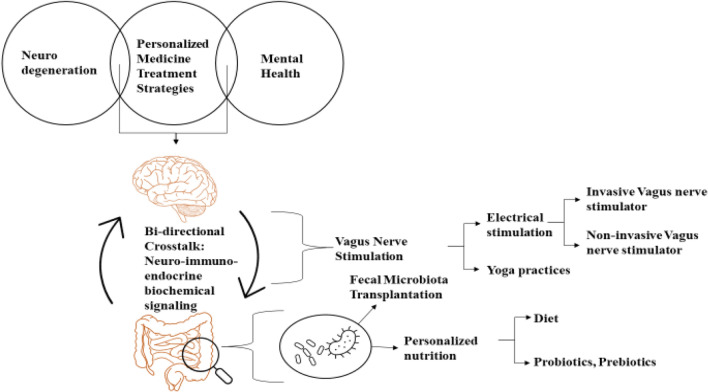
A holistic approach, termed personalized medicine, has opened a new door in medical science approaches, and the close interrelationship between microbiome and personalized medicine seems to hold a pivotal key in the treatment of ND and mental health. Since no two persons' microbiome is found to be similar, personalized treatment is found to be an essential and a necessary method for precisely treating and thereby preventing these disorders from becoming critical or life-threatening. An approach to healthcare is to understand the complex individualized mechanisms underlying a disorder. Sometimes, a single disorder yields various phenotypes in various people, hence implying its complexity. This brings the necessity of tailoring the therapeutic interventions by taking into consideration genetics, age, medical history, and other biomarkers. Predictive technologies also play a critical role in early identification of risks of developing a disease. Understanding complex gene environment interactions models in neurodegenerative and behavioral disorders are essential for developing personalized therapeutics for these diseases [5]. Modifying the external environmental risk factors from an early age through tailored nutritional needs, exercise, or at the accurate stage with treatments like probiotics, fecal microbiota transplant (FMT), VNS, etc. should help modulate the gene environment interaction, hence achieving personalized medicine. Figure 2 represents the treatment of neurodegenerative and mental health disorders through personalized treatment of disruptions of GBA.
Fig. 2.
Utilizing gut–brain axis for personalized treatment of ND and mental health disorders
In recent years, there has been an evolving appreciation for targeting GBA as a target for ND and mental health conditions. Gut is highly adaptable and modifiable for GBA-based treatments such as probiotics [15] and FMT [36, 51]; these treatments are used to recolonize the gut with healthy gut bacteria. Hence, it is evident that the use of the GBA as a target for conditions including mood disorders, and for development of personalized treatment methods could be beneficial [56]. Probiotics implementation administers one or two healthy bacterial strains to the gut [48], whereas in FMT many bacterial strains from faeces are transferred from healthy person to recipient [51]. An alternative approach for FMT is Microbial Ecosystem Therapeutics-2 (MET-2), which is currently being studied as a potential method in GAD and Major Depressive Disorder (MDD). In this approach the gut is repopulated with healthy bacteria by oral administration of lyophilized bacterial species which are initially isolated from the stool of a healthy person [57]. Prior to these treatments, the stool samples are collected from the patients. These samples are compared and analyzed for diversity and abundance of bacteria and thus the dissimilarities are recorded and the patients lacking bacterial species are administered. For this, one has to know what makes a "healthy microbiome" and use this in aforementioned personalized treatments. These treatments suggest that there is great potential for personalized treatments in mental health. Metagenomics approaches can be crucial in studying gut microbiota profile [58].
Personalizing nutrition through various diet patterns is also a beneficial strategy. A Mediterranean diet which contains large quantities of unsaturated fats, proteins, and fibers is also proved to be efficacious in preventing AD. It was also explored that across a person's life modifiable environmental factors also contribute to one-third of AD cases. These AD risk factors include diet, smoking and alcohol consumption, physical and cognitive activity, early-stage education, and social interactions at later stages [59]. Probiotics treatment has been found to reverse the PD conditions. For instance, a bacillus sp. has been found to convert L-tyrosine to L-DOPA, and thus it can restore the lost dopamine. FMT is also found to improve the non-GI symptoms of neurologic patients. It was found that administration of FMT increases DA and 5-HT levels, restores motor impairment, and improves gut dysbiosis [60]. Also, prebiotics and antibiotics were also found to result in restoring the gut ecosystem and improving psychological and cognitive brain processes. Evidence suggests that people who have undergone exposure to pesticides, industrial compounds like polychlorinated biphenyls, manganese and other metals, traumatic brain injury (TBI) and frequent concussions, and vitamin D deficiency have an elevated risk of developing PD. FMT could have the potential for treating AD and PD while taking into consideration their genetic and environmental factors, in the future [61].
Gut microbiota modifications are leading to negative variations in the proper functioning of the brain and hence leading to neurological conditions. The dysbiotic changes due to increase in biological age can make the nutrient signaling pathways ineffective and provoke pro-inflammatory innate immunity and other pathological conditions, thereby promoting unhealthy aging [62]. Maintaining gut microbiota diversity by personalizing nutrition through probiotics and prebiotics consumption from an early age can help avoid or delay the progression of diseases.
Conclusions and Future Prospects
We attempt to discuss the gut–brain axis, accentuating how a disruption in its components lead to ND and mental health conditions. An important step in having a good, diverse microbiota profile is the hands of lifestyle decisions, as microbiota is associated with brain. Consumption of high sugar, high fat-based diet can lead to disruption of the homeostasis of gut microbiota, thereby negatively impacting the integrity of gut and brain by modifying neurotransmitter metabolism, among other effects. Probiotics, prebiotics as seen in many cases in previous sections also supplement in maintaining gut health. Akkermansia is being researched as a potential treatment strategy for restoring the microbiota–gut–brain axis [63]. Manipulating gut microbiota profiles considering certain factors/biomarkers can help in personalized medicine. There are challenges surrounding the personalized medicine. The conditions that we considered in this review are multifactorial and the disruptions in gut–brain axis components is one of the contributors. Studying the unique gut microbiota profiles of patients is essential for considering this to be a personalized medicine strategy. Researchers have been investigating a number of sequencing methods with the hope of developing a novel diagnostic and therapeutic method that deciphers the relationship between the gut microbiome and neurodegenerative diseases. These include whole-genome shotgun sequencing, 16S rRNA sequencing, metatranscriptomics, metaproteomics, metabolomics, and multi-omics [64]. However, these have a number of drawbacks, such as their expense, the difficulty of assessing the sample in low abundance, and the intricacy of the data. Interpreting the data, identifying new disease biomarkers, and exploring potential therapy methods remain extremely difficult tasks. Therefore, more technological advancement is required to explore the connection between the gut microbiome and neurodegenerative disorders that aids in developing a prominent diagnostic and therapeutic approach. Although, translational significance development of such treatments in humans is still a challenge, they can possibly be a part of paradigm shift in the future treatments.
Acknowledgements
The authors thank the Principal and Management of CBIT, Hyderabad for their constant support and encouragement in carrying out this work. The authors greatly acknowledge Dr Prerana Bhan from Act Genomics and Dr Vidyani Suryadevara from Rush University for their generous inputs.
Author contributions
All authors had full access to all the contents of review paper and take responsibility for the integrity and accuracy of the text and analysis. Study concept and design: AC and CNR. Acquisition of data: AC, SDMR and JM. Analysis and interpretation of data: AC and CNR. Drafting of the manuscript: AC, SDMR, JM and CNR. Study supervision: CNR.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest to this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mintzer J, Donovan KA, Kindy AZ, et al. Lifestyle choices and brain health. Front Med. 2019;6:204. doi: 10.3389/fmed.2019.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong R. What causes neurodegenerative disease? Folia Neuropathol. 2020;58:93–112. doi: 10.5114/fn.2020.96707. [DOI] [PubMed] [Google Scholar]
- 3.Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2017 doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCammon JM, Sive H. Addressing the genetics of human mental health disorders in model organisms. Annu Rev Genomics Hum Genet. 2015;16:173–197. doi: 10.1146/annurev-genom-090314-050048. [DOI] [PubMed] [Google Scholar]
- 5.Tsuang MT, Bar JL, Stone WS, Faraone SV. Gene-environment interactions in mental disorders. World Psychiatry. 2004;3:73–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Kida S, Kato T. Microendophenotypes of psychiatric disorders: phenotypes of psychiatric disorders at the level of molecular dynamics, synapses, neurons, and neural circuits. Curr Mol Med. 2015;15:111–118. doi: 10.2174/1566524015666150303002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amani M, Shokouhi G, Salari AA. Minocycline prevents the development of depression-like behavior and hippocampal inflammation in a rat model of Alzheimer’s disease. Psychopharmacology. 2019;236:1281–1292. doi: 10.1007/s00213-018-5137-8. [DOI] [PubMed] [Google Scholar]
- 8.Goetz LH, Schork NJ. Personalized medicine: motivation, challenges, and progress. Fertil Steril. 2018;109:952–963. doi: 10.1016/j.fertnstert.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Sanzo M, Cipolloni L, Borro M, et al. Clinical applications of personalized medicine: a new paradigm and challenge. Curr Pharm Biotechnol. 2017;18:194–203. doi: 10.2174/1389201018666170224105600. [DOI] [PubMed] [Google Scholar]
- 10.Wood JD. The first nobel prize for integrated systems physiology: Ivan Petrovich Pavlov, 1904. Physiology. 2004;19:326–330. doi: 10.1152/physiol.00034.2004. [DOI] [PubMed] [Google Scholar]
- 11.Foster JA. Modulating brain function with microbiota. Science. 2022;376:936–937. doi: 10.1126/SCIENCE.ABO4220. [DOI] [PubMed] [Google Scholar]
- 12.Ahlawat S, Asha SKK. Gut–organ axis: a microbial outreach and networking. Lett Appl Microbiol. 2021;72:636–668. doi: 10.1111/lam.13333. [DOI] [PubMed] [Google Scholar]
- 13.Vicario M, Alonso C, Guilarte M, et al. Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin-releasing factor receptor type-1 upregulation in the rat intestine and IBS-like gut dysfunction. Psychoneuroendocrinology. 2012;37:65–77. doi: 10.1016/j.psyneuen.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Bhuiyan P, Wang YW, Sha HH, et al. Neuroimmune connections between corticotropin-releasing hormone and mast cells: novel strategies for the treatment of neurodegenerative diseases. Neural Regen Res. 2021;16:2184–2197. doi: 10.4103/1673-5374.310608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maguire M, Maguire G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev Neurosci. 2019;30:179–201. doi: 10.1515/revneuro-2018-0024. [DOI] [PubMed] [Google Scholar]
- 16.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 17.Bonaz B, Sinniger V, Pellissier S. Vagus nerve stimulation at the interface of brain–gut interactions. Cold Spring Harb Perspect Med. 2019 doi: 10.1101/cshperspect.a034199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS™) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–728. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 19.Ellrich J. Transcutaneous auricular Vagus nerve stimulation. J Clin Neurophysiol. 2019;36:437–442. doi: 10.1097/WNP.0000000000000576. [DOI] [PubMed] [Google Scholar]
- 20.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Kalia VC, Shim WY, Patel SKS, et al. Recent developments in antimicrobial growth promoters in chicken health: opportunities and challenges. Sci Total Environ. 2022;834:155300. doi: 10.1016/J.SCITOTENV.2022.155300. [DOI] [PubMed] [Google Scholar]
- 22.Meng C, Bai C, Brown TD, et al. Human Gut microbiota and gastrointestinal cancer. Genom. Proteom. Bioinforma. 2018;16:33–49. doi: 10.1016/j.gpb.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ait-Belgnaoui A, Payard I, Rolland C, et al. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J Neurogastroenterol Motil. 2018;24:138–146. doi: 10.5056/jnm16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 25.Strandwitz P, Kim KH, Terekhova D, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4:396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao K, Mu CL, Farzi A, Zhu WY. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr. 2020;11:709–723. doi: 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8:238–252. doi: 10.1080/19490976.2017.1290757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spielman LJ, Gibson DL, Klegeris A. Unhealthy gut, unhealthy brain: the role of the intestinal microbiota in neurodegenerative diseases. Neurochem Int. 2018;120:149–163. doi: 10.1016/j.neuint.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018 doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambrosini YM, Borcherding D, Kanthasamy A, et al. The gut–brain axis in neurodegenerative diseases and relevance of the canine model: a review. Front Aging Neurosci. 2019 doi: 10.3389/fnagi.2019.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyal D, Ali SA, Singh RK. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog Neuro-Psychopharmacol Biol Psychiatry. 2021 doi: 10.1016/j.pnpbp.2020.110112. [DOI] [PubMed] [Google Scholar]
- 32.Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. npj Park Dis. 2021;7:1–13. doi: 10.1038/s41531-021-00156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang D, Zhao D, Ali Shah SZ, et al. The role of the gut microbiota in the pathogenesis of Parkinson’s disease. Front Neurol. 2019 doi: 10.3389/fneur.2019.01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janeiro MH, Ramírez MJ, Solas M. Dysbiosis and Alzheimer’s disease: Cause or treatment opportunity? Cell Mol Neurobiol. 2022;42:377–387. doi: 10.1007/s10571-020-01024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badshah H, Ali T, Rehman S, ur, , et al. Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J Neuroimmune Pharmacol. 2016;11:48–60. doi: 10.1007/s11481-015-9623-z. [DOI] [PubMed] [Google Scholar]
- 36.Dumitrescu L, Popescu-Olaru I, Cozma L, et al. Oxidative stress and the microbiota–gut–brain axis. Oxid Med Cell Longev. 2018 doi: 10.1155/2018/2406594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu S, Hu M, Liu J, et al. Phosphorylation of Tau and α-synuclein induced neurodegeneration in MPTP mouse model of Parkinson’s disease. Neuropsychiatr Dis Treat. 2020;16:651–663. doi: 10.2147/NDT.S235562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varian BJ, Poutahidis T, DiBenedictis BT, et al. Microbial lysate upregulates host oxytocin. Brain Behav Immun. 2017;61:36–49. doi: 10.1016/j.bbi.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin TW, Harward SC, Huang YZ, McNamara JO. Targeting BDNF/TrkB pathways for preventing or suppressing epilepsy. Neuropharmacology. 2020 doi: 10.1016/j.neuropharm.2019.107734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lima Giacobbo B, Doorduin J, Klein HC, et al. Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol. 2019;56:3295–3312. doi: 10.1007/s12035-018-1283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkar A, Lehto SM, Harty S, et al. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Gianferante D, Hanlin L, et al. HPA-axis and inflammatory reactivity to acute stress is related with basal HPA-axis activity. Psychoneuroendocrinology. 2017;78:168–176. doi: 10.1016/j.psyneuen.2017.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clapp M, Aurora N, Herrera L, et al. Gut microbiota’s effect on mental health: the gut–brain axis. Clin Pract. 2017;7:987. doi: 10.4081/cp.2017.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cimpianu CL, Strube W, Falkai P, et al. Vagus nerve stimulation in psychiatry: a systematic review of the available evidence. J Neural Transm. 2017;124:145–158. doi: 10.1007/s00702-016-1642-2. [DOI] [PubMed] [Google Scholar]
- 46.Zheng ZH, Tu JL, Li XH, et al. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav Immun. 2021;91:505–518. doi: 10.1016/j.bbi.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Lasselin J, Schedlowski M, Karshikoff B, et al. Comparison of bacterial lipopolysaccharide-induced sickness behavior in rodents and humans: relevance for symptoms of anxiety and depression. Neurosci Biobehav Rev. 2020;115:15–24. doi: 10.1016/j.neubiorev.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Trudeau F, Gilbert K, Tremblay A, et al. Bifidobacterium longum R0175 attenuates post-myocardial infarction depressive-like behaviour in rats. PLoS ONE. 2019 doi: 10.1371/journal.pone.0215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Partrick KA, Rosenhauer AM, Auger J, et al. Ingestion of probiotic (Lactobacillus helveticus and Bifidobacterium longum) alters intestinal microbial structure and behavioral expression following social defeat stress. Sci Rep. 2021;11:1–12. doi: 10.1038/s41598-021-83284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Venegas DP, De La Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iob E, Kirschbaum C, Steptoe A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol Psychiatry. 2020;25:1130–1140. doi: 10.1038/s41380-019-0501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Müller N. COX-2 inhibitors, aspirin, and other potential anti-inflammatory treatments for psychiatric disorders. Front Psychiatry. 2019 doi: 10.3389/fpsyt.2019.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim KO, Gluck M. Fecal microbiota transplantation: an update on clinical practice. Clin Endosc. 2019;52:137–143. doi: 10.5946/ce.2019.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Palma G, Lynch MDJ, Lu J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 55.Li N, Wang Q, Wang Y, et al. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress. 2019;22:592–602. doi: 10.1080/10253890.2019.1617267. [DOI] [PubMed] [Google Scholar]
- 56.Bruce-Keller AJ, Salbaum JM, Luo M, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77:607–615. doi: 10.1016/j.biopsych.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyyappan AC, Milev R. The safety, efficacy, and tolerability of microbial ecosystem therapeutic-2 in people with major depression and/or generalized anxiety disorder: protocol for a phase 1, open-label study. JMIR Res Protoc. 2020 doi: 10.2196/17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandal RS, Saha S, Das S. Metagenomic surveys of gut microbiota. Genom Proteom Bioinform. 2015;13:148–158. doi: 10.1016/j.gpb.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kashyap PC, Chia N, Nelson H, et al. Microbiome at the frontier of personalized medicine. Mayo Clin Proc. 2017;92:1855–1864. doi: 10.1016/j.mayocp.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahman MA, Rahman MS, Uddin MJ, et al. Emerging risk of environmental factors: insight mechanisms of Alzheimer’s diseases. Environ Sci Pollut Res. 2020;27:44659–44672. doi: 10.1007/s11356-020-08243-z. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, Yang F, Zhang S, et al. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. Dnpj Park Dis. 2021;7:1–10. doi: 10.1038/s41531-021-00213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Badal VD, Vaccariello ED, Murray ER, et al. The gut microbiome, aging, and longevity: a systematic review. Nutrients. 2020;12:1–25. doi: 10.3390/nu12123759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalia VC, Gong C, Shanmugam R, et al. The Emerging Biotherapeutic Agent: Akkermansia. Indian J Microbiol. 2022;62:1–10. doi: 10.1007/S12088-021-00993-9/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Z, Li J, Gui S, et al. Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. NeuroReport. 2018;29:417–425. doi: 10.1097/WNR.0000000000000985. [DOI] [PubMed] [Google Scholar]