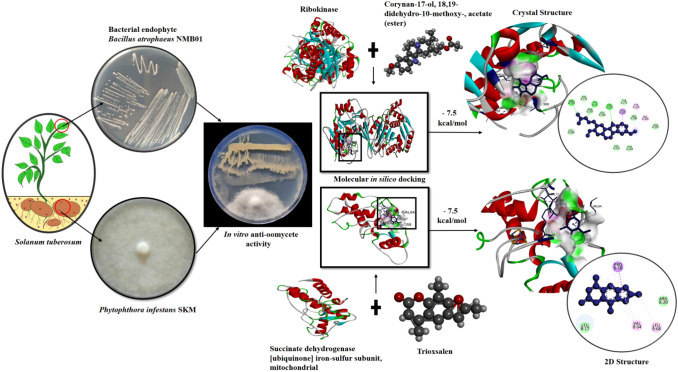
Abstract
The antagonistic Bacillus spp. is known well for the production of versatile antimicrobial biomolecules with broad spectrum of action against different types of plant pathogens. Considering the significance of metabolically active biomolecules, attempts were made to decipher the anti-oomycete nature of biomolecules produced by Bacillus atrophaeus NMB01 during di-trophic interaction with Phytophthora infestans. Ten biomolecules produced by B. atrophaeus NMB01 during di-trophic interaction with P. infestans were docked against the twelve target proteins of P. infestans. Molecular docking of biomolecules reported trioxsalen and corynan-17-ol,18,19-didehydro-10-methoxy-acetate(ester) as best hits with highest binding energy in the range of − 7.5 to − 5 kcal/mol against target proteins of P. infestans. Comparatively less binding energy was observed for commercially available fungicides mandipropamid and metalaxyl on docking against the target proteins of P. infestans. We also confirmed the direct impact of trioxsalen andcorynan-17-ol, on P. infestans under in vitro with 66% and 50% inhibition of mycelial growth of P. infestans, respectively. This is the first study attempted to untangle the role of bioactive anti-oomycete compounds produced by B. atrophaeus strain NMB01 during di-trophic interaction with P. infestans against late blight pathogen P. infestans infecting potato. From the present study, we conclude that the biomolecules, trioxsalen and corynan-17-ol, can be explored for the management of P. infestans, the incitant of late blight of potato.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-022-01044-7.
Keywords: Bacillus atrophaeus NMB01; Phytophthora infestans; Trioxsalen; Corynan-17-ol,18,19-didehydro-10-methoxy-, acetate (ester); Molecular docking
Introduction
Globally, potato (Solanumtuberosum L.) is consumed as the third most important food crop next to wheat and rice. Potato, being the staple food in most of the countries, it also serves as a repository of nutrients and minerals. However, the yield and productivity of potato is hampered due to late blight of potato incited by oomycete P. infestans. In severe cases of infection by P. infestans, the yield losses of upto 100% have been reported [1]. In this juncture, several chemical fungicides have been used to manage P. infestans. However, in due course of time the efficacy of fungicide was declined due to the development of resistance in the pathogen. Further, existence of both mating types (A1 and A2) of P. infestans allows for sexual reproduction and results in new gene combination and production of oospores as survival structures and thus renders the disease more difficult to manage [2].
Leading to the emergence of fungicide resistance and occurrence of sexual forms among the strains of P. infestans, it is necessary to discover new anti-oomycete molecules for the management of P. infestans [3]. In this regard, environmental-friendly method of biological control can provide a distinct line of defence. Bacterial endophytes Bacillus spp. are the potent inhibitors of P. infestans through their versatile mode of action and by the production of antifungal biomolecules [4]. Among the various endophytic bacterial strains, antifungal biomolecules produced by the antagonist B. atrophaeus NMB01 during di-trophic interaction with P. infestans were docked to decipher the mode of action against P. infestans. The cyclic lipopeptides (CLPs), Iturin A and Fengycin A produced by B.megaterium WL-3 strain have anti-oomycete effect against P. infestans [5]. B. atrophaeus strain S2BC-2 hadchitinolytic and antifungal activityagainst oomycetous fungus causing rhizome rot of ginger [6]. B. atrophaeus GBSC56 had the ability to promote plant growth and also possessed strong nematicidal activity against Meloidogyne incognita [7]. Lipopeptides and volatile compounds from B. atrophaeus CAB-1 had inhibitory activity against Botrytis cinerea and Sphaerothecafuliginea [8]. Similarly, Huang et al. [9] reported the antagonistic properties of B. atrophaeus XW2 against Colletotrichum gloeosporioides. Furthermore, the disease incidence of apple ring rot caused by Botryosphaeria dothidea was reduced due to the antifungal nature of B. atrophaeus J-1 [10]. Screening of potential bioactive compounds using in vitro and in vivo evaluation is very difficult and time consuming. As an outcome, molecular modelling and docking would facilitate to trace powerful anti-oomycete biomolecules. Singh et al. [11] reported S-adenosyl-L-homocysteine (SAH) and nicotinamide adenine dinucleotide (NAD) bound to the active regions of RXLR proteins of P. infestans. Similarly, Wang et al. [12] reported the anti-oomycete activity of pyrazolyl oxime ethers by the binding ability of these compound to the protein succinate dehydrogenase. Considering the significance, in silico analysis was carried out to dock ten antifungal biomolecules produced during di-trophic interaction between B. atrophaeusstrainNMB01 and P. Infestans against twelve protein targets of P. infestans to discover the biomolecule with anti-oomycete action.
Materials and Methods
Source of P. infestans and Bacterial Endophyte Strain
The cultures of plant pathogenic fungus P. infestans isolate SKM and six bacterial antagonist B. atrophaeusstrain NMB01, B. subtilis NM01, B. subtilis NM02, B. subtilis NM03, B. subtilis NM05, B. subtilis NM06 were collected from the culture collection center, Department of Plant Pathology, Centre for Plant Protection Studies, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India pertaining to the NCBI accession No. MW285753, ON101255, ON411601, ON411602, ON411603, ON411604 and ON411607respectively.
Screening for Anti-oomycete Activity of Bacterial Endophytes Against P. infestans
Six endophytic bacterial strains were tested for their antagonistic activity against mycelial growth of P. infestans in vitro. The anti-oomycete bioassay using dual culture method and the percent inhibition of pathogen was calculated as per the protocol described by kim et al. [13].
Extraction and Characterization of Bioactive Compounds from the Zone of Inhibitionduring Di-trophic Interaction and Produced by B. atrophaeus NMB01 Alone
The biomolecules produced during di-trophic interaction between P. infestans and B. atrophaeus NMB01 and produced by B. atrophaeus NMB01 alone were extracted and characterized as per the protocol described byCawoy et al.[14].
Identification of Target Proteins of P. infestans
Based on a literature review, twelve effective protein targets of P. infestans were identified and used to assess the efficacy. The target proteins include autophagy-related protein 8 [15], bZIP transcription factor 1 [16], calmodulin [17], calreticulin [18], cytochrome c oxidase subunit 1 [19], glucanase inhibitor protein 3 [20], methionine aminopeptidase [21], ribokinase [22], RXLR effector protein Avr 1 [23], serine/threonine—protein kinase PKZ1 [24], succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial [19] and uncharacterized protein (PITG_15862) [25]. Through UniProt database, the protein sequence of the targets of P. infestans were retrieved and used for molecular docking studies.
Molecular Modelling and Structure Validation of P. infestans Target Proteins
The crystal structure of the selected targets of P. infestans was not found in the Protein Data Bank Database (PDB, https://www.rcsb.org/). Hence, molecular modelling was done using SWISS-MODEL (Method: Rigid-body assembly), Phyre2 (Method: Profile based alignment), and ROBETTA (Meta server, https://robetta.bakerlab.org/). FASTA sequences of the targets were subjected to BLAST to compare the protein query from protein database. Based on query coverage performance and percentage identity obtained from Blastp, SWISS-Model, Phyre2 and ROBETTA software were used. Modelled target proteins were validated using the Ramachandran plot of the PROCHECK tool from the Structural Analysis and Verification Server (SAVES, Meta server) (https://saves.mbi.ucla.edu/). The torsional angles psi and phi of the aminoacid residues were checked using the Ramachandran plot [26] which provide outline on the 3D chemistry of a protein structure.
Preparation of Ligands for the Biomolecules Produced During Di-trophic Interaction by B. atrophaeus against P. infestans
For docking analysis, based on the peak area percentage ten antifungal compounds produced during di-trophic interaction by B. atrophaeusNMB01towards the suppression of P. infestans were used for docking analysis, which includesCorynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester)); Trioxsalen; 3-Methyladipic acid; Furazanamine, 4-azido; 2-Benzyl-2-methyl-1,3-oxathiolane; Deoxyspergualin; 2-Methylbutanoic anhydride; Diethylene glycol monolaurate; Pentadecanoic acid and Benzeneacetic acid. All two-dimensional (2D) and three dimensional (3D) structures of ten ligands were retrieved from PubChem chemical data base (https://pubchem.ncbi.nlm.nih.gov/) as SDF format. The commercial fungicides mandipropamid and metalaxylwereused as a reference ligand molecule to ascertain the efficiency of test molecules againstP.infestans.
Virtual screening and Ligand–Protein Docking of Biomolecules with Target Proteins of P. infestans
Molecular docking study was carried out using PyRx 0.8AutoDockvina module [27]. Proteins were made using PyRx's make macromolecule option. All ligand structures were minimized using conjugate gradient, 200-step optimization, using commercial molecular mechanics parameters-unified force field (UFF). Target binding site pockets were found using CASTp3.0 [28]. Using AutoDock4 and autogrid4, ligands were configured for grid and docking. During docking, ligands could adopt variable conformations and orientations with an exhaustiveness of 8. Target proteins and ligand structures were translated to PDBQT format. BIOVIA discovery studio client 2021 was used to visualize docked protein–ligand complex interactions.
Effects of Trioxsalen and Corynan-17-ol, 18,19-Didehydro-10-Methoxy-, Acetate (ester) on Mycelial growth of P. infestans in vitro
The antifungal activity of corynan-17-ol, 18, 19-didehydro-10-methoxy-, acetate (ester) and trioxsalen were assessed against P. infestans as per the protocol described by Islam et al. [29].
Scanning Electron Microscopy
The mycelium of P. infestans treated with trioxsalen, corynan-17-ol, 18, 19-didehydro-10-methoxy-, acetate (ester) and mycelium grown in untreated control plates were transferred to carbon stubs after sputter coating and observed under scanning electron microscope [30].
Statistical analysis
The experiment was conducted in a completely randomized design. The mean values of the treatments were analyzed with ANOVA at 5% level of significance. All data were explained using the statistical analysis tool Web Agri Stat Package 2.0 (WASP).
Results
Antagonistic activity of six bacterial endophytes against P. infestans
The antagonistic activity ofB. atrophaeus NMB01, B. subtilis NM01, B. subtilis NM02,B. subtilis NM03, B. subtilis NM05 and B. subtilis NM06 against P. infestans revealed that the mycelial reduction of P. infestanswas 78.10%, 51.30%, 50.13%, 42.06%, 39.00% and 33.30% respectively over untreated control (Fig. S1 and Table S1).
Anti-Oomycete Activity of Bioactive Compounds Against P. infestansand Identification of Anti-Oomycetousbio Molecules Produced During Di-trophic Interaction
Among all the six bacterial endophytes tested, B. atrophaeus NMB01 produced the highest mycelial reduction percentage when compared to other endophytes. VOC/NVOC bioactive metabolites extracted from the zone of inhibition, produced by NMB01 during the interaction with P. infestans revealed the presence of ten antimicrobial compounds. The compounds were identified as corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester), trioxsalen, deoxyspergualin, benzeneacetic acid, 2-Benzyl-2-methyl-1,3-oxathiolane, 2-Methylbutanoic anhydride, furazanamine, 4-azido-, diethylene glycol monolaurate, pentadecanoic acid,3-Methyladipic acid.The GC–MS analysis depicts the chromatogram of theseten compounds(Fig. S2 andTable S2). These compounds were further taken for docking analysis.
Similarly, twelve biomolecules viz., Azafrin, Acifluorfen, N-Nitrosopyrrolidine, Clindamycin, Phenalenone, 7-Gamabufotalin, 2(3H)-Furanone, 5-heptyldihydro-, 5-Hydroxymethylfurfural, Aziridine, 2-methyl-2-(2,2,4,4-tetramethylpentyl)-, 6''-O-Acetyldaidzin, Papaverine and Bacillibactin were produced by B.atrophaeus NMB01 in the absence of P. infestans (Fig. S3). The biomolecules produced by P. infestans were identified as 3H-Pyrazol-3-one, 2,4-dihydro-2,4,5-trimethyl, 1-Nitro-2-acetamido-1,2-dideoxy-d-mannitol, Oleic Acid, Butanoic acid,2-ethyl-2,3,3-trimethyl- and Melezitose(Fig. S4).However, the compounds produced by P. infestans were not produced by B. atrophaeus NMB01 and during its interaction withP. infestans.
Molecular Modelling and Validation of Target Proteins
The sequence identity between template and 3D model structure using SWISS- MODEL server for autophagy-related protein 8 (PDB ID-6WY6), calreticulin (PDB ID-6ENY), cytochrome c oxidase subunit 1 (PDB ID-6PW1), ribokinase (PDB ID-6ILR), methionine aminopeptidase (PDB ID-4IU6) and succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial (PDB ID-3ABV) proteins of P.infestans had 77.39%, 52.16%, 65.97%, 43.51%, 55.41% and 67.22% identity, respectively (TableS3). Furthermore, Ramachandran plot validation of the modelled 3D structure of autophagy-related protein 8, calreticulin, cytochrome c oxidase subunit 1, ribokinase, methionine aminopeptidase and succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial showed 96.0%, 84.7%, 93.3%, 91.7%, 94.5% and 85.6% residues in the allowed region of the plot, respectively (Fig. S5).
As no template structure were found forglucanase inhibitor protein 3 and serine/threonine—protein kinase PKZ1 of P. infestans. The3D structure was modelled using Phyre2 software. The Phyre2 based modelled structurehad 100% confidence score and the percent coverage was 94% and 65% for glucanase inhibitor protein 3 and serine/threonine—protein kinase PKZ1 respectively (TableS4). Similarly, ROBETTA was used for predicting 3D structure of bZIP transcription factor 1, calmodulin, RXLR effector protein Avr 1 and uncharacterized protein (PITG_15862).The predicted 3 D structure of bZIP transcription factor 1, calmodulin, RXLR effector protein Avr 1 and uncharacterized protein had the confidence score of 50%, 65%, 69% and 42% sequentially (TableS5). Similarly, validation of the 3 D structure of glucanase inhibitor protein 3, serine/threonine—protein kinase PKZ1, bZIP transcription factor 1, calmodulin, RXLR effector protein Avr 1 and uncharacterized protein through Ramachandran plot had 82.9%, 67.7%, 84.0%, 91.7%, 93.5% and 77.8%ofaminoacid residues in the most favored region, respectively(Fig. S6).
Molecular Docking of Biomolecules Produced from the Zone of Inhibition Against Target Proteins of P. infestans
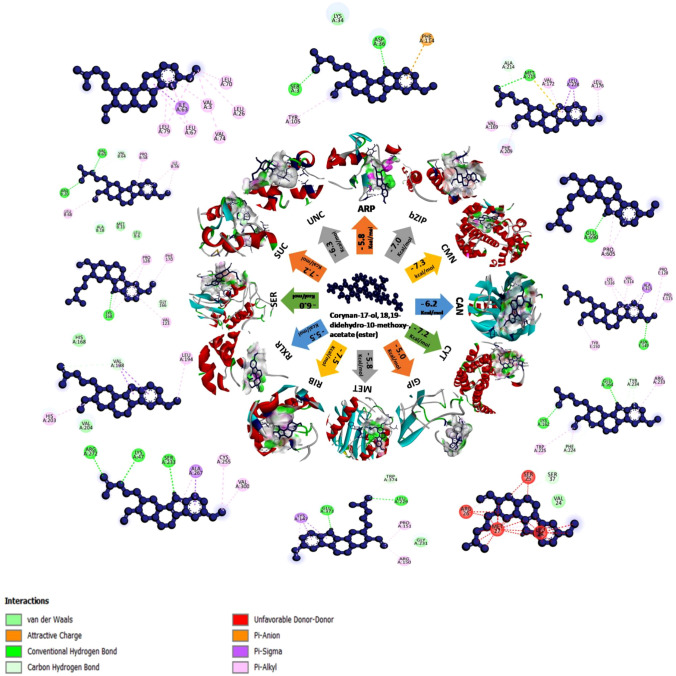
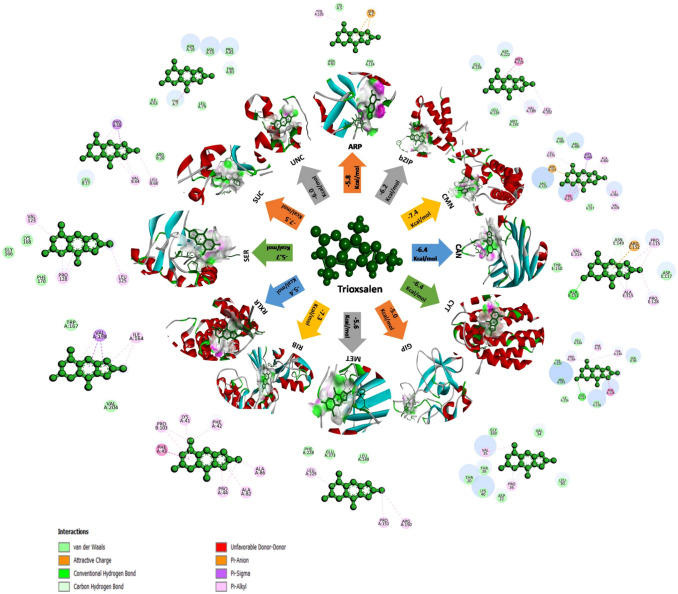
Molecular docking (120 docking runs) were performed to understand the interaction betweenten antifungal compounds of B. atrophaeusNMB01( Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester), Trioxsalen, Deoxyspergualin, Benzeneacetic acid, 2-Benzyl-2-methyl-1,3-oxathiolane, 2-Methylbutanoic anhydride, Furazanamine, 4-azido-, Diethylene glycol monolaurate, Pentadecanoic acid, 3-Methyladipic acid) produced during di-trophic interaction and twelve receptor proteins (autophagy-related protein 8, bZIP transcription factor 1, calmodulin, calreticulin, cytochrome c oxidase subunit 1, glucanase inhibitor protein 3, methionine aminopeptidase, ribokinase, RXLR effector protein Avr 1, serine/threonine—protein kinase PKZ1, succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial and uncharacterized protein (PITG_15862)) of P.infestans.From the ten compounds, corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) and trioxsalen had the highest binding energy in the range of − 7.5 to − 5 kcal/mol which was shown in the heat map (Fig. S7). In the following section, we discussed all the high affinity molecules and information of H-bonding, hydrophobic interactions, Van der waals force, Pi-Alkyl and Carbon hydrogen bond between ligands and target proteins were illustrated by the interacting aminoacids (Table S6). Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) and trioxsalen had the highest binding energy of − 7.5 kcal/mol with the target ribokinase and succinate dehydrogenase respectively. Corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) had the binding affinity of − 7.3 kcal/mol with target calmodulin, − 7.2 kcal/mol for cytochrome c oxidase subunit 1and succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial, − 7.0 kcal/mol for bZIP transcription factor, − 6.3 kcal/mol for uncharacterized protein (PITG_15862), − 6.2 kcal/mol for calreticulin, − 6.0 kcal/mol for serine/threonine—protein kinase PKZ1, − 5.8 kcal/mol for autophagy-related protein 8and methionine aminopeptidase, − 5.5 kcal/mol for RXLR effector protein Avr 1 and − 5.0 kcal/mol for glucanase inhibitor protein 3(Fig. 1). Similarly, trioxsalen had the maximum binding affinity of − 7.4 kcal/mol with target calmodulin followed by − 7.3 kcal/mol for ribokinase, − 6.4 kcal/mol for cytochrome c oxidase subunit 1 and calreticulin, − 6.2 kcal/mol for bZIP transcription factor 1, − 6 kcal/mol for uncharacterizedprotein (PITG_15862), − 5.8 kcal/mol for autophagy-related protein, − 5.7 kcal/mol for serine/threonine—protein kinase PKZ1, − 5.6 kcal/mol for methionine aminopeptidase, − 5.5 kcal/mol for RXLR effector protein Avr 1 and − 5.0 kcal/mol for glucanase inhibitor protein 3 (Fig. 2).These two biomolecules had greater binding affinity than deoxyspergualin, benzeneacetic acid, 2-Benzyl-2-methyl-1,3-oxathiolane, 2-Methylbutanoic anhydride, furazanamine, 4-azido-, diethylene glycol monolaurate, pentadecanoicacidand 3-Methyladipic acid.
Fig. 1.
Illustrative diagram representing the protein–ligand interaction among twelve target proteins of P. infestans and the bioactive compound corynan-17-ol,18,19-didehydro-10-methoxy-,acetate(ester). 2D structure which is in blue colour depicts the various types of interaction between them. [ARP- Autophagy-related protein 8; bZIP- bZIP transcription factor 1; CMN- Calmodulin; CAN- Calreticulin; CYT- Cytochrome c oxidase subunit 1; GIP- Glucanase inhibitor protein 3; MET- Methionine aminopeptidase; RIB- Ribokinase; RXLR- RxLR effector protein Avr1; SER- Serine/threonine-protein kinase PKZ1; SUC- Succinate dehydrogenase [ubiquinone] iron-sulfursubunit,mitochondrial; UNC-Uncharacterized protein (PITG_15862)]
Fig. 2.
Illustrative diagram representing the protein–ligand interaction among twelve target proteins of P. infestans and the bioactive compound trioxsalen. 2D structure which is in green colour depicts the various types of interaction between them. [ARP- Autophagy-related protein 8; bZIP- bZIP transcription factor 1; CMN- Calmodulin; CAN- Calreticulin; CYT- Cytochrome c oxidase subunit 1; GIP- Glucanase inhibitor protein 3; MET- Methionine aminopeptidase; RIB- Ribokinase; RXLR- RxLR effector protein Avr1; SER- Serine/threonine-protein kinase PKZ1; SUC- Succinate dehydrogenase [ubiquinone] iron-sulfursubunit,mitochondrial; UNC-Uncharacterized protein (PITG_15862)]
The binding affinity of these novel biomolecules were compared with the fungicides mandipropamid and metalaxyl, which were used for the management of P. infestans. Out of twelve targets, succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial had the highest binding affinity of − 7.1 kcal/mol and − 5.7 kcal/mol respectively (Fig. S8). But the binding affinity of these fungicides was less than corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) and trioxsalen. Analysis on the number of hydrogen bonds formed by corynan-17-ol, trioxsalen and mandipropamid with twelve target proteins of P. infestansthrough Circos plot demonstrate the strength of docked complex(Fig. S9).Thus, docking score confirmed that, corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) and trioxsalen can be explored for the management of P.infestans.
Anti-oomycete Activity of Trioxsalen and Corynan-17-ol, 18,19-Didehydro-10-Methoxy-, Acetate (Ester) against p. infestans
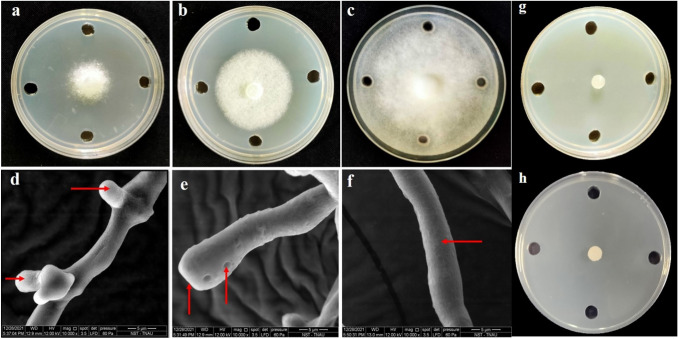
Anti-oomycete activity of trioxsalen and corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) against P. infestans revealed that, mycelial growth of P. infestans was reduced upto 66% and 50% respectively over untreated control. In a similar manner, mandipropamid and metalaxyl were able to totally suppress the mycelial growth of P. infestans at 1000 ppm concentration (Fig. 3 and Fig. S10).Examination of the mycelium exposed to the biomolecules induced swellings, dissolution of cell wall, formation of hyphal protrusions and holes on the hyphal wall and thus resulted in hyphal abnornamalities (Fig. 3).
Fig. 3.
Inhibitory effect of trioxsalena and corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester) b on the mycelial growth of P. infestans, untreated control c and the abnormalities in the morphology of P. infestans under scanning electron microscopy. d hypha with protrusions under the effect of trioxsalen, e swollen and hypha with hole due to the effect of corynan-17-ol, 18,19-didehydro-10-methoxy-, acetate (ester),f healthy hypha of P. infestans, ginhibitory effect of mandipropamid and hmetalaxyl on the mycelial growth of P. infestans
Discussion
Late blight caused by P. infestans hinders the potato production overseas. Metalaxyl, inhibit ribosomal RNA synthesis and suppress P. infestans [31]. Similarly, CAA fungicides mandipropamid hinder phospholipid and cellulose biosynthesis. Continuous use of metalaxyl in Ireland, Netherlands, and Switzerland develop resistance in P. infestans [32]. Thus, it warrants eco-friendly management. Bacterial endophytes are a promising alternative. Bacillus genus produce antifungal biomolecules and suppress P. infestans [33, 34].
Molecular docking, is used to discover novel biomolecules against the target proteins of P. infestans. In silico docking with twelve protein targets of P. infestans and ten biomolecules of B. atrophaeus led to the discovery corynan-17-ol, and trioxsalencompared to the fungicides mandipropamid and metalaxyl. The maximum binding energy of corynan-17-ol, and trioxsalen with the target protein ribokinase inhibited the formation of ribose-5-phosphate [22]. Likewise, maximum binding energy of trioxsalenand corynan-17-ol, with succinate dehydrogenase, mitochondrial and cytochrome c oxidase might hamper the availability of ATP required for pathogenicity [19]. Similarly, interaction of trioxsalen and corynan-17-ol,with calmodulin, bZIP transcription factor 1 and RXLR effector protein Avr 1 could block signaling pathway during plant–microbe interaction [17], appressorial formation[16]and host immunity [23] respectively. Thus, interaction of ligand with 12 different target proteins of P. infestansmight prevent host pathogen interaction and emphasize the multiple mode of action leading to suppression of resistant development. Furthermore, binding of both corynan-17-ol, and trioxsalen with uncharacterized protein (PITG_15862), calreticulin, glucanase inhibitor protein 3, serine/threonine—protein kinase PKZ1, autophagy-related protein 8 and methionine aminopeptidase might suppress oospore formation.[25], hamperpathogenesis [18], defense response of pathogen [20], zoospore development [24], virulence [15] and pathogenicity [21].Moreover, Amorosa et al.[35] and Zhang et al. [36] reported the antifungal nature of biomolecules against P. infestans. Similarly, corynan-17-ol, and trioxsalen produced by B. Atrophaeus also suppressed P. infestans. However, based on the available literatures, anti-oomycete activity of corynan-17-ol, and trioxsalen has not been reported yet. Hence, this is the first report, explaining the anti-oomycetes nature against P. infestans.
Conclusion
The result obtained from the study confirmed the anti-oomycete nature of bioactive compounds of B. atrophaeus NMB01 produced during di-trophic interaction against P. infestans. The binding energies of the protein–ligand interactions and the in vitro observations also confirmed that the ligands corynan-17-ol, 18,19-didehydro-10-methoxy-acetate(ester) and trioxsalen served as a potential inhibitors of the target proteins autophagy-related protein 8, bZIP transcription factor 1, calmodulin, calreticulin, cytochrome c oxidase subunit 1, glucanase inhibitor protein 3, methionine aminopeptidase, ribokinase, RXLR effector protein Avr 1, serine/threonine—protein kinase PKZ1, succinate dehydrogenase [ubiquinone] iron-sulfur subunit, mitochondrial and uncharacterized protein (PITG_15862).To conclude,the current study is unique in the sense that, corynan-17-ol, 18,19-didehydro-10-methoxy-,acetate(ester) and trioxsalenare bestowed with diverse mode of action resulting in the suppression of host pathogen relationship.Thus, corynan-17-ol, 18,19-didehydro-10-methoxy-,acetate(ester) and trioxsalencan be explored as novel biomolecule to manage P. infestans.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Department of Plant Pathology, Department of Plant Biotechnology, DBT-BTIS facility at Department of Plant Molecular Biology and Bioinformatics, Department of Nanoscience and Technology, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India for providing facilities.
Author Contributions
NS, SA and SN conceptualized the research; JR performed molecular docking and lab experiments; MK guided for data analysis and interpretation; SN and RV have supported in the analysis of bioinformatics data.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sudha Appusami, Email: sudha.a@tnau.ac.in.
Nakkeeran Sevugapperumal, Email: nakkeeranayya@tnau.ac.in.
References
- 1.Nowicki M, Foolad MR, Nowakowska M, Kozik EU. Potato and tomato late blight caused by Phytophthora infestans: an overview of pathology and resistance breeding. Plant Dis. 2012;96:4–17. doi: 10.1094/PDIS-05-11-0458. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Pavia SP, Grunwald NJ, Diaz-Valasis M, Cadena-Hinojosa M, Fry WE. Soilborne oospores of Phytophthora infestans in central Mexico survive winter fallow and infect potato plants in the field. Plant Dis. 2004;88:29–33. doi: 10.1094/PDIS.2004.88.1.29. [DOI] [PubMed] [Google Scholar]
- 3.Fry WE, Birch PR, Judelson HS, Grunwald NJ, Danies G, Everts KL, Gevens AJ, Gugino BK, Johnson DA, Johnson SB, McGrath MT. Five reasons to consider Phytophthora infestansa reemerging pathogen. Phytopathology. 2015;105:966–981. doi: 10.1094/PHYTO-01-15-0005-FI. [DOI] [PubMed] [Google Scholar]
- 4.Caulier S, Gillis A, Colau G, Licciardi F, Liepin M, Desoignies N, Modrie P, Legreve A, Mahillon J, Bragard C. Versatile antagonistic activities of soil-borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and other potato pathogens. Front Microbiol. 2018;9:143. doi: 10.3389/fmicb.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Liang J, Zhang C, Wang L, Gao W, Jiang J. Bacillus megaterium WL-3 lipopeptides collaborate against Phytophthora infestans to control potato late blight and promote potato plant growth. Front Microbiol. 2020;11:1–14. doi: 10.3389/fmicb.2020.01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanmugam V, Thakur H, Gupta S. Use of chitinolytic Bacillus atrophaeus strain S2BC-2 antagonistic to Fusarium spp for control of rhizome rot of ginger. Ann Microbiol. 2013;63:989–996. doi: 10.1007/s13213-012-0552-2. [DOI] [Google Scholar]
- 7.Ayaz M, Ali Q, Farzand A, Khan AR, Ling H, Gao X. Nematicidal volatiles from Bacillus atrophaeusGBSC56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita. Int J Mol Sci. 2021;22:5049. doi: 10.3390/ijms22095049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Li B, Wang Y, Guo Q, Lu X, Li S, Ma P. Lipopeptides, a novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeusCAB-1. Appl Microbiol Biotechnol. 2013;97:9525–9534. doi: 10.1007/s00253-013-5198-x. [DOI] [PubMed] [Google Scholar]
- 9.Huang H, Wu Z, Tian C, Liang Y, You C, Chen L. Identification and characterization of the endophytic bacterium Bacillus atrophaeus XW2, antagonistic towards Colletotrichum gloeosporioides. Annals of microbiology. 2015;65:1361–1371. doi: 10.1007/s13213-014-0974-0. [DOI] [Google Scholar]
- 10.Mu Y, Yue Y, Gu G, Deng Y, Jin H, Tao K. Identification and characterization of the Bacillus atrophaeus strain J-1 as biological agent of apple ring rot disease. J Plant Dis Prot. 2020;127:367–378. doi: 10.1007/s41348-020-00309-x. [DOI] [Google Scholar]
- 11.Singh Y, Patil VU, Dhasmana A, Chakraborty SK, Shukla PK, Rawat S (2017) In silico study of RxLR effectors of Phytophthora infestans HP-10–31, A2 mating type potato late blight pathogen. International Journal of Advanced Biotechnology and Research 8(2):398. https://scholarworks.utrgv.edu/cgi/viewcontent.cgi?article=1157&context=som_pub
- 12.Wang M, Du Y, Ling C, Yang Z, Jiang B, Duan H, An J, Li X, Yang X. Design, synthesis and antifungal/anti-oomycete activity of pyrazolyl oxime ethers as novel potential succinate dehydrogenase inhibitors. Pest Manag Sci. 2021;77:3910–3920. doi: 10.1002/ps.6418. [DOI] [PubMed] [Google Scholar]
- 13.Kim MJ, Shim CK, Park JH. Control efficacy of Bacillus velezensis afb2–2 against potato late blight caused by Phytophthora infestans in organic potato cultivation. Plant Pathol J. 2021;37:580. doi: 10.5423/PPJ.FT.09.2021.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cawoy H, Debois D, Franzil L, De Pauw E, Thonart P, Ongena M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb Biotechnol. 2015;8:281–295. doi: 10.1111/1751-7915.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Zhang X, Wang W, Geng X, Shi Y, Na R, Dou D, Li H. Network and role analysis of autophagy in Phytophthora sojae. Sci Rep. 2017;7:1–2. doi: 10.1038/s41598-017-01988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco FA, Judelson HS. A bZIP transcription factor from Phytophthora interacts with a protein kinase and is required for zoospore motility and plant infection. Mol Microbiol. 2005;56:638–648. doi: 10.1111/j.1365-2958.2005.04575.x. [DOI] [PubMed] [Google Scholar]
- 17.Zheng X, Wagener N, McLellan H, Boevink PC, Hua C, Birch PR, Brunner F. Phytophthora infestans RXLR effector SFI 5 requires association with calmodulin for PTI/MTI suppressing activity. New Phytol. 2018;219:1433–1446. doi: 10.1111/nph.15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ah-Fong A, Kim KS, Judelson HS. RNA-seq of life stages of the oomycete Phytophthora infestans reveals dynamic changes in metabolic, signal transduction, and pathogenesis genes and a major role for calcium signaling in development. BMC Genom. 2017;18:1–21. doi: 10.1186/s12864-017-3585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Y, Ye T, Gao Z. The succinate dehydrogenase PsSDHB is involved in hyphal morphology, chemical stress response and pathogenicity of Phytophthora sojae. Physiol Mol Plant Pathol. 2018;102:8–16. doi: 10.1016/j.pmpp.2017.10.008. [DOI] [Google Scholar]
- 20.Damasceno CM, Bishop JG, Ripoll DR, Win J, Kamoun S, Rose JK. Structure of the glucanase inhibitor protein (GIP) family from Phytophthora species suggests coevolution with plant endo-β-1, 3-glucanases. Mol Plant Microbe Interact. 2008;21:820–830. doi: 10.1094/MPMI-21-6-0820. [DOI] [PubMed] [Google Scholar]
- 21.Grenville-Briggs LJ, Avrova AO, Bruce CR, Williams A, Whisson SC, Birch PR, van West P. Elevated amino acid biosynthesis in Phytophthora infestans during appressorium formation and potato infection. Fungal Genet Biol. 2005;42:244–256. doi: 10.1016/j.fgb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Villalobos-Pina L, Rojas A, Acosta H (2021) First In silico Structural Model of Glucokinase-1 from Phytophthora infestansreveals a Possible Pyrophosphate Binding Site. Archives of Proteomics and Bioinformatics 2(1):39–46. https://www.scientificarchives.com/admin/assets/articles/pdf/first-in-silico-structural-model-of-glucokinase-1-from-phytophthora-infestans-reveals-a-possible-pyrophosphate-binding-site-20211221031209.pdf
- 23.Boevink PC, Wang X, McLellan H, He Q, Naqvi S, Armstrong MR, Zhang W, Hein I, Gilroy EM, Tian Z, Birch PR (2016) A Phytophthora infestans RXLR effector targets plant PP1c isoforms that promote late blight disease. Nature communications 7(1):1–4.10.1038/ncomms10311 [DOI] [PMC free article] [PubMed]
- 24.Overdijk EJ, Putker V, Smits J, Tang H, Bouwmeester K, Govers F, Ketelaar T. Phytophthora infestans RXLR effector AVR1 disturbs the growth of Physcomitrium patens without affecting Sec5 localization. PLoS ONE. 2021;16:e0249637. doi: 10.1371/journal.pone.0249637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo T, Wang XW, Shan K, Sun W, Guo LY. The loricrin-like protein (LLP) of Phytophthora infestans is required for oospore formation and plant infection. Front Plant Sci. 2017;8:142. doi: 10.3389/fpls.2017.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramachandran GN. Protein structure and crystallography. Science. 1963;141:288–291. doi: 10.1126/science.141.3577.288. [DOI] [PubMed] [Google Scholar]
- 27.Dallakyan S, Olson AJ (2015) Small-molecule library screening by docking with PyRx. In: Chemical biology, Humana Press, New York, NY 243-250. 10.1007/978-1-4939-2269-7_19 [DOI] [PubMed]
- 28.Tian W, Chen C, Lei X, Zhao J, Liang J (2018) CASTp 3.0: computed atlas of surface topography of proteins. Nucleic acids research 46(W1):W363–7. 10.1093/nar/gky473 [DOI] [PMC free article] [PubMed]
- 29.Islam MR, Jeong YT, Lee YS, Song CH. Isolation and identification of antifungal compounds from Bacillus subtilis C9 inhibiting the growth of plant pathogenic fungi. Mycobiology. 2012;40:59–65. doi: 10.5941/MYCO.2012.40.1.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen JN, Harbison AM (2017) Scanning electron microscopy sample preparation and imaging. In: Molecular profiling, Humana Press, New York, NY pp. 71–84. http://dx.doi.org/10.1007/978-1-4939-6990-6_5 [DOI] [PubMed]
- 31.Ivanov AA, Ukladov EO, Golubeva TS. Phytophthora infestans: An overview of methods and attempts to combat late blight. Journal of Fungi. 2021;7:1071. doi: 10.3390/jof7121071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gisi U, Cohen Y. Resistance to phenylamide fungicides: a case study with Phytophthora infestans involving mating type and race structure. Annu Rev Phytopathol. 1996;34:549–572. doi: 10.1146/annurev.phyto.34.1.549. [DOI] [PubMed] [Google Scholar]
- 33.Lal M, Yadav S, Singh V, Nagesh M. The use of bio-agents for management of potato diseases. Plant Growth. 2016 doi: 10.5772/64853. [DOI] [Google Scholar]
- 34.Ajay S, Sunaina V (2013) Direct inhibition of Phytophthora infestans, the causal organism of late blight of potato by Bacillus antagonists. Potato J 32:3–4. https://agris.fao.org/agris-search/search.do?recordID=IN2022016708
- 35.Amoroso VB, Mendez RA, Junio HA, Molino RJ, Pescadero IR, Villalobos AP (2021) Characterization of a natural fungicide from an indigenous plant Tasmannia piperita (Hook. f.) Miers Extract: Stability, Metabolomics, and In silico Studies. Philipp. J. Sci 150:355–70. https://philjournalsci.dost.gov.ph/images/pdf/pjs_pdf/vol150no2/plant_characterization_of_a_natural_fungicide_from_T_piperita_.pdf
- 36.Zhang S, Zheng X, Reiter RJ, Feng S, Wang Y, Liu S, Jin L, Li Z, Datla R, Ren M. Melatonin attenuates potato late blight by disrupting cell growth, stress tolerance, fungicide susceptibility and homeostasis of gene expression in Phytophthora infestans. Front Plant Sci. 2017;8:1993. doi: 10.3389/fpls.2017.01993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.