Abstract
Study Design
Meta-analysis.
Objectives
Our objective is to review the randomized controlled trials (RCTs) that have been conducted previously on the topic of osteoarthritis of the knee to assess and compare the efficacy and safety of autologous and allogeneic sources of mesenchymal stromal cells (MSCs) in the treatment of osteoarthritis.
Materials and methods
We searched the electronic databases PubMed, Embase, Web of Science, and the Cochrane Library until August 2021 for randomised controlled trials (RCTs) analysing the efficacy and safety of autologous and allogeneic sources of MSCs in the management of knee osteoarthritis. These searches were conducted independently and in duplicate. The outcomes that were taken into consideration for analysis were the visual analogue score (VAS) for pain, the Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), the Lysholm score, and adverse events. The OpenMeta [Analyst] software was utilised to carry out the analysis in the R platform.
Results
In total, 21 studies with a total of 936 patients were considered for this analysis. Because none of the studies made a direct comparison of the autologous and allogeneic sources of MSCs, we pooled the results of all of the included studies of both sources and made a comparative analysis of how the two types of MSCs fared in their respective applications. Although both allogeneic and autologous sources of MSCs demonstrated significantly better VAS improvement after 6 months (p = 0.006, p = 0.001), this trend was not maintained after 1 year for the allogeneic source (p = 0.171, p = 0.027). When compared to their respective controls based on WOMAC scores after 1 year, autologous sources (p = 0.016) of MSCs performed better than allogeneic sources (p = 0.186).A similar response was noted between the sources at 2 years in their Lysholm scores (p = 0.682, p = 0.017), respectively. Moreover, allogeneic sources (p = 0.039) of MSCs produced significant adverse events than autologous sources (p = 0.556) compared to their controls.
Conclusion
Our analysis of literature showed that autologous sources of MSCs stand superior to allogeneic sources of MSC with regard to their consistent efficacy for pain, functional outcomes, and safety. However, we strongly recommend that further studies be conducted that are of a high enough quality to validate our findings and reach a consensus on the best source of MSCs for use in cellular therapy treatments for knee osteoarthritis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s43465-022-00751-z.
Keywords: Mesenchymal stromal cell, Bone marrow-derived mesenchymal stromal cell, Adipose-derived mesenchymal stromal cell, Cartilage regeneration, Knee osteoarthritis, Meta-analysis
Introduction
Articular cartilage injuries pose a major health hazard among adults resulting in joint pain, stiffness, joint effusion, and limitation of range of movements, and finally leading to progressive joint destruction [1–3]. Chondral and osteochondral injuries are of three types, namely (a) invisible articular cartilage disruption; (b) mechanical disruption of the articular surface limited to articular cartilage; and (c) mechanical disruption of articular cartilage and subchondral bone [1]. The restoration of articular cartilage and subchondral bony defects require augmentation of cell transplants either autologous or allogeneic in nature [4, 5]. Various preclinical and clinical studies have proven the functional benefits in the usage of artificial scaffolds, matrices, and growth factors to regenerate new articular cartilage [6, 7]. The natural history of articular cartilage injuries defines the line of management in terms of quality and quantity of cells to be transplanted [8].
The origin of cell sources plays a major role in cellular and regenerative therapies. The usage of mesenchymal stromal cells of either autologous or allogeneic origin is still a debate among all researchers. Bone marrow- and adipose tissue-derived mesenchymal stromal cells (MSCs) are the most commonly used MSCs of autologous origin [9], whereas placenta, amniotic fluid, and Wharton’s jelly-derived MSCs are allogeneic in origin. Autologous sources of MSCs need a minimally invasive procedure to retrieve MSCs from their source and are devoid of immunogenic reactions, and carry no risk for the disease transmission, whereas in an allogeneic source of MSCs the immunogenic reactions and the possible risk for the disease transmission are uncertain, although it does not require any procedure to retrieve MSCs from its source [10, 11].
There have been a number of studies on autologous and allogeneic sources of MSCs on knee osteoarthritis (OA), and the results of these studies have shown conflicting evidence in terms of the safety and effectiveness of the treatment. When AD-MSCs of 108 cells were administered as an intra-articular injection in the patients with OA knee at the end of 6 months follow-up, Jo et al. reported that hyaline-like cartilage was regenerated. Patients with OA knee were followed up for 6 months [12]. The combination of autologous activated peripheral blood stem cells with growth factors and hyaluronic acid, along with arthroscopic micropuncture, demonstrated significant cartilage regeneration in patients with early osteoarthritis of the knee [13]. When autologous mononuclear cells derived from peripheral blood are injected into the infrapatellar fat pad of an arthritic knee, total MSC migration is increased by a factor of 25 in just 24 h, and as a result the activity of chondrogenic genes is upregulated by a factor of 15 [14].
de Windt et al. concluded that the mixture of allogeneic MSCs along with re-cycled autologous chondrocytes for the management of patients with cartilage defects to be a safe and single-stage cartilage repair [15]. The composite of allogeneic MSCs mixed with re-cycled autologous chondrocytes demonstrated neochondrogenesis at second-look arthroscopy, maintenance of cartilage thickness in MRI, patient’s DNA in DNA short tandem repeat analysis, and increased concentrations of proteoglycans and type 2 collagen [16]. The composite of culture-expanded allogeneic umbilical cord-derived MSCs admixed with hyaluronate demonstrated cartilage-like tissue in the articular cartilage defects in patients with OA knee in the MRI at 1, 3, and 7 years without any complications [17]. Vega et al. concluded that 40 × 10 [6] allogeneic MSCs are logistically more convenient than autologous MSCs in OA knee [18].
We aim to determine whether the use of currently available allogeneic MSC sources of MSCs matches the clinical efficacy and patient safety of that of the autologous sources in the management of osteoarthritis of the knee based on the promising evidence in the literature favouring these sources.
Materials and Methods
This meta-analysis was conducted in accordance with the Back Review Group of the Cochrane Collaboration's recommendations19, and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was followed in its reporting. [20]
Search Strategy
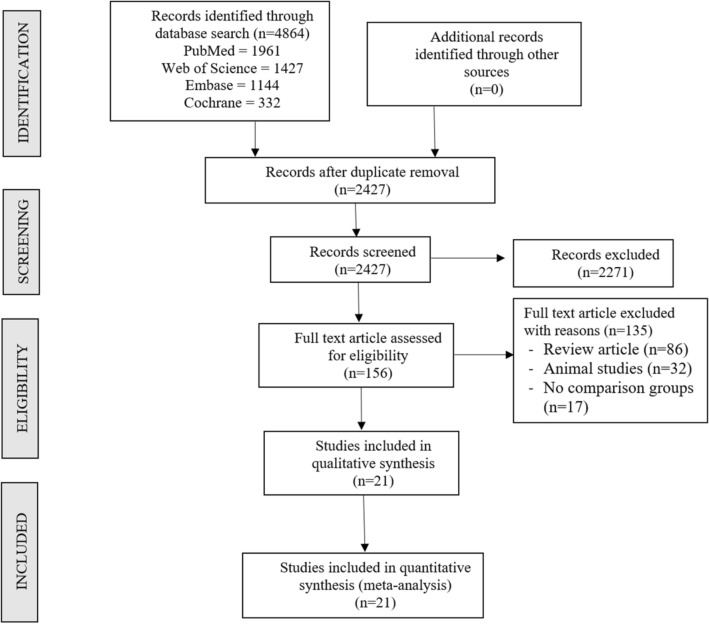
Two different reviewers independently searched the electronic literature for studies that assessed the efficacy and security of scaffold-based MSC delivery in the treatment of osteoarthritis of the knee. We searched through the information in PubMed, Embase, Web of Science, and the Cochrane Library databases up until August 2021. The language or the date were not constrained in any way. Some of the search terms included "Knee Osteoarthritis," "Knee Degeneration," "Stem Cell Therapy," "Mesenchymal Stromal Cells," "Bone marrow," "Adipose," "Autologous," and "Allogeneic." You can see an illustration of a search method that was applied in one of the included databases in Supplementary File 1. The chosen articles' reference lists were also thoroughly searched for any additional studies that had not been located through the initial search. According to the inclusion and exclusion criteria, studies that met the requirements for inclusion in the meta-analysis were added. The authors' differences were eventually resolved through discussion, which persisted until an agreement was reached. A detailed flowchart of the study selection process is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram of the included studies
Inclusion Criteria
If studies met the following PICOS requirements, they were included in the quantitative review:
Population patients with osteoarthritis of the knee.
Intervention MSC treatment using autologous cells.
Comparator MSC therapy from an allogeneic source.
Outcomes Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), Lysholm Knee Scale (Lysholm), and Adverse Events Visual Analogue Score (VAS) for Pain
Study Design Randomized Controlled Trials.
Exclusion Criteria
Trials were disqualified if any of the following conditions were met:
RCTs on MSC-based treatment for OA knee without mentioning where the MSCs were obtained for the study.
Studies on stem cell therapy conducted in vitro.
Without a suitable comparison group, observational studies and interventional studies.
Studies investigating stem cell therapy using knee osteoarthritis animal models.
Studies using stem cells in vitro and review articles.
Data Extraction
Two reviewers independently gathered pertinent information from the articles that were included for analysis. The following information was taken:
1. Study characteristics include the authors, country, level of evidence, and the total number of participants.
2. Baseline characteristics include the mean age, gender ratios, osteoarthritis grade according to Kellgren–Lawrence, source of the MSCs used, intervention for both groups, MSCs' delivery method, the length of the follow-up period, and the assessment criteria used.
3. VAS for pain, functional outcomes like the WOMAC score, and Lysholm score are examples of efficacy outcomes.
4. Adverse events in the included studies are safety outcomes.
Any discrepancy during data collection was settled through discussion until an agreement was reached.
Risk of Bias and Quality Assessment
Using the ROB2 tool developed by The Cochrane Collaboration for randomised studies, two reviewers independently evaluated the methodological quality of the included studies. The randomisation process, deviation from the intended intervention, missing outcome data, and measurement of the outcome are among the tool's five bias assessment domains, and selection of the reported results. Both of these domains were used to evaluate the included studies [21].
Statistical Analysis
The R platform with the OpenMeta [Analyst] package was used to carry out the meta-analysis [22]. We used the risk ratio (RR) with a confidence interval (CI) of 95% for the outcomes that were dichotomous variables and the weighted mean difference (WMD) with a confidence interval of 95% for the outcomes that were continuous variables. The I2 test was utilised to evaluate the presence of heterogeneity [23]. If the value of I [2] was below 50% and p was greater than 0.1, we evaluated using a model with fixed effects; otherwise, we used a model with random effects. A p value of less than 0.05 was considered to be statistically significant. We carried out sensitivity analyses to investigate the factors that led to the existence of heterogeneity. Egger's regression test as well as a funnel plot and normal quantile plot were utilised to investigate the possibility of publication bias in the studies that were considered (Table 1).
Table 1.
Characteristics of included studies
| Sl No | Authors | Year | Country | Nature of study | Kellgren–Lawrence grade | Sample size | Treatment/control | Mean age (SD) | Male/female | MSC type | MSC Source | Follow-up (months) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | Control group | Treatment group | Control group | |||||||||||
| 1 | A Vega | 2014 | Spain | RCT | II, III, IV | 30 | 15/15 | 56.6 ± 9.24 | 57.3 ± 9.09 | 06/09 | 05/10 | BM | Allo | 12 |
| 2 | CT Vangness Jr | 2014 | USA | RCT | NR | 55 | 36/19 | 44.6 ± 9.82 | 47.8 ± 8 | 25/11 | 13/06 | BM | Allo | 24 |
| 3 | D Garay Mendoza | 2017 | Mexico | RCT | NR | 61 | 30/31 | 55.57 ± 12.02 | 59.32 ± 10.85 | 07/23 | 09/22 | BM | Auto | 6 |
| 4 | D Kuah | 2018 | Australia | RCT | I, II, III | 20 | 16/4 | 50.8 ± 7.29 | 55.0 ± 10.42 | 11/05 | 01/03 | AD | Allo | 12 |
| 5 | E Estrada | 2020 | Argentina | RCT | I, II, III | 89 | 60/29 | 61 ± 12 | 61 ± 12 | NR | NR | BM / AD | Auto | 12 |
| 6 | J Freitag | 2019 | Australia | RCT | II, III | 30 | 20/10 | 54.6 ± 6.3 | 51.5 ± 6.1 | 11/09 | 01/09 | AD | Auto | 12 |
| 7 | JJ Ruane | 2021 | USA | RCT | I, II, III | 32 | 17/15 | 58.06 ± 9.14 | 58.6 ± 8.05 | 09/08 | 10/05 | BM | Auto | 12 |
| 8 | JM Lamo-Espinosa | 2016 | Spain | RCT | II, III, IV | 30 | 20/10 | 65.9 | 60.3 | 12/08 | 07/03 | BM | Auto | 12 |
| 9 | JR Garza | 2020 | USA | RCT | II, III | 39 | 26/13 | 60.5 ± 7.9 | 57.1 ± 9.1 | 15/11 | 7/6 | AD | Auto | 12 |
| 10 | KL Wong | 2013 | Singapore | RCT | NR | 56 | 28/28 | 53 | 49 | 15/13 | 14/14 | BM | Auto | 24 |
| 11 | L Lu | 2019 | China | RCT | I, II, III | 53 | 27/26 | 55.03 ± 9.19 | 59.64 ± 5.97 | 03/24 | 03/23 | AD | Auto | 12 |
| 12 | Lv Xiaoxia | 2015 | Huang | RCT | I, II | 80 | 40/40 | 55.9 ± 8.1 | 55.1 ± 6.8 | 14/26 | 13/27 | BM | Auto | 12 |
| 13 | M Emadedin | 2018 | Iran | RCT | II, III, IV | 43 | 19/24 | 51.7 ± 9.2 | 54.7 ± 5.3 | 12/07 | 15/09 | BM | Auto | 6 |
| 14 | PK Gupta | 2016 | India | RCT | II, III | 60 | 40/20 | 58.10 ± 8.23 | 54.90 ± 8.27 | 12\28 | 4\16 | BM | Allo | 12 |
| 15 | R Bastos | 2019 | Brazil | RCT | I, II, III, IV | 47 | 30/17 | 55.7 ± 7.8 | 55.9 ± 13.4 | 15/15 | 09/08 | BM | Auto | 12 |
| 16 | S Wakitani | 2002 | Japan | I, II | 24 | 12/12 | NR | NR | NR | NR | BM | Auto | 16 | |
| 17 | TDX Tran | 2019 | Taiwan | RCT | II, III | 33 | 15/18 | 58.2 ± 5.70 | 59.0 ± 6.04 | 03/12 | 05/13 | AD | Auto | 24 |
| 18 | WS Lee | 2019 | South Korea | RCT | II, III, IV | 24 | 12/12 | 62.2 ± 6.5 | 63.2 ± 4.2 | 03/09 | 03/09 | AD | Auto | 6 |
| 19 | YG Koh | 2012 | South Korea | RCT | IV | 50 | 25/25 | 54.2 ± 9.3 | 54.4 ± 11.3 | 08/17 | 08/17 | AD | Auto | 16 |
| 20 | YG Koh | 2014 | South Korea | RCT | I, II, III | 44 | 23/21 | 52.3 ± 4.9 | 54.2 ± 2.9 | 06/17 | 05/16 | AD | Auto | 24 |
| 21 | Z Hong | 2018 | China | RCT | II, III | 32 | 16/16 | 51 ± 5.95 | 53 ± 10.97 | 03/13 | 03/13 | AD | Auto | 12 |
AD adipose derived, Allo Allogenic, Auto autologous, BM bone marrow derived, MSC mesenchymal stem cell, NR not reported, RCT randomized controlled trial, SD standard deviation, USA United States of America
Results
Search Results
A search conducted using an electronic database produced a total of 2427 articles after an initial screening to remove duplicates reduced the number of articles to 4864. After reviewing the titles and abstracts of those 2427 articles, we decided not to include 2271 of them in our analysis. There were 156 articles that were eligible for full-text review, but 135 of them were disqualified. In the treatment of knee osteoarthritis, none of the studies that were reviewed conducted a head-to-head comparison of the use of MSCs derived from autologous versus allogeneic sources. As a result, we made a combined comparative quantitative analysis by combining the findings of all of the included studies that used autologous sources of MSCs in one group, and we used allogeneic sources of MSCs in another group. Both groups were comprised of studies. In our meta-analysis, we used data from 936 patients across 21 studies that were included in the analysis. Figure 1 presents the PRISMA flow diagram of study selection in its entirety. 9 out of the 21 studies [24, 27, 28, 30, 32, 37–41] used MSC derived from adipose tissue. One of these studies utilised an allogeneic source of MSCs, while the remaining 8 studies used an autogenous source of MSCs. 12 out of the 21 studies [18, 24–26, 29, 31, 33–36, 42, 43] used MSCs derived from bone marrow. Two of these studies used allogeneic sources of MSCs, while the remaining ten studies used autogenous sources of MSCs. In addition, there was no uniformity across the included studies with regard to the outcome measures that were applied. The table that follows provides an overview of the studies that were taken into consideration. The intervention protocol that was used with both the case and control groups, as well as the measures that were used to evaluate the outcomes, are presented in Table 2.
Table 2.
Stem cell transplantation protocol of the included studies
| Study | MSC type | MSC source | MSC preparation | MSC count (107 cells) | Treatment group intervention | Control group intervention | Outcome measures |
|---|---|---|---|---|---|---|---|
| A Vega | BM | Allo | CE-BMMSC | 4 | sIA injection of MSC | sIA injection of 60 mg HA | VAS, WOMAC |
| CT Vangness Jr | BM | Allo | CE-BMMSC | 5/15 | sIA injection of MSC + 20 mg HA | sIA injection of 20 mg HA | VAS, Lysholm score |
| D Garay Mendoza | BM | Auto | BMC | NA | 600 µg/day G-CSF for 3 consecutive days before the procedure + sIA Injection of MSC |
Oral acetaminophen 500 mg every 8 h for 6 months |
VAS, WOMAC |
| D Kuah | AD | Allo | CE-ADMSC | 0.39–0.67 | sIA injection of MSC | Placebo sIA injection of cell culture media and cryopreservative | VAS, WOMAC, MRI assessment |
| E Estrada | AD | Auto | BMC | NA | sIA injection of bone marrow concentrate | sIA injection of PRP | IKDC, Lysholm score, KOOS |
| E Estrada | BM | Auto | SVF | NA | sIA injection of lipoaspirate | sIA Injection of PRP | |
| J Freitag | AD | Auto | CE-ADMSC | 10 | sIA injection of MSC ± 2nd injection at 6 months | Conservative management | VAS, WOMAC, KOOS, MRI assessment |
| JJ Ruane | BM | Auto | BMC | NA | sIA injection of bone marrow concentrate + PRP |
Gel-One® cross-linked hyaluronate injection |
VAS, KOOS |
| JM Lamo-Espinosa | BM | Auto | CE-BMMSC | 1 | sIA injection of MSC + 60 mg HA | sIA injection of 60 mg HA | VAS, WOMAC, MRI assessment |
| JR Garza | AD | Auto | SVF | NA | sIA injection of MSC | Placebo injection without cells | WOMAC, MRI assessment |
| KL Wong | BM | Auto | CE-BMMSC | 1.46 |
HTO + microfracture + sIA injection of MSC + 20 mg HA |
HTO + microfracture + sIA injection of 20 mg HA |
Tegner score, Lysholm score |
| L Lu | AD | Auto | CE-ADMSC | 5 | 2 IA injection of MSC at 0,3 weeks and sham injection at 1,2 weeks | 4 IA injection of 25 mg HA at 0,1,2,3 weeks | VAS, WOMAC |
| Lv Xiaoxia | BM | Auto | CE-BMMSC | 3.82 | 3 × monthly IA injection of MSC + 20 mg HA | sIA injection of 20 mg HA | Tegner score, Lysholm score |
| M Emadedin | BM | Auto | CE-BMMSC | 4 | sIA injection of MSC | Placebo sIA injection of Normal Saline | VAS, WOMAC |
| PK Gupta | BM | Allo | CE-BMMSC | 2.5–15 | sIA injection of MSC + 20 mg HA | Placebo sIA injection of 20 mg HA | VAS, WOMAC, MRI assessment |
| R Bastos | BM | Auto | CE-BMMSC | 4 | sIA injection of MSC in 10 ml of PRP | sIA injection of 4 mg Dexamethasone | KOOS, MRI assessment |
| S Wakitani | BM | Auto | CE-BMMSC | 1 | HTO + Microfracture + sIA injection of MSC | HTO + microfracture + placebo injection | MRI assessment, HSS knee rating scale |
| TDX Tran | AD | Auto | SVF | NA | Arthroscopic micro fracture + sIA injection of MSC | Arthroscopic micro fracture | WOMAC, MRI assessment |
| WS Lee | AD | Auto | CE-ADMSC | 10 | sIA injection of MSC | Placebo injection with Normal Saline | WOMAC, MRI assessment |
| YG Koh | AD | Auto | SVF | 0.189 | Arthroscopic debridement + sIA injection of MSC + PRP | Arthroscopic debridement + PRP | VAS, Tegner score, Lysholm score |
| YG Koh | AD | Auto | CE-ADMSC | 0.411 |
HTO + sIA injection of MSC + PRP |
HTO + PRP | VAS, Lysholm Score |
| Z Hong | AD | Auto | SVF | 0.745 | sIA injection of MSC | sIA injection of 40 mg HA | VAS, WOMAC, MRI assessment |
AD adipose derived, Allo allogenic, Auto autologous, BM bone marrow derived, BMC bone marrow concentrate, CE-ADMSC culture-expanded adipose derived MSC, CE-BMMSC culture-expanded bone marrow MSC, HA hyaluronic acid, HSS Hospital for Special Surgeries, HTO high tibial osteotomy, IA intra-articular, IKDC International Knee Documentation Committee, KOOS Knee Osteoarthritis Outcome Score, PRP platelet-rich plasma, MRI magnetic resonance imaging, MSC mesenchymal stem cells, sIA single intra-articular, SVF stromal vascular fraction, VAS visual analogue score, WOMAC Western Ontario McMaster Universities Osteoarthritis Index
Quality Assessment
Figure 2 displays the results of an analysis conducted using the RoB2 tool to assess the level of methodological rigour present in the studies that were included. There was not enough of a concern about bias in any of the studies that were included for them to be disregarded.
Fig. 2.

Methodological quality and risk of bias assessment of all the included studies
Efficacy Outcomes
Visual Analogue Scale for Pain
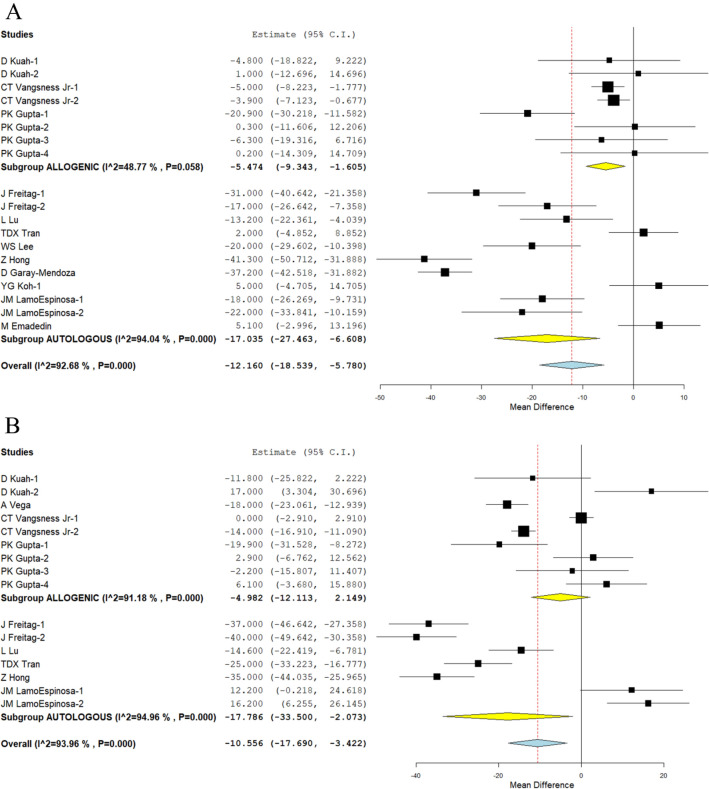
We looked at three different studies [25, 27, 34], and nine other studies [26, 28, 29, 32, 33, 37–39, 41] have reported the VAS outcome of allogeneic and autologous sources of MSCs, respectively, at the 6-month time point. There was a substantial amount of heterogeneity among the studies that were included. (I2 > 80%, p < 0.001). As a result, the analysis was conducted using the random-effects model. On analysis, a significant reduction in VAS score was noted compared to their controls at 6 months in both allogeneic sources (WMD = − 5.474, 95 percent CI [− 9.343, − 1.605], p = 0.006) and autologous sources (WMD = − 17.035, 95 percent CI [− 27.463, − 6.608], p = 0.001) compared to their controls, as shown in Fig. 3A.
Fig. 3.
Forest plot of the included studies comparing allogenic and autologous sources of MSCs compared to their controls. A VAS at 6 months; B VAS at 12 months
In a similar manner, we looked at four different studies. 18, 25, 27, and 5 studies, 28 studies, and 29, 32, 37, and 41 studies, respectively, reported the VAS outcome of allogeneic and autologous sources of MSCs at 12 months. There was a substantial amount of heterogeneity among the studies that were included (I2 > 80 percent, p < 0.001). As a result, the analysis was conducted using the random-effects model. On analysis, a significant reduction in VAS score was noted compared to their controls at 12 months in autologous sources (WMD = − 17.786, 95% CI [− 33.500, − 2.073], p = 0.027); however, allogeneic sources (WMD = − 17.035, 95% CI [− 27.463, − 6.608], p = 0.001; Fig. 3B) were unable to produce a significant difference compared to their controls.
Although both sources were able to significantly reduce pain in comparison to their controls in the short term (6 months), only autologous sources demonstrated significant results consistently across all of the time points that were analysed in comparison to their controls. Symptomatic relief from pain following MSC injections was observed from 3 months to 24 months later in the studies that were included, and this was true regardless of the source of the MSCs that were used in the studies.
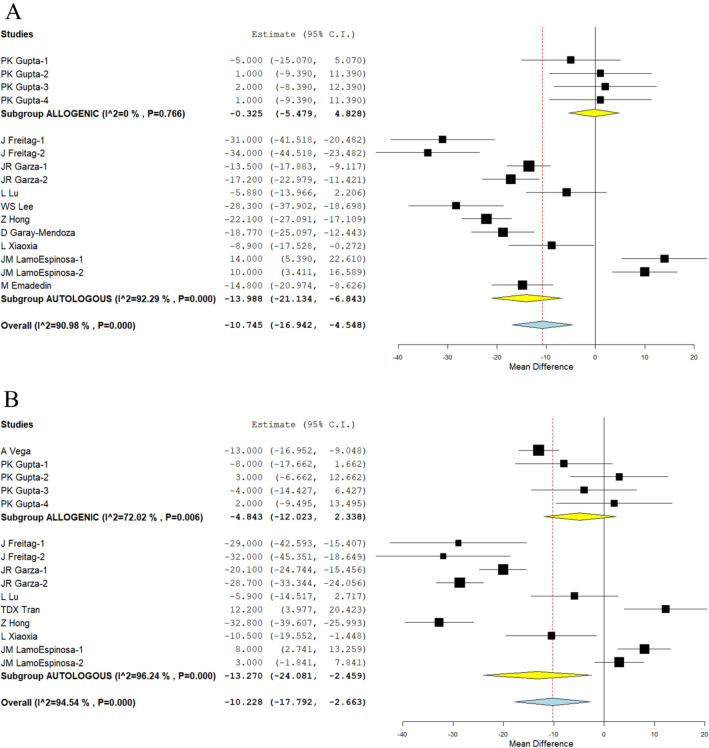
WOMAC Score
We analysed one study [34] as well as nine other studies reporting the WOMAC scores of allogeneic and autologous sources of MSCs at 6 months [26, 28, 30, 32, 33, 38, 41, 43]. The studies included were found to have a significant amount of heterogeneity. (I2 > 80%, p 0.001). As a result, the random-effects model was used to conduct the analysis. In the analysis, allogeneic sources of MSCs did not result in any significant change compared to their controls (WMD = − 0.325, 95% CI [− 5.479, 4.828], p = 0.902), whereas autologous sources only showed a significant improvement in WOMAC score (WMD = − 13.988, 95% CI [− 21.134, − 6.843], p 0.001) compared to their controls at 6 months.
The WOMAC scores of allogeneic and autologous sources of MSCs at 12 months were analysed in two studies [18, 34] and seven studies [28–30, 32, 37, 41, 43], respectively. The studies that were included were found to have a significant amount of heterogeneity (I2 > 80%, p 0.001). As a result, the random-effects model was used to conduct the analysis. In the analysis, WOMAC score in autologous sources was significantly lower than in controls at 12 months (WMD = − 13.270, 95% CI [− 24.081, − 2.459], p = 0.016). On the other hand, as can be seen in Fig. 4B, allogeneic sources failed to significantly differ from their controls (WMD = − 4.843, 95% CI [− 12.023, 2.338], p = 0.186).
Fig. 4.
Forest plot of the included studies comparing allogenic and autologous sources of MSC therapy compared to their controls. A WOMAC at 6 months; B WOMAC at 12 months
As shown in Fig. 4, which was discovered through an analysis of the WOMAC score reduction potential of both sources, most studies that used allogeneic sources of MSCs did not report any significant improvement in comparison to their controls, as depicted in the figure. On the other hand, studies that used autologous sources of MSCs showed a consistent improvement in WOMAC scores in comparison to their controls across all of the time points that were analysed. As a dependable source of MSCs that can consistently produce better functional outcomes across both time points, autologous sources of mesenchymal stromal cells (MSCs) are superior to allogeneic sources of MSCs. This is so because the WOMAC score emphasises more on the functional effectiveness of the intervention than pain relief.
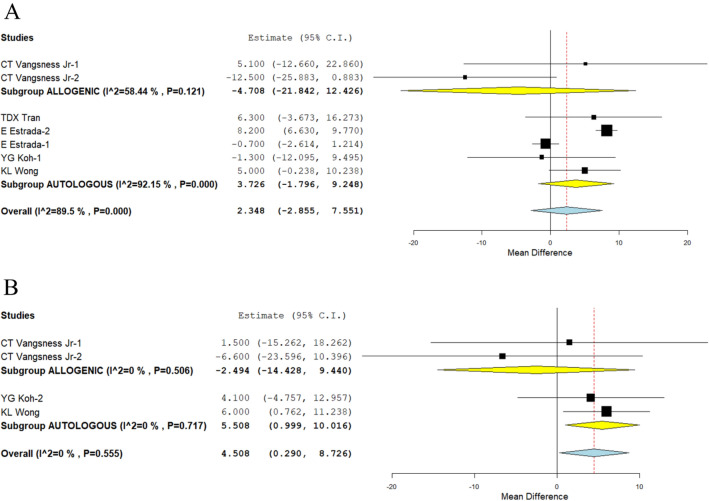
Lysholm Knee Score
We looked at one study [25] and four studies [24, 31, 37, 39] that reported the Lysholm score at 12 months and used allogeneic and autologous sources of MSCs, respectively. There was a significant amount of heterogeneity among the included studies. As a result, the random-effects model was used in the analysis at all time points. According to analysis, neither allogeneic (WMD = − 4.708, 95% CI [− 21.842, 12.426], p = 0.590) nor autologous sources (WMD = 3.726, 95%t CI [− 1.796, 9.248], p = 0.186) led to any significantly higher scores than their controls at 12 months. This is shown in Fig. 5A. Figure 5B demonstrates that while allogeneic sources of MSCs showed no improvement at all at 24 months compared to their controls (WMD = -2.494, 95% CI [− 14.428, 9.440], p = 0.682), autologous sources showed a significant improvement in scores.
Fig. 5.
Forest plot of the included studies comparing allogenic and autologous sources of MSC therapy compared to their controls. A Lysholm at 12 months; B Lysholm at 24 months
Studies using autologous MSC sources that show a significant improvement in functional outcomes when the improvement of the Lysholm score of both sources is critically analysed, supporting the WOMAC score findings.
Safety
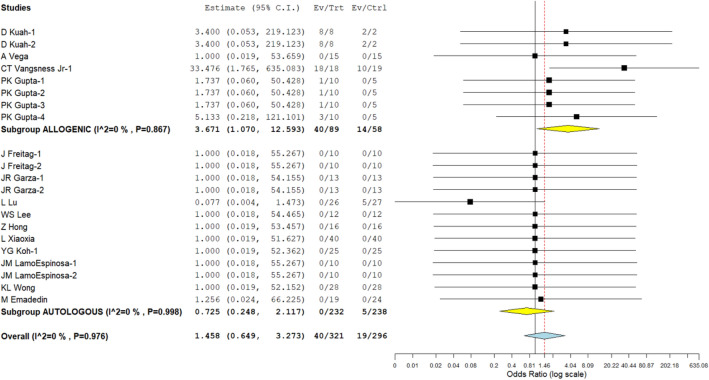
Four studies involving 147 patients that used an allogeneic source of MSC to treat knee osteoarthritis reported side effects with little variation between the studies (I2 = 0.0%, p = 0.867). The data were analysed using a fixed-effects model. After analysis, we found that, in comparison to the controls, adverse events had significantly increased. Figure 6 shows the results (RR = 3.671, 95% CI [1.070, 12.593], p = 0.039). Similar results were found in our analysis of ten studies for knee osteoarthritis using an autologous source of MSCs, which included 470 patients reporting adverse events with little heterogeneity among the included studies. (p = 0.998; I2 = 0.0%). The data were analysed using a fixed-effects model. There was no discernible increase in the adverse events when compared to the controls (RR = 0.725, [0.248, 2.117] at 95% confidence level, p = 0.556; Fig. 6). Neither of the MSC sources experienced any significant, long-lasting adverse effects from the intervention, such as tumours, death, or immune responses. The analysis shows that autologous sources of MSCs are safer for treating knee osteoarthritis than allogeneic sources.
Fig. 6.
Forest plot of the included studies comparing adverse events upon using allogenic and autologous sources of MSC therapy compared to their controls
Sensitivity Analysis
Each analysis underwent a sensitivity analysis. After one study at a time was taken out of the meta-analysis, there was no discernible difference in any of the conclusions (VAS for pain, WOMAC, Lysholm, or adverse events). However, the use of the random-effects model ensured that, even after the reanalysis, the overall pattern of the results remained the same. We did not perform a subgroup analysis of the intervention based on the nature and type of scaffolds used, because the majority of the outcomes that were examined did not produce statistically significant results.
Publications Bias
In the course of the meta-analysis that was conducted to look into publication bias, the funnel plot, normal quantile plot, and Egger's regression test were used. Additionally, publication bias was investigated using Egger's funnel plot. Based on the funnel plot, the normal quantile plot, shown in Fig. 7, and Egger's regression test, which produced a result of p = 0.519, there was no evidence of publication bias. Additionally, based on the test's outcomes, there was no evidence of publication bias. The distribution of the axes-related studies revealed no discernible heterogeneity, and each study was located relatively close to the 95% confidence interval (CI). This demonstrates that publication bias is at a minimum level.
Fig. 7.
Publication bias assessment with funnel plot and quantile plot for visual analogue score at 6 months in the included studies
Discussion
The decision to use autologous or allogeneic sources of MSCs to regenerate the cartilage is a fundamental clinical decision that rests upon the hands of the patients opting for MSC-based therapy for any regenerative procedure. Although affordability is a concern with the allogeneic sources, studies on the functional outcomes of allogeneic sources on par with the autologous counterparts were conflicting in the literature [44, 45]. To improve the efficacy of cellular therapy for both patients and clinicians, Murray et al. laid the consensus report in terms of “DOSES” (D—donor; O—origin of tissues; S—separation methods; E—exhibited characteristics; and S—site of delivery) [46].
To overcome the donor site morbidity for the autologous MSCs retrieval, researchers show more interest toward allogeneic MSCs. Various studies have demonstrated superior results in the use of allogeneic MSCs in animal models for the management of chondral and osteochondral injuries [47, 48], whereas Frisbie et al. demonstrated inferior results with their use in chondral lesions of the medial femoral condyle in the equine model [49].
Due to the inconsistent cell counts in the autologous preparation, allogeneic preparation has gained popularity among regenerative medicine professionals in which they will be able to deliver the target number of cell counts in the desired area. Allogeneic preparations are available readily as an off-label product and with quality assurance, but the main concerns with an allogeneic product are in terms of their safety, efficacy, cell viability, and vitality. Various countries provide strict guidelines and regulations in the usage of allogeneic MSC products given their safety and efficacy. Various studies have demonstrated that allogeneic MSCs are hypoimmune responsive, as they do not elicit alloreactive lymphocyte proliferative responses in vitro, but there is still a debate on the immunogenicity of allogeneic MSCs in vivo. Huang et al. reported increased expression of MHC-1a and 2 in the rat model after allogeneic MSC differentiation with the expression of a specific anti-donor alloantibody in the serum. This led to faster elimination and reduced efficacy and duration of the transplanted MSCs [50].
Main Finding
We comprehensively and critically reviewed all the available literature to identify whether allogeneic sources give comparable results to that of the autologous sources of MSCs for OA knee and found that
Although both allogeneic and autologous sources of MSCs demonstrated significantly better VAS improvement after 6 months (p = 0.006, p = 0.001), this trend was not maintained after 1 year for the allogeneic source (p = 0.171, p = 0.027). In a similar vein, the autologous source (p = 0.016) of MSCs outperformed the allogeneic source (p = 0.186) when compared to their control based on WOMAC scores at 1 year. At 2 years, the Lysholm scores of the sources were found to have responded in a manner that was comparable to one another (p = 0.682, p = 0.017, respectively).
Compared to their corresponding controls, adipose tissue-derived MSCs demonstrated a statistically significant and consistent improvement in all functional outcome measures such as VAS for pain, WOMAC, and Lysholm scores at varying time intervals. Allogeneic sources, on the other hand, did not demonstrate any functional benefits when evaluated in the long term using VAS, WOMAC, and Lysholm scores. This is despite the fact that the VAS for pain improved in the short term (after 6 months).
Moreover, allogeneic sources (p = 0.039) of MSCs produced significant adverse events than autologous sources (p = 0.556) compared to their controls.
It is simple to harvest MSCs from autologous sources, and doing so results in significant cost savings while eliminating all potential risks of graft rejection or disease transmission [51]. Autologous mesenchymal stem cell products, on the other hand, require a two-staged procedure for cartilage regeneration while simultaneously planning for culture expansion. Allogeneic mesenchymal stem cell preparations, on the other hand, can be delivered in a single-staged procedure with the desired dosage of MSCs [52]. MSC-derived allogeneic products, such as CARTISTEM (allogeneic cord blood-derived MSCs—2.5 × 106 cells/500 L/cm2 area of knee cartilage) [17], Stempeucell (allogeneic ex vivo cultured pooled human BM-MSCs—2 × 108 cells cryopreserved and stored in 15 mL cryo-bags) [34], and JointStem (autologous AD-MSCs—10 × 107 cells) [53], have been with a definite dosage for cartilage injuries. However, there is a lack of evidence in the literature regarding allogeneic MSC preparation in terms of long-term safety, efficacy, and other research-related potential pitfalls [10]. The dynamic fate of allogeneic MSCs that have been implanted is a topic of ongoing debate.
When the analysis is performed using different scenarios, the results generated by autologous sources of the MSC cocktail are inconsistent. This is due to the cellular heterogeneity as well as the subjective qualities of the MSCs that are collected. On the other hand, the allogeneic pool of MSC contains homogeneous cells and has the theoretical grounds to deliver consistent results. However, these cells also suffer from immune reactions because of their unfamiliarity with the recipient's immune system [10, 11, 54]. Therefore, the clinical outcome is dependent on the harvesting and cultural characteristics of the MSCs isolated from either an autologous or an allogeneic source. This can be either the patient's own tissue or tissue from another person. As people get older, there are fewer mesenchymal stem cells (MSCs) found in their own autologous sources [55]. To achieve the desired cartilage regeneration by MSCs, it is possible to isolate and culture expand MSCs derived from allogeneic sources. This will result in a greater number of MSCs as well as off-the-shelf products that can be used for emergency applications. This is something that can be done to get the desired results. To achieve the cartilage regeneration that is desired, there has not been any standardisation of the optimal dose of cells, adjuvants, and source of MSC harvest.
Cell-based regenerative therapies are dependent on having access to MSC sources that are reliable, powerful, and effective. Embryonic stem cells and induced pluripotent stem cells have both been the subject of clinical tests conducted by a variety of researchers (iPSCs). Cost, the capacity for long-term culture and storage, and tumorigenic potential are the factors that influence whether or not iPSCs will be made available for commercial use [56]. Cell engraftment and retention can be achieved using autologous cell sources without the risk of the host immune system rejecting the cells. The isolation of cells, their subsequent expansion, and subsequent transplantation to target sites all require multiple stages of procedure in autologous cell therapy. The subjective nature of individual patients is a primary barrier to the reliability and quality control of the product, which contribute to the wide range of clinical outcomes that can be achieved through the utilisation of autologous cell sources [51]. An allogeneic source of cells is something that can be tried as a promising next-generation cell therapy option to overcome all of these obstacles.
To properly choose an allogeneic MSC product for cartilage regeneration, it is important to take into consideration a number of different parameters first. The number of MSC passages, the desired number of MSCs to be injected, the immunogenicity of allogeneic MSCs, the shelf life of an allogeneic MSC product, as well as the protocols for injection and rehabilitation are all included in these parameters [54]. The allogeneic preparation of MSCs is required to take place in a laboratory that has been validated as complying with the GMP standards. Additionally, the laboratory must strictly adhere to all regulatory guidelines and protocols that are currently in effect [54]. As a result of the presence of a double-negative cell population, an allogeneic pool of mesenchymal stromal cells (MSCs) is regarded as the most desirable therapeutic product in the field of regenerative medicine (cells that are both HLA-1 negative and HLA-2 negative) [57]. The temporal relationship between the efficacy of the mesenchymal stromal cells (MSCs) delivered in cartilage regeneration and the number of cellular passages, dosage and frequency, cell of origin, usage of scaffolds, and bio-micromolecules has not yet been established. This is because the number of cellular passages, dosage and frequency, cell of origin, and usage of scaffolds are all important factors. It is anticipated that the number of cellular passages, dosage, and frequency, as well as the cell of origin, and the utilisation of scaffolds will all have an effect on this relationship.
Future Directives
Despite all the attempts to refine the treatment of cartilage lesions in osteoarthritis with MSC injections, effective regeneration of cartilage could not be demonstrated, which led to the next generation of surgical techniques to place the cells in a scaffold in a prepared subchondral bed of cartilage defect as in autologous chondrocyte implantation and matrix-assisted chondrocyte implantation techniques [58–60]. With a limited potential for cartilage to heal, engineered chondrogenesis plays a major role in regenerating a cartilaginous tissue. The engineered cartilage tissue maintains the joint homeostasis and provides a better biomechanical strength of the joint. The basis of cartilage engineering are mesenchymal stromal cells (autologous or allogeneic in origin), growth factors, and scaffolds [61]. Successful cartilage engineering depends on the tissue ergonomics with isolation and culture expansion of appropriate cells, optimum level of growth factors, and provision of 3D scaffolds for the cells to be anchored till the healing gets completed [62]. Although the autologous cellular precursors are the ideal candidates, improvising the acceptability and functionality of the allogeneic sources makes the procedure more readily available without the need for additional harvesting procedure to the patient. Moreover, the subjective functional limitations of the individual autologous cells could be counteracted with the utility of allogeneic sources of identified and established proliferative potential to better establish the procedure results with certainty.
The cartilage phenotype that was produced through the use of a PLGA–gelatin/chondroitin/hyaluronate scaffold was significantly superior when compared to the cartilage phenotype that was produced through the use of PLGA on its own [63]. The structure-specific regeneration of hyaline cartilage with autologous MSCs and a poly-lactic-co-glycolic acid and ACECM scaffold was demonstrated using a rabbit model with cartilage defects. Using an ACECM scaffold allowed for the regeneration of hyaline cartilage in this manner [64]. The porous fabricated BM-MSCs–dECM/marrow clot composite scaffold has the potential to support chondrogenesis. Marrow cells can be preserved, multiplied, and differentiated into chondrocytes by using this scaffold as a support structure. Magnesium-containing bioactive glass nanospheres (Mg-BGN) admixed with decellularized cartilage extracellular matrix show a lot of promise as a potential tool for cartilage regeneration in articular cartilage defects [66] (DCECM) [66]. Aside from the specific origin of the MSCs that are used in cartilage regeneration techniques, other aspects, such as the scaffolds that provide support and the nutrients that are required to maintain the cells at the site of injury, are all being investigated to locate the optimal composite complex that can regenerate damaged cartilage.
In addition, biomimetic 3D-printed nanocomposite scaffold and water-based polyurethane 3D-printed scaffold have been developed to improve the quality of scaffolds that are used in cartilage engineering. The microarchitecture, shape, and structure of the tissue that is supposed to be regenerated can be preserved by using these scaffolds [63, 68, 69]. Research must be done to improve the viability of autologous or allogeneic sources of MSCs, their proliferation, and the quantification of collagen in articular cartilage that has been regenerated. This can be accomplished through research. [69, 70]
Limitations
Our investigation does have a few drawbacks. The majority of the studies did not establish blinding, which may have increased the likelihood of treatment bias on the part of either patients or observers. The majority of the outcomes that were reported across the studies showed a degree of heterogeneity, which may be explained by the fact that the treatment protocols that were used in each study were different, as can be seen in Table 2. In addition, patients with varying stages of the disease process were included in the studies, which may be another factor that contributed to the variability of the results of the studies. We believe that a large trial that involves multiple centres is necessary to provide additional confirmation of the results of our research. This clinical trial should investigate both autologous and allogeneic sources of MSCs, have a standardised dosage and intervention protocol, be evaluated with established outcome measures both in the short term and long term, and not include any adjuvant procedures.
Conclusion
According to the findings of our review of the relevant research, autologous sources of mesenchymal stromal cells (MSCs) are superior to allogeneic sources of MSC in terms of their consistent efficacy with regard to pain, functional outcomes, and safety. However, we strongly recommend that further studies be conducted that are of a high enough quality to validate our findings and reach a consensus on the best source of MSCs for use in cellular therapy treatments for knee osteoarthritis.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buckwalter JA. Articular cartilage injuries. Clinical Orthopaedics. 2002;402:21–37. doi: 10.1097/00003086-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hsu H, Siwiec RM. Knee Osteoarthritis. In: StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov/books/NBK507884/. Retrieved 23 Oct 2021
- 3.Primorac D, Molnar V, Rod E, et al. Knee osteoarthritis: A review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes. 2020;11(8):E854. doi: 10.3390/genes11080854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Żylińska B, Silmanowicz P, Sobczyńska-Rak A, Jarosz Ł, Szponder T. Treatment of articular cartilage defects: Focus on tissue engineering. In Vivo. 2018;32(6):1289–1300. doi: 10.21873/invivo.11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim S, Nagesh HY, Pandey V Allogeneic chondrocyte implantation: What is stopping it from being a standard of care? J Arthrosc Surg Sports Med. 10.25259/JASSM_8_2021
- 6.Stampoultzis T, Karami P, Pioletti DP. Thoughts on cartilage tissue engineering: A 21st century perspective. Current Research in Translational Medicine. 2021;69(3):103299. doi: 10.1016/j.retram.2021.103299. [DOI] [PubMed] [Google Scholar]
- 7.Jiang S, Guo W, Tian G, et al. Clinical application status of articular cartilage regeneration techniques: Tissue-engineered cartilage brings new hope. Stem Cells International. 2020;2020:5690252. doi: 10.1155/2020/5690252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal D. Recent advances and future trends in articular cartilage repair. Journal of Arthroscopic Surgery and Sports Medicine. 2020;1(1):159–173. doi: 10.25259/JASSM_11_2020. [DOI] [Google Scholar]
- 9.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells—current trends and future prospective. Bioscience Reports. 2015;35(2):e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells International. 2019;2019:e9628536. doi: 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musiał-Wysocka A, Kot M, Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplantation. 2019;28(7):801–812. doi: 10.1177/0963689719837897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells Dayton Ohio. 2014;32(5):1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 13.Turajane T, Chaweewannakorn U, Larbpaiboonpong V, et al. Combination of intra-articular autologous activated peripheral blood stem cells with growth factor addition/ preservation and hyaluronic acid in conjunction with arthroscopic microdrilling mesenchymal cell stimulation Improves quality of life and regenerates articular cartilage in early osteoarthritic knee disease. Journal of the Medical Association of Thailand Chotmaihet Thangphaet. 2013;96(5):580–588. [PubMed] [Google Scholar]
- 14.Hopper N, Wardale J, Howard D, Brooks R, Rushton N, Henson F. Peripheral blood derived mononuclear cells enhance the migration and chondrogenic differentiation of multipotent mesenchymal stromal cells. Stem Cells International. 2015;2015:e323454. doi: 10.1155/2015/323454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Windt TS, Vonk LA, Slaper-Cortenbach ICM, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells Dayt Ohio. 2017;35(1):256–264. doi: 10.1002/stem.2475. [DOI] [PubMed] [Google Scholar]
- 16.de Windt TS, Vonk LA, Slaper-Cortenbach ICM, Nizak R, van Rijen MHP, Saris DBF. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: A first-in-man trial in 35 patients. Stem Cells Dayton Ohio. 2017;35(8):1984–1993. doi: 10.1002/stem.2657. [DOI] [PubMed] [Google Scholar]
- 17.Park Y, Ha C, Lee C, Yoon YC, Park Y. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: Results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Translational Medicine. 2017;6(2):613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega A, Martín-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation. 2015;99(8):1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 19.Furlan AD, Malmivaara A, Chou R, et al. 2015 updated method guideline for systematic reviews in the cochrane back and neck group. Spine. 2015;40(21):1660–1673. doi: 10.1097/BRS.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLOS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. Journal of Statistical Software. 2012;49(5):1–15. doi: 10.18637/jss.v049.i05. [DOI] [Google Scholar]
- 23.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estrada E, Décima JL, Rodríguez M, Di Tomaso M, Roberti J. Patient-reported outcomes after platelet-rich plasma, bone marrow aspirate, and adipose-derived mesenchymal stem cell injections for symptomatic knee osteoarthritis. Clinical Medicine Insights: Arthritis Musculoskeletal Disorders. 2020;13:1179544120931086. doi: 10.1177/1179544120931086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vangsness CT, Farr J, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: A randomized, double-blind, controlled study. Journal of Bone and Joint Surgery. American Volume. 2014;96(2):90–98. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- 26.Garay-Mendoza D, Villarreal-Martínez L, Garza-Bedolla A, et al. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. International Journal of Rheumatic Diseases. 2018;21(1):140–147. doi: 10.1111/1756-185X.13139. [DOI] [PubMed] [Google Scholar]
- 27.Kuah D, Sivell S, Longworth T, et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: A randomized double-blind placebo-controlled single ascending dose study. Journal of Translational Medicine. 2018;16(1):49. doi: 10.1186/s12967-018-1420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freitag J, Bates D, Wickham J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regenerative Medicine. 2019;14(3):213–230. doi: 10.2217/rme-2018-0161. [DOI] [PubMed] [Google Scholar]
- 29.Lamo-Espinosa JM, Mora G, Blanco JF, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II) Journal of Translational Medicine. 2016;14(1):246. doi: 10.1186/s12967-016-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garza JR, Campbell RE, Tjoumakaris FP, et al. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: A double-blinded prospective randomized controlled clinical trial. American Journal of Sports Medicine. 2020;48(3):588–598. doi: 10.1177/0363546519899923. [DOI] [PubMed] [Google Scholar]
- 31.Wong KL, Lee KBL, Tai BC, Law P, Lee EH, Hui JHP. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: A prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy: The Journal of Arthroscopic and Related Surgery. 2013;29(12):2020–2028. doi: 10.1016/j.arthro.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 32.Lu L, Dai C, Zhang Z, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: A prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Research & Therapy. 2019;10(1):143. doi: 10.1186/s13287-019-1248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emadedin M, Labibzadeh N, Liastani MG, et al. Intra-articular implantation of autologous bone marrow-derived mesenchymal stromal cells to treat knee osteoarthritis: A randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20(10):1238–1246. doi: 10.1016/j.jcyt.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Gupta PK, Chullikana A, Rengasamy M, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Research & Therapy. 2016;18(1):301. doi: 10.1186/s13075-016-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastos R, Mathias M, Andrade R, et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: A controlled, double-blind clinical trial. Knee Surgery, Sports Traumatology, Arthroscopy. 2020;28(6):1989–1999. doi: 10.1007/s00167-019-05732-8. [DOI] [PubMed] [Google Scholar]
- 36.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10(3):199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 37.Tran TDX, Wu CM, Dubey NK, et al. Time- and Kellgren-Lawrence grade-dependent changes in intra-articularly transplanted stromal vascular fraction in osteoarthritic patients. Cells. 2019;8(4):E308. doi: 10.3390/cells8040308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Translational Medicine. 2019;8(6):504–511. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. The Knee. 2012;19(6):902–907. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Koh YG, Kwon OR, Kim YS, Choi YJ. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: A prospective study. Arthroscopy: The Journal of Arthroscopy and Related Surgery. 2014;30(11):1453–1460. doi: 10.1016/j.arthro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 41.Hong Z, Chen J, Zhang S, et al. Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. International Orthopaedics. 2019;43(5):1123–1134. doi: 10.1007/s00264-018-4099-0. [DOI] [PubMed] [Google Scholar]
- 42.Ruane JJ, Ross A, Zigmont V, McClure D, Gascon G. A single-blinded randomized controlled trial of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee with active control. Journal of Stem Cells and Regenerative Medicine. 2021;17(1):3–17. doi: 10.46582/jsrm.1701002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv X, Huang C, Yin Z, Hong B, Jiang H, Huang X. Effectiveness of autologous bone marrow mesenchymal stem cell transplant for knee osteoarthritis. Chinese Journal of Cell Stem Cell. 2015;5(2):28–32. [Google Scholar]
- 44.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Engineering. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 45.Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: Cell biology to clinical progress. Npj Regenerative Medicine. 2019;4(1):1–15. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray IR, Chahla J, Safran MR, et al. International expert consensus on a cell therapy communication tool: DOSES. Journal of Bone and Joint Surgery. American Volume. 2019;101(10):904–911. doi: 10.2106/JBJS.18.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Zheng Q, Xiao Y, Feng J, Shi Z, Pan Z. Rat cartilage repair using nanophase PLGA/HA composite and mesenchymal stem cells. Journal of Bioactive and Compatable Polymers. 2009;24(1):83–99. doi: 10.1177/0883911508100655. [DOI] [Google Scholar]
- 48.Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy: The Journal of Arthroscopy and Related Surgery. 2007;23(2):178–187. doi: 10.1016/j.arthro.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Frisbie DD, Trotter GW, Powers BE, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Veterinary Surgery. 1999;28(4):242–255. doi: 10.1053/jvet.1999.0242. [DOI] [PubMed] [Google Scholar]
- 50.Huang XP, Sun Z, Miyagi Y, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122(23):2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 51.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: Past, present, and future. Stem Cell Research & Therapy. 2019;10(1):68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.García-Bernal D, García-Arranz M, Yáñez RM, et al. The current status of mesenchymal stromal cells: Controversies, unresolved issues and some promising solutions to improve their therapeutic efficacy. Frontiers in Cell and Developmental Biology. 2021;9:650664. doi: 10.3389/fcell.2021.650664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss JN. A Phase 3 Study to Evaluate the Efficacy and Safety of JointStem in the Treatment of Osteoarthritis. In: Weiss JN, ed. Orthopedic Stem Cell Surgery. Springer International Publishing, NewYork; 2021:199–203. 10.1007/978-3-030-73299-8_38
- 54.Zhang J, Huang X, Wang H, et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Research & Therapy. 2015;6(1):234. doi: 10.1186/s13287-015-0240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu JM, Wu X, Gimble JM, Guan X, Freitas MA, Bunnell BA. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell. 2011;10(1):66–79. doi: 10.1111/j.1474-9726.2010.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moradi S, Mahdizadeh H, Šarić T, et al. Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Research & Therapy. 2019;10(1):341. doi: 10.1186/s13287-019-1455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. Journal of Inflammation London, England. 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roseti L, Desando G, Cavallo C, Petretta M, Grigolo B. Articular cartilage regeneration in osteoarthritis. Cells. 2019;8(11):1305. doi: 10.3390/cells8111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flanigan DC, Sherman SL, Chilelli B, et al. Consensus on rehabilitation guidelines among orthopedic surgeons in the United States following Use of Third-Generation Articular Cartilage Repair (MACI) for Treatment of Knee Cartilage Lesions. Cartilage. 2021;13(1 Suppl):1782S–1790S. doi: 10.1177/1947603520968876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beck A, Wood D, Vertullo CJ, et al. Morphological assessment of MACI grafts in patients with revision surgery and total joint arthroplasty. Cartilage. 2021;13(1 Suppl):526S–539S. doi: 10.1177/1947603519890754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ikada Y. Challenges in tissue engineering. Journal of the Royal Society, Interface. 2006;3(10):589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nikolova MP, Chavali MS. Recent advances in biomaterials for 3D scaffolds: A review. Bioactive Materials. 2019;4:271–292. doi: 10.1016/j.bioactmat.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.H F, Y H, C Z, et al. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials. 2006;27(26). 10.1016/j.biomaterials.2006.04.013 [DOI] [PubMed]
- 64.Guo W, Zheng X, Zhang W, et al. Mesenchymal stem cells in oriented PLGA/ACECM composite scaffolds enhance structure-specific regeneration of hyaline cartilage in a rabbit model. Stem Cells Int. 2018;2018:e6542198. doi: 10.1155/2018/6542198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei B, Guo Y, Xu Y, et al. Composite scaffolds composed of bone marrow mesenchymal stem cell-derived extracellular matrix and marrow clots promote marrow cell retention and proliferation. Journal of Biomedical Materials Research. Part A. 2015;103(7):2374–2382. doi: 10.1002/jbm.a.35373. [DOI] [PubMed] [Google Scholar]
- 66.Yuan Z, Lyu Z, Liu X, Zhang J, Wang Y. Mg-BGNs/DCECM composite scaffold for cartilage regeneration: A preliminary in vitro study. Pharmaceutics. 2021;13(10):1550. doi: 10.3390/pharmaceutics13101550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Do AV, Khorsand B, Geary SM, Salem AK. 3D printing of scaffolds for tissue regeneration applications. Advanced Healthcare Materials. 2015;4(12):1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chung JJ, Im H, Kim SH, Park JW, Jung Y. Toward biomimetic scaffolds for tissue engineering: 3D printing techniques in regenerative medicine. Frontiers in Bioengineering and Biotechnology. 2020;8:1251. doi: 10.3389/fbioe.2020.586406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan BP, Leong KW. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. European Spine Journal. 2008;17(Suppl 4):467–479. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pina S, Ribeiro VP, Marques CF, et al. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials (Basel, Switzerland). 2019;12(11):E1824. doi: 10.3390/ma12111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.