Abstract
The histone-like protein (HlpA) is highly conserved among streptococci. After lysis of streptococci in infected tissues, HlpA can enter the bloodstream and bind to proteoglycans in the glomerular capillaries of kidneys, where it can react with antibodies or stimulate host cell receptors. Deposits of streptococcal antigens in tissues have been associated with localized acute inflammation. In this study, we measured the ability of purified HlpA (5 to 100 μg/ml), from Streptococcus mitis, to induce the production of proinflammatory cytokines by cultured, murine peritoneal macrophages. The release of tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) was time and concentration dependent and was not diminished by the presence of polymyxin B. Exposure of macrophages to a mixture of HlpA and lipoteichoic acid resulted in a synergistic response in the production of both TNF-α and IL-1. Stimulation with a mixture of HlpA and heparin resulted in reduced cytokine production (50% less IL-1 and 76% less TNF-α) compared to that by cells incubated with HlpA alone. The inclusion of antibodies specific to HlpA in macrophage cultures during stimulation with HlpA did not affect the quantity of TNF-α or IL-1 produced. These observations suggest that streptococcal histone may contribute to tissue injury at infection sites by promoting monocytes/macrophages to synthesize and release cytokines that initiate and exacerbate inflammation. Streptococcus pyogenes, which can infect tissues in enormous numbers, may release sufficient amounts of HlpA to reach the kidneys and cause acute poststreptococcal glomerulonephritis.
The histone-like protein (HlpA) of Streptococcus pyogenes and viridans group streptococci is considered a possible virulence factor in the pathogenesis of streptococcus-associated nephritides (SAN), including acute poststreptococcal glomerulonephritis and the nephritis that often accompanies infective endocarditis caused by streptococci (19, 38). Release of HlpA from streptococci at localized sites of infection (pharyngitis, pyoderma, endocarditis) is presumed to be a consequence of bacteriolysis caused by host defenses (9, 35). Extracellular HlpA can form soluble complexes, through its cationic domain, with lipoteichoic acid (LTA), a polyanionic surface antigen of these bacteria and a known nephritotoxin (23, 25, 26). The streptococcal components can enter the bloodstream directly from valvular lesions or through absorption by capillaries surrounding infected tissue and can be carried to the kidneys where HlpA binds selectively to heparan sulfate proteoglycans (HSPG) in basement membranes of glomerular capillaries and collecting tubules. Focal deposits of HlpA and LTA or their complexes can act as a nidus for the formation of in situ immune complexes (3, 8, 9, 39) that induce the inflammation and immunopathology typical of SAN (14, 29, 38). Nephritogenic amounts of HlpA are expected to arise only from enormous numbers of bacteria in tissues, a condition which occurs in pharyngitis and pyoderma caused by group A streptococci, and from colonies of viridans group streptococci growing on heart valves.
The inflammation of renal tissue, observed in SAN, is generally believed to result from the action of anaphylatoxins generated by the classical complement pathway (12, 27, 29, 38); however, certain streptococcal components, including LTA, can directly induce monocytes (2, 5, 24, 28, 31) and endothelial cells (37) to synthesize and secrete the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6. These cytokines activate T and B lymphocytes, stimulate fibroblast proliferation, and induce local vascular endothelial cells to synthesize adhesion receptors that mediate extravasation of leukocytes (21, 36). The present study was undertaken to evaluate the ability of HlpA and HlpA-LTA complexes to induce murine macrophages to produce IL-1 and TNF-α. Our findings indicate that the streptococcal protein is an effective modulator of cytokine production and that it acts synergistically with LTA, properties that enhance its credibility as a virulence factor of streptococci.
MATERIALS AND METHODS
Reagents.
Recombinant human TNF-α was obtained from R & D Systems, Inc. (Minneapolis, Minn.). Recombinant human IL-1β was purchased from Genzyme (Cambridge, Mass.). Polymyxin B sulfate, heparin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and LTA of S. pyogenes were obtained from Sigma Chemical Co. (St. Louis, Mo.). Actinomycin D was from Calbiochem, Boehringer Diagnostics (La Jolla, Calif.). RPMI 1640 medium, CMRL-1066 medium, antibiotic-antimycotic, l-glutamine, minimal essential medium nonessential amino acids solution, and pyruvate were purchased from Gibco BRL Life Technologies (Grand Island, N.Y.). Fetal bovine serum was obtained from Atlanta Biologicals (Norcross, Ga.). Lipopolysaccharide (LPS) of Escherichia coli J5 was obtained from List Laboratories (Campbell, Calif.). Goat polyclonal antibodies to mouse TNF-α, IL-1α, and IL-1β were purchased from R & D Systems. Rabbit antiserum to HlpA (enzyme immunoassay titer = 500,000) was obtained from previous studies (39). All other reagents were purchased from local vendors and were reagent grade.
Streptococcal HlpA.
Streptococcus mitis (ATCC 9811) was grown at 37°C for 18 h, without shaking, in Trypticase soy broth supplemented with 1 g of yeast extract per liter. Early-stationary-phase bacteria were harvested, and the histone-like protein was extracted as previously described (35). HlpA was purified to homogeneity by affinity chromatography on a column of heparin-agarose as previously described (39). Purity of the isolated protein was verified by silver nitrate staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, by Western immunoblot assay with rabbit antiserum to S. pyogenes or to S. mitis, and by N terminus amino acid sequencing. Previous studies have shown that the HlpA proteins of S. pyogenes and viridans group streptococci have >90% amino acid sequence identities, are immunologically cross-reactive, and have identical binding activities to animal cells (8, 39). Preliminary experiments indicated that murine macrophages responded equally well to HlpA from S. pyogenes M6 strain D471 and S. mitis. The latter species was the preferred source of HlpA for this study because of its superior growth rate and larger protein yields.
Macrophage cultures.
Specific-pathogen-free, female, strain CBA/J mice (Harlan Sprague-Dawley Inc., Indianapolis, Ind.) were injected intraperitoneally with 0.5 ml of complete Freund adjuvant (diluted 1:1 with sterile saline). After 14 days, the macrophages were harvested from the peritoneal cavities of two mice, washed three times with sterile, serum-free RPMI 1640 culture medium supplemented with 1 mM l-glutamine, 25 mM HEPES, 100 U of penicillin/ml, and 100 μg of streptomycin/ml, and suspended to 106 cells/ml in the same medium. The pooled macrophage suspension (0.1 ml per well) was dispensed to 96-well culture plates (Costar, Cambridge, Mass.) and incubated in 5% CO2 at 37°C. After 2 h, the adherent cells were washed three times with RPMI 1640 medium and overlaid with 0.1 ml of fresh medium containing 10% fetal calf serum with or without streptococcal components. Polymyxin B sulfate (25 μg/ml) was added routinely to macrophage cultures to prevent possible cell induction by trace amounts of LPS that might contaminate the bacterial reagents (30). At the times indicated in Fig. 2, the culture medium was harvested, clarified by centrifugation, and assayed for cytokine content. To determine the cellular content of IL-1 the macrophage monolayer was overlaid with 0.1 ml of fresh culture medium and the cells were lysed by two cycles of freezing and thawing. The extract was clarified by centrifugation before bioassay.
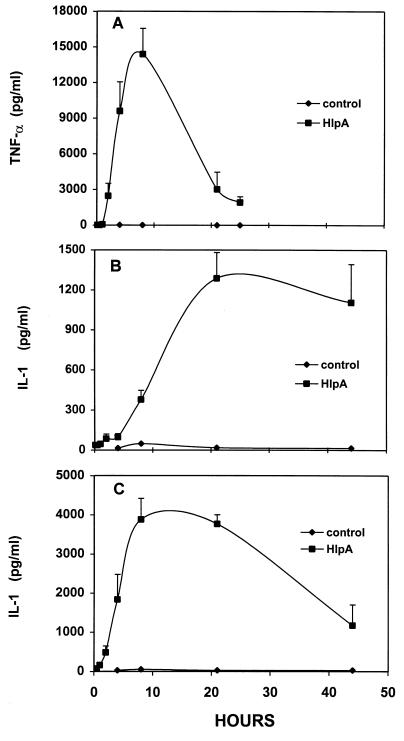
FIG. 2.
Time course of cytokine production by cultured macrophages incubated with 50 μg of streptococcal HlpA/ml. Culture supernatants were collected and tested for TNF-α (A) and IL-1 (B) at the indicated times. To measure intracellular pools of IL-1, macrophages were washed and lysed at the indicated times and the cell extracts were tested for cytokines (C). Data represent means of 6 to 12 samples, and error bars indicate SD. Control data were obtained from parallel assays using culture supernatants and lysates of macrophages not treated with HlpA.
TNF-α analysis.
TNF-α activity in cell-free specimens of each macrophage culture was determined by means of the WEHI 164 subclone 13 cytotoxicity assay (16). WEHI cells (American Type Culture Collection, Manassas, Va.) were cultured in 96-well culture plates (104 cells in 0.1 ml) with 0.1 ml of medium containing the amounts of test samples indicated in the text and actinomycin D (0.5 μg/ml) per well. Serial twofold dilutions of macrophage culture fluids were tested in triplicate. After 20 h at 37°C in 5% CO2, 20 μl of a 5-mg/ml solution of MTT was added to the wells, followed by incubation at 37°C for an additional 4 h. Supernatant fluid (150 μl) was removed from each well and discarded and replaced with 100 μl of 0.04 N HCl in isopropanol. The plates were then stored overnight in the dark at room temperature, and the absorbance at 540 nm was measured with a Titertek Autoreader. The responsiveness of the WEHI cells was standardized with recombinant human TNF-α.
IL-1 analysis.
The concentration of IL-1 in supernatant fluids of cultured macrophages was determined by bioassay using RINm5F cells according to the method of Hill et al. (22). RINm5F, an insulin-secreting cell line derived from a radiation-induced rat islet cell tumor, was a gift from M. L. McDaniel (Washington University School of Medicine, St. Louis, Mo.). The cells were dispensed in 96-well culture plates at a concentration of 2 × 105 cells/0.2 ml of CMRL-1066 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (complete CMRL) and incubated at 37°C in 5% CO2 for 24 h. The culture medium was replaced prior to the addition of test samples obtained from macrophage cultures. Each macrophage sample was tested in triplicate. After incubation at 37°C in 5% CO2 for 24 h, the supernatants from the treated monolayers were collected for measurement of nitrite concentrations. Fifty microliters of RINm5F culture supernatants was mixed with 50 μl of Griss reagent (1 part 0.1% naphthylethylenediamine dihydrochloride in H2O plus 1 part 1.32% sulfanilamide in 60% acetic acid) (18) in wells of a 96-well plate, and the absorbance at 540 nm was measured on a Titertek Autoreader. The IL-1 concentration was extrapolated from a standard curve obtained from RINm5F cells treated with recombinant human IL-1β at 1 to 100 pg/ml.
Statistical analysis.
Student's t test was used for statistical analyses, and P values <0.05 were considered significant. All data are expressed as means ± standard deviations (SD) of 3 to 16 independent observations.
RESULTS
HlpA induces cytokine production.
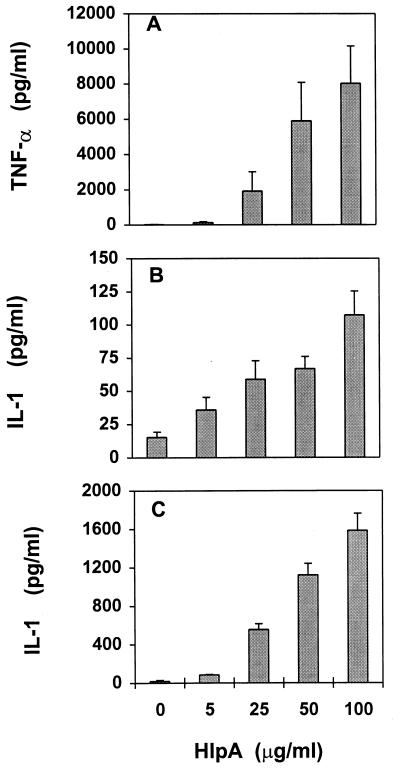
The addition of streptococcal HlpA to cultured murine macrophages resulted in a concentration-dependent induction of TNF-α and IL-1 secretion (Fig. 1). The largest amounts of extracellular cytokines were induced by HlpA at 100 μg/ml (10 μM). Larger quantities of inducer did not cause significantly more cytokine production (data not shown) and were not evaluated further because they were not considered relevant to HlpA concentrations at sites of streptococcus infection; at 60,000 copies/cell, 109 streptococci contain 1 μg of HlpA. The amount of TNF-α produced at 4 h postinduction (Fig. 1A) greatly exceeded that of IL-1 (Fig. 1B); however, secretion of IL-1 increased 15-fold over the next 17 h (Fig. 1C) whereas that of TNF-α did not (data not shown). The viabilities of macrophages, as indicated by trypan dye exclusion activity, were 96% ± 3% after 4 h and 93% ± 4% after 21 h, regardless of the presence of inducer. The identities of TNF and IL-1 were verified by neutralization of their activities (>90%) by goat anti-mouse TNF-α and by a mixture of anti-mouse IL-1α and IL-1β antibodies, respectively, added to diluted macrophage culture supernatant 10 min prior to the bioassay (data not shown).
FIG. 1.
Secretion of TNF-α and IL-1 by murine macrophages induced with streptococcal HlpA. Culture supernatants were tested for TNF-α (A) after 4 h of induction and for IL-1 after 4 (B) and 21 h (C) of induction. Data represent means of 9 to 13 experiments, and error bars indicate SD.
The kinetics of cytokine production by macrophage cultures induced with HlpA at 50 μg/ml (5 μM) is shown in Fig. 2. Although biologically active TNF-α was produced rapidly in the first 8 h, its activity decreased by 86% during the ensuing 18 h (Fig. 2A). In contrast, the maximum concentration of IL-1 was not attained until 21 h after addition of HlpA and remained at 85% of peak activity during the next 24 h (Fig. 2B). Macrophages incubated in culture medium free of inducer produced less than 20 pg of TNF-α/ml and less than 50 pg of IL-1/ml. In comparison, 10 ng of LPS/ml was used as a positive control and induced 7.3 ng of TNF-α/ml and 1.1 ng of IL-1/ml in parallel cultures. The ability of HlpA to induce production of cytokines was not diminished by the presence of polymyxin B sulfate at 25 μg/ml in the culture medium (data not shown), indicating that the induction mechanism is not related to the LPS type. Because IL-1 has been shown by others (6, 17, 34) to accumulate intracellularly before and during secretion, macrophages were washed with medium and lysed and the resulting extract was tested for the cytokine at the indicated time intervals (Fig. 2C). Figure 2C shows that the maximum amount of intracellular IL-1 was observed approximately 8 h after HlpA addition and 13 h before secreted IL-1 reached maximum levels as shown in Fig. 2B. WEHI and RINm5F indicator cells were incubated with 50 μg of HlpA/ml and were found not to show cytotoxicity; results were equivalent to those for untreated controls shown in Fig. 1 and 2.
Induction time.
The effects of varying the length of the induction period were examined because the presence of streptococcus components at infection sites may be ephemeral. Macrophages were induced with 5 nM HlpA for 10, 30, 60, or 120 min, washed three times, overlaid with fresh culture medium, and incubated for 4 h to allow for the production of cytokines. TNF-α production increased proportionately with induction time up to 60 min (4,589 ± 1,571 pg/ml [mean ± SD]; P < 0.05; the difference between the values after 60 and 120 min of induction (7,654 ± 2,942 pg/ml [mean ± SD]) was not statistically significant, indicating that full induction occurred within 1 to 2 h. The quantity of IL-1 also reflected the duration of macrophage exposure to streptococcal HlpA. Maximal production of extracellular (150 ± 48 pg/ml [mean ± SD]) and intracellular IL-1 (3,279 ± 1,519 pg/ml [mean ± SD]) occurred after a 120-min incubation with HlpA. Longer exposure to HlpA did not result in significantly more cytokine production, as shown in Fig. 2.
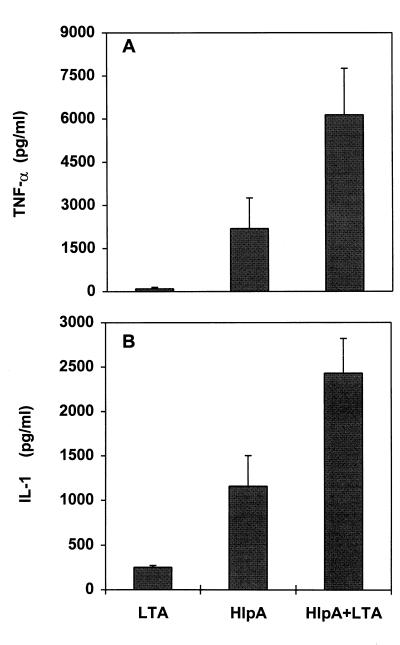
Synergism of HlpA and LTA.
The tendency of extracellular HlpA to form soluble, ionic complexes with streptococcal LTA (35) suggests that macrophages probably interact simultaneously with these substances in vivo. LTA is also a potent inducer of TNF-α and IL-1 production (5). To determine whether the induction of macrophages with a mixture of HlpA and LTA would result in additive or synergistic effects on cytokine production, cells were incubated with each inducer alone and with preformed complexes of LTA-HlpA. Previous studies have shown that a weight ratio of HlpA to LTA of 5:1 yields soluble complexes with strong binding properties for host cells (35). Figure 3 shows that the complexes caused synergistic induction of both TNF-α and IL-1. The TNF-α level was 269% of the sum of cytokine levels in HlpA- and LTA-treated cultures (P < 0.01), and the IL-1 level was 172% of the sum value (P < 0.01). Higher inducer concentrations (>50 μg of HlpA/ml or >20 μg of LTA/ml) did not exhibit synergistic effects on cytokine production (data not shown).
FIG. 3.
Synergistic effects of HlpA and LTA on TNF-α and IL-1 secretion by cultured murine macrophages. Cells were incubated with 25 μg of HlpA or 5 μg of LTA/ml or a mixture of HlpA and LTA. Supernatants were tested for TNF-α after 4 h (A) and for IL-1 after 21 h (B). Data are the means of four to six experiments, and error bars represent SD.
Inhibition by heparin and antiserum.
Previous studies have shown that the binding of HlpA to HSPG on epithelial cells and renal basement membranes is competitively inhibited by heparin (8, 35). To discern whether heparin could also inhibit the induction of cytokines by HlpA, macrophage cultures were incubated with 50 μg of HlpA/ml, followed by an equivalent amount of heparin added at the indicated times (Table 1). When these data were normalized to the quantities of cytokines produced by cells incubated with only HlpA, it was found that heparin inhibited 77% of TNF-α and 68% of IL-1 when added at zero time and 53 and 0%, respectively, when added 1 h after the HlpA. Macrophages treated with only heparin did not produce cytokines (data not shown).
TABLE 1.
Inhibition by heparin of HlpA induction of murine macrophage
| Inhibitora | Mean concn
(pg/ml) ± SD ofb:
|
|
|---|---|---|
| TNF-α | IL-1 | |
| None | 9,752 ± 1,461 (100) | 2,535 ± 1,014 (100) |
| Heparin at 0 min | 2,243 ± 585 (23) | 811 ± 177 (32) |
| Heparin after 10 min | 2,438 ± 1,072 (25) | 940 ± 228 (37) |
| Heparin after 60 min | 4,583 ± 488 (47) | 2,611 ± 684 (103) |
Murine macrophages were incubated at 37°C for 4 h with 50 μg of HlpA/ml. Heparin was added to a final concentration of 50 μg/ml at the indicated times during the incubation period.
Cytokine concentrations are based on data from six experiments. Values in parentheses are percentages of the cytokine concentration produced by HlpA-induced macrophages in the absence of inhibitor. Culture supernatants were tested for TNF-α, and cell lysates were used for the determination of IL-1.
The addition of HlpA-specific rabbit antiserum, at a dilution range of 1:100 to 1:50,000, to 10 μg of HlpA/ml prior to or 30 min after its addition to macrophage cultures did not significantly affect the quantity of cytokines produced (data not shown).
DISCUSSION
Our results show that purified streptococcal HlpA can induce macrophages/monocytes to produce the proinflammatory cytokines TNF-α and IL-1. This property was enhanced synergistically by the presence of streptococcal LTA, a polyanionic surface antigen and a potent inducer of cytokine production (5). Although HlpA and LTA are known to form soluble complexes in vitro, it is not clear whether they bind to host cell surfaces individually or as preformed complexes. HlpA-LTA complexes accumulating on host cell surfaces or forming in situ might serve to bridge several membrane receptors, resulting in a synergistic signal for the induction of cytokine synthesis and secretion. Indeed, cross-linking of LTA on the surfaces of monocytes by F(ab)2 fragments of immunoglobulin G has been reported to aggregate receptors and enhance cytokine production (28). It was also reported that once the LTA receptors on the monocytes were fully aggregated, the quantities of cytokines produced were similar to those produced by monocytes induced by LPS. In the present study, LPS was 52-fold more potent as an inducer of IL-1 production than were the HlpA-LTA complexes, on a weight-to-weight basis. In addition, the action of pore-forming bacterial toxins (lysins) has been shown to enhance the release of preformed pools of IL-1 by monocytes (6). Monocytes incubated sequentially with LTA and lysin produced a synergistic effect on IL-1 release but did not affect production of TNF-α (5). This lytic phenomenon does not explain the synergism between HlpA and LTA observed in the present study because these streptococcal components, individually or as complexes, do not cause lysis of animal cells (10, 32, 35) and because HlpA-LTA complexes also exerted synergistic effects on TNF-α production by murine macrophages.
The known receptors for HlpA and LTA on host cells are distinct. LTA binds through its fatty acid moiety to fibronectin in pericellular matrices and to unknown integral membrane proteins (10, 11, 20, 32), whereas HlpA binds ionically to HSPG (8, 39), such as the integral membrane polymer families syndecan and glypican (4, 13). The stimulatory effects of LTA on monocytes are competitively inhibited by polycations (poly-l-lysine and poly-l-arginine), which are believed to act at the initial cell binding step by coating LTA micelles and preventing its dissociation to monomeric LTA, the membrane-inserting form of the polymer (5). Polyanions (heparin and dextran sulfate) were found to competitively inhibit the binding of HlpA to animal cells (8, 35) as well as the induction of cytokine release by macrophages in the present study. HSPG polymers on animal cell surfaces have also been shown to serve as adhesin receptors for a variety of bacteria, viruses, and parasites (33). The coupling of HSPG components on epithelial cell surfaces by antibody-coated beads has been reported to induce phagocytosis (15). The signal transduction mechanism involved in cytokine induction by HlpA-HSPG binding has not been defined.
Streptococcus-induced nephritides are characterized by inflammation in glomeruli that may lead to a transient renal insufficiency (14). The disease usually resolves gradually over a few weeks but can lead to more chronic renal injury (7). Although the exact pathogenic mechanism has not been resolved, several immunopathogenetic models have been postulated (12, 29, 38); in these models, localized immune complexes cause inflammation through the activation of the complement cascade and generation of the anaphylatoxins C3a, C4a, and C5a (27). In addition, our data indicate that the release of HlpA and LTA by streptococci at sites of infection and their absorption into the bloodstream, distribution to kidneys, and deposition in the walls of glomerular capillaries and the mesangium can result in the induction of local monocytes to produce TNF-α and IL-1. These cytokines can, in turn, induce the synthesis of other mediators such as the chemotactic cytokine IL-8, cell lipid-derived prostanoids and leukotrienes, and endothelial cell adhesion molecules (21, 36), all of which are potent signals for the initiation and amplification of localized tissue inflammation. Further experiments with an animal model are necessary to resolve the nephritogenic potential of HlpA.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant R01-DE05696 from the National Institute for Dental and Craniofacial Research.
We thank Susan Alder for her excellent technical assistance.
REFERENCES
- 1.Bayne E K, Rupp E A, Limjuco G, Chin J, Schmidt J A. Immunocytochemical detection of interleukin-1 within stimulated human monocytes. J Exp Med. 1986;163:1267–1280. doi: 10.1084/jem.163.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benabdelmoumene S, Dumont S, Petit C, Poindron P, Wachmann W, Klein J P. Activation of human monocytes by Streptococcus mutansserotype f polysaccharide: immunoglobulin G Fc receptor expression and tumor necrosis factor and interleukin-1 production. Infect Immun. 1991;59:3261–3266. doi: 10.1128/iai.59.9.3261-3266.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergey E J, Stinson M W. Heparin-inhibitable basement membrane-binding protein of Streptococcus pyogenes. Infect Immun. 1988;56:1715–1721. doi: 10.1128/iai.56.7.1715-1721.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernfield M, Kokenyesi R, Kato M, Hinkes M T, Spring J, Gallo R L, Lose E J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 5.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhakdi S, Muhly M, Korom S, Huho F. Release of interleukin-1β associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron J S. The long term outcome of glomerular diseases. In: Schrier R W, Gottschalk C W, editors. Diseases of the kidney. Boston, Mass: Little, Brown & Co.; 1988. pp. 2155–2161. [Google Scholar]
- 8.Choi S H, Stinson M W. Binding of a Streptococcus mutanscationic protein to kidney in vitro. Infect Immun. 1991;59:537–543. doi: 10.1128/iai.59.2.537-543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi S H, Zhang X, Stinson M W. Dynamics of streptococcus histone retention by mouse kidneys. Clin Immunol Immunopathol. 1995;76:68–74. doi: 10.1006/clin.1995.1089. [DOI] [PubMed] [Google Scholar]
- 10.Courtney H S, Simpson W A, Beachey E H. Binding of streptococcal lipoteichoic acid to fatty acid-binding sites on human plasma fibronectin. J Bacteriol. 1983;153:763–770. doi: 10.1128/jb.153.2.763-770.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courtney H S, Stanislawski L, Ofek I, Simpson W A, Hasty D L, Beachey E H. Localization of a lipoteichoic acid binding site to a 24-kilodalton NH2-terminal fragment of fibronectin. Rev Infect Dis. 1988;10:S360–S362. doi: 10.1093/cid/10.supplement_2.s360. [DOI] [PubMed] [Google Scholar]
- 12.Couser W G. Mechanisms of glomerular injury in immune complex disease. Kidney Int. 1985;28:569–583. doi: 10.1038/ki.1985.167. [DOI] [PubMed] [Google Scholar]
- 13.David G. Integral membrane heparan sulfate proteoglycans. FASEB J. 1993;7:1023–1030. doi: 10.1096/fasebj.7.11.8370471. [DOI] [PubMed] [Google Scholar]
- 14.Davison A M. Infection-associated glomerulonephritis. In: Cameron S, Davison A M, Grunfeld J-P, Kerr D, Ritz E, editors. Oxford textbook of clinical nephrology. Oxford, United Kingdom: Oxford University Press; 1992. pp. 456–475. [Google Scholar]
- 15.Dehio C, Freissler E, Lanz C, Gomez-Duarte O G, David G, Meyer T F. Ligation of cell surface heparan sulfate proteoglycans by antibody-coated beads stimulates phagocytic uptake into epithelial cells: a model for cellular invasion by Neisseria gonorrhoeae. Exp Cell Res. 1998;242:528–539. doi: 10.1006/excr.1998.4116. [DOI] [PubMed] [Google Scholar]
- 16.Eskandari M K, Nguyen D T, Kunkel S L, Remick D G. WEHI 164 subclone 13 assay for TNF: sensitivity, specificity and reliability. Immunol Investig. 1990;19:69–79. doi: 10.3109/08820139009042026. [DOI] [PubMed] [Google Scholar]
- 17.Giri J G, Lomedico P T, Mizel S B. Studies on the synthesis and secretion of interleukin-1. A 33,000 molecular weight precursor for interleukin-1. J Immunol. 1985;134:343–349. [PubMed] [Google Scholar]
- 18.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 19.Gutman R A, Striker G E, Gilliland B C, Cuter R E. The immune complex glomerulonephritis of bacterial endocarditis. Medicine. 1972;51:1–25. doi: 10.1097/00005792-197201000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Hasty D L, Ofek I, Courtney H S, Doyle R J. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill J R, Corbett J A, Baldwin A C, McDaniel M L. Nitric oxide production by the rat insulinoma cell line, RINm5F, is specific for Il-1: a spectrophotometric IL-1 assay. Anal Biochem. 1996;236:14–19. doi: 10.1006/abio.1996.0125. [DOI] [PubMed] [Google Scholar]
- 23.Hyzy J, Sciotti V, Albini B, Stinson M W. Deposition of circulating streptococcal lipoteichoic acid in mouse tissues. Microb Pathog. 1992;13:123–132. doi: 10.1016/0882-4010(92)90072-v. [DOI] [PubMed] [Google Scholar]
- 24.Katsuta S, Kaneki Y, Tsutsui K, Tamaki S, Ibata H, Chen D R, Suzuki S, Natsuume-Sakai S, Yamamoto A. Interleukin-1 inducing activity of a streptococcus preparation OK-432 and its fractions by human monocytes. Clin Lab Immunol. 1989;28:129–136. [PubMed] [Google Scholar]
- 25.Leon O, Panos C. Effect of streptococcal lipoteichoic acid on prolyl hydroxylase activity as related to collagen formation in mouse fibroblast monolayers. Infect Immun. 1985;50:745–752. doi: 10.1128/iai.50.3.745-752.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon O, Panos C. An electron microscope study of kidney basement membrane changes in the mouse by lipoteichoic acid from Streptococcus pyogenes. Can J Microbiol. 1987;33:709–717. doi: 10.1139/m87-124. [DOI] [PubMed] [Google Scholar]
- 27.Liszewski M R, Farries T C, Lublin D M, Rooney I A, Atkinson J P. Control of the complement system. Adv Immunol. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 28.Mancuso G, Tomasello F, Ofek I, Teti G. Anti-lipoteichoic acid antibodies enhance release of cytokines by monocytes sensitized with lipoteichoic acid. Infect Immun. 1994;62:1470–1473. doi: 10.1128/iai.62.4.1470-1473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michael A F, Kim Y. Pathogenesis of acute post-streptococcal glomerulonephritis. In: Reed S E, Zabriskie J B, editors. Streptococcal diseases and the immune response. New York, N.Y: Academic Press; 1980. pp. 79–92. [Google Scholar]
- 30.Morrison D, Jacobs D. Binding of polymyxin B to the lipid portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 31.Muller-Alouf H, Alouf J E, Gerlach D, Ozegowski J-H, Fitting C, Cavaillon J-M. Comparative study of cytokine release by human peripheral blood mononuclear cells stimulated with Streptococcus pyogenessuperantigenic erythrogenic toxins, heat-killed streptococci and lipopolysaccharide. Infect Immun. 1994;62:4915–4921. doi: 10.1128/iai.62.11.4915-4921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ofek I, Beachey E H, Jefferson W, Campbell G I. Cell membrane binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975;141:990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rostand K S, Esko J D. Microbial adhesins to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer I I, Scott S, Hall G L, Limjuco G, Chin J, Schmidt J A. Interleukin-1β is located in the cytoplasmic ground substance but is largely absent from the Golgi apparatus and plasma membranes of stimulated human monocytes. J Exp Med. 1988;167:389–407. doi: 10.1084/jem.167.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stinson M W, McLaughlin R, Choi S H, Juarez Z E, Barnard J. Streptococcal histone-like protein: primary structure of hlpAand protein binding to lipoteichoic acid and epithelial cells. Infect Immun. 1998;66:259–265. doi: 10.1128/iai.66.1.259-265.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tracey K J, Cerami A. Tumor necrosis factor: an updated review of its biology. Crit Care Med. 1993;21:S415–S422. [PubMed] [Google Scholar]
- 37.Vernier A, Diab M, Soell M, Haan-Archipoff G, Beretz A, Wachsmann D, Klein J P. Cytokine production by human epithelial and endothelial cells following exposure to oral viridans streptococci involves lectin interactions between bacteria and cell surface receptors. Infect Immun. 1996;64:3016–3022. doi: 10.1128/iai.64.8.3016-3022.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogt A, Batsford S, Rodriguez-Iturbe B, Garcia R. Cationic antigens in poststreptococcal glomerulonephritis. Clin Nephrol. 1983;20:271–279. [PubMed] [Google Scholar]
- 39.Winters B D, Rammasubbu N, Stinson M W. Isolation and characterization of a Streptococcus pyogenesprotein that binds to basal laminae of human cardiac muscle. Infect Immun. 1993;61:3259–3264. doi: 10.1128/iai.61.8.3259-3264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]