Abstract
The coronavirus disease 2019 (COVID-19) pandemic has become a public health emergency of global concern. In China, traditional Chinese medicine has been widely administered to COVID-19 patients without sufficient evidence. To evaluate the efficacy of Shenhuang Granule (SHG) for treating critically ill patients with COVID-19, we included in this study 118 patients who were admitted to the ICU of Tongji Hospital between January 28, 2020 and March 28, 2020. Among these patients, 33 (27.9%) received standard care plus SHG (treatment group) and 85 (72.1%) received standard care alone (control group). Enrolled patients had a median (IQR) age of 68 (57-75) years, and most (79 [67.1%]) were men. At end point of this study, 83 (70.3%) had died in ICU, 29 (24.5%) had been discharged from ICU, and 6 patients (5.2%) were still in ICU. Compared with control group, mortality was significantly lower in treatment group (45.4% vs. 80%, p < .001). Patients in treatment group were less likely to develop acute respiratory distress syndrome (ARDS) (12 [36.3%] vs. 54 [63.5%], p = 0.012) and cardiac injury (5 [15.1%] vs. 32 [37.6%], p = 0.026), and less likely to receive mechanical ventilation (22 [66.7%] vs. 72 [84.7%], p = 0.028) than those in control group. The median time from ICU admission to discharge was shorter in treatment group (32 [20–73] days vs. 76 [63–79] days, p = 0.0074). These findings suggest that SHG treatment as a complementary therapy might be effective for critically ill adults with COVID-19 and warrant further clinical trials.
Keywords: Coronavirus disease 2019, traditional Chinese medicine, Shenhuang Granule, inflammation
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the novel coronavirus first detected in Wuhan, China, that causes coronavirus disease 2019 (COVID-19) [1]. Since initial detection of the virus, more than 26,760,000 cases and 876,000 deaths have been reported worldwide as of September 2020 (World Health Organization, COVID-19 outbreak situation 2020, accessed 7 September 2020). Unfortunately, the number of cases and deaths continues to rise every day. So far, the COVID-19 pandemic remains a global challenge.
Manifestations of SARS-CoV-2 infection are broad and variable, from asymptomatic carriers to pneumonia and fulminant disease, including acute respiratory distress syndrome (ARDS), sepsis, and ultimately, multisystem organ failure with many patients being admitted to intensive care unit (ICU) [2, 3]. At present however, there are no specific therapeutic drugs available for COVID-19. Currently, supportive therapies including oxygen support are the cornerstone for management of COVID-19 [4, 5]. In addition, antiviral drugs, immune modulators, and convalescent plasma are under investigation [6-9]. In China, greater than 85% of SARS-CoV-2-infected patients have received Traditional Chinese Medicine (TCM) treatment [10]. However, reports evaluating the efficacy of TCM herbal formula in treating patients hospitalized with COVID-19, especially in critically ill patients, are limited.
TCM has been used effectively to combat epidemic infectious diseases for thousands of years. To date, hundreds of herbal TCM formulae have been developed to prevent and treat epidemic infections. Based on “Truncation and Reversion” strategy and the theoretical understanding of the herbal properties within TCM, we developed the Shenhuang Granule (SHG) [11]. As described in our previous paper, SHG is composed of Panax ginseng, Rheum palmatum L. stem, Sargentodoxa cuneate, Taraxacum mongolicum, Aconiti Lateralis Radix Praeparata, and Whitmania pigra Whitman [12]. Panax ginseng and Rheum palmatum L. stem have been found to show antiviral activity [13-15]. Jinhong Decoction, composed of Rheum palmatum L. stem, Sargentodoxa cuneata, and Taraxacum mongolicum, could inhibit inflammatory cytokines such as TNF-α, IL-6, and IL-8 and protect against excessive inflammatory response in severe infectious diseases [16, 17]. Aconiti Lateralis Radix Praeparata shows a significant effect in rheumatoid arthritis by inhibiting the concentrations of IL-1β, TNF-α, and IFN-γ [18]. Whitmania pigra Whitman has been widely employed in decoction for the treatment of blood stasis syndrome for many years in China and its enzyme extracts suppress LPS-induced upregulation of inflammatory factors in rat vascular smooth muscle cells [19]. These pre-clinical and clinical studies indicated that some herbs of SHG could directly kill bacteria and inhibit viruses while other herbs could regulate inflammation response and maintain a balance between pro-inflammatory and anti-inflammatory mediators, with no serious adverse reactions observed [13-19]. Therefore, SHG treatment can be expected to have several beneficial effects on clinical outcome in patients with severe COVID-19. In this report, we evaluate the efficacy of combined SHG and standard care for treating critically ill adults with COVID-19.
Methods
Study Design and Participants
This single-center, retrospective, observational study was done at Tongji Hospital (Wuhan, China), which has two, large ICUs and was designated to treat patients with severe COVID-19. We recruited all of the adult (aged ≥18 years) inpatients admitted to ICU from January 28 to March 28, 2020, who were diagnosed with laboratory-confirmed COVID-19, according to World Health Organization interim guidance. All patients received standard care according to the National Guideline of Diagnosis and Treatment of COVID-19 (Trial Version 7). The standard care included timely provision of effective oxygen therapy, circulatory support, renal failure and renal replacement therapy, blood purification treatment, immunotherapy and other therapeutic measures such as short-term use of glucocorticoids. The patients recruited from one of the ICUs at Tongji Hospital received two sachets of SHG per day, from ICU admission to death or discharge, together with standard care. Patients with severe primary disease including malignant tumor, blood diseases, severe liver disease and known allergies to one or more substances in SHG, as well as women during pregnancy or lactation, were excluded from SHG treatment. We compared outcomes in patients who received standard care plus SHG (treatment group) with those in patients who received standard care alone (control group). Patients who either died within 48 h after being admitted to ICU, or those who received other herbs and participated in other clinical trials, or those admitted without key information, were excluded from the analysis. Follow-up continued through April 22, 2020. The study was approved by the Ethics Committee of Tongji Hospital(HSZY-PJ-2020-001-01). Informed consent was obtained from each patient or the patient’s legally authorized representative.
Study Medications
The SHG comprises 50 g of Panax ginseng C. A. Mey, 40 g of Rheum palmatum L. stem, 30 g of Sargentodoxa cuneata stem, 30 g of Taraxacum mongolicum, 50 g of Aconiti Lateralis Radix Praeparata and 6 g of Whitmania pigra Whitman, which are packaged into two sachets. (The detailed formula and medicinal product certificate were provided in a separate document for reviewing purposes). SHG was manufactured based on the Pharmacopedia of the People’s Republic of China and provided by Beijing Tcmages Pharmaceutical Co., Ltd. The granule was dissolved in warm water and taken orally by patients. For those critical patients with difficulty taking medication, the granule solution was administered through feed tube.
Data Collection
Data was extracted independently by two researchers using a standardized form. The data obtained included demographic characteristics, chronic medical histories, vital signs at ICU admission, laboratory finding, chest radiographs or computed tomography (CT) scan, treatment, and complications as well as outcomes of each patient. Laboratory tests for IL-6, IL-10, and TNF-a were not available for all patients.
Outcomes
The primary outcome was mortality after ICU admission. Secondary outcomes were incidence of organ dysfunction/failure including ARDS, acute kidney injury, acute hepatic injury, acute cardiac injury, coagulopathy and shock, the proportion of patients requiring mechanical ventilation, and length of ICU stay. ARDS was defined according to the Berlin definition [20]. Acute kidney injury was diagnosed according to the KDIGO Clinical Practice Guideline [21]. Acute liver injury was defined as ALT and/or AST ≥ 2 times the upper limit of normal, with total bilirubin ≥ 2 times the upper limit of normal and/or international normalized ratio ≥ 1.7. Acute cardiac injury was defined by troponin elevation above the 99th percentile. Coagulopathy was defined as prothrombin time (PT) and/or activated partial thromboplastin time (APTT) more than 1.5 times the normal control.
Statistical Analysis
Continuous variables were expressed in mean ± SD or median (IQR), as appropriate. Categorical variables were presented as number (percentage). Comparisons between continuous variables used Student‘s t-test or Mann-Whitney test. Comparisons for categorical variables were performed by using the chi-squared test or Fisher exact test. Kaplan-Meier method was used for survival data and the log-rank test was used to compare the survival times between both groups. A two-sided p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using Graphpad Prism, version 8.0.
Results
Baseline Characteristics
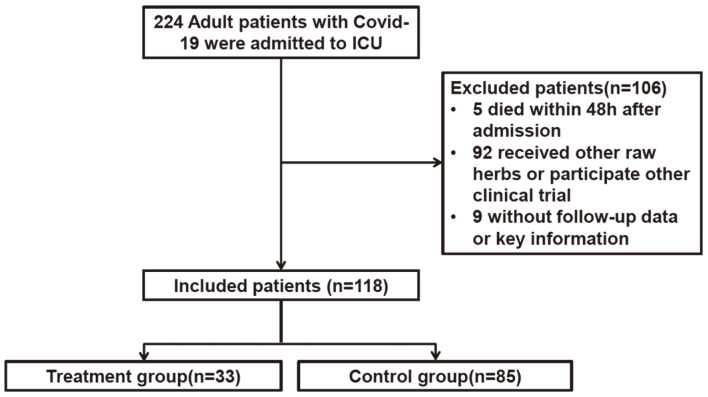
From January 28, 2020 to March 28, 2020, 224 patients with COVID-19 were admitted to ICU of Tongji Hospital. Of these patients, a total of 106 patients were excluded from this study. Five patients died within 48 h after being admitted to the ICU; 92 patients received other raw herbs or participated in other clinical trials. For 9 patients, follow-up data or key information were not available. Thus, 118 patients were included in the analysis (Fig. 1).
Fig. 1.
CONSORT flow diagram.
Of the118 patients, 33 (27.9%) received standard care plus SHG and 85 (72.1%) received standard care alone. The median duration of administration of SHG was 9 days (IQR, 6-14 days). Overall and per-group baseline characteristics are presented in Table 1. Baseline characteristics show an overall median age of 68 years (IQR, 57-75 years) and a predominance of men (79 [67.1%]). Hypertension (52 of 118 [44.0%]), diabetes (25 of 118 [21.1%]), and coronary heart disease (21 of 51 [17.7%]) were the most frequent comorbidities. The demographics, clinical characteristics, laboratory findings and concomitant treatment were comparable in both groups. (p > 0.05, Table 1).
Table 1.
Demographic, clinical, laboratory and radiographic findings of patients on admission to ICU.
| Variable | No. (%) | p value | ||
|---|---|---|---|---|
|
| ||||
| Total N=118 | Treatment group N=33 | Control group N=85 | ||
| Age, median (IQR), y | 68(57-75) | 65(56-73) | 69(58-75) | 0.196 |
| Female gender, No. (%) | 39(32.9) | 12(36.3) | 27(31.7) | 0.633 |
| Duration from onset of symptoms to ICU admission, median (IQR), d | 10(6-15.2) | 13(6.5-17) | 10(6-15) | 0.571 |
| Comorbidities | ||||
| Hypertension | 52(44.0) | 16(48.4) | 36(42.3) | 0.679 |
| Coronary heart disease | 21(17.7) | 3(9.0) | 18(21.1) | 0.18 |
| Chronic pulmonary disease | 9(7.5) | 1(3.1) | 8(9.3) | 0.441 |
| Diabetes | 25(21.1) | 7(21.2) | 18(21.1) | >0.999 |
| Cerebrovascular disease | 7(5.7) | 4(12.1) | 3(3.5) | 0.094 |
| Chronic kidney disease | 3(2.4) | 2(6.0) | 1(1.1) | 0.188 |
| Liver diseases | 0(0) | 0(0) | 0(0) | >0.999 |
| Malignancy | 1(0.8) | 0(0) | 1(1.1) | >0.999 |
| Smoking | 12(10.1) | 3(9.0) | 9(10.5) | >0.999 |
| Vital signs | ||||
| Body temperature, °C(T>37.5°C) | 33(27.9) | 7(21.2) | 26(30.5) | 0.366 |
| Heart rate, mean (SD), bpm | 94.8(20.4) | 90(13.2) | 96(22.4) | 0.115 |
| Mean blood pressure mean (SD), mmHg | 94.5(20.8) | 92.9(7.5) | 95.1(24.1) | 0.62 |
| Respiratory rate, median (IQR), rpm | 23(20-27) | 22(20-25) | 22(20-32) | 0.093 |
| Oxygen saturation median (IQR), % | 93(86-96) | 92.5(87.2-95) | 93(85-96) | 0.81 |
| Laboratory tests median (IQR) | ||||
| White blood cell count, × 109/L | 10.5(7.1-14.3) | 11.5(7.3-16.0) | 9.6(6.6-13.3) | 0.144 |
| Lymphocyte count, × 109/L | 0.64(0.42-0.96) | 0.84(0.42-1.14) | 0.58(0.42-0.85) | 0.08 |
| Platelet count, ×109/L | 167(112-243) | 164(95-235) | 168(114-248) | 0.751 |
| Alanine aminotransferase, U/L | 28(18-44) | 25(16-45) | 28(18-43) | 0.701 |
| Aspartate aminotransferase, U/L | 34(23-57) | 28(19-43) | 38(25-67) | 0.132 |
| Total bilirubin, mmol/l | 11.9(7.8-16.5) | 11.1(7.0-16.0) | 12.2(8.3-19.2) | 0.196 |
| Creatinine, μmol/l | 86(64-116) | 77(58-128) | 87(69-114) | 0.262 |
| Blood urea nitrogen, mmol/l | 8(5.2-12.6) | 6.9(4.1-10.7) | 8.4(5.7-13.3) | 0.094 |
| Serum sodium (mmol/l) | 140(137-144) | 141(139-144) | 139(136-144) | 0.184 |
| Serum potassium (mmol/l) | 4.43(4.0-5.0) | 4.43(4.1-5.0) | 4.48(4.0-4.9) | 0.648 |
| Lactate dehydrogenas (U/L) | 482(366-673) | 492(348-736) | 481(366-593) | 0.418 |
| Creatine kinase (U/L) | 134(74-398) | 135(78-419) | 132(70-360) | 0.421 |
| CK-MB Creatine kinase–MB, U/L | 1.7(0.8-2.5) | 1.6(0.6-1.9) | 1.8(1.1-7.1) | 0.204 |
| Fibrinogen, g/l | 4.7(3.2-5.9) | 4.4(3.0-5.2) | 4.7(3.2-6.1) | 0.274 |
| Prothrombin time, s | 15.6(14.5-17.8) | 16.1(14.6-18.3) | 15.5(14.5-17.4) | 0.251 |
| Activated partial thromboplastin time, s | 40.7(35.7-45.7) | 40(35.6-46.1) | 41(35.9-45.6) | 0.97 |
| D-dimmer, mg/l | 3.4(1.4-12.6) | 4.6(1.6-8.1) | 3.2(1.3-14.8) | 0.996 |
| C-reactive protein, mg/l | 87(37-144) | 67(12-134) | 97(41-148) | 0.117 |
| Procalcitonin, ng/ml | 0.2(0.1-1.1) | 0.2(0.1-2.2) | 0.2(0.1-0.87) | 0.559 |
| Imaging features | ||||
| Consolidation | 48(40.6) | 14(42.4) | 34(40.0) | 0.836 |
| Ground-glass opacity | 42(35.5) | 10(30.3) | 32(37.6) | 0.524 |
| Bilateral pulmonary infiltration | 93(78.7) | 25(75.7) | 68(80.0)) | 0.622 |
Abbreviations: ICU, intensive care unit, IQR, interquartile range.
Chronic pulmonary disease was defined as chronic obstructive pulmonary disease, asthma, or chronic bronchitis.
Outcomes
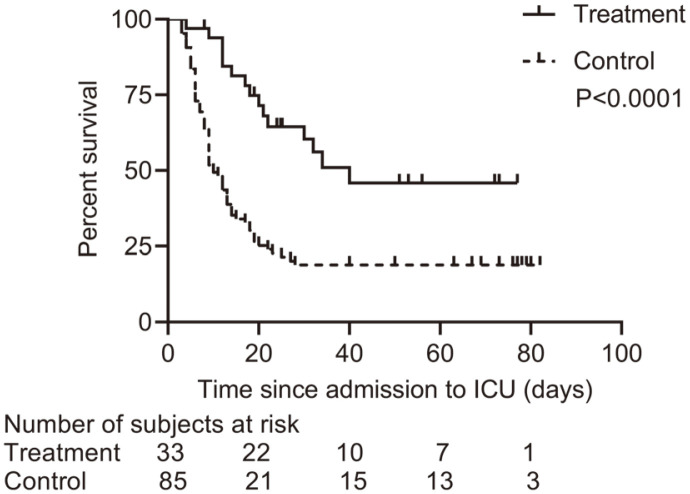
As of April 22, 2020, among the 118 patients, 83 (70.3%) had died in the ICU, 29 (24.5%) had been discharged from the ICU, and 6 patients (5.2%) were still in the ICU, with a median follow-up time of 13.5 days (Table 2). ICU mortality in treatment group was significantly lower compared with control group (45.4% vs 80%; p < .001). Moreover, among patients who died in the ICU (n = 83), the median survival time was longer in treatment group than that in control group (18 [12-30] days vs. 9 [6-13] days; p < 0.001); while among patients discharged from the ICU, the median length of stay in ICU was significantly shorter in treatment group as compared with control group (32 [20-73] days vs. 76 [63-79] days, p = 0.0074) (Table 2). Kaplan-Meier analysis showed that the survivor probability of patients with COVID-19 in treatment group was significantly higher than that of patients in control group (p < 0.001) (Fig. 2).
Table 2.
Complications, treatments and outcomes of patients.
| No. (%) | p value | |||
|---|---|---|---|---|
|
| ||||
| Total N=118 | Treatment group N=33 | Control group N=85 | ||
| Complications | ||||
| ARDS | 66(55.8) | 12(36.3) | 54(63.5) | 0.012 |
| Liver dysfunction | 18(14.2) | 4(12.1) | 14(16.4) | 0.555 |
| Acute kidney injury | 36(30.5) | 8(24.2) | 28(32.9) | 0.357 |
| Cardiac injury | 37(31.3) | 5(15.1) | 32(37.6) | 0.026 |
| Coagulopathy | 29(24.5) | 9(27.2) | 20(23.5) | 0.812 |
| Shock | 27(22.8) | 5(15.1) | 22(25.8) | 0.328 |
| Treatments | ||||
| High-flow nasal cannula oxygen therapy | 20(16.9) | 9(27.2) | 11(12.9) | 0.098 |
| Mechanical ventilation | 94(79.6) | 22(66.7) | 72(84.7) | 0.028 |
| Non-invasive | 23(19.3) | 4(12.1) | 19(22.3) | 0.301 |
| Invasive | 71(60.1) | 18(54.5) | 53(62.3) | 0.436 |
| ECMO | 5(4.2) | 2(6.0) | 3(3.5) | 0.618 |
| Antiviral therapy | 71(60.1) | 21(63.6) | 50(58.8) | 0.679 |
| Antibiotics | 112(94.8) | 31(93.9) | 82(95.2) | 0.671 |
| Glucocorticoid therapy | 92(77.9) | 24(72.7) | 68(80) | 0.459 |
| Renal replacement therapy | 23(19.4) | 7(21.2) | 16(18.8) | 0.798 |
| Outcomes | ||||
| Discharged from ICU | 29(24.5) | 14(42.4) | 15(17.6) | 0.008 |
| Died in ICU | 83(70.3) | 15(45.4) | 68(80.0) | 0.0002 |
| Still in ICU as of 4/22/2020a | 6(5.2) | 4(12.2) | 2(2.4) | 0.051 |
| Length of ICU stay at study end pointb | ||||
| Died, median (IQR) (d) | 9(6-17) | 18(12-30) | 9(6-13) | 0.0002 |
| Discharged alive, median (IQR), (d) | 67(27-76) | 32(20-73) | 76(63-79) | 0.0074 |
Abbreviations: ICU, intensive care unit; ARDS, acute respiratory distress syndrome;
ECMO, extracorporealmembrane oxygenation; IQR, interquartile range.
aPatients were admitted between 1/28/2020 and 3/28/2020, with follow-up through 4/22/2020.
bLength of stay begins with admission time and ends with discharge time, time at death, or on the last day of data collection for the study.
Boldface values were considered statistically significant.
Fig. 2.
Kaplan–Meier curves of survival rate according to the treatment (log rank test p < 0.0001).
Of the 118 critically ill patients, about half of patients had organ dysfunction, including 66 (55.8%) with ARDS, 37 (31.3%) with cardiac injury, 36 (30.5%) with acute kidney injury, 29 (24.5%) with coagulopathy, 27 (22.8%) with shock, and 18 (14.2%) with liver dysfunction. Compared with control group, treatment group patients were less likely to develop ARDS (12 [36.3%] vs 54 [63.5%], p = 0.012) and cardiac injury (5 [15.1%] vs 32 [37.6%], p = 0.026). The occurrence rate of other complications was similar in both groups (p > 0.05). Twenty (16.9%) patients were treated with high-flow nasal cannula oxygen therapy, 94 (79.6%) with mechanical ventilation, 5 (4.2%) with extracorporeal membrane oxygenation (ECMO), and 23 (19.4%) with renal replacement therapy. Additionally, 112 (94.8%) patients received antibiotics, 71 (60.1%) received antiviral agents such as lopinavir/ritonavir and 92 (77.9%) patients received glucocorticoids (Table 2). The comparisons of treatment for patients in both groups were shown in the Table 2. Compared with control group, patients in treatment group were less likely to receive mechanical ventilation (66.7% vs 84.7%; p = 0.028).
Some laboratory markers of inflammation were analyzed during ICU stay. We found that WBC increase and lymphopenia occurred less frequently in the treatment group than control group ((14 [42.4%] vs. 65 [76.4%] and 20 [60.6%] vs. 78 [91.7%]; both p < 0.001) (Table 3). Compared with the control group, patients in the treatment group were less likely to have elevated C-reactive protein (CRP) (21 [63.6%] vs. 75 [88.2%], p = 0.003). Elevated TNF-a, IL-6, and IL-10 were observed in most patients. Except for TNF-a, elevated IL-6 and IL-10 were less frequently observed in treatment group as compared with control group (19 of 26 [73%] vs. 65 of 68 [95.6%], p = 0.004 and 14 of 26 [53.8%] vs. 45 of 66 [80.3%], p = 0.018) (Table 3).
Table 3.
Inflammatory markers in patients during ICU stay.
| No. / total No. (%)a | p value | |||
|---|---|---|---|---|
|
| ||||
| Total N=118 | Treatment group N=33 | Control group N=85 | ||
| White blood cell count, × 109/L | ||||
| <3.5 | 11(9.2) | 3(9.0) | 8(9.4) | >0.999 |
| >9.5 | 79(66.8) | 14(42.4) | 65(76.4) | 0.0009 |
| Lymphocyte count, × 109/L | ||||
| <1.1 | 98(83.0) | 20(60.6) | 78(91.7) | 0.0002 |
| >3.2 | 1(0.8) | 0(0) | 1(1.1) | >0.999 |
| Procalcitonin, ng/ml | ||||
| increased | 53(44.8) | 14(42.4) | 39(45.8) | 0.837 |
| C-reactive protein, mg/l | ||||
| increased | 96(81.3) | 21(63.6) | 75(88.2) | 0.003 |
| TNF-a, pg/ml | ||||
| increased | 67/93(71.9) | 22/26(84.6) | 45/67(67.1) | 0.123 |
| IL-6, pg/ml | ||||
| increased | 87/94(92.5) | 19/26(73.0) | 65/68(95.6) | 0.004 |
| IL-10, pg/ml | ||||
| increased | 59/82(72.8) | 14/26(53.8) | 45/66(80.3) | 0.018 |
Abbreviations: ICU, intensive care unit.
aFor some variables, not available for all patients.
Boldface values were considered statistically significant.
Discussion
This study showed that the mortality rate was significantly lower in patients who received SHG treatment than those who did not. Our results also revealed a lower occurrence rate of organ dysfunction such as ARDS and cardiac injury, lower proportion of patients requiring mechanical ventilation, and shorter time to discharge from ICU in treatment group than control group.
Severe coronavirus disease (COVID-19) is characterized by pulmonary hyper-inflammation and potentially life-threatening systemic inflammatory cytokine storms [22]. Some patients, especially those who are older or have existing chronic medical conditions, may progress rapidly with ARDS, extra-pulmonary organ dysfunction such as acute kidney injury and acute liver injury and need to be admitted to ICU [23,24] . In our cohort, a high occurrence rate of ARDS and other organ dysfunction was observed, indicating that excessive systemically inflammatory response was common in critically ill patients with COVID-19. The mortality rate was higher than that recently reported in other case series and cohort studies [25, 26]. This could reflect the possible lack of critical care resources, especially where facilities are not adequately staffed, given the large number of COVID-19 cases in a short period of time, and the longer follow-up time.
According to current research, a higher pro-inflammatory cytokine storm existed in critical COVID-19 patients inducing excessive systemic inflammatory response, leading to ARDS and other organ dysfunction, and ultimately, death [24-22]. Thus, controlling the local and systemic inflammatory response presumably could reduce the severity and mortality rate of COVID-19 [27, 28]. SHG mainly consists of Panax ginseng, which has been shown to have antiviral activity, and Rheum palmatum L. stem, which could effectively suppress massive release of inflammatory mediators by inhibition of interactions between spike protein and the angiotensin converting enzyme 2 [29]. Historically, traditional herbal medicines have been effectively used to prevent and control epidemic outbreaks including SARS and H1N1influenza [30, 31]. These findings provide a rationale for clinical application of SHG treatment for severe COVID-19 patients.
In patients with COVID-19, elevated inflammatory cytokines were consistently reported. In our study, we measured the level of some inflammatory cytokines and found that the level of inflammatory cytokines such as TNF-a, IL-6 and IL-10 were significantly increased in most patients. Moreover, the occurrence rates of elevated IL-6 and IL-10 were lower in SHG treatment group as compared to control group. Consistently, the occurrence rate of elevated CRP, which is an inflammatory biomarker, was also lower in treatment group. These results indicated that SHG treatment could decrease the excessive release of inflammatory cytokines and suppress inflammatory response in severe patients with COVID-19. Indeed, Panax ginseng, a key component of SHG, was shown to significantly reduce DNA damages induced by oxidative stress in bone marrow cells and peripheral lymphocyte cells by its anti-oxidative stress and anti-inflammatory properties [32]. The SARS-CoV-2 infection activates innate and adaptive immune response. On the other hand, the virus infection might cause deficiency of immune system, as lymphopenia occurred in 35% of non-critical patients and more than 80% of critical patients infected with SARS-CoV-2, respectively [23]. In the current study, lymphopenia occurred in 98 (83%) patients. In addition, lymphopenia was less frequent in treatment group than control group, suggesting that SHG treatment might reverse lymphopenia in severe and critical patients with COVID-19. Moreover, elevated IL-6 and CRP level and lymphopenia were found to be positively associated with disease severity and prognosis of patients with COVID-19 [23, 24, 33]. Indeed, patients in treatment group, who were less likely to have elevated IL-6 and CRP level and lymphopenia, had a lower occurrence rate of organ dysfunction and better prognosis. Our results demonstrated SHG treatment potentially improves outcomes of severe and critical patients with COVID-19 partly through enhancing anti-viral immunity while inhibiting systemic inflammation.
This study has several limitations. First, the present study was a single-center, retrospective, observational study, a type that has a lower standard of evidence and is prone to confounding results and bias. Second, our study did not collect viral load data to determine the efficacy of antiviral effects of SHG treatment. Third, safety data related to the combination treatment with conventional medicine were not evaluated in our study, although these herbs have been used widely in TCM to control infectious diseases. Fourth, due to the retrospective nature of the study, methodologically the two groups had matching and non-matching sample size. Actually, the number of severe patients with COVID-19 dropped dramatically in China after March 2020. Thus, a degree of bias may have been introduced into our two groups. Last, interpretation of our findings might be limited by the sample size. Therefore, future controlled clinical trials are required to validate the findings of our study.
Conclusion
In summary, our findings suggest that by combination with conventional medicine, SHG treatment might have some beneficial effects such as mortality decrease, reduction in occurrence rate of organ dysfunction, decrease in proportion of patients requiring mechanical ventilation, as well as shorter median length of stay in ICU for severe patients with COVID-19, and warrant further controlled clinical trials to better evaluate the efficacy and safety of SHG treatment for severe COVID-19.
Acknowledgments
Thanks to all medical and management staff for providing care to COVID-19 patients and assistance with data collection from electronic medical records.
The work was supported by the Natural Science Foundation of Hubei Province (2017 CFB400) and the National Key Research and Development Program of China (2018YFC1705900, 2020YFC08845600). The funding sources had no role in the study design, analysis, or reporting.
Footnotes
Conflict of Interest
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1.Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020;9:1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5.Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA. 2020;323:1839–1841. doi: 10.1001/jama.2020.4914. [DOI] [PubMed] [Google Scholar]
- 6.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMc2008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Fang BJ, Wang G. The basic theory and practical application of catharsis large intestine and truncating and reversing in the prevention and treatment of sepsis. CJITWM. 2020;40:102–5. doi: 10.7661/j.cjim.20191009.224. https://doi.org/10.7661/j.cjim.20191009.224. [DOI] [Google Scholar]
- 12.Fang BJ, Zhang W, Wu XX, Huang TR, Li HC, Zheng Y, et al. Shenhuang granule in the treatment of severe coronavirus disease 2019 (COVID-19): study protocol for an open-label randomized controlled clinical trial. Trials. 2020;21:568. doi: 10.1186/s13063-020-04498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang BJ, Sun LH, Bu JH. The discussion of acute deficiency syndrome theory and its clinical application in emergency medicine. JETCM. 2017;26:1724–1726. doi: 10.3969/j.issn.1004-745X.2017.10.010. https://doi.org/10.3969/j.issn.1004-745X.2017.10.010. [DOI] [Google Scholar]
- 14.Xi XT, Zeng RF, Li J. The new thinking of integrated traditional Chinese and Western medicine from the core pathogenesis of sepsis. JETCM. 2018;27:105–109. doi: 10.3969/j.issn.1004-745X.2018.1.032. https://doi.org/10.3969/j.issn.1004-745X.2018.1.032. [DOI] [Google Scholar]
- 15.Wang Y, Jung Y-J, Kim K-H, Kwon Y, Kim Y-J, Zhang Z, et al. Antiviral activity of fermented ginseng extracts against a broad range of influenza viruses. Viruses. 2018;10:471. doi: 10.3390/v10090471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Zhang JZ, Zhang PT. Clinical research on impact of jinhong decoction on sepsis. Acad. J. Shanghai Univ. Tradit. Chin. Med. 2002;16:43–44. [Google Scholar]
- 17.Zhu PT, Zhang JZ, Gao J, Xl Z, Wang ZG, Shen P, et al. Experimental study on "Jinhong Pill" in regulating cytokine and preventing intestinal mucosal barrier of acute biliary tract infection in rats. Shanghai J. Tradit. Chin. Med. 2001;4:39–42. [Google Scholar]
- 18.Xie YF, Feng WW, Liu MC, Xie J, Yu L, Gong XH, et al. Investigation of efficacy enhancing and toxicity reducing mechanism of combination of aconiti lateralis radix praeparata and paeoniae radix alba in adjuvant-induced arthritis rats by metabolomics. Evid. Based Complement. Alternat. Med. 2019;2019:9864841. doi: 10.1155/2019/9864841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li SS, Cheng L, An DK, Song SL, Liang H, Chu FL, et al. Whitmania pigra whitman extracts inhibit lipopolysaccharide induced rat vascular smooth muscle cells migration and their adhesion ability to THP-1 and RAW 264.7 cells. J. Atheroscler. Thromb. 2017;24:301–311. doi: 10.5551/jat.36558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 21.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012;120:c179–184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhama K, Patel SK, Pathak M, Yatoo MI, Tiwari R, Malik YS, et al. An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med. Infect. Dis. 2020;37:101755. doi: 10.1016/j.tmaid.2020.101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho TY, Wu SL, Chen JC, Li CC, Hsiang CY. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, Lim CE, Kang HJ, Liu J. Chinese herbal medicines for the treatment of type A H1N1 influenza: a systematic review of randomized controlled trials. PLoS One. 2011;6:e28093. doi: 10.1371/journal.pone.0028093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Manheimer E, Shi Y, Gluud C. Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. J. Altern. Complement. Med. 2004;10:1041–1051. doi: 10.1089/acm.2004.10.1041. [DOI] [PubMed] [Google Scholar]
- 32.Zhang QH, Wu CF, Duan L, Yang JY. Protective effects of total saponins from stem and leaf of Panax ginseng against cyclophosphamide-induced genotoxicity and apoptosis in mouse bone marrow cells and peripheral lymphocyte cells. Food Chem. Toxicol. 2008;46:293–302. doi: 10.1016/j.fct.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]