Abstract
Our laboratory previously demonstrated that group C streptococcal isolates from humans and horses secrete streptokinases that preferentially activate plasminogens reflecting the origin of the isolates. To analyze the significance of these findings, series of streptokinase-producing Streptococcus equisimilis isolates recovered from humans and horses were examined. Southern blot analysis revealed that chromosomal DNA of the streptococcal isolates from humans reacted exclusively with a skchu probe and that chromosomal DNA of streptococcal isolates from horses reacted preferentially with an skceq probe in a distinct pattern. The streptococcal isolates were examined for the ability to acquire surface-bound plasmin-like activity when grown in the presence of human or equine plasma. Each of eight isolates from humans acquired significant enzymatic activity only when grown in the presence of human plasma, while each of eight isolates from horses acquired activity only when grown in the presence of equine plasma. Analysis of bacterial and host protein requirements indicated critical roles for streptokinase, activatable plasminogen, and fibrinogen. These requirements may explain why certain streptococcal isolates cause disease only in a limited number of mammalian hosts.
It is well established that the plasmin(ogen) system is responsible for the degradation of fibrin, a major constituent of blood clots (10). Recently, evidence indicating that it also plays an important role in a variety of biological processes characterized by tissue remodeling and migration of cells has accumulated (25, 27–31). Plasminogen is a glycoprotein found in circulating blood and in extravascular spaces (10, 35). The cleavage of plasminogen by specific plasminogen activators generates plasmin, a serine protease with broad substrate specificity. Plasmin can hydrolyze fibrin and extracellular matrix proteins as well as activate latent metalloproteinases. Under normal physiological conditions the generation of plasmin and the expression of its enzymatic activity are tightly controlled (10, 35). Specific host inhibitors of plasminogen activators and plasmin are particularly important in limiting plasmin activity (8, 14, 28). For a variety of mammalian cells, it has been demonstrated that assembly of plasmin(ogen) system components on the surface leads to the generation of cell-associated plasmin activity even in the presence of physiological inhibitors (27, 30).
Our laboratory has demonstrated that clinical and laboratory strains of group A streptococci, when grown in the presence of human plasma, acquired cell surface-associated enzymatic activity dependent on both the cellular production of streptokinase, a secreted plasminogen activator, and the presence of plasminogen in the culture medium (9, 18, 37). A role for human fibrinogen as a cofactor has also been documented (9, 38). The plasmin-like activity was captured by the bacteria prior to consumption of α2-antiplasmin, the primary physiological inhibitor of plasmin (18, 37). By comparing an isogenic mutant lacking streptokinase production to wild-type group A or group C streptococci, a number of investigators demonstrated that there was an absolute requirement for streptokinase to generate this type of surface-associated enzymatic activity (9, 20).
Streptococcus pyogenes group A bacteria are pathogens with a host range essentially limited to humans, whereas group C streptococci infect a variety of mammals including humans. Previously we demonstrated that group C streptococcal isolates produce streptokinases that preferentially activate plasminogens reflecting the origin of the isolate (24). For example, group C isolates recovered from horses efficiently activate equine plasminogen but do not activate human or porcine plasminogens efficiently (24). Furthermore, we purified streptokinase produced by Streptococcus equisimilis organisms isolated from a horse and demonstrated that the molecule was structurally distinct from streptokinase produced by streptococci isolated from humans (26). One would predict that if the streptokinase-dependent generation of cell surface-associated proteolytic activity is important in the infectious process, group C streptococci from various hosts would be capable of acquiring this enzymatic activity also in a species-specific manner. In this study, we examined the ability of group C streptococci isolated from humans and horses to capture plasmin-like activity when grown in plasma and addressed the species specificity of streptokinase in the interaction of bacteria with plasminogen and other host plasma proteins.
MATERIALS AND METHODS
Reagents.
Todd-Hewitt broth (THB) was obtained from Difco Laboratories (Detroit, Mich.) and prepared according to the formulation of the manufacturer. Chemically defined medium for streptococci was obtained from Hazelton Research Products (Lenexa, Kans.) and was prepared according to the formulation of van de Rijn and Kessler (34). Ultrafiltration of THB was performed by utilizing a PM-10 Diaflo membrane (Amicon Corp., Danvers, Mass.), and the filtrate (Mr < 10,000) was added to the chemically defined medium at 10% of the total volume. Human fresh-frozen plasma was obtained from Civitan Regional Blood Center, Gainesville, Fla., and aliquots were stored at −20°C. Equine plasma was collected from normal, healthy horses by venipuncture. Blood was placed immediately into 1/10 volume of 3.8% sodium citrate and centrifuged to prepare plasma, and aliquots were stored at −20°C. Plasminogen-depleted plasma was prepared by passage over a lysine-Sepharose column. The plasmin-selective chromogenic substrate H-d-valyl-l-leucyl-lysine-paranitroaniline dihydrochloride (S-2251) was obtained from Pharmacia/Hoefer (Franklin, Ohio). All other reagents were from Sigma Chemical Company (St. Louis, Mo.).
Bacterial isolates.
The study comprised 16 group C S. equisimilis isolates of human or equine origin (Table 1). Sugar fermentation reactions with trehalose, sorbitol, and lactose confirmed the species identification (12). Among the eight S. equisimilis isolates of human origin, six were obtained from Shands Hospital, Gainesville, Fla.; 26RP66 was obtained from Rockefeller University, New York, N.Y.; and 12449 was obtained from the American Type Culture Collection, Manassas, Va. Seven of the eight S. equisimilis isolates of equine origin were kindly provided by John Timoney, Gluck Equine Research Center, Lexington, Ky., and VM-45 was from the University of Florida College of Veterinary Medicine, Gainesville, Fla.
TABLE 1.
List of group C S. equisimilis isolates from humans and horses
| Isolatea | Source | Providerb |
|---|---|---|
| (A) 26RP66 | Human | Rockefeller University |
| (B) SHS-15 | Human | Shands Hospital |
| (C) 12449 | Human | ATCC |
| (D) C-521 | Human | Shands Hospital |
| (E) SHS-2 | Human | Shands Hospital |
| (F) C-350 | Human | Shands Hospital |
| (G) C-287 | Human | Shands Hospital |
| (H) CH | Human | Shands Hospital |
| (I) Hot & Restless | Horse | Gluck Equine Research Center |
| (J) VM-45 | Horse | UF College of Veterinary Medicine |
| (K) Hipkins | Horse | Gluck Equine Research Center |
| (L) 312092A | Horse | Gluck Equine Research Center |
| (M) 298393A | Horse | Gluck Equine Research Center |
| (N) Irish Spring | Horse | Gluck Equine Research Center |
| (O) 306073 | Horse | Gluck Equine Research Center |
| (P) 296243A | Horse | Gluck Equine Research Center |
The letters in parentheses are the isolate identifiers used in this study.
ATCC, American Type Culture Collection; UF, University of Florida.
Plasminogens and fibrinogens.
Human Glu-plasminogen was purchased from American Diagnostica, Greenwich, Conn. Human and equine fibrinogen was purchased from Sigma and further purified by lysine-Sepharose chromatography to remove any contaminating plasminogen. Equine plasminogen was purified from plasma by lysine-Sepharose chromatography, followed by molecular sieving chromatography on a Sephadex G-100 column as previously described (19). The concentrations of the purified proteins were determined by measuring the absorbance at 280 nm by using an absorptivity of 1.7 ml mg−1 cm−1.
Streptokinases.
A highly purified group C streptokinase that efficiently activates human plasminogen but not equine plasminogen (SKhu), Kabikinase, was a gift from KabiVitrum, A.B., Stockholm, Sweden. There was no carrier protein in this preparation, and the streptokinase was found to be homogeneous by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis analysis (16). Group C streptokinase that efficiently activates equine plasminogen but not human plasminogen (SKeq) was prepared from a group C streptococcal isolate of equine origin as described by Nowicki et al. (26). Briefly, S. equisimilis isolate 87-542-W was grown as a standing culture in 200 ml of chemically defined medium. The pH of the culture was monitored and maintained above 7.0. The supernatant was concentrated by passing it through a PM 10 filter (Amicon), discarding the eluate, and applying the concentrate (Mr > 10,000) to a column of immobilized human plasminogen as described previously (26). After the column was washed, bound streptokinase was eluted with 8 M urea. The streptokinase was extensively dialyzed into 20 mM HEPES and then stored as aliquots at −20°C. The concentration of the SKeq was determined with BCA protein assay reagent (Pierce, Rockford, Ill.), with bovine serum albumin as the standard. The plasminogen activator activity of SK preparations was determined by a standard assay (19).
Iodination of fibrinogen and measurement of bacterial fibrinogen binding.
Fifty micrograms of human or equine fibrinogen was radiolabeled with 125I as previously described (32). The binding of radiolabeled fibrinogen to group C streptococci was performed as follows. Individual cultures of eight group C S. equisimilis isolates recovered from either humans or horses were grown in 100 ml of THB overnight at 37°C as stationary cultures. Cells were then pelleted by centrifugation at 10,000 × g for 20 min and washed twice in 100 ml of 10 mM Veronal-buffered saline containing 0.1% gelatin, pH 7.35 (VBS-gel). Bacteria were resuspended at 6% wet weight/volume in VBS-gel. Approximately 50,000 cpm of radiolabeled human or equine fibrinogen was added to the bacteria in a final volume of 200 μl to yield a final bacterial concentration of 3% wet weight/volume. The bacteria were incubated for 2 h at 37°C and then washed three times with 2 ml of VBS-gel. Bound fibrinogen was quantified in a Beckman 4000 gamma counter.
Streptococcal genomic DNA isolation.
Streptococci were grown from starter cultures, inoculated into 100 ml of THB (Difco Laboratories) supplemented with 0.3% yeast extract (Difco) and 120 mM glycine (Fisher Scientific), and grown overnight at 37°C as stationary cultures. Cells were harvested by centrifugation and washed once with 20 mM Tris-HCl, pH 8.2. After centrifugation, the pellet was resuspended in a mixture of 3.2 ml of 20 mM Tris-HCl, pH 8.2, and 7 ml of 24% polyethylene glycol 20000 (Fisher Scientific) in distilled H2O. After the mixture was mixed, 3.5 ml of lysozyme (from a 20-mg/ml solution in distilled H2O; Sigma Chemical Co.) was added and the mixture was incubated at 37°C for 1 h with occasional shaking. The solution was centrifuged at 17,500 × g for 10 min, and the pellet was resuspended in 10 mM Tris-HCl–1 mM EDTA, pH 7.6. Next, 300 μl of 20% SDS was added, and the mixture was incubated at 65°C for 15 min. Two hundred microliters of RNase (10 mg/ml in distilled H2O) was added, and the solution was mixed and then incubated at 37°C for 15 min. Next, 200 μl of proteinase K (10 mg/ml in distilled H2O; Calbiochem, La Jolla, Calif.) was added, and the solution was mixed and incubated at 37°C for 30 min. Cells were harvested by centrifugation at 17,500 × g for 10 min, and the pellet was discarded. The DNA was purified from the supernatant by ultracentrifugation in cesium chloride (Gibco BRL, Gaithersburg, Md.).
DNA hybridization studies.
DNA probes for hybridization by the technique of Southern (22) were generated by the following methods. Plasmid pNC1, containing a 1,056-bp EcoRI/PstI DNA insert with an internal coding sequence of the streptokinase gene cloned from S. equisimilis H46A representing approximately 85% of the open reading frame (ORF; designated skchu) (13, 21) was digested with EcoRI and PstI, and the resulting DNA fragments were subjected to agarose gel electrophoresis. The 1-kb EcoRI/PstI fragment was excised, labeled, and used as a probe. Plasmid pBSTK22 contains a 2.9-kb BamHI/SauIIIA DNA insert harboring the coding sequence of the streptokinase gene cloned from S. equisimilis 87-542-W (designated skceq). The DNA fragment of interest was amplified by PCR. Primers 5′-GTAGGGCTATGTTTATTTTGCTAA-3′ and 5′-GGTTTGCTTTTAGAAGCGCGTTATT-3′ were used to amplify a 1,589-kb fragment containing the entire ORF of the gene comprising skceq. The PCR product was purified with the Wizard PCR Preps DNA purification system according to instructions of the manufacturer (Promega Corporation, Madison, Wis.). Both fragments were labeled with [α-32P]dCTP by using a random priming kit according to the instructions of the manufacturer (Amersham, Arlington Heights, Ill.).
Approximately 1 to 2 μg of genomic DNA from each of eight group C streptococcal isolates from humans and eight group C isolates from horses was digested to completion with HindIII, and DNA fragments were separated in parallel 0.7% (wt/vol) agarose gels. The DNA fragments were transferred to GeneScreen Plus nylon membranes by the capillary blotting procedure according to the instructions of the manufacturer (Du Pont, Boston, Mass.). One membrane was hybridized with the skchu probe, while the other membrane was hybridized with the skceq probe. Hybridization was performed overnight at 45°C in 10% dextran sulfate–50% formamide-0.5% SDS. Membranes were washed once at room temperature with excess 2× SSC (150 mM NaCl–15 mM Na3C6H5O7 · 2H2O) for 10 min. Two washes were then performed at the hybridization temperature with 2× SSC containing 1% SDS for 20 min, followed by two washes at room temperature with 0.2× SSC–1% SDS. Membranes were exposed to X-ray film overnight at room temperature, and hybridizing bands were visualized by automated film developing.
Determination of bacterium-associated enzymatic activity.
Bacterial isolates were grown at 37°C overnight as stationary cultures either in THB or a chemically defined medium containing 10% THB ultrafiltrate (CDM). One hundred microliters of the stationary culture was added to a mixture containing 1.4 ml of medium and 0.6 ml of human or equine plasma (final plasma concentration of 30%) or VBS-gel. After incubation at 37°C for 6 h, the cells were harvested by centrifugation, washed three times in VBS-gel, and resuspended in 1 ml of the same buffer. The number of bacteria was normalized by measuring the optical density at 550 nm and adjusting with VBS-gel to an optical density of approximately 2.0. S-2251 was added to yield a final concentration of 450 μM in a final volume of 400 μl. After incubation at 37°C, an equal volume of a 10% acetic acid in VBS-gel solution was added to stop the reaction. The bacteria were pelleted by centrifugation, and the absorbance of the bacterium-free supernatant was determined spectrophotometrically at 405 nm.
The effects of exogenous streptokinase on bacterium-associated enzymatic activity.
S. equisimilis isolates were grown in THB or CDM to stationary phase at 37°C, as standing cultures. Bacterial cultures were concentrated by centrifugation and washed in sterile VBS-gel, and the numbers of bacteria were normalized by measuring the optical density at 550 nm. In studies in which exogenous SK was added to the culture medium, 100 μg of chloramphenicol/ml was also added. Chloramphenicol allowed the effects of endogenous and exogenous SK to be distinguished. The dose of chloramphenicol utilized was found to be sufficient to prevent SK synthesis under these assay conditions. The bacteria were washed three times in VBS-gel and then incubated with SKhu for 6 h at 37°C in CDM containing 100 μg of chloramphenicol/ml and supplemented with either 30% human plasma, 30% equine plasma, or buffer alone. The final concentration of the exogenous SKhu was 200 U/ml in a final volume of 2 ml. The bacteria were washed twice in VBS-gel, and cell surface-associated enzymatic activity was determined as previously described.
RESULTS
Characterization of the streptokinase genes in human and equine group C isolates.
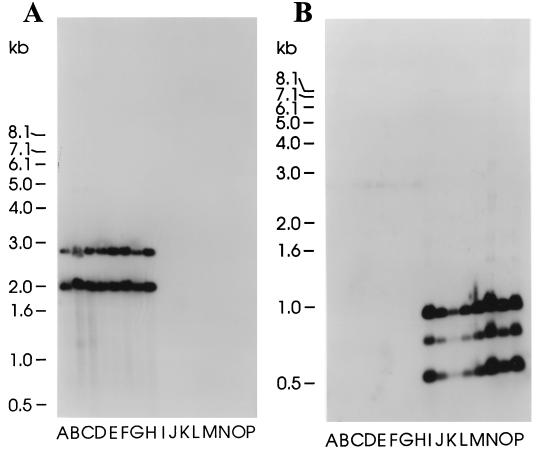
The initial studies were designed to compare the skc genes in group C S. equisimilis isolates recovered from humans and horses. Chromosomal DNA was isolated and incubated with HindIII, and fragments were separated on agarose gels as described in Materials and Methods. Separated fragments were transferred to nylon membranes by the method of Southern and probed with a streptokinase gene probe cloned from S. equisimilis H464A (skchu), isolated from a human, representing approximately 85% of the ORF (21), or the corresponding probe derived from 87-542-W (skceq), an isolate from a horse. (For precise details of these probes see Materials and Methods.) Chromosomal DNA from all group C isolates recovered from human patients reacted with the skchu gene probe and demonstrated reactivity with a similar series of bands in all of the enzyme digests (Fig. 1). Similar blots of fragmented chromosomal DNA from group C isolates recovered from equine sources demonstrated no reactivity with the skchu gene probe (Fig. 1).
FIG. 1.
DNA hybridization studies. Eight S. equisimilis isolates recovered from humans (A to H) and eight S. equisimilis isolates recovered from horses (I to P) were analyzed. For details of isolates see Table 1. (A) The membrane was reacted with a probe consisting of the internal coding sequence (85% of the ORF) of skchu. (B) The membrane was reacted with a probe consisting of the gene comprising skceq and its flanking sequence. For details of the probe sequences, see Materials and Methods.
Studies utilizing the skceq probe derived from isolate 87-542-W demonstrated reactivity with a similarly sized fragment from each of the equine isolates; however, this probe showed limited reactivity with the chromosomal DNA from any group C S. equisimilis organism isolated from humans. The pattern of reactive bands in the HindIII digests of chromosomal DNA from isolates recovered from humans or horses was distinct (Fig. 1). These studies demonstrate that the gene encoding streptokinase in isolates recovered from either humans or horses is well conserved but, in agreement with earlier studies, that the genes encoding the plasminogen activator in isolates recovered from humans differ markedly from those in isolates recovered from horses (6). This fundamental difference may explain the reason for the species-specific plasminogen activation potential of these distinct gene products.
Analysis of the ability of human and equine group C streptococcal isolates to acquire surface enzymatic activity when grown in homologous plasma.
Previous studies had demonstrated that isolates recovered from humans of group A and C streptococci could acquire a plasminogen-dependent surface enzymatic activity when grown in human plasma (18, 37). In preliminary studies we demonstrated that human plasminogen was activated efficiently by SKhu, a highly purified streptokinase from an isolate of human origin, whereas equine plasminogen was activated efficiently only by SKeq, a streptokinase purified from culture supernatant produced by S. equisimilis 87-542-W, an isolate from a horse (data not shown). Based on the difference in the streptokinase gene between isolates recovered from humans and horses shown in Fig. 1 and the reported difference in activation of the plasminogen activator between isolates recovered from humans and horses (24), the next series of studies were designed to compare the abilities of representative isolates to acquire enzymatic activity when grown in plasma obtained from the same species (homologous) from which the bacteria were originally recovered.
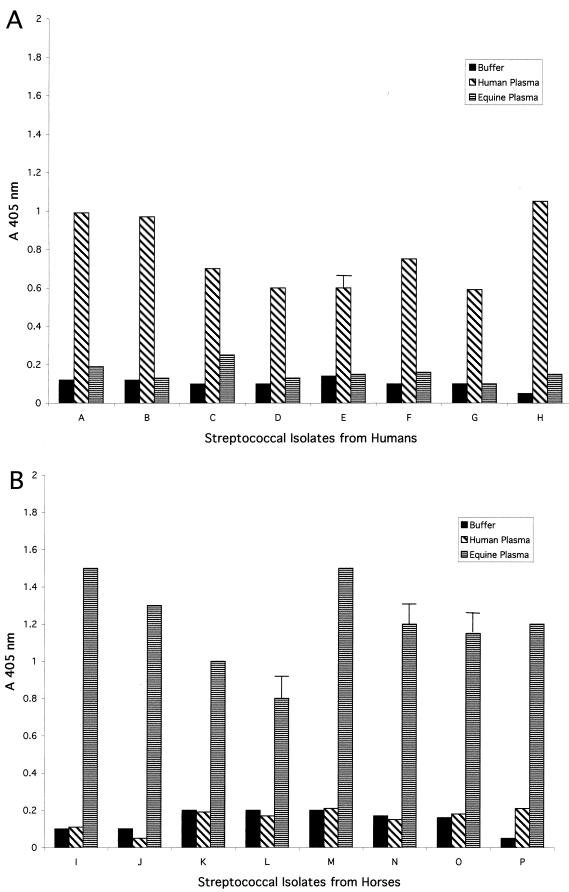
To determine if the ability to capture cell-associated enzymatic activity, when grown in plasma from the same species as that of the host from which the bacteria were isolated, was a common characteristic of group C streptococci, eight isolates recovered from humans and eight isolates recovered from horses were studied. The results presented in Fig. 2A demonstrate that all of the isolates recovered from humans, when grown in the presence of human plasma, acquired significant enzymatic activity, as measured by the ability of washed bacteria to cleave the synthetic substrate S-2251. The same isolates grown in the presence of equine plasma were markedly less efficient in acquiring enzymatic activity. In a similar series of studies using streptococcal isolates recovered from horses, only isolates grown in the presence of equine plasma acquired measurable enzymatic activity. When the same isolates were grown in human plasma, acquisition of enzymatic activity was markedly reduced (Fig. 2B).
FIG. 2.
Surface-associated enzymatic activity acquired by S. equisimilis isolates recovered from humans or horses following incubation in either human or equine plasma. S. equisimilis isolates were grown in CDM containing buffer, 30% human plasma, or 30% equine plasma. Cell-associated enzymatic activity was determined as described in Materials and Methods. (A) Results for S. equisimilis isolates recovered from humans. (B) Results for S. equisimilis isolates recovered from horses. Error bars are not depicted for standard deviations <0.1 A405 unit.
Bacterium-associated enzymatic activity was measured in all of these assays by hydrolysis of the chromogenic substrate S-2251. This substrate is sensitive to cleavage by plasmin or streptokinase-plasmin(ogen) complexes; however, it can also be hydrolyzed by other serine proteases that could potentially be produced either by the bacteria or as a result of activation of a nonplasminogen plasma component(s). Consequently, in the next series of experiments the role of plasminogen in the acquisition of surface enzymatic activity was determined. For these studies, representative group C streptococcal isolates from human or equine sources were grown in the presence of either homologous plasma or homologous plasma depleted of plasminogen. Plasminogen-depleted plasma was prepared by passage of plasma over a lysine-Sepharose column. A control sample of plasminogen-depleted plasma reconstituted with purified plasminogen was also included to ensure that the lysine-Sepharose treatment did not deplete any critical component in addition to plasminogen.
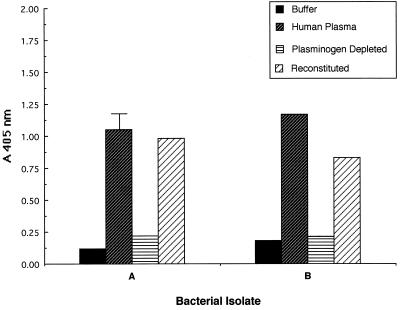
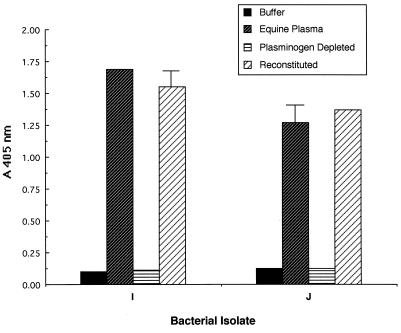
The results of these studies for two representative isolates recovered from humans (isolates A and B) and two representative isolates recovered from horses (isolates I and J) are presented in Fig. 3 and 4, respectively. In all cases, efficient acquisition of enzymatic activity was observed only when bacteria were incubated with a homologous source of plasma containing plasminogen.
FIG. 3.
Contribution of the presence of plasminogen in the growth medium to the ability of S. equisimilis isolates from humans to acquire surface-associated enzymatic activity. S. equisimilis isolates A and B were grown in CDM containing either buffer, 30% human plasma, 30% human plasminogen-depleted plasma, or 30% human plasminogen-depleted plasma reconstituted with 2 μg of human plasminogen/ml. Cell-associated enzymatic activity was determined as described in Materials and Methods. Error bars are not depicted for standard deviations <0.1 A405 unit.
FIG. 4.
Contribution of the presence of plasminogen in the growth medium to the ability of S. equisimilis isolates from horses to acquire surface-associated enzymatic activity. S. equisimilis isolates I and J were grown in CDM containing either buffer, 30% equine plasma, 30% equine plasminogen-depleted plasma, or 30% equine plasminogen-depleted plasma reconstituted with 2 μg of equine plasminogen/ml. Cell-associated enzymatic activity was determined as described in Materials and Methods. Error bars are not depicted for standard deviations <0.1 A405 unit.
Contribution of streptokinase and species of plasminogen to acquisition of enzymatic activity by group C streptococci.
The results presented in Fig. 2 to 4 suggest that the ability of streptokinase from different isolates to activate plasminogen could account for the observed acquisition of cell-associated enzymatic activity only when cells are grown in homologous plasma. Previous studies have suggested that SK demonstrates a unique profile of species specificity in its ability to activate different sources of mammalian plasminogen (23, 24, 42). Consequently, the next series of studies were designed to determine whether the ability of group C streptococci to acquire enzymatic activity in heterologous plasma was solely a function of SK or represented differences in plasmin-binding potential between species. For these experiments a representative human isolate (isolate A) and a representative equine isolate (isolate J) were incubated in heterologous plasma, with or without the addition of a source of Skhu. To distinguish the effect of endogenously generated SK from that attributable to the addition of exogenous SKhu, experiments were performed with both untreated bacteria and bacteria pretreated with chloramphenicol to inhibit de novo synthesis of the plasminogen activator. In preliminary studies a concentration (100 μg/ml) of chloramphenicol significantly inhibited acquisition of surface enzymatic activity for bacteria incubated with homologous plasma (data not shown).
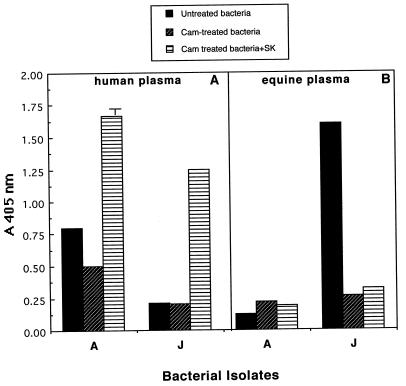
Untreated or chloramphenicol-treated bacteria were incubated in either 30% human or 30% equine plasma in the presence or absence of purified SKhu (200 U), and the quantity of cell-associated enzymatic activity, following a 1-h incubation at 37°C, was determined by the ability of washed bacteria to cleave the synthetic substrate S-2251. The results of these studies are presented in Fig. 5.
FIG. 5.
Effects of the addition of exogenous SKhu on the ability of S. equisimilis isolates grown in human or equine plasma to acquire surface-associated enzymatic activity. An S. equisimilis isolate from a human (isolate A) and an S. equisimilis isolate from a horse (isolate J) were incubated in CDM containing 30% plasma alone, 30% plasma plus chloramphenicol (Cam), or 30% plasma plus Cam and 200 U of SKhu. (A) Results obtained for bacteria grown in the presence of 30% human plasma; (B) results for bacteria grown in the presence of 30% equine plasma. Cell-associated enzymatic activity was determined as described in Materials and Methods. Error bars are not depicted for standard deviations <0.1 A405 unit.
In agreement with the previous results, the human isolate grown in human plasma acquired enzymatic activity. This activity was significantly reduced by addition of chloramphenicol to the reaction mixture (Fig. 5). Addition of exogenous SKhu to chloramphenicol-treated bacteria incubated in the presence of human plasma enhanced the acquisition of enzymatic activity. This result indicates that production of SK is the limiting factor in the acquisition of cell-surface enzymatic activity. In studies in which equine plasma was substituted for human plasma, no enzymatic activity was acquired by the human isolate in the presence or absence of SKhu (Fig. 5).
When these studies were carried out with a representative group C isolate recovered from a horse, a distinct pattern of acquisition of enzymatic activity was observed. As expected, enzymatic activity could be captured when the isolate was incubated in equine plasma. This activity was inhibited by addition of chloramphenicol (Fig. 5). When incubated in human plasma the isolate from a horse failed to acquire any enzymatic activity; however, when incubated with human plasma and SKhu, enzymatic activity could be captured (Fig. 5). These studies indicate that an isolate recovered from a horse can bind either human or equine plasmin.
In a similar series of studies using SKeq in place of SKhu, human group C isolates were found to be capable of binding equine plasmin, and once again there was an absolute requirement for a combination of SK and a species of activatable plasminogen for any isolate to acquire surface enzymatic activity. (data not shown).
These studies indicated that group C isolates could acquire enzymatic activity only when incubated with a combination of plasma and streptokinase which would enable plasminogen activation to occur. Based on these results, group C isolates were capable of binding either human or equine plasmin and their ability to acquire enzymatic activity is predictable based on the species-specific activation preference of streptokinase.
Is streptokinase alone responsible for the species specificity of acquisition of plasmin(ogen)-dependent cell-associated enzymatic activity?
The studies presented to date indicate that in this system, the species-specific acquisition of cell surface enzymatic activity is dependent on both streptokinase and plasminogen. It is not clear, however, whether these two factors alone can account for all of the species-specific properties of the system. Previous studies of the ability of group A streptococci to acquire enzymatic activity when grown in homologous plasma had identified a key role for fibrinogen (9, 38). Consequently the ability of a representative group C isolate of either human or equine origin to acquire enzymatic activity when grown in homologous plasminogen-depleted serum reconstituted with either human or equine plasminogen and/or human or equine fibrinogen was studied. This experimental design permitted the roles of both plasminogen and fibrinogen to be determined in a single assay.
The results of these preliminary experiments demonstrated that acquisition of surface enzymatic activity for an isolate of human origin (isolate A) required a source of both human plasminogen and human fibrinogen. In similar experiments using a group C organism isolated from a horse (isolate J), efficient enzymatic activity was observed only when the plasminogen-depleted equine serum was reconstituted with both equine plasminogen and fibrinogen (data not shown).
The next series of studies were conducted to assess the role of fibrinogen in the species-selective acquisition of enzymatic activity by group C isolates of human and equine origin. Preliminary results indicated that growth of any bacterial isolate in homologous serum demonstrated a marked reduction in acquisition of enzymatic activity compared with growth in plasma (data not shown). In order to assess the contribution of fibrinogen in these reactions, chloramphenicol-treated group C isolates recovered from either humans or horses were tested for their ability to acquire enzymatic activity when grown in human or equine fibrinogen in the presence of homologous plasminogen and a source of SK that would result in plasminogen activation. The results of these studies for one S. equisimilis isolate recovered from a human (Fig. 5A) and one S. equisimilis isolate recovered from a horse (Fig. 5B) demonstrate that either human or equine isolates were effective in capturing enzymatic activity when grown with homologous fibrinogen and plasminogen and a source of SK that would activate the plasminogen. There was evidence for a species-specific contribution of fibrinogen for the isolate recovered from a human; however, this effect was not observed with an isolate recovered from a horse (Table 2). For the isolate recovered from a horse, the presence of either human or equine fibrinogen would facilitate the acquisition of enzymatic activity from a reaction mixture which also contained SKeq and equine plasminogen. For these studies excess SK was added to enable the contribution of fibrinogen-SK-plasmin(ogen) binding to be distinguished from direct binding of plasmin. In the presence of excess SK no plasmin generated can bind directly to the bacteria (Table 2).
TABLE 2.
Effect of different species of fibrinogen on the ability of streptococcal group C isolates to acquire plasminogen-dependent enzymatic activity
| Isolate | Origin | Reactant(s)b | Source of streptokinase | Bacterium-associated enzymatic activitya |
|---|---|---|---|---|
| A | Human | Human plasma | Human isolate | 1.30 |
| Equine plasma | 0.21 | |||
| Fibhu + Plghu | 1.23 | |||
| Fibhu + PBS | 0.10 | |||
| Fibeq + Plghu | 0.22 | |||
| PBS + Plghu | 0.08 | |||
| J | Horse | PBS | Equine isolate | 0.09 |
| Human plasma | 0.24 | |||
| Equine plasma | 1.40 | |||
| Fibhu + PBS | 0.10 | |||
| Fibhu + Plgeq | 0.58 | |||
| Fibhu + PBS | 0.10 | |||
| Fibeq + Plgeq | 0.78 | |||
| PBS + Plgeq | 0.09 |
Bacterium-associated enzymatic activity was determined following 6 h of incubation with the reactants shown at 37°C as described in Materials and Methods. Values are A405 units.
Fibeq, equine fibrinogen; Fibhu, human fibrinogen; Plgeq, equine plasminogen; Plghu, human plasminogen; PBS, phosphate-buffered saline.
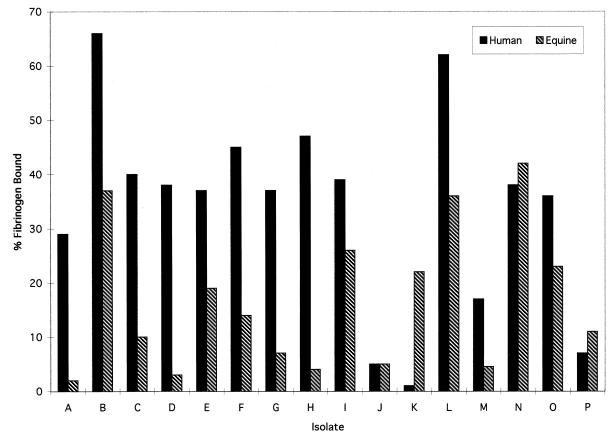
Parallel studies of the binding of radiolabeled human and equine fibrinogen revealed that all of the group C S. equisimilis isolates recovered from humans demonstrated significant binding of human fibrinogen but that binding of equine fibrinogen was comparatively less for all isolates (Fig. 6). By contrast, the fibrinogen-binding potential of the equine isolates was variable and demonstrated no definitive species preference. Some isolates (isolates L, M, and O) recovered from horses bound human plasminogen more efficiently than equine plasminogen. These results suggest that some of the observed species specificity for the binding of plasmin(ogen)-dependent enzymatic activity for the isolates of human origin (Table 2) may be attributable to the fibrinogen-binding properties of each organism.
FIG. 6.
Binding of human and equine fibrinogen to group C S. equisimilis isolates from humans and horses. 125I-labeled human or equine fibrinogen was added to each group C isolate, and the quantity of fibrinogen bound was determined following a 1-h incubation at 37°C. All estimates were carried out in duplicate, and variations between samples were <5%.
DISCUSSION
The plasminogen activator streptokinase expressed by the majority of groups A, C, and G streptococci demonstrates species specificity in the range of mammalian plasminogen sources that can be activated (23, 24, 42). Of particular interest has been the observation that the plasminogen species activation profile predicts the mammalian hosts that can be infected by a given organism (24). Streptococcal isolates recovered from humans secrete SK which demonstrates efficient activation of human plasminogen but not equine plasminogen, while isolates from horses produce a plasminogen activator that is efficient in activating equine plasminogen but lacks the ability to efficiently activate human plasminogen (24). The epidemiological associations between SK activity and the species of mammal subject to infection have suggested a possible cause-and-effect relationship. Plasmin(ogen) activation results in the generation of the potent serine proteinase plasmin. This enzyme, although most frequently associated with clot lysis, has a substrate range broader than that of fibrin and is capable of degrading fibronectin, laminin, and other components of the extracellular matrix, as well as being capable of activating a number of proenzymes including collagenases and matrix metalloproteinases (10, 29–31).
These properties of plasmin have been associated with a number of normal and pathogenic processes in humans including tissue remodeling, trophoblast implantation, wound healing, and the metastatic spread of tumor cells (2, 29–31). Because of the diverse range of potentially destructive activities of plasmin on normal tissue, the generation and activity of this enzyme in the human host are subject to extremely tight regulation by protease inhibitors. These include PAI 1 and PAI 2, which regulate the major eukaryotic plasminogen activator proteins (14), as well as α2-antiplasmin, which regulates the enzymatic activity of any plasmin generated (8). As a consequence of these efficient regulatory systems, fluid phase plasmin activity is not normally observed under physiological conditions. The action of plasmin on fibrin clots and other substrates is only noted when plasmin is localized to a clot or to a cell surface which facilitates escape from normal regulatory control.
Detailed biochemical analysis of the mechanism of activation of plasminogen by SK has only been carried out for a streptokinase molecule purified from the culture medium of a group C isolate recovered from a human (7). This form of streptokinase is a unique plasminogen activator in that it is not enzymatic in its activator function. Rather, a 1:1 stoichiometric complex of SK and plasminogen is formed (7). It is this complex that mediates the catalytic conversion of plasminogen to plasmin. This activation process cannot be regulated by host inhibitors. The only mechanism for prevention of SK-dependent activation is to prevent the complex from forming by the action of a specific anti-SK neutralizing antibody. Despite this difference in the activation process, any fluid phase plasmin generated by SK is quickly inactivated by α2-antiplasmin, although the activator complex itself cannot be regulated (8). Only when all of the α2-antiplasmin is consumed will free plasmin be detected in plasma. This so called “lytic state” has been achieved by administration of high doses of SK to patients undergoing “clot busting” therapy to treat myocardial infarctions and venous thromboembolism (36). However, the concentration of SK required to achieve a lytic state is 4 to 5 orders of magnitude greater than the concentration of SK produced by 1010 streptococci. Thus, it would be unrealistic to expect that this level of plasminogen activation mediated by SK secretion could occur during a streptococcal infection.
Recent observations from our laboratory and others have demonstrated that groups A, C, and G streptococci can bind human plasmin to a high-affinity surface plasmin binding protein and that, once bound, the enzyme cannot be regulated by α2-antiplasmin (15, 17, 18, 37, 38). These findings suggested that the activation of plasminogen by SK might be part of a more complex pathway that facilitated acquisition of an unregulatable host enzymatic activity by the bacteria. These properties could enhance bacterial colonization through the action of plasmin-unmasking adhesions on host cells or by permitting invasion by degradation of basement membranes. Studies of the interaction of group A streptococci with human plasma have identified a number of different pathways by which these bacteria can acquire surface enzymatic activity. These include (i) direct binding of preformed plasmin (4, 5, 17, 33), (ii) a complex interaction between a bacterial fibrinogen-binding protein and human fibrinogen, which provides a cell surface anchor site for an SK-plasminogen complex (9, 38), and (iii) a unique plasminogen-binding M protein (PAM) expressed by a limited number of serotype isolates (1, 41). PAMs can bind plasminogen directly, and, once bound, the protein can be activated by host plasminogen activators or SK. In all cases, the resulting bacterium-associated plasmin activity cannot be regulated by host α2-antiplasmin or other regulatory serpins. For a detailed review of all of these pathways see reference 3.
In this study we have examined the contribution of the species-specific activation of plasminogen to the ability of group C S. equisimilis isolates recovered from human patients and horses to acquire enzymatic activity when the isolates were grown in either human or equine plasma, in serum, or with purified plasma proteins. The initial studies compared the respective skc genes in each isolate and confirmed that there was a marked difference between the skc genes of isolates from different sources. The genes encoding SKhu, a plasminogen activator that efficiently activates human plasminogen to plasmin, were closely related in all of the isolates recovered from humans. However, skchu was markedly different from the skc present in the isolates recovered from horses. The skceq sequence encodes a plasminogen activator that activates equine plasminogen efficiently but fails to activate human plasminogen efficiently. Among S. equisimilis isolates recovered from either humans or horses, the skc genes were closely related.
When each group C isolate was grown in homologous plasma (i.e., isolates recovered from humans and grown in the presence of human plasma or isolates recovered from horses and grown in the presence of an equine plasma), the ability to acquire surface enzymatic activity was observed for all SK-secreting isolates. In all cases this activity was dependent on the presence of an activatable source of plasminogen. No isolate could acquire enzymatic activity when incubated in any source of plasminogen-depleted plasma. Isolates from humans grown in equine plasma failed to acquire significant enzymatic activity, while all of the isolates from horses acquired surface enzymatic activity and vice versa. The ability to acquire enzymatic activity was also shown to be dependent on the production of a plasminogen activator by the bacteria. Inhibition of protein synthesis prevented any isolate grown in homologous plasma from acquiring significant surface-associated enzymatic activity, and this property could be restored by addition of the appropriate source of purified SK.
Studies of the ability of bacteria to acquire enzymatic activity when incubated in homologous serum rather than plasma suggested a potential role for plasma proteins in addition to plasminogen. Based on our previous studies of group A streptococci (9, 37, 38) fibrinogen was considered to be a potentially important cofactor. Other studies of group A streptococcal virulence have also suggested that fibrinogen binding is important (11, 39, 40). In the studies evaluating a role for fibrinogen, a series of experiments were performed with a purified protein system. Different combinations of human or equine fibrinogen and/or plasminogen and SKhu or SKeq indicated that an isolate recovered from a human required a source of human fibrinogen to efficiently acquire enzymatic activity. By contrast, isolates from horses could acquire enzymatic activity in the presence of either human or equine fibrinogen and an appropriate combination of homologous plasminogen and SK that would result in plasminogen activation. The binding of fibrinogen was variable among isolates. All of the isolates recovered from the humans studied demonstrated preferential reactivity with human fibrinogen, while for isolates recovered from horses there was no clear evidence for a species-specific fibrinogen-binding preference.
Taken together all the data presented in the study indicate that the species specificity of SK results in a predictable pattern of acquisition of cell-associated enzymatic activity for isolates grown in different plasma sources. These studies are consistent with secreted SK being part of a coupled system that is plasminogen and fibrinogen dependent and that results in the ability of bacteria to acquire surface enzymatic activity despite the presence of efficient host regulators, such as α2-antiplasmin. These pathways are highly efficient in allowing the organism to acquire surface enzymatic activity following secretion of low levels of the plasminogen activator SK compared with the levels of SK required to generate fluid phase plasmin. From a comparison of the abilities of isolates from different mammalian hosts to participate in these pathways, the species specificity of SK-mediated plasminogen activation is apparent in all of the experimental systems studied, and this finding provides further support for the concept that bacterial plasminogen activators may be key virulence factors in determining the host range for pathogenic streptococcal infections.
ACKNOWLEDGMENTS
We thank John Timoney, Gluck Equine Research Center, for providing group C S. equimilis isolates from horses; Joseph Feretti, University of Oklahoma, Oklahoma City, for providing the skchu probe; and Kenneth Johnston, Louisiana State University Medical Center, New Orleans, for providing the skceq probe.
This work was supported by grants from the National Institutes of Health (HL 41898 and AI 43474) and a grant-in-aid from the American Heart Association, Florida Affiliate.
REFERENCES
- 1.Berge A, Sjöbring U. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J Biol Chem. 1993;268:25417–25424. [PubMed] [Google Scholar]
- 2.Blasi F, Verde P. Urokinase-dependent cell surface proteolysis and cancer. Cancer Biol. 1990;1:117–126. [PubMed] [Google Scholar]
- 3.Boyle M D P, Lottenberg R. Plasminogen activation by invasive human pathogens. Thromb Haemost. 1997;77:1–10. [PubMed] [Google Scholar]
- 4.Broder C C, Lottenberg R, Boyle M D P. Mapping of the human plasmin domain recognized by the unique plasmin receptor of group A streptococci. Infect Immun. 1989;57:2597–2605. doi: 10.1128/iai.57.9.2597-2605.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broeseker T A, Boyle M D P, Lottenberg R. Characterization of the interaction of human plasmin with its specific receptor on a group A streptococcus. Microb Pathog. 1988;5:19–27. doi: 10.1016/0882-4010(88)90077-0. [DOI] [PubMed] [Google Scholar]
- 6.Caballero A, Johnston K H, Lottenberg R. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Cloning, expression and sequence analysis of streptokinases from Streptococcus equisimilis which preferentially activate equine and porcine plasminogens, abstr. B-42; p. 172. [Google Scholar]
- 7.Castellino F J, Sodetz J M, Brockway W J, Sefring G E., Jr Streptokinase. Methods Enzymol. 1976;45:244–257. doi: 10.1016/s0076-6879(76)45024-3. [DOI] [PubMed] [Google Scholar]
- 8.Cederholm-Williams S A, Decock F, Linjen H R, Collen D. Kinetics of the reactions between streptokinase, plasmin and alpha-2 antiplasmin. Eur J Biochem. 1979;100:125–132. doi: 10.1111/j.1432-1033.1979.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 9.Christner R, Li Z, Raeder R, Podbielski A, Boyle M D P. Identification of key gene products required for acquisition of plasmin-like enzymatic activity by group A streptococci. J Infect Dis. 1997;175:1115–1120. doi: 10.1086/516450. [DOI] [PubMed] [Google Scholar]
- 10.Collen D. On the regulation and control of fibrinolysis. Thromb Haemost. 1980;43:77–89. [PubMed] [Google Scholar]
- 11.Courtney H S, Li Y, Dale J B, Hasty D L. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect Immun. 1994;62:3937–3946. doi: 10.1128/iai.62.9.3937-3946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Facklam R R, Rutledge L. Physiologic and in vitro virulence differences among four beta-hemolytic group C streptococcus species. In: Kimura Y, Kotami S, Shiokawa Y, editors. Proceedings of the IXth International Symposium on Streptococci and Streptococcal Diseases. Berkshire, United Kingdom: Reedbooks; 1984. pp. 64–69. [Google Scholar]
- 13.Huang T T, Malke H, Ferretti J J. Heterogeneity of the streptokinase gene in group A streptococci. Infect Immun. 1989;57:502–506. doi: 10.1128/iai.57.2.502-506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruithof E K. Plasminogen activator inhibitors—a review. Enzyme. 1988;40:113–121. doi: 10.1159/000469153. [DOI] [PubMed] [Google Scholar]
- 15.Kuusela P, Ullberg M, Saksela O, Kronvall G. Tissue-type plasminogen activator-mediated activation of plasminogen on the surface of group A, C, and G streptococci. Infect Immun. 1992;60:196–201. doi: 10.1128/iai.60.1.196-201.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lottenberg R, Broder C C, Boyle M D P. Identification of a specific receptor for plasmin on a group A streptococcus. Infect Immun. 1987;55:1914–1928. doi: 10.1128/iai.55.8.1914-1918.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lottenberg R, Desjardin L E, Wang H, Boyle M D P. Streptokinase-producing streptococci grown in human plasma acquire unregulated cell-associated plasmin activity. J Infect Dis. 1992;166:436–440. doi: 10.1093/infdis/166.2.436. [DOI] [PubMed] [Google Scholar]
- 19.Lottenberg R, Dolly F R, Kitchens C S. Recurrent thromboembolic disease and pulmonary hypertension associated with severe hypoplasminogenemia. Am J Hematol. 1985;19:181–193. doi: 10.1002/ajh.2830190211. [DOI] [PubMed] [Google Scholar]
- 20.Malke H, Mechold U, Gase K, Gerlach D. Inactivation of the streptokinase gene prevents Streptococcus equisimilisH46A from acquiring cell-associated plasmin activity in the presence of plasminogen. FEMS Microbiol Lett. 1994;116:107–112. doi: 10.1111/j.1574-6968.1994.tb06683.x. [DOI] [PubMed] [Google Scholar]
- 21.Malke H, Roe B, Ferretti J J. Nucleotide sequence of the streptokinase gene from streptococcus equisimilisH46A. Gene. 1985;34:357–362. doi: 10.1016/0378-1119(85)90145-3. [DOI] [PubMed] [Google Scholar]
- 22.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 23.Marcum J A, Kline D L. Species specificity of streptokinase. Comp Biochem Physiol. 1983;75:389–394. doi: 10.1016/0305-0491(83)90345-0. [DOI] [PubMed] [Google Scholar]
- 24.McCoy H E, Broder C C, Lottenberg R. Streptokinases produced by pathogenic group C streptococci demonstrate species-specific plasminogen activation. J Infect Dis. 1991;164:515–521. doi: 10.1093/infdis/164.3.515. [DOI] [PubMed] [Google Scholar]
- 25.McNeill H, Jensen P J. A high-affinity receptor for urokinase plasminogen activator on human keratinocytes: characterization and potential modulation during migration. Cell Regul. 1990;1:843–852. doi: 10.1091/mbc.1.11.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowicki S, Minning-Wenz D, Johnston K H, Lottenberg R. Characterization of a novel streptokinase produced by Streptococcus equisimilisof non-human origin. Thromb Haemost. 1994;72:595–603. [PubMed] [Google Scholar]
- 27.Ossowski L. Invasion of connective tissue by human carcinoma cell lines: requirement for urokinase, urokinase receptor and interstitial collagenase. Cancer Res. 1992;52:6754–6760. [PubMed] [Google Scholar]
- 28.Plow E F, Herren T, Redlitz A, Miles L A, Hoover-Plow J L. The cell biology of the plasminogen system. FASEB J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- 29.Pöllänen J, Stephens R W, Vaheri A. Directed plasminogen activation at the surface of normal and malignant cells. Adv Cancer Res. 1991;57:273–328. doi: 10.1016/s0065-230x(08)61002-7. [DOI] [PubMed] [Google Scholar]
- 30.Reich R, Thompson E W, Iwamoto Y, Martin G R, Deason J R, Fuller G C, Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988;48:3307–3312. [PubMed] [Google Scholar]
- 31.Strickland S, Reich E, Sherman M I. Plasminogen activator in early embryogenesis: enzyme production by trophoblast and parietal endoderm. Cell. 1976;9:231–240. doi: 10.1016/0092-8674(76)90114-8. [DOI] [PubMed] [Google Scholar]
- 32.Thorell J I, Johnsson B G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971;251:363–368. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- 33.Ullberg M, Kronvall G, Karlsson I, Wiman B. Receptors for human plasminogen on gram-negative bacteria. Infect Immun. 1990;58:21–25. doi: 10.1128/iai.58.1.21-25.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Rijn I, Kessler R E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980;27:444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vassalli J D, Sappino A P, Belin D. The plasminogen activator/plasmin system. J Clin Investig. 1991;88:1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verstraete M, Lijnen H R, Collen D. Thrombolytic agents in development. Drugs. 1995;50:29–42. doi: 10.2165/00003495-199550010-00003. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Lottenberg R, Boyle M D P. Analysis of plasmin(ogen) acquisition by clinical isolates of group A streptococci incubated in human plasma. J Infect Dis. 1994;169:143–149. doi: 10.1093/infdis/169.1.143. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Lottenberg R, Boyle M D P. A role for fibrinogen in the streptokinase-dependent acquisition of plasmin(ogen) by group A streptococci. J Infect Dis. 1995;171:85–92. doi: 10.1093/infdis/171.1.85. [DOI] [PubMed] [Google Scholar]
- 39.Whitnack E, Beachey E H. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J Clin Investig. 1982;69:1042–1045. doi: 10.1172/JCI110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitnack E, Beachey E H. Biochemical and biological properties of the binding of human fibrinogen to M protein in group A streptococci. J Bacteriol. 1985;164:350–358. doi: 10.1128/jb.164.1.350-358.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wistedt A C, Ringdahl U, Muller-Esteri W, Sjöbring U. Identification of a plasminogen-binding motif in PAM, a bacterial surface protein. Mol Microbiol. 1995;18:569–578. doi: 10.1111/j.1365-2958.1995.mmi_18030569.x. [DOI] [PubMed] [Google Scholar]
- 42.Wohl R C, Sinio L, Summaria L, Robbins K C. Comparative activation kinetics of mammalian plasminogens. Biochim Biophys Acta. 1983;745:20–31. doi: 10.1016/0167-4838(83)90165-6. [DOI] [PubMed] [Google Scholar]