Abstract
A new homocysteine thiolactone derivative, thiolactomide (1), was isolated along with a known compound, N-acetyl homocysteine thiolactone (2), from a culture extract of soil-derived Streptomyces sp. RK88-1441. The structures of these compounds were elucidated by detailed NMR and MS spectroscopic analyses with literature study. In addition, biological evaluation studies revealed that compounds 1 and 2 both exert neuroprotective activity against 6-hydroxydopamine (6-OHDA)-mediated neurotoxicity by blocking the generation of hydrogen peroxide in neuroblastoma SH-SY5Y cells.
Keywords: Thiolactomide, Streptomyces sp., N-acetyl homocysteine thiolactone, neuroprotective activity, 6-hydroxydopamine
Introduction
Natural products isolated from microorganisms serve as useful chemical templates for the development of various lead compounds essential for clinical applications [1, 2]. Recently, various secondary metabolites of actinomycetes have been attracting particular interest, as many of them have unique structural features and interesting biological properties [3, 4] that make them useful as bioprobes, i.e., biochemical tools for investigating cell functions in chemical biology studies [5, 6]. As part of an ongoing research program focused on novel bioactive secondary metabolites of actinomycetes, we recently reported the isolation and structure elucidation of a new benadrostin derivative (RK-144171) and two known compounds, 3-indolylcarbonyl α-L-rhamnopyranoside and 2-aminobenzoyl α-L-rhamnopyranoside, from a fermentation broth of Streptomyces sp. RK88-1441 [7]. Here, we investigated the minor fraction of this fermentation broth and isolated a new homocysteine thiolactone derivative, thiolactomide (1), along with a known compound, N-acetyl homocysteine thiolactone (2). Also in this study, we describe the isolation, structural elucidation, and biological activity of these compounds.
Materials and Methods
General Experimental Procedures
The specific rotations were measured on a JASCO P-1020 polarimeter (JASCO Corporation, Japan) that uses a 100 mm glass microcell. UV spectra were recorded on an Optizen 2120 UV spectrophotometer (Mecasys, Korea). The IR spectra were recorded on a Bruker VERTEX80V FT-IR spectrometer (Bruker, Germany). The NMR spectra were recorded on a Bruker Avance HD 800 NMR spectrometer (Bruker) at the Korea Basic Science Institute (KBSI) in Ochang, Korea. Chemical shifts were referenced to a residual solvent signal (DMSO-d6δH 2.50, δC 39.51). High-resolution electrospray ionization mass spectrometry (HRESIMS) data were acquired with a Q-TOF mass spectrometer (Waters, USA) on a SYNAPT G2. Column chromatography was performed on reversed-phase silica gel (0.075 mm; Cosmosil, Japan). Analytical C18 (Cosmosil, 5 μm, 4.6 × 150 mm) and semipreparative C18 (Cosmosil, 10 μm, 10 × 250 mm) columns were used for reversed-phase HPLC on a YL900 HPLC system (Young Lin, Korea) equipped with a YL9120 UV/Vis detector (Young Lin) that used HPLC grade solvents (Burdick & Jackson, USA). Open column chromatography was performed with a silica gel (silica gel 60, 0.063-0.200 mm, Merck). Semi preparative C18 (Cosmosil 5C18-MS-II, 5 μm, 10 × 250 mm) columns were used for HPLC on a YL9100 HPLC system equipped with a photodiode array detector (YL9160) that uses HPLC grade solvents (Burdick & Jackson). Neuroblastoma SH-SY5Y (#CRL-2266) cells were purchased from American Type Culture Collection (ATCC, USA). Finally, 6-OHDA was purchased from Sigma-Aldrich (USA), and a ROS-Glo H2O2 Assay Kit was purchased from Promega (UK).
Cultivation and Extraction of the Strain RK88-1441
A BLAST search revealed that the 16S rRNA sequence of the strain RK88-1441 could make it an actinomycete of the genus Streptomyces. Therefore, RK88-1441 [8], was cultured in a medium consisting of soluble starch (10 g), yeast extract (1 g), NZ-amine (1 g), and agar (15 g) in 1.0 L of distilled water at pH 7.0. The stock culture was cultured in a 250 ml Erlenmeyer flask containing 50 ml of seed culture medium (soluble starch 1%, yeast extract 0.1%, and tryptone 0.1%) for 3 days at 28°C on a rotary shaker with agitation at 125 rpm. For a large culture (10 L), 1% of the preculture broth was inoculated into 40 × 1,000-ml baffled Erlenmeyer flasks containing 250 ml of modified CDY broth (glucose 2%, soluble starch 1%, meat extract 0.3%, yeast extract 0.25%, K2HPO4 0.005%, NaCl 0.05%, CaCO3 0.05%, and MgSO4·7H2O 0.05%), which were cultured for 8 days at 28°C on a rotary shaker with agitation at 125 rpm. The mixture was then centrifuged, and the supernatant was extracted with EtOAc, while the mycelium was extracted with acetone. After concentrating the residual solvents under reduced pressure, the two portions were combined and dried to yield 2.2 g of the Streptomyces sp. RK88-1441 extract.
Isolation of Compounds 1 and 2
The dried extract (2.2 g) was separated by silica gel column chromatography (CHCl3/MeOH, gradient 50:1–0:1 (v/v)) into seven fractions. Fraction 2 was further purified by HPLC with isocratic elution using 20% aqueous MeOH to yield compounds 1 (1.8mg) and 2 (12mg).
Thiolactomide (1): white amorphous powder; [α]D26–16 (c 0.1, MeOH); UV (MeOH) λmax (log e) 233 (3.37); IR (ATR) νmax (cm-1) 3272, 1779, 1700, 1648, 1137, 1056; 1H and 13C NMR data, Table 1; HRESIMS m/z 188.0749 [M + H]+ (calcd for C8H14NO2S, 188.0745).
Table 1.
NMR data for thiolactomide (1) in DMSO-d6
| Position | Thiolactomide (1) | |
|---|---|---|
|
| ||
| δC | δH (J in Hz) | |
| 1 | 205.8 | |
| 2 | 58.4 | 4.59 (m) |
| 3 | 30.6 | a 2.07 (m) |
| b 2.40 (m) | ||
| 4 | 27.1 | a 3.28 (m) |
| b 3.39 (m) | ||
| 5-NH | 8.07 (d, 8.3) | |
| 6 | 176.5 | |
| 7 | 34.4 | 2.38 (quint, 7.0) |
| 8 | 20.0 | 1.03 (3H, d, 7.0) |
| 9 | 19.8 | 1.01 (3H, d, 7.0) |
1H and 13C data were recorded at 800 and 200 MHz, respectively.
N-acetyl homocysteine thiolactone (2): white needles; [α]D26–21 (c 0.1, MeOH); HRESIMS m/z 160.0420 [M + H]+ (calcd for C6H10NO2S, 160.0420).
Cell Culture
SH-SY5Y cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Welgene, Korea) supplemented with 10% fetal bovine serum (Welgene) and 1% penicillin/streptomycin (Gibco, USA) in a humidified incubator at 37°C with 5% CO2.
Cell Viability Assay
The proliferation of the SH-SY5Y cells was estimated using the WST colorimetric assay. Briefly, SH-SY5Y cells were seeded at a density of 1 × 104 in 96-well plates and treated with various concentrations of 6-OHDA (0-30 μM), and compounds 1 and 2 (0-10 mM) for 48 h. The wells were then treated with various concentrations of 1 and 2 in the presence or absence of 6-OHDA for 48 h. After incubation, 10 μl of EZ-Cytox solution (DoGen, Korea) was added to each well, followed by incubation for 2 h. The treated cells were then measured at 450 nm using a microplate spectrophotometer (SpectraMax 190, Molecular Devices, USA). N-acetylcysteine (NAC) (Sigma-Aldrich, USA) was used as a positive control (5 mM).
Cellular H2O2 Generation Assay
The generation of H2O2 in SH-SY5Y cells was measured using the ROS-Glo H2O2 assay (Promega, UK). Briefly, SH-SY5Y cells were seeded at a density of 1 × 104 in 96-well plates and incubated overnight. The wells were then treated with various concentrations of 1 and 2 in the presence or absence of 6-OHDA for 48 h, and then ROS-Glo H2O2 detection substrate was added to the test wells for 20 min. The samples were measured using a luminescence plate reader (Victor X2, Perkin Elmer, USA). ROS-Glo H2O2 detection solution was prepared according to the manufacturer’s protocol.
Statistical Analysis
All data are presented as mean ± standard error of the mean (SEM) of at least three independent results. The average and relative SEM were calculated on GraphPad Prism, version 8.4.3 (GraphPad Software, USA). Differences less than 0.05 (p < 0.05) were statistically significant.
Results
Structural Determination of Compounds
Compound 1 was isolated as a white amorphous powder and its molecular formula was established as C8H13NO2S based on HR-ESI-MS analysis. The mass of 1 was 28 amu (e.g., two CH3 moieties) higher than that of compound 2, while its planar structure was similar to that of compound 1 based on their 1H and 13C NMR spectra (Table 1). In particular, only the signals of the methyl [H3-8 (δH 1.03) and H3-9 (δH 1.01)] and methine [H-7 (δH 2.38)] groups and the lack of a methylene proton were different from the spectroscopic data of 2. The structure of 1 was further clarified by 2D-NMR spectroscopy (COSY, HSQC, and HMBC) (Fig. 2). The COSY spectrum revealed that compound 1 consists of two partial structures. In the first partial structure, the exchangeable NH proton (δH 8.07) correlated with the methine proton H-2 (δH 4.59), which also correlated with H2-3 (δH 2.40 and 2.07). Moreover, the protons at position 3 correlated with H2-4 (δH 3.39 and 3.28). In the second partial structure, the methine proton H-7 (δH 2.38) correlated with the two methyl groups (H3-8, δH 1.03 and H3-9, δH 1.01). The two structures were further confirmed by HMBC, where H3-8 and H3-9 correlated with the methine carbon C-7 (δC 34.4) and the amide carbonyl carbon C-6 (δC 176.5), and H-2 (δH 4.59) and H2-3 (δH 2.40 and 2.07) correlated with the carbonyl carbon C-1 (δC 205.8). In addition, the HMBC correlations of the NH proton (δH 8.07) to C-2 (δC 58.4) and C-6 (δC 176.5) confirmed the connectivity of the two partial structures. Based on these data, we concluded that the structure of 1 was that of an isobutyryl homocysteine thiolactone (Fig. 1).
Fig. 2. Key COSY and HMBC correlations of compound 1.
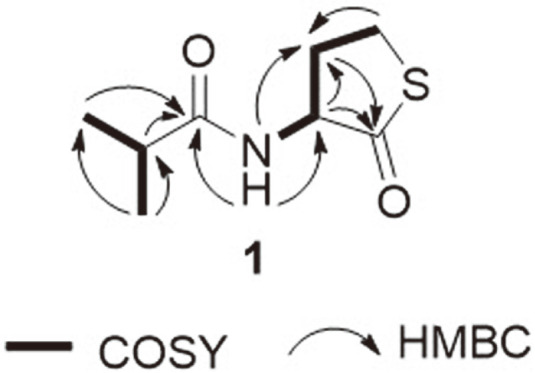
Fig. 1. Chemical structures of compounds 1 and 2.
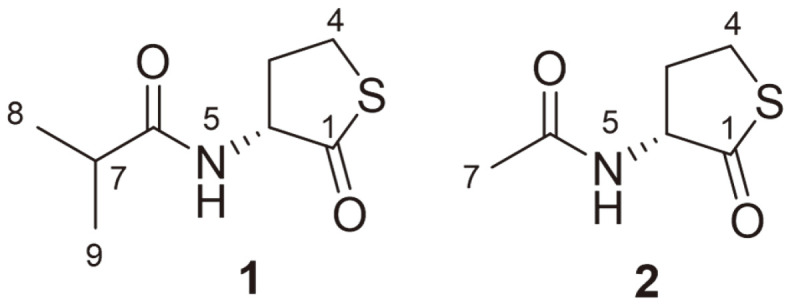
Furthermore, compound 1 displayed a negative optical rotation ([α]D26–16), similar to compound 2, indicating that the absolute configuration at C-2 was R. Therefore, compound 1 was designated as thiolactomide. Compound 2 was identified as N-acetyl homocysteine thiolactone through comparison with previously reported data [9, 10].
Biological Evaluation
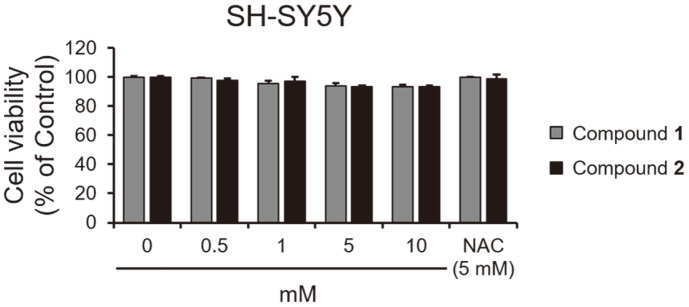
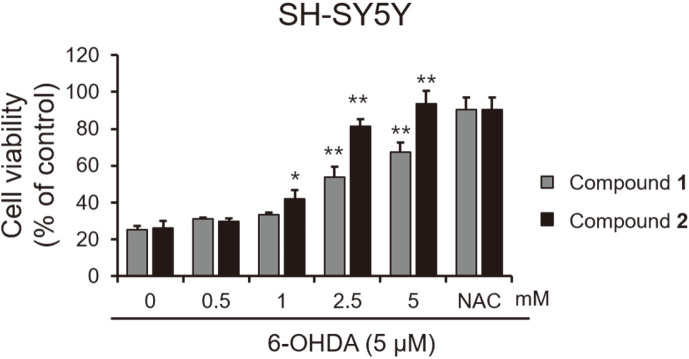
Neuroexcitotoxicity and oxidative stress play a significant role in neurodegenerative disorders such as Alzheimer’s disease, ischemic stroke, and Parkinson’s disease [11-14]. The neurotoxic agent 6-hydroxydopamine (6-OHDA) is known to induce dementia and brain injury by generating ROS [15, 16]. In our results, 6-OHDA exhibited cytotoxicity to SH-SY5Y neuroblastoma cells at above 3 μM (Fig. 3). Furthermore, the cell viability tests showed that compounds 1 and 2 were non-toxic to SH-SY5Y cells (Fig. 4). Both exhibited potent neuroprotective activity in SH-SY5Y neuroblastoma cells with EC50 values of 2.96 ± 0.7 and 1.71 ± 0.32 mM, respectively, as both compounds inhibited 6-OHDA-induced neurotoxicity (Fig. 5).
Fig. 3. Cytotoxic effects of 6-OHDA in SH-SY5Y cells.
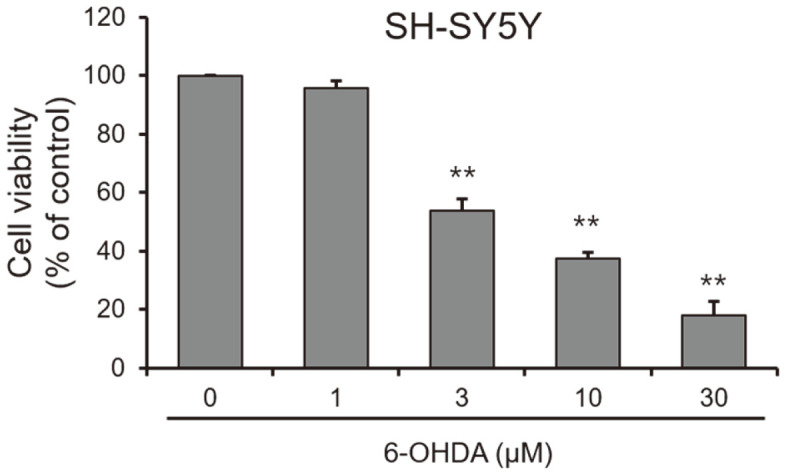
Cells were treated with the indicated concentration of 6-OHDA for 48 h. Cell viability was determined by the EZ-Cytox assay. *p < 0.05 and **p < 0.01 vs. control (DMSO: 0).
Fig. 4. Cytotoxic effects of compounds 1 and 2 in SH-SY5Y cells.
Cells were treated with the indicated concentration of compounds 1 and 2 for 48 h. Cell viability was determined by the EZ-Cytox assay. *p < 0.05 and **p < 0.01 vs. control (DMSO: 0). NAC was used as a positive control.
Fig. 5. Protective effects of compounds 1 and 2 against 6-OHDA-induced neurotoxicity in SH-SY5Y cells.
Cells were treated with 6-OHDA (5 μM) and the indicated concentration of compounds 1 and 2 for 48 h. Cell viability was determined by the EZ-Cytox assay. NAC was used as a positive control. *p< 0.05 and **p < 0.01 vs. control (DMSO: 0).
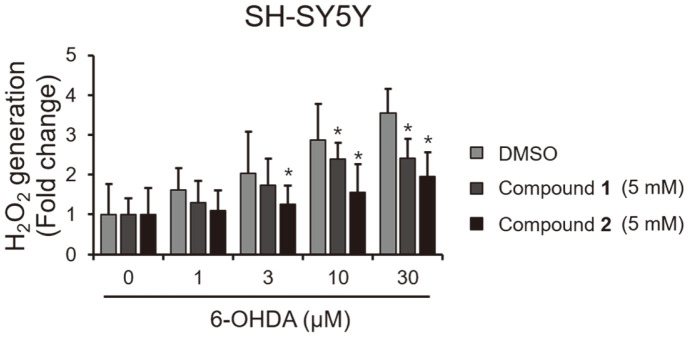
To further confirm the mechanism of action of the isolated compounds, we investigated the level of 6-OHDA-mediated H2O2 generation in the presence or absence of 1 or 2. As shown in Fig. 6, compounds 1 and 2 effectively blocked the level of H2O2 induced by 6-OHDA, but treatment with 6-OHDA alone favored the generation of H2O2 in a dose-dependent manner.
Fig. 6. Inhibitory effects of compounds 1 and 2 against 6-OHDA-induced H2O2 generation in SH-SY5Y cells.
Cells were treated with indicated concentration of 6-OHDA and 5 mM of compounds 1 and 2 for 48 h. Intracellular H2O2 levels were measured using the ROS-Glo H2O2 Assay Kit. *p< 0.05 vs. control (DMSO: 0).
Discussion
We isolated a new homocysteine thiolactone derivative, thiolactomide (1), and a known compound, N-acetyl homocysteine thiolactone (2), from the minor fraction of a fermentation broth of Streptomyces sp. RK88-1441.
N-acetyl homocysteine thiolactone has been found to perform many beneficial physiological roles [17, 18], as well as having several interesting pharmaceutical applications. For example, N-acetyl homocysteine thiolactone has been used as a mucolytic and muco-regulating drug [19]. It is also frequently used for the treatment of chronic hepatitis [20].
In the present study, we evaluated the neuroprotective effects of isolated compounds 1 and 2 against 6-OHDA-mediated ROS stress. Both analogues were non-toxic toward neuroblastoma SH-SY5Y cells and exhibited neuroprotective activities with EC50 values of 3.96 ± 0.7 and 1.71 ± 0.32 mM, respectively. Further tests also showed that both compounds 1 and 2 can effectively reduce the generation of H2O2 induced by 6-OHDA, indicating that they may serve as neuroprotective agents for the treatment of Parkinson’s and Alzheimer’s diseases.
Acknowledgments
We would like to thank Dr. T Nakamura at RIKEN for the HRESIMS measurements. This work was supported by the National Research Foundation of Korea (NRF) (Grant No. NRF-2021M3H9A1037439) and the KRIBB Research Initiative Program (KGM5292113 and JHM0022111) funded by the Ministry of Science ICT (MSIT) of the Republic of Korea. We thank the Korea Basic Science Institute, Ochang, Korea, for providing the NMR (700 and 800MHz) and HR-ESI-MS.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1.Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 2.Butler MS, Robertson AA, Cooper MA. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014;31:1612–1661. doi: 10.1039/C4NP00064A. [DOI] [PubMed] [Google Scholar]
- 3.Berdy J. Bioactive microbial metabolites. J. Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 4.Genilloud O, Gonzalez I, Salazar O, Martin J, Tormo JR, Vicente F. Current approaches to exploit actinomycetes as a source of novel natural products. J. Ind. Microbiol. Biotechnol. 2011;38:375–389. doi: 10.1007/s10295-010-0882-7. [DOI] [PubMed] [Google Scholar]
- 5.Osada H. in Bioprobes. Ed. Springer; Berlin: 2000. pp. 1–14. [DOI] [Google Scholar]
- 6.Osada H. Chemical biology based on small molecule-protein interaction. Ed. Wiley; New Jersey: 2009. pp. 1–10. [DOI] [Google Scholar]
- 7.Jang JP, Takahashi S, Futamura Y, Nogawa T, Jang JH, Ahn JS, et al. RK-144171, a new benadrostin derivative produced by Streptomyces sp.RK88-1441. J. Antibiot. 2017;70:102–104. doi: 10.1038/ja.2016.65. [DOI] [PubMed] [Google Scholar]
- 8.Osada H, Ishinabe K, Yano T, Kajikawa K, Isono K. New pyrrolobenzodiazepine antibiotics, RK-1441A and B.I. Biological properties. Agric. Biol. Chem. 1990;54:2875–2881. doi: 10.1080/00021369.1990.10870396. [DOI] [PubMed] [Google Scholar]
- 9.Jizba JV, Sedmera P, Vanek Z, Drautz H, Zahner H. Two thiolactones from Streptomyces Tu 2476. J. Antibiot. 1985;38:111–112. doi: 10.7164/antibiotics.38.111. [DOI] [PubMed] [Google Scholar]
- 10.Meguro H, Konno T, Tuzimura K. Circular dichroism of thiolo-g-lactones and their configurations and conformations. Tetrahedron Lett. 1972;31:3165–3168. doi: 10.1016/S0040-4039(01)93993-0. [DOI] [Google Scholar]
- 11.Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, et al. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer's disease: understanding the therapeutics strategies. Mol. Neurobiol. 2016;53:648–661. doi: 10.1007/s12035-014-9053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyrent E, Gomez G. Oxidative stress differentially induces tau dissociation from neuronal microtubules in neurites of neurons cultured from different regions of the embryonic Gallus domesticus brain. J. Neurosci. Res. 2020;98:734–747. doi: 10.1002/jnr.24541. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Han Z, Ji X, Luo Y. Epigenetic regulation of oxidative stress in ischemic stroke. Aging Dis. 2016;7:295–306. doi: 10.14336/AD.2015.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson's disease. J. Parkinsons Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson's disease. Neurotox. Res. 2007;11:151–167. doi: 10.1007/BF03033565. [DOI] [PubMed] [Google Scholar]
- 16.Jagmag SA, Tripathi N, Shukla SD, Maiti S, Khurana S. Evaluation of models of Parkinson's disease. Front. Neurosci. 2015;9:503. doi: 10.3389/fnins.2015.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leanza WJ, Chupak LS, Tolman RL, Marburg S. Acidic derivatives of homocysteine thiolactone: utility as anionic linkers. Bioconjug. Chem. 1992;3:514–518. doi: 10.1021/bc00018a009. [DOI] [PubMed] [Google Scholar]
- 18.McCully KS, Vezeridis MP. Homocysteine thiolactone in arteriosclerosis and cancer. Res. Commun. Chem. Pathol. Pharmacol. 1988;59:107–119. [PubMed] [Google Scholar]
- 19.de Barrio M, Tornero P, Prieto A, Sainza T, Zubeldia JM, Herrero T. Recurrent fixed drug eruption caused by citiolone. J. Investig. Allergol. Clin. Immunol. 1997;7:193–194. [PubMed] [Google Scholar]
- 20.Miglio F, D'Ambro A, Stefanini GF, Corazza GR, Pesa O, Flacco L, et al. Use of citiolone in chronic hepatitides. Results of a research with clinical and laboratory controls. Minerva Med. 1977;68:3177–3192. [PubMed] [Google Scholar]