Abstract
Current international consensuses on Helicobacter pylori eradication therapy recommend that only regimens that reliably produce eradication rates of ⩾90% should be used for empirical treatment. The Real-world Practice & Expectation of Asia-Pacific Physicians and Patients in Helicobacter Pylori Eradication Survey also showed that the accepted minimal eradication rate in H. pylori-infected patients was 91%. According to efficacy prediction model, the per-protocol eradication rates of 7-day and 14-day standard triple therapies fall below 90% when clarithromycin resistance rate ⩾5%. Several strategies including bismuth-containing, non-bismuth-containing quadruple therapies (including sequential, concomitant, hybrid and reverse hybrid therapies), high-dose dual therapy and vonoprazan-based triple therapy have been proposed to increase the eradication rate of H. pylori infection. According to efficacy prediction model, the eradication rate of 14-day concomitant therapy, 14-day hybrid therapy and 7-day vonoprazan-based triple therapy is less than 90% if the frequency of clarithromycin-resistant strains is higher than 90%, 58% and 23%, respectively. To meet the recommendation of the consensus report and patients’ expectation, local surveillance networks for resistance of H. pylori to clarithromycin are required to select appropriate eradication regimens in each geographic region. In areas with low (<5%) clarithromycin resistance (e.g. Sweden, Philippine, Myanmar and Bhutan), 7-day and 14-day standard triple therapies can be adopted for the first-line treatment of H. pylori infection with eradication rates of ⩾90%. In areas with high (⩾5%) clarithromycin resistance (most other countries worldwide) or unknown clarithromycin resistance, 14-day hybrid, 14-day reverse hybrid, 14-day concomitant and 10- to 14-day bismuth quadruple therapy can be used to treat H. pylori infection.
Keywords: clarithromycin resistance, Helicobacter pylori, quadruple therapy, triple therapy, vonoprazan
Introduction
Helicobacter pylori (H. pylori) infection is the principal cause of chronic gastritis, gastric ulcer, duodenal ulcer, gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue lymphoma (MALToma).1,2 H. pylori elimination has become the standard therapy to cure peptic ulcer disease.3,4 In addition, eradication therapy is also strongly recommended in the treatment of H. pylori-related gastric MALToma.5 In regions with high incidence of gastric adenocarcinoma, eradication of H. pylori is advocated as a preventative measure for gastric cancer.6,7 With the rising prevalence of clarithromycin resistance in H. pylori, the eradication rates of standard triple therapy consisting of a proton pump inhibitor (PPI), amoxicillin and clarithromycin infection have declined to less than 80% in most countries.8–10 Recently, several strategies including bismuth-containing, non-bismuth-containing quadruple therapies (including sequential, concomitant, hybrid and reverse hybrid therapies), high-dose dual therapy and vonoprazan-based triple therapy have been proposed to increase the eradication rate of H. pylori infection.11,12
However, the Real-world Practice & Expectation of Asia-Pacific Physicians and Patients in Helicobacter Pylori Eradication (REAP-HP) Survey showed that 7-day standard triple therapy remained the most popular regimen in the Asia-Pacific region.13 In addition, data from the European Registry on Helicobacter pylori management (Hp-EuReg) also showed that despite the high pretreatment clarithromycin resistance rate of 23% in Europe, the standard triple therapy containing both amoxicillin and clarithromycin remains as the most commonly prescribed (39%) H. pylori infection treatment, achieving only an 81.5% modified intention-to-treat eradication rate.14,15
Therefore, in this study, we aimed to (1) review current first-line eradication regimens with a per-protocol eradication rate exceeding 90% in most geographic area, and (2) identify appropriate empirical H. pylori eradication strategies in regions with high and low clarithromycin resistances.
Minimal accepted eradication rates of international consensuses and infected patients
The Report Card scheme proposed by Graham et al. suggests that only regimen ⩾90% eradication rate is considered to be good and can be used in clinical practice.16 The Kyoto Consensus Report on Helicobacter Pylori Gastritis also recommended that only regimens which reliably produce eradication rates of ⩾90% in that population should be used for empirical treatment.17 In the Bangkok consensus report, only the regimens proven to reliably achieve the threshold cure rates of 95% for per-protocol analysis or 90% for intention-to-treat analysis are recommended to use in the first-line treatment of H. pylori infection.18 Current medicine practice emphasizes shared decision-making with patients.19,20 It is important for physicians to know the expectations of patients and try to meet patients’ expectations on eradication therapy when they prescribe anti-H. pylori regimen. The REAP-HP survey showed that the expected minimal eradication rate of H. pylori-infected patients was 91%.13 Nonetheless, the most popular regimen adopted by physicians in the Asia-Pacific region was 7-day standard triple therapy that achieves an eradication rate less than 80% in most Asian countries.21,22 The data suggest that there is a gap in cure rate between that achieved by the eradication regimens of physicians and that expected by infected patients.
Cutoff value of clarithromycin resistance rates in first-line treatment of H. pylori infection by 7-day and 14-day standard triple therapies
The primary causes for eradication failure of standard triple therapy include antibiotic resistance, poor compliance and rapid metabolism of PPI.8,10,11,23 Clarithromycin resistance has been identified as a major cause of failure of standard triple therapy consisting of a PPI, amoxicillin and clarithromycin.8,10 The antibacterial action of clarithromycin relies on its interaction with the peptidyl transferase loop in the V domain of the 23S ribosomal RNA molecule, which may inhibit bacterial protein synthesis. Point mutations in the V domain of the 23S ribosomal RNA hinder the affinity between clarithromycin and the peptidyl transferase loop, leading to the inhibition of the interaction between clarithromycin and the 23S ribosomal RNA.24 The 23S ribosomal RNA A2142G and A2143G mutations were the most frequent mutations responsible for clarithromycin resistance.25
Currently, the primary antibiotic resistance pattern of H. pylori is increasing worldwide.26 A recent observation study confirmed the positive correlation between macrolide and quinolone consumption in the community and corresponding H. pylori resistance in European countries.27 In this study, we determine the breakpoint of 90% eradication rate of clarithromycin-containing regimen by prediction model according to the prevalence of clarithromycin resistance. The efficacy of a clarithromycin-containing regimen can be predicted in a region with clarithromycin resistance rate of p as long as we know the eradication rates in strains susceptible (S) and resistant (R) to clarithromycin. The predicted eradication was S × (1 − p) + R × p. The eradication rates of clarithromycin-containing regimens in strains susceptible and resistant to clarithromycin were obtained from the pooled data of randomized controlled trials included in the current study after a systematic search and quality assessment as detailed below. The Hp-normogram to assess the prevalence of clarithromycin resistance was constructed accordingly.28
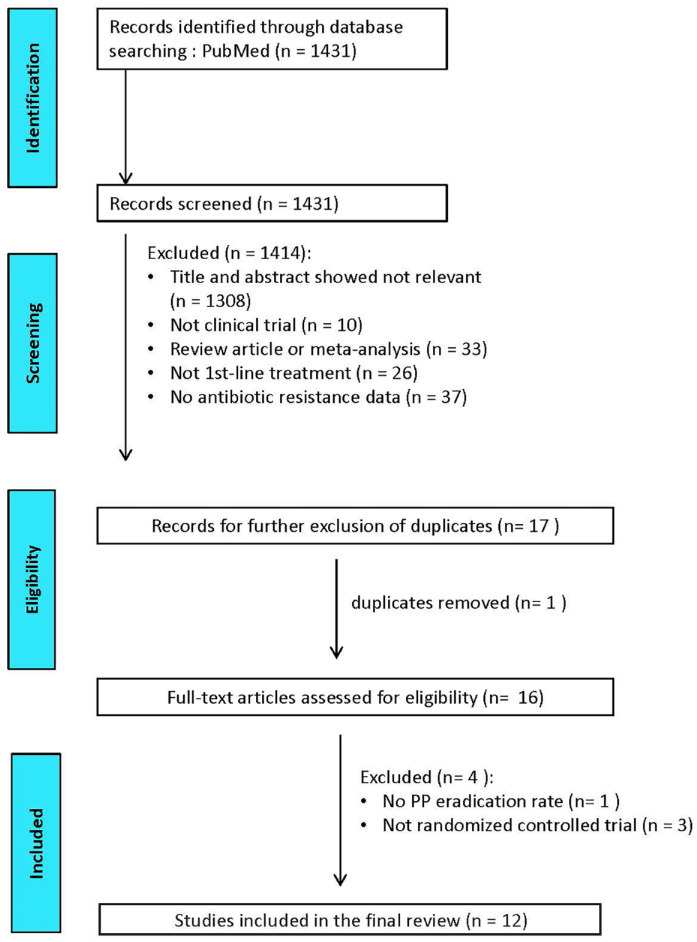
Randomized prospective trials published in English language articles reporting the efficacies of 7-day clarithromycin–amoxicillin standard triple therapy, 14-day clarithromycin–amoxicillin standard triple therapy, 14-day concomitant therapy, 14-day hybrid therapy or 7-day vonoprazan–clarithromycin–amoxicillin triple therapy for strains susceptible and resistant to clarithromycin in the first-line treatments in adults were eligible for literature search. The status of H. pylori infection before and after treatment should be verified by at least one of the following tests: rapid urease test, histology, culture or urea breath test. The post-treatment H. pylori status should be examined at least 4 weeks after eradication treatments. Medical literatures were searched from PubMed (1 January 2011 to 1 January 2022). Search field limited to ‘title and abstract’, we identified the eligible literatures with the keywords of ‘Helicobacter pylori’ or ‘H. pylori’ or ‘H pylori’, and ‘therapy’ or ‘treatment’, and ‘resistance’ or ‘resistant strains’. The titles and abstracts were screened by CAS and CBS to exclude irrelevant studies. The references in related meta-analysis investigating the efficacies of aforementioned therapies were also reviewed for eligibility. The full articles of potentially eligible studies were further reviewed by CAS and CBS. Literatures published in the abstract form only were excluded. Studies that did not report the results according to the per-protocol analysis were also excluded. In addition, studies that did not investigate the eradication rates for clarithromycin-susceptible and clarithromycin-resistant strains were also excluded. Two investigators, CAS and CBS, reviewed the literatures under the above criteria independently using pre-designed data extraction form. Disagreements were resolved through discussion with PIH to reach consensus. Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the literature search.
Figure 1.
PRISMA diagram of the literature search.
PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Table 1 summarizes the per-protocol eradication rates of 7-day and 14-day standard triple therapies for H. pylori infection according to clarithromycin resistance in randomized controlled trials.29–34 In patients treated by 7-day triple standard therapy, the eradication rates of those harboring clarithromycin-susceptible strains and clarithromycin-resistant strains were 92.5% (223/241) and 42.9% (12/28), respectively. According to efficacy prediction model, the expected eradication rate is less than 90% when the frequency of clarithromycin-resistant strains is higher than 5% (Figure 2). In patients treated by 14-day triple standard therapy, the eradication rates of those harboring clarithromycin-susceptible strains and clarithromycin-resistant strains were 90.2% (724/803) and 46.2% (48/104), respectively. The expected eradication rate is less than 90% when the frequency of clarithromycin-resistant strains is higher than 4% (Figure 2). The data indicate that per-protocol eradication rates of 7-day and 14-day standard triple therapies fall below 90% if clarithromycin resistance rate is >5%. Therefore, health providers must adopt more efficacious anti-H. pylori regimens to meet the recommendation of consensus recommendation and patients’ expectation when community clarithromycin resistance rate for H. pylori is greater than 5%. Most consensuses recommend that only regimens which reliably produce eradication rates of ⩾90% in that population should be used for empirical treatment, and use 15% as a breakpoint of using standard triple therapy. In fact, the eradication rates of 7-day and 14-day standard triple therapies fall below 90% in areas with 5–15% clarithromycin resistance.
Table 1.
The pooled per-protocol eradication rates of anti-H. pylori treatments for clarithromycin-susceptible and clarithromycin-resistant strains in randomized controlled trials.
| Regimen | Eradication rate | |
|---|---|---|
| CLA-susceptible (95% CI) | CLA-resistant (95% CI) | |
| 7-day standard triple therapy | 92.5% (223/241) (89.2–95.9%) | 42.9% (12/28) (24.5–61.2%) |
| 14-day standard triple therapy | 90.2% (724/803) (88.1–92.2%) | 46.2% (48/104) (36.6–55.7%) |
| 14-day concomitant therapy | 98.2% (55/56) (94.8–100%) | 94.1% (16/17)a,b (82.9–100%) |
| 14-day hybrid therapy | 99.1% (106/107)c,d (97.2–100%) | 83.3% (15/18)e,f (66.1–100%) |
| 7-day vonoprazan-based triple therapy | 93.2% (164/176) (89.5–96.9%) | 79.5% (66/83)g,h (70.8–88.2%) |
p < 0.001 for 14-day concomitant therapy versus 7-day standard triple therapy.
p < 0.001 for 14-day concomitant therapy versus 14-day standard triple therapy.
p = 0.013 for 14-day hybrid therapy versus 7-day standard triple therapy.
p = 0.002 for 14-day hybrid therapy versus 14-day standard triple therapy.
p = 0.007 for 14-day hybrid therapy versus 7-day standard triple therapy.
p = 0.004 for 14-day hybrid therapy versus 14-day standard triple therapy.
p < 0.001 for 7-day vonoprazan-based triple therapy versus 7-day standard triple therapy.
p < 0.001 for 7-day vonoprazan-based triple therapy versus 14-day standard triple therapy.
CLA, clarithromycin; CI, confidence interval.
Figure 2.
Predicted efficacies of 7-day standard triple therapy, 14-day standard triple therapy, 14-day concomitant therapy, 14-day hybrid therapy and 7-day vonoprazan triple therapy for H. pylori infection according to the frequency of clarithromycin-resistant strains.
Non-bismuth quadruple therapies
Non-bismuth quadruple therapies include sequential therapy, concomitant therapy, hybrid therapy and reverse hybrid therapy. Figure 3 summarizes the regimens of these non-bismuth quadruple therapies and other novel treatments for H. pylori infection.
Figure 3.
A summary of current anti-H. pylori regimens.
Sequential therapy designed by Zullo et al., consists of a 5-day dual therapy with a PPI (standard dose, b.i.d.) and amoxicillin (1 g, b.i.d.), followed by a 5-day triple therapy with a PPI (standard dose, b.i.d.), clarithromycin (500 mg, b.i.d.) and metronidazole (500 mg, b.i.d.).35 In a recent prospective randomized controlled trial, 10-day sequential therapy resulted in eradication rates of 95%, 70%, 78% and 43% for non-resistance, single clarithromycin resistance, single metronidazole resistance and dual resistances, respectively.30 Several randomized controlled trials showed that 10-day sequential therapy was superior to 7-day clarithromycin-based standard triple therapy.30,36 However, sequential therapy was only marginally better than 10-day clarithromycin-based triple therapy and not superior to 14-day clarithromycin-based triple therapy or 10- to 14-day bismuth-based quadruple therapy.37 Furthermore, this therapy is complex and requires changing antibiotics during treatment, potentially reducing patient compliance. Current international consensus guidelines therefore do not recommend 10-day sequential therapy as the preferred option in treating H. pylori infection.38,39
Concomitant therapy consisted of a four-drug regimen consisting of PPI (standard dose, b.i.d.), clarithromycin (500 mg, b.i.d.), amoxicillin (1 g, b.i.d.) and a nitroimidazole (tinidazole or metronidazole 500 mg, b.i.d.) administered together for 7–14 days.40 A meta-analysis of 19 studies including 2070 patients revealed that the mean H. pylori cure rate by intention-to-treat analysis for 3-day to 10-day concomitant therapy was 88%.41 A tendency toward better results with longer treatments (7–10 days versus 3–5 days) was observed in the meta-analysis. In addition, 7-day concomitant therapy has been proven more effective than 7-day triple therapy.30 A retrospective multicentre study from Italy compared the efficacy of bismuth quadruple therapy and concomitant therapy in areas with high clarithromycin resistance.42 A total of 203 patients without previous exposure to clarithromycin received concomitant therapy, 100 patients for 10 days and 103 for 14 days, and 201 with previous exposure to clarithromycin received 10-day bismuth quadruple therapy. The results showed that the eradication rates by intention-to-treat analysis of concomitant and bismuth quadruple therapy were comparable (88.2% versus 91.5%). Subgroup analysis demonstrated that eradication rates were significantly higher with 14-day concomitant therapy compared with 10-day concomitant therapy (96.1% versus 80.0%). The Maastricht Consensus Report recommends that the duration of concomitant therapy should preferably be optimized to 14 days, unless the shorter 10-day treatment is locally effective.43 A recent randomized controlled trial revealed that the eradication rates of 14-day concomitant therapy for clarithromycin-susceptible and clarithromycin-resistant strains were 100% (30/30) and 89% (8/9), respectively.44 Another randomized controlled trial showed that the eradication rates of 14-day concomitant therapy for clarithromycin-susceptible and clarithromycin-resistant strains were 96% (25/26) and 100% (8/8), respectively.45 Taken together, 14-day concomitant therapy can achieve an eradication rate of 98.2% (55/56) for clarithromycin-susceptible strains and 94.1% (16/17) for clarithromycin-resistant strains. According to efficacy prediction model, the eradication rate of 14-day concomitant therapy is less than 90% only when the frequency of clarithromycin-resistance is higher than 90% (Figure 2).
Hybrid therapy developed by Hsu et al. consists of a dual therapy with a PPI and amoxicillin for 7 days followed by a quadruple regimen with a PPI, amoxicillin, clarithromycin and metronidazole for 7 days.46,47 It achieved an eradication rate of 97.4% by intention-to-treat analysis and 99.1% by per-protocol analysis in a Taiwan population with clarithromycin resistance rate of 7%.46 A subsequent randomized controlled trial from Iran demonstrated that 14-day hybrid therapy achieved a higher eradication rate than 14-day standard triple therapy.48 A recent large multicentre randomized controlled trial documented that 14-day hybrid and 14-day concomitant therapies had comparable efficacy in the treatment of H. pylori infection, and both could cure more than 90% of patients with H. pylori infections in areas of high clarithromycin and metronidazole resistance.45 The overall data of recent randomized controlled trials45,47,49 showed that the eradication rates of 14-day hybrid therapy for clarithromycin-susceptible and clarithromycin-resistant strains were 99.1% (106/107) and 83.3% (15/18), respectively. According to efficacy prediction model, the eradication rate of 14-day hybrid therapy is less than 90% only when the frequency of clarithromycin-resistant strains is higher than 58% (Figure 2). Currently, 14-day hybrid therapy is recommended as one of the first-line therapies in the American College of Gastroenterology guideline on the treatment of H. pylori infection.50 In addition, it is also a recommended first-line treatment option on the Bangkok Consensus Report on H. pylori management in ASEAN.18 In the Taiwan H. pylori Consensus Report, hybrid therapy is a recommended treatment in areas with either high or low clarithromycin resistance.51
Nonetheless, hybrid therapy is a two-stage regimen, and patients have to take additional two antibiotics in the last 7 days of therapy. The complicated drug administration may confuse patients and dampen enthusiasm for its use. Reversing the sequence of drug administration (a quadruple regimen followed by a dual regimen) can simplify hybrid therapy so that it is not necessary for patients to take additional medications during the course of treatment.47 A recent retrospective cohort study showed that 14-day reverse hybrid therapy had a comparable eradication rate as 14-day hybrid therapy.52 It had a higher eradication rate than standard triple therapy.53 In addition, 14-day reverse hybrid therapy achieved a higher eradication rate than 7-day concomitant therapy.46 A recent multicentre randomized controlled trial showed that 14-day reverse hybrid therapy and 14-day concomitant therapy were equivalent in efficacy in the first-line treatment of H. pylori infection. However, the former has fewer adverse events than the latter.54 Another randomized controlled trial also demonstrated that 14-day reverse hybrid and 14-day bismuth quadruple therapies produce comparable efficacy in the treatment of H. pylori infection, and both can cure more than 90% of patients with H. pylori infections in Taiwan.55
Bismuth quadruple therapy
The Maastricht V/Florence consensus report recommends bismuth-containing quadruple therapy with PPI, bismuth, metronidazole and tetracycline as the choice treatment for H. pylori infection in areas of either low or high clarithromycin resistance.43 A recent network meta-analysis of H. pylori regimens demonstrated that 10–14 days of bismuth quadruple therapy is superior to 7-day clarithromycin triple therapy (85% versus 73%1).56 Currently, the optimal treatment duration of bismuth-containing quadruple therapy remains unclear. The efficacy of bismuth quadruple therapy for 1–3 days, 4 days or 7 days was less effective than when given for 10–14 days.57 10- to 14-day bismuth quadruple therapy achieves ⩾85% eradication rate, even in areas with a high prevalence of metronidazole resistance.57 A large randomized trial by Malfertheiner and colleagues has demonstrated that 10-day bismuth quadruple therapy was superior to 7-day standard triple therapy in Europe.58 Another multicentre randomized trial from Taiwan also showed that 10-day bismuth quadruple therapy was preferable to 14-day triple therapy in the first-line treatment in the face of rising prevalence of clarithromycin resistance.34 However, the frequency of adverse events of bismuth quadruple therapy lasting 10–14 days is extremely high, ranging from 48% to 67% in large randomized trials.34,49,55,58 In addition, the complex administration of bismuth quadruple therapy might reduce the adherence of patients. A recent randomized controlled trial also documented that 14-day bismuth quadruple therapy had comparable efficacy in the treatment of H. pylori infection as 14-day hybrid therapy. However, the former had more adverse events than the latter.49 Pylera is a ‘three-in-one pill’ formulation which is licensed in some European countries. Each capsule of Pylera® contains metronidazole 125 mg, tetracycline 125 mg and bismuth potassium subcitrate 140 mg (Aptalis Pharma US, Inc. 100 Somerset Corporate Boulevard Bridgewater, NJ 08807 USA). A retrospective database, multicentre observational study from Italy showed that the three-in-one bismuth quadruple therapy is highly effective (intention-to-treat eradication rate: 91.4%) and well tolerated (good compliance rate: 94.9%) in the first-line treatment of H. pylori infection.59 However, the widespread use of bismuth quadruple therapy is limited since neither bismuth nor Pylera are not universally available. Data from the Hp-EuReg also showed that over 90% eradication was reliably obtained only with 10-day bismuth quadruple or 14-day concomitant treatments in the Europe.14
High-dose dual therapy
High-dose dual therapy is an emerging treatment for H. pylori infection.60 The new therapy consists of high-dose PPI and amoxicillin, which keep the intragastric pH at a value higher than 6.5 regardless of CYP2C19 genotype61 and maintain steady plasma concentration of amoxicillin above the minimal inhibitory concentration for H. pylori.62 A recent randomized controlled trial by Yang et al. showed that 14-day high-dose dual therapy containing rabeprazole 20 mg and amoxicillin 750 mg 4 times/day achieved a higher eradication rate than 7-day standard triple therapy (intention-to-treat analysis: 95% versus 81%) in Taiwan.60 The novel modality is simple for administration, has fewer adverse events than standard triple therapy and can avoid use of unnecessary antibiotics. Another randomized controlled trial by Tai et al. showed that a 14-day esomeprazole- and amoxicillin-containing high-dose dual therapy achieved a comparable eradication rate as 7-day concomitant therapy (intention-to-treat analysis: 92% versus 87%) in Taiwan, and the former fewer adverse events than the latter.63 However, this novel therapy was less effective as a first-line therapy for eradicating H. pylori in both Korea64 and the United States.65
Vonoprazan-based triple therapy
Vonoprazan is a novel first-in-class potassium-competitive potent acid blocker, which is a drug that inhibits H+, K+-adenosine triphosphate (ATPase) through reversible potassium-competitive ion binding, thereby inhibiting gastric acid secretion. The inhibitory effect (pKa 9.4) is largely unaffected by ambient pH and accumulates in parietal cells under both secretory and resting conditions.66 It has been used to treat H. pylori infection in Japan since February 2015. Vonoprazan is currently approved in Japan for first-line H. pylori eradication with clarithromycin-containing triple therapy and for second-line therapy with metronidazole and amoxicillin. The eradication rate of vonoprazan-based first-line triple therapy (combined with clarithromycin and amoxicillin) was reported to be 97.6% in patients with clarithromycin-susceptible H. pylori in a phase III study.12 A multicenter, prospective, randomized trial comparing the eradication rate between the 7-day vonoprazan-based triple therapy and 7-day PPI-based triple therapy in the clarithromycin-susceptible H. pylori-infected patients revealed 87.3% versus 76.5% in intention-to-treat and 88.9% versus 86.7% in per-protocol analyses.67 Another randomized clinical trial found that 7-day vonoprazan triple therapy was significantly more effective than 14-day omeprazole-based standard triple therapy for H. pylori infection.68 A meta-analysis of five Japanese studies including 1599 patients reported that vonoprazan-based and PPI-based standard triple therapies had comparable eradication rates for clarithromycin-susceptible H. pylori stains. However, the former was significantly more effective than the latter for clarithromycin-resistant strains (82% versus 40%).69 The overall data of recent randomized controlled trials67,70 showed that the eradication rates of 7-day vonoprazan-based triple therapy for clarithromycin-susceptible and clarithromycin-resistant strains were 93.2% (164/176) and 79.5% (66/83), respectively. According to efficacy prediction model, the eradication rate of 7-day vonoprazan-based triple therapy is less than 90% when the frequency of clarithromycin-resistant strains is higher than 23% (Figure 2). However, data from studies conducted outside Japan are awaited to establish the global benefits of vonoprazan-based eradication therapies.
Conclusion
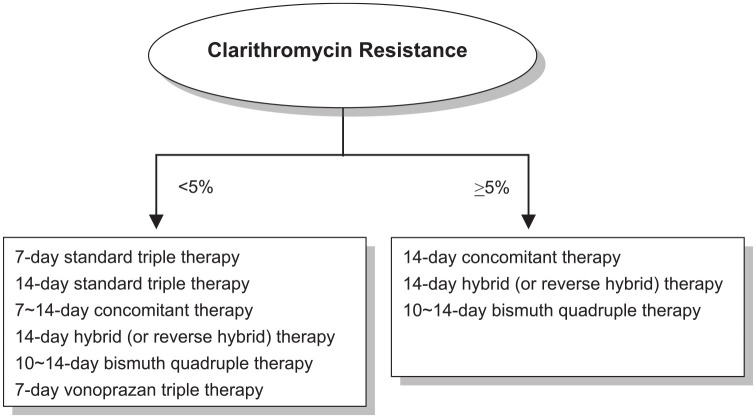
According to the REAP-HP survey, the accepted minimal eradication rate of H. pylori-infected patients is 91%. Current international consensuses on H. pylori eradication therapy also recommend that only regimens that reliably produce eradication rates of ⩾90% should be used for empirical treatment. In general, the per-protocol eradication rate of 7-day and 14-day standard triple therapies fall below 90% when clarithromycin resistance rate > 5%. To meet the recommendation of the consensus report and patients’ expectation, local surveillance networks for resistance of H. pylori to clarithromycin are required to aid regional physicians in selecting appropriate treatment. Figure 4 summarizes the recommended anti-H. pylori therapies in regions with high and low clarithromycin resistances.8,71,72 In areas with low (<5%) clarithromycin resistance (e.g. Sweden, Philippine, Myanmar and Bhutan), 7-day and 14-day standard triple therapies can be adopted for the first-line treatment of H. pylori infection with eradication rates of ⩾90%. In areas with high (⩾5%) clarithromycin resistance (most countries worldwide including the United States, Mexico, Brazil, France, Germany, UK, Italy, Spain, Netherland, Bulgaria, Turkey, Iran, Israel, China, Japan, Korea, Taiwan, Hong Kong, Thailand, Singapore, Australia, New Zealand and Cameroon) or unknown clarithromycin resistance, 14-day hybrid, 14-day reverse hybrid, 14-day concomitant and 10- to 14-day bismuth quadruple therapy can be used to treat H. pylori infection.
Figure 4.
Recommended anti-H. pylori therapies in regions with high and low clarithromycin resistances.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848221138168 for Update on the first-line treatment of Helicobacter pylori infection in areas with high and low clarithromycin resistances by Chih-An Shih, Chang-Bih Shie and Ping-I Hsu in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iD: Ping-I Hsu  https://orcid.org/0000-0003-3905-4674
https://orcid.org/0000-0003-3905-4674
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Chih-An Shih, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Antai Medical Care Corporation, Antai Tian-Sheng Memorial Hospital, Pingtung County; Department of Nursing, Meiho University, Pingtung County.
Chang-Bih Shie, Division of Gastroenterology, Department of Internal Medicine, An Nan Hospital, China Medical University, No. 66, Sec. 2, Changhe Rd., Annan Dist., Tainan City, 70965.
Ping-I Hsu, Division of Gastroenterology and Hepatology, Department of Internal Medicine, An Nan Hospital, China Medical University, No. 66, Sec. 2, Changhe Rd., Annan Dist., Tainan City, 70965.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Chih-An Shih: Writing – original draft; Writing – review & editing.
Chang-Bih Shie: Writing – review & editing.
Ping-I Hsu: Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was funded by the An Nan Hospital (Grant Numbers: ANHRF 109-13, ANHRF 109-38, ANHRF110-18, ANHRF110-43), China Medical University and Ministry of Science and Technology, Executive Yuan, Taiwan, ROC (Grant numbers: MOST110-2314-B039-045 and MOST109-2314-B039-053).
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology 2007; 133: 985–1001. [DOI] [PubMed] [Google Scholar]
- 2. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002; 347: 1175–1186. [DOI] [PubMed] [Google Scholar]
- 3. Graham DY, Lew GM, Klein PD, et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized controlled study. Ann Intern Med 1992; 116: 705–708. [DOI] [PubMed] [Google Scholar]
- 4. Sung JJ, Chung SC, Ling TK, et al. Antibacterial treatment of gastric ulcer associated with Helicobacter pylori. N Eng J Med 1995; 332: 139–142. [DOI] [PubMed] [Google Scholar]
- 5. Zucca E, Dreyling M. Gastric marginal zone lymphoma of MALT type: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009; 20: 113–114. [DOI] [PubMed] [Google Scholar]
- 6. Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer 2013; 132: 1272–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liou JM, Malfertheiner P, Lee YC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut 2020; 69: 2093–2112. [DOI] [PubMed] [Google Scholar]
- 8. Mégraud F. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 2004; 53: 1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luther J, Higgins PD, Schoenfeld PS, et al. Empiric quadruple vs triple therapy for primary treatment of Helicobacter pylori infection: systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol 2010; 105: 65–73. [DOI] [PubMed] [Google Scholar]
- 10. De Francesco V, Margiotta M, Zullo A, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med 2006; 144: 94–100. [DOI] [PubMed] [Google Scholar]
- 11. Huang CC, Tsai KW, Tsai TJ, et al. Update on the first-line treatment for Helicobacter pylori infection - a continuing challenge from an old enemy. Biomark Res 2017; 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016; 65: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chuah YY, Wu DC, Chuah SK, et al. Real-world practice and expectation of Asia-pacific physicians and patients in Helicobacter pylori eradication (REAP-HP survey). Helicobacter 2017; 22: e12380. [DOI] [PubMed] [Google Scholar]
- 14. Nyssen OP, Bordin D, Tepes B, et al. European registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021; 70: 40–54. [DOI] [PubMed] [Google Scholar]
- 15. Nyssen OP, Vaira D, Tepes B, et al. Room for improvement in the treatment of Helicobacter pylori infection: lessons from the European registry on H. Pylori management (Hp-EuReg). J Clin Gastroenterol 2022; 56: e98–e108. [DOI] [PubMed] [Google Scholar]
- 16. Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007; 12: 275–278. [DOI] [PubMed] [Google Scholar]
- 17. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64: 1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahachai V, Vilaichone RK, Pittayanon R, et al. Helicobacter pylori management in ASEAN: the Bangkok consensus report. J Gastroenterol Hepatol 2018; 33: 37–56. [DOI] [PubMed] [Google Scholar]
- 19. Kon AA, Davidson JE, Morrison W, et al. Shared decision making in ICUs: an American college of critical care medicine and American thoracic society policy statement. Crit Care Med 2016; 44: 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dahlerus C, Quinn M, Messersmith E, et al. Patient perspectives on the choice of dialysis modality: results from the empowering patients on choices for renal replacement therapy (EPOCH-RRT) study. Am J Kidney Dis 2016; 68: 901–910. [DOI] [PubMed] [Google Scholar]
- 21. Kawai T, Takahashi S, Suzuki H, et al. Changes in the first line Helicobacter pylori eradication rates using the triple therapy-a multicenter study in the Tokyo metropolitan area (Tokyo Helicobacter pylori study group). J Gastroenterol Hepatol 2014; 29: 29–32. [DOI] [PubMed] [Google Scholar]
- 22. Tai WC, Liang CM, Lee CH, et al. Seven-day nonbismuth containing quadruple therapy could achieve a grade “a” success rate for first-line Helicobacter pylori eradication. Biomed Res Int 2015; 2015: 623732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Furuta T, Shirai N, Sugimoto M, et al. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet 2005; 20: 153–167. [DOI] [PubMed] [Google Scholar]
- 24. Stone GG, Shortridge D, Flamm RK, et al. Identification of a 23S rRNA gene mutation in clarithromycin-resistant Helicobacter pylori. Helicobacter 1996; 1: 227–228. [DOI] [PubMed] [Google Scholar]
- 25. Taylor DE, Ge Z, Purych D, et al. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pylori and association of clarithromycin resistance with 23S rRNA mutations. Antimicrob Agents Chemother 1997; 41: 2621–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasahun GG, Demoz GT, Desta DM. Primary resistance pattern of Helicobacter pylori to antibiotics in adult population: a systematic review. Infect Drug Resist 2020; 13: 1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Megraud F, Bruyndonckx R, Coenen S, et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021; 70: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 28. Chen PY, Wu MS, Chen CY, et al. Systemic review with meta-analysis: the efficacy of levofloaxacin triple therapy as the first- or second-line treatments of Helicobacter pylori infection. Aliment Pharmacol Ther 2016; 44: 427–437. [DOI] [PubMed] [Google Scholar]
- 29. Liou JM, Lin JT, Chang CY, et al. Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomised comparative trial with crossover design. Gut 2010; 59: 572–578. [DOI] [PubMed] [Google Scholar]
- 30. Hsu PI, Wu DC, Chen WC, et al. Randomized controlled trial comparing 7-day triple, 10-day sequential, and 7-day concomitant therapies for Helicobacter pylori infection. Antimicrob Agents Chemother 2014; 58: 5936–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tepeš B, Vujasinović M, Šeruga M, et al. Randomized clinical trial comparing 10-day sequential, 7-day concomitant and 7-day standard triple therapies for Helicobacter pylori eradication. Eur J Gastroenterol Hepatol 2016; 28: 676–683. [DOI] [PubMed] [Google Scholar]
- 32. Liou JM, Chen CC, Chen MJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2013; 381: 205–213. [DOI] [PubMed] [Google Scholar]
- 33. Liou JM, Chen CC, Chang CY, et al. Sequential therapy for 10 days versus triple therapy for 14 days in the eradication of Helicobacter pylori in the community and hospital populations: a randomised trial. Gut 2016; 65: 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liou JM, Fang YJ, Chen CC, et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2016; 388: 2355–2365. [DOI] [PubMed] [Google Scholar]
- 35. Zullo A, Rinaldi V, Winn S, et al. A new highly effective short-term therapy schedule for Helicobacter pylori eradication. Aliment Pharmacol Ther 2000; 14: 715–718. [DOI] [PubMed] [Google Scholar]
- 36. Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med 2007; 146: 556–563. [DOI] [PubMed] [Google Scholar]
- 37. Gatta L, Vakil N, Leandro G, et al. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol 2009; 104: 3069–3079. [DOI] [PubMed] [Google Scholar]
- 38. Georgopoulos SD, Michopoulos S, Rokkas T, et al. Hellenic consensus on Helicobacter pylori infection. Ann Gastroenterol 2020; 33: 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fallone CA, Moss SF, Malfertheiner P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology 2019; 157: 44–53. [DOI] [PubMed] [Google Scholar]
- 40. Treiber G, Ammon S, Schneider E, et al. Amoxicillin/metronidazole/omeprazole/clarithromycin: a new, short quadruple therapy for Helicobacter pylori eradication. Helicobacter 1998; 3: 54–58. [DOI] [PubMed] [Google Scholar]
- 41. Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol 2012; 5: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romano M, Gravina AG, Nardone G, et al. Non-bismuth and bismuth quadruple therapies based on previous clarithromycin exposure are as effective and safe in an area of high clarithromycin resistance: a real-life study. Helicobacter 2020; 25: e12694. [DOI] [PubMed] [Google Scholar]
- 43. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/florence consensus report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 44. Hsu PI, Tsay FW, Kao JY, et al. Equivalent efficacies of reverse hybrid and concomitant therapies in first-line treatment of Helicobacter pylori infection. J Gastroenterol Hepatol 2020; 35: 1731–1737. [DOI] [PubMed] [Google Scholar]
- 45. Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013; 145: 121–128. [DOI] [PubMed] [Google Scholar]
- 46. Hsu PI, Wu DC, Wu JY, et al. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter 2011; 16: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hsu PI, Lin PC, Graham DY. Hybrid therapy for Helicobacter pylori infection: a systemic review and meta-analysis. World J Gastroenterol 2015; 21: 12954–12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sardarian H, Fakheri H, Hosseini V, et al. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter 2013; 18: 129–134. [DOI] [PubMed] [Google Scholar]
- 49. Tsay FW, Wu DC, Yu HC, et al. A randomized controlled trial shows that both 14-day hybrid and bismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with moderate antibiotic resistance. Antimicrob Agents Chemother 2017; 61: e00140–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017; 112: 212–239. [DOI] [PubMed] [Google Scholar]
- 51. Sheu BS, Wu MS, Chiu CT, et al. Consensus on the clinical management, screening-to-treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter 2017; 22: e12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lin TF, Wu DC, Tsay FW, et al. Reverse hybrid therapy achieves a similar eradication rate as standard hybrid therapy for Helicobacter pylori infection. J Chin Med Assoc 2020; 83: 233–237. [DOI] [PubMed] [Google Scholar]
- 53. Hsu PI, Kao SS, Wu DC, et al. A randomized controlled study comparing reverse hybrid therapy and standard triple therapy for Helicobacter pylori infection. Medicine (Baltimore) 2015; 94: e2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chang SN, Shih YH, Lee WC, et al. 14-day reverse hybrid therapy vs 7-day concomitant therapy in the first-line treatment of Helicobacter pylori infection. Advances Dig Med 2022; 9: 38–43. [Google Scholar]
- 55. Hsu PI, Tsay FW, Graham DY, et al. Equivalent efficacies of reverse hybrid and bismuth quadruple therapies in eradication of Helicobacter pylori infection in a randomized controlled trial. Clin Gastroenterol Hepatol 2018; 16: 1427–1433. [DOI] [PubMed] [Google Scholar]
- 56. Li BZ, Threapleton DE, Wang JY, et al. Comparative effectiveness and tolerance of treatments for Helicobacter pylori: systematic review and network meta-analysis. BMJ 2015; 351: h4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther 2007; 26: 343–357. [DOI] [PubMed] [Google Scholar]
- 58. Malfertheiner P, Bazzoli F, Delchier JC, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011; 377: 905–913. [DOI] [PubMed] [Google Scholar]
- 59. Zagari RM, Romiti A, Ierardi E, et al. The “three-in-one” formulation of bismuth quadruple therapy for Helicobacter pylori eradication with or without probiotics supplementation: efficacy and safety in daily clinical practice. Helicobacter 2018; 23: e12502. [DOI] [PubMed] [Google Scholar]
- 60. Yang JC, Lin CJ, Wang HL, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2015; 13: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sugimoto M, Furuta T, Shirai N, et al. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther 2004; 76: 290–301. [DOI] [PubMed] [Google Scholar]
- 62. Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998; 26: 1–10. [DOI] [PubMed] [Google Scholar]
- 63. Tai WC, Liang CM, Kuo CM, et al. A 14 day esomeprazole- and amoxicillin-containing high-dose dual therapy regimen achieves a high eradication rate as first-line anti-Helicobacter pylori treatment in Taiwan: a prospective randomized trial. J Antimicrob Chemother 2019; 74: 1718–1724. [DOI] [PubMed] [Google Scholar]
- 64. Kwack W, Lim Y, Lim C, et al. High dose ilaprazole/amoxicillin as first-line regimen for Helicobacter pylori infection in Korea. Gastroenterol Res Pract 2016; 2016: 1648047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Attumi TA, Graham DY. High-dose extended-release lansoprazole (dexlansoprazole) and amoxicillin dual therapy for Helicobacter pylori infections. Helicobacter 2014; 19: 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Matsumoto H, Shiotani A, Graham DY. Current and future treatment of Helicobacter pylori infections. Adv Exp Med Biol 2019; 1149: 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sue S, Ogushi M, Arima I, et al. Vonoprazan- vs proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: a multicenter, prospective, randomized trial. Helicobacter 2018; 23: e12456. [DOI] [PubMed] [Google Scholar]
- 68. Bunchorntavakul C, Buranathawornsom A. Randomized clinical trial: 7-day vonoprazan-based versus 14-day omeprazole-based triple therapy for Helicobacter pylori. J Gastroenterol Hepatol 2021; 36: 3308–3313. [DOI] [PubMed] [Google Scholar]
- 69. Li M, Oshima T, Horikawa T, et al. Systematic review with meta-analysis: vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter 2018; 23: e12495. [DOI] [PubMed] [Google Scholar]
- 70. Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut 2020; 69: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kuo YT, Liou JM, El-Omar EM, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017; 2: 707–715. [DOI] [PubMed] [Google Scholar]
- 72. Savoldi A, Carrara E, Graham DY, et al. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018; 155: 1372–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848221138168 for Update on the first-line treatment of Helicobacter pylori infection in areas with high and low clarithromycin resistances by Chih-An Shih, Chang-Bih Shie and Ping-I Hsu in Therapeutic Advances in Gastroenterology