Abstract
Neuropathic pain is a distressing medical condition with few effective treatments. The role of Vascular endothelial growth factor A (VEGFA) in inflammation pain has been confirmed in many researches. However, the mechanism of VEGFA affects neuropathic pain remains unclear. In this study, we demonstrated that VEGFA plays an important role in spare nerve injury (SNI)-induced neuropathic pain, which is mediated by enhanced expression and colocalized of VEGFA, p-AKT and TRPV1 in SNI-induced neuropathic pain model. Soluble VEGFR1 (sFlt1) not only relieved mechanical hyperalgesia and the expression of inflammatory markers, but ameliorated the expression of VEGFA, VEGFR2, p-AKT, and TRPV1 in spinal cord. However, these effects of sFlt1 can be blocked by rpVEGFA and by 740 Y-P. Therefore, our study indication that targeting VEGFA with sFlt1 reduces neuropathic pain development via the AKT/TRPV1 pathway in SNI-induced nerve injury. This study elucidates a new therapeutic target for neuropathic pain.
Keywords: VEGFA, Spare nerve injury, neuropathic pain, vascular endothelial growth factor receptor2, AKT, transient receptor potential vanilloid type 1
Introduction
Neuropathic pain (NP), caused by sensory system damage or disease in the peripheral or central nervous system, has become the most challenging neurological disease worldwide1 and seriously affects patients’ quality of life and causes severe economic burdens.2 The maladaptive immune reaction has been recognized as a vital component promoting the development of neuropathic pain.3 Many studies found that the induction and maintenance of neuropathic pain is closed related to excessive inflammatory response of glia and enhanced electrical activity of neurons in both PNS and CNS after nerve damage.4–7 Sensory neurons and microglia can produce cytokines and chemokines directly, such as IL-1β, IL-6 and TNFα, from damaged nerve,8–13 to reshape immune microenvironment in the somatosensory nervous system, enhancing information transfer,14,15 therefore to direct activation and sensitization of nociceptors.16 IL-1β and tumor necrosis factor (TNF)-α can induce hyperalgesia and central sensitization through N-methyl-d-aspartate receptors (NMDARs) via cyclic adenosine monophosphate response element–binding protein (CREB)-mediated gene transcription regulation.17 Moreover, TNF-α, IL-1β, IL-6 in the spinal dorsal horn can be released by the activated astrocytes18–20 and by the activated JAK2–STAT3 cascade in nerve damage induced pain.21,22 In addition, spinal IL-33 promotes spare nerve injury (SNI)-induced neuropathic pain through neuronal CaMKII/CREB and astroglial JAK2/STAT3 pathway.23 All above results demonstrated the vigorous neuroimmune response facilitate the generation of behavioral hypersensitivity caused by peripheral nerve injury.
Inflammation response in damaged nerve, including leakage and migration of the immune cells from the lesional vascular endothelial is involved in the initial nociceptive processes and secondary hypersensitivity.24 Vascular endothelial growth factor (VEGF), a multifunctional cytokine, was initially discovered as a tumor-secreted protein that promotes vascular permeability.25,26 It reported that Vascular endothelial growth factor A (VEGFA) plays an important role in angiogenic related pathologies, such as diabetes, arthritis and cancer, all of which are associated with chronic pain development.25,27,28 Besides, accumulating scholars have focused on the mechanism of VEGFA in neuroinflammation.29–31 Along with the vital role of inflammation in neuropathic pain, the studies showed that VEGFA is upregulated in CCI model and facilitated NP,32–34 while intrathecal blockade of VEGFA or VEGFR2, the pain responses has been inhibited.35,36 The systemic anti-VEGFR2 was a consequence of direct sensory neuronal inhibition.37 In addition, the common downstream pathways of VEGFA mainly involved in Mitogen-activated protein kinase (MAPK), Phosphoinositide 3-kinase/Protein kinase B (PI3K/AKT) and calcium signaling pathways38,39, and the MAPK and PI3K/AKT signaling pathways are widely reported in NP.40,41 Identifying the specific pathway by which VEGFA is involved in neuropathic pain may elucidate a useful therapeutic target.
Therefore, in this study, we aimed to clarify the role of VEGFA in neuropathic pain and the common downstream molecules of the VEGFA signaling pathway were screened after nerve injury induced by spared nerve injury (SNI). Moreover, neutralization of VEGFA by soluble vascular endothelial growth factor receptor 1 (VEGFR1) -soluble Fms-like tyrosine kinase 1(sFlt1) in an SNI-induced neuropathic pain model was assessed by mechanical and thermal pain investigation, and the effects of sFlt1 on VEGFA and the inflammatory pathway were also determined.
Materials and methods
Animals and drugs
Male C57BL/6J mice, weight 20–25 g, 6–8 weeks of age, provided by Guangdong Medical Laboratory Animal Center, were housed in 21 ± 1°C with a 12-h light/12-h dark room, and access to chow and water ad libitum. All experiments were performed in accordance with the International Association for the Study of Pain, and the animal experiments were approved by the Animal Care and Use Committee of Guangzhou Medical University. The mouse VEGFA recombinant protein (rpVEGFA) and VEGFR1 (sFlt1) recombinant protein were purchased from R&D Systems (China), and 740 Y-P was obtained from MedChemExpress (China).
Nociceptive behavioral tests
The behavioral tests were performed in a blinded manner. All mice were accommodated in the testing chambers for 30 min successively 3 days before the test. The mechanical threshold was assessed using von Frey filaments as described by Zhang. et al.42 Briefly, the mice were placed in brown plastic cylinders located on a wire mesh table and allowed to acclimate for 20 min until they calm down. von Frey filaments were applied at a gradually increasing force in increments of 0.04 g. The filament was applied until it buckled, and the pressure was then maintained for 3 s. Sudden paw withdrawal, flinching, and paw licking was considered positive reactions, and a result was recorded if there were more than 3 positive responses in 5 trials. Moreover, the paw withdrawal mechanical threshold (PWMT) was calculated from the averaged results of 5 repeated experiments.
The mouse PWTL was measured with a radiant heat stimulator (UGO, Italy) as reported by Hargreaves et al.43 The mouse was placed on a transparent platform, and the light was focused on the plantar region. The time of the mouse withdrawal was recorded, and the light was automatically turned off after 20 s to avoid harm the plantar tissue. The PWTL was calculated from the averaged results of 5 repeated experiments.
VEGFA intrathecal injection model
Mice were randomly divided into the saline group, rpVEGFA group (100pg,1 ng,10 ng,100 ng), and each group include 6 mice. The mice were acclimated, and the basal PWMT and PWTL were determined before the drug application. Then, the mice received 10 μL for different doses (100pg,1 ng,10 ng,100 ng) rpVEGFA or an equal volume (10 µl) of saline via intrathecal (i.t.) injection, and the PWMT and PWTL were measured 30 min later. All injection procedures were performed under awake.
Establishment of the SNI model
The SNI-induced NP model was prepared according to the protocol provided by Decosterd.44 In brief, the mouse was first anesthetized with isoflurane and shaved on the left thigh, followed by sterilization of the exposed skin with 75% alcohol. The skin on the lateral middle thigh was cut with a scalpel, and the bicep femoris muscle was dissected bluntly with scissor to expose the left sciatic nerve and its three terminal branches: the sural, common peroneal, and tibial nerve. Then, the common peroneal nerve and the tibial nerve were separated with a small glass rod, tightly ligated with 6-0 silk, and transected distal to the ligation site, removing 2–4 mm from each nerve. Care was taken to avoid damage or stretch the intact sural nerve. Finally, the incision was sutured with 5-0 silk layer by layer. The wounds of mice in the sham group mice were exposed, and the nerves were not ligated or cut.
Drug administration for the SNI model
A total of 42 mice were randomly divided into the following seven groups (n=6 in each group): Sham group, SNI group, SNI+ sFlt1 group, SNI + rpVEGFA group, SNI + rpVEGFA+sFlt1 group, SNI + 740 Y-P and SNI + 740 Y-P group+sFlt1 group. Except for the SHAM group only expose the nerve rather than cut, the other 5 group were all set up the SNI model first, then i.t. injection of drugs in each group as follows: 10 µl saline, 50 ng sFlt1, 50 ng rpVEGFA, 50 ng sFlt1 plus 50 ng rpVEGFA and 50 ng sFlt1 plus 250 ng 740 Y-P, respectively, according to the experiment design. All injection were performed under awake, the i.t. injection volume of each injection is 10ul. The drugs were administered daily at 8:00–10:00 a.m. for 14 days, and nociceptive behavioral tests were conducted from 9:00–14:00. The mice were sacrificed and the tissue were collected at the 14th day after behavioral tests.
Western blot
The mouse was deeply anesthetized and sacrificed, and the L4-6 spinal cord was extracted quickly on ice and frozen in liquid nitrogen. All proteins were extracted from the spinal cord, and the total protein concentration was measured by the bicinchoninic acid protein assay (BCA; Pierce) according to the manufacturer’s instructions. Then, the standard WB protocol was executed.45 In brief, 30 μg of total protein in each lane were separated by SDS-PAGE and then transferred to a membrane. The membrane was blocked with 5% bovine serum albumin (BSA) for 1 h at room temperature and incubated overnight at 4°C with primary antibodies against VEGFA, IL-6, TNF-α, IL-1β (1:2000, rabbit; Proteintech), VEGFR2, AKT, p-p38 (1:2000, rabbit; Cell Signaling), p-AKT (1:2000, rabbit, Affinity), and transient receptor potential vanilloid type 1 (TRPV1, 1:2000, rabbit, Alomone Labs). For the loading control, the blots were probed with a GAPDH antibody (1:5000, mouse; Cell Signaling) or β-Actin antibody (1:5000, rabbit, Affinity). The membrane was washed three times with Tris-buffered saline in Tween 20 (TBST) and incubated (1 h, room temperature) with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10000) or goat anti-mouse IgG (1:10000, Jackson ImmunoResearch) secondary antibodies. The membrane was visualized using an enhanced chemiluminescence (ECL) kit (Clinx) after another three washes as described above. The band intensities were quantified using Image-Pro Plus 6.0 and analyzed with GraphPad Prism 8.
Immunofluorescence staining
Mice were deep anesthetized with ether before perfused transcardially with saline, followed by 4% PFA. The L4-6 spinal cord segments were dissected after perfusion and postfixed in 4% PFA overnight. The samples were sequentially dehydrated with a gradient series of sucrose solutions (10%, 20%, and 30%), and the lumbar spinal cords were cut into 20-μm-thick sections with a freezing microtome (Leica). Multiple-immunofluorescence (IF) staining was conducted according to the standard protocol.46,47 In brief, sections were blocked with commercial blocking buffer and 0.3% Triton-100 for 60 min at room temperature, then incubated with the following primary antibodies overnight at 4°C: VEGFA (1:100, rabbit; Proteintech), TRPV1 (1:100, rabbit; Alomone Labs), and p-AKT (1:200, rabbit; Affinity). The sections were washed three times with PBS for 10 min each time and incubated with the corresponding horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit IgG (1:1000), Jackson ImmunoResearch) for 60 min; after three washes, the sections were stained with Alexa Fluor 488 tyramide. The conjugated antibody was then removed with eluent (Absin), and the sections were stained with another target protein as described above together with Alexa Fluor 594 tyramide. Finally, the sections were mounted onto glass slides and sealed with an anti-fluorescence quencher. Images were acquired in a dark room with a Leica scanning microscope (Olympus, Japan). Image-Pro Plus 6.0 and GraphPrism 8 were used for merging and analysis, and the average optical density (AOD) was used for the statistical analysis.
Statistical analysis
All statistical analyses were performed by GraphPad Prism version 8.0 (GraphPad Software Inc., San Diego, CA). The data are presented as the mean ± standard error of the mean (SEM). The statistical significance between two groups was analyzed using unpair t-test. For multiple comparisons, One-way analysis of variance (ANOVA) was conducted, followed by Fisher’s post hoc test for multiple comparisons. P<0.05 was considered statistically significant.
Results
VEGFA induces pain sensation directly and is relates to SNI-induced hyperalgesia
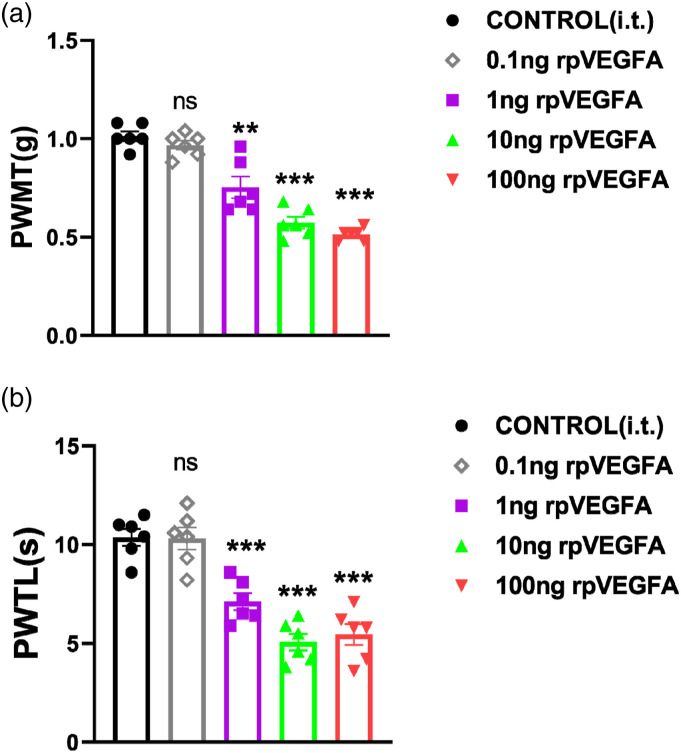
VEGFA is frequently mentioned in neuropathic pain research33,48 and is known to play a critical role in partial sciatic nerve ligation (PSL)-induced and in diabetic neuropathic pain; however, whether VEGFA directly or indirectly evokes pain sensation is unclear. In this study, we i.t. injected rpVEGFA to observe pain behavior in C57BL/6J mice. The doses of rpVEGFA administered were comparable to those reported in the previous studies.49 Interestingly, the PWMT was decreased dramatically in the rpVEGFA treatment mice compared to the saline control group at the 1 ng–100 ng doses (Figure 1(a)), Moreover, the thermal pain threshold showed a similar decreased trend to the heat beam stimulation (Figure 1(b)). These results suggest that VEGFA can directly affect mechanical pain and thermal pain sensation, and exerts allogenic functions.
Figure 1.
rpVEGF induces mechanical and thermal hyperalgesia. (A). Effect of Vascular endothelial growth factor A (rpVEGFA) on the paw withdrawal mechanical threshold (PWMT). After 1 ng, 10 ng and 100 ng rpVEGFA i.t. injection, the PWMT was significantly decreased when compared to control mice; (B). Effect of rpVEGFA on the paw withdrawal thermal latency (PWTL). Compared with the control group, the PWTL was dramatically reduced after 1 ng, 10 ng and 100 ng rpVEGFA i.t. injection. The results were analyzed by One-way analysis of variance (ANOVA) and by multiple comparisons. (n = 6). * p < 0.05, ** p < 0.01, and *** p < 0.001.
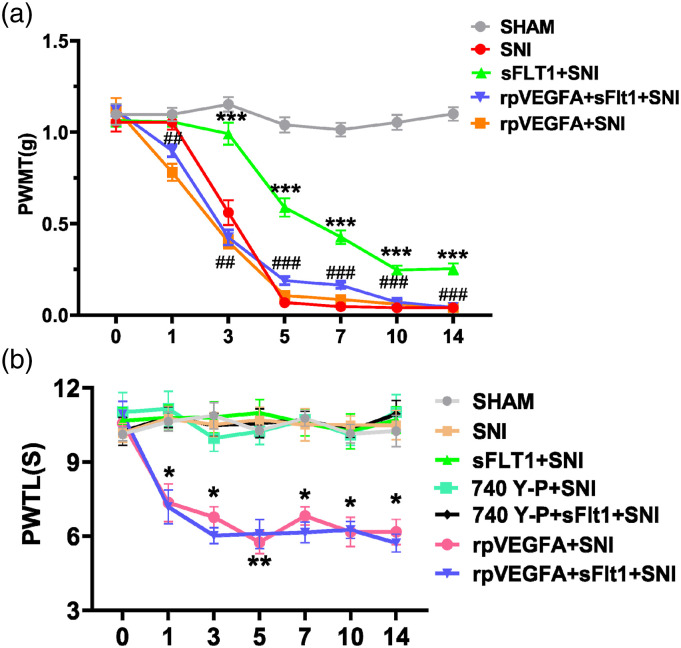
Previously, researchers have investigated the role of VEGFA in neuropathic pain, such as trigeminal NP and diabetic neuropathic pain.33,35 They found that VEGFA is involved in neuropathic pain, and inhibiting VEGFA can relieve NP. To further clarify whether VEGFA is plays a role in SNI-induced NP, we established an SNI model to detect the expression of VEGFA and the PWMT and thermal pain threshold were recorded. In line with our expectation, the PWMT of the SNI mice decreased significantly from the 3rd day to the 14th day, followed by the significantly increased expression of VEGFA in the SNI group compared with the sham group, but the PWMT of the Sham group mice remained stable compared to that before surgery (Figure 2(a)). However, the paw withdrawal latency of the SNI group mice showed comparable to that of the sham group mice, and this result is consistent with that reported by Decosterd et al.44 Our results suggest that SNI-induced mechanical hyperalgesia is related to VEGFA activation.
Figure 2.
Differential nociception in each group. (a) Mechanical hyperalgesia was assessed by von Frey hairs. The paw withdrawal threshold was significantly reduced on 3rd to 14th day after spared nerve injury (red curve) when compared to sham group, sFlt1 ameliorate the reduced PWMT from 5th to 14th day (green curve); rpVEGFA reversed the sFlt1 improved PWMT, when compared to sFlt1 group, the difference was very significant (blue curve). (b) Thermal hyperalgesia was induced by a radiant heat stimulator and recorded as the PWTL. There was no significant change in paw withdrawal thermal latency test after SNI surgery when compared to sham surgery mice; Both or single sFlt1 and 740Y-P had no effect on PWTL in SNI mice; rpVEGFA induced significantly decreased PWTL from 1st day to 14th day after SNI (pink curve), but cannot be reversed by sFlt1 treatment (blue curve). In Figure 2(a), differences between the SNI group and sFlt1+SNI group are shown as ⁎, and differences between the sFlt1+SNI group and rpVEGFA+sFlt1+ SNI group are shown as #. In figure 2(b), differences between the SNI group and rpVEGFA +SNI group are shown in *. ⁎ and # indicate p < .05, ⁎⁎ and ## indicate p < .01, and ⁎⁎⁎ and ### indicate p < .001. The data are shown as the mean ± SEM (n = 6). The results were analyzed by One-way analysis of variance ANOVA and by multiple comparisons. PWMT, paw withdrawal mechanical threshold; PWLT, paw withdrawal thermal latency; SNI: spare nerve injury
VEGFA mediates SNI-induced hyperalgesia via AKT/TRPV1 pathway
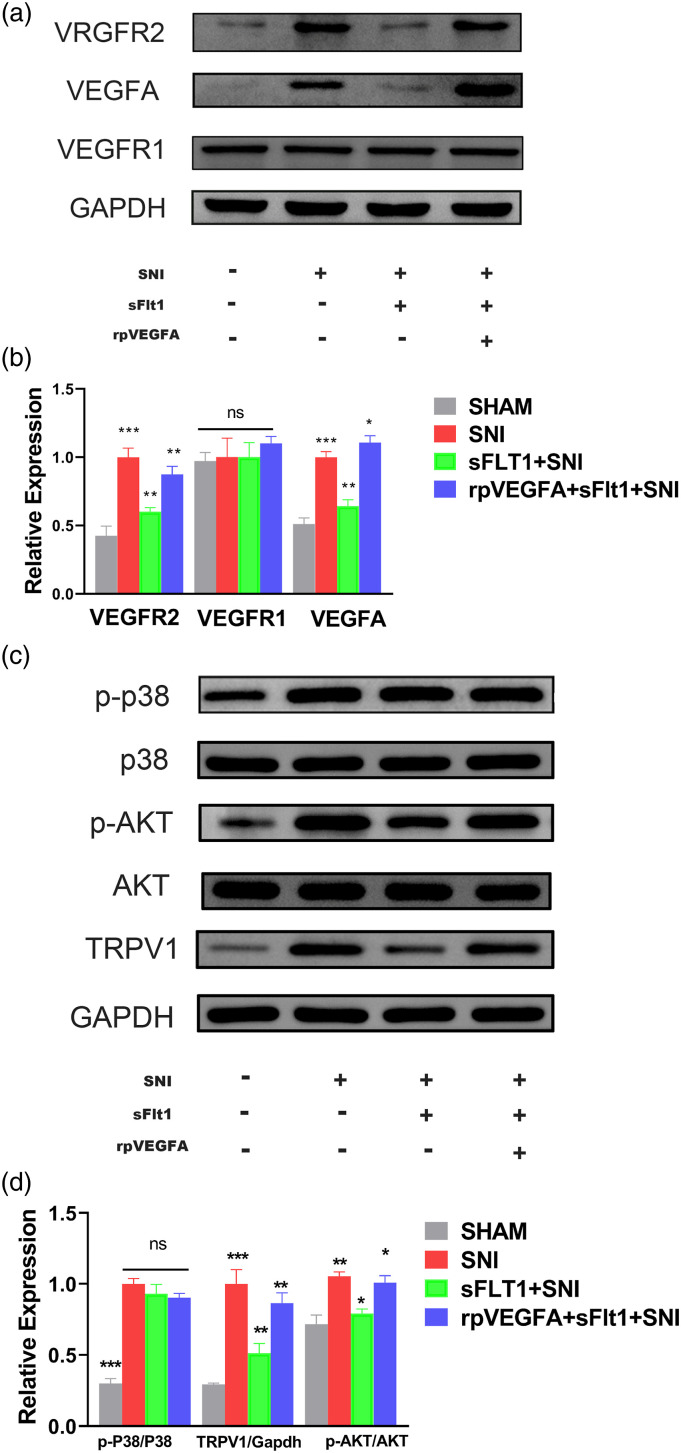
The protein expression in spinal cord was analyzed after 14 days of SNI model. As shown in Figures 3(a) and (b), the expression of VEGFA was increased significantly after SNI model. Same increased trend of the expression also showed in VEGF receptor subunit 2 (VEGFR2), while that of VEGF receptor subunit 1 (VEGFR1) was not altered (Figures 3(a) and (b)). These results indicated that VEGFA and VEGFR2 are involved in nerve injury-induced NP. We further detected the expression of the molecules which downstream of VEGFA, and the levels of p-p38 and p-AKT were all robust enhanced in SNI mice (Figures 3(c) and (d)).
Figure 3.
Differential spinal cord VEGFA pathway expression in each group as determined by Western blot. (a–d) Molecular expression and quantification in each group. The expression of VEGFR1 (a, b) and AKT (c, d) were not different in each group, while that of p-p38 was increased after SNI surgery compared with the SHAM group, but did not different from that in the sFlt1+SNI and in rpVEGFA+sFlt1+ SNI group (c, d). The increased expression of VEGFA, VEGFR2, p-AKT, and TRPV1 was ameliorated by sFIt1 treatment (green bar, B and D), and rpVEGFA inhibited the sFIt1-induced downregulation (blue bar, B and D). The data are shown as the mean ± SEM (n = 3). The results were analyzed by One-way analysis of variance ANOVA and by multiple comparisons. ⁎ p < 0.05, ⁎⁎ p < .01, and ⁎⁎⁎ p < .001. SNI: spare nerve injury.
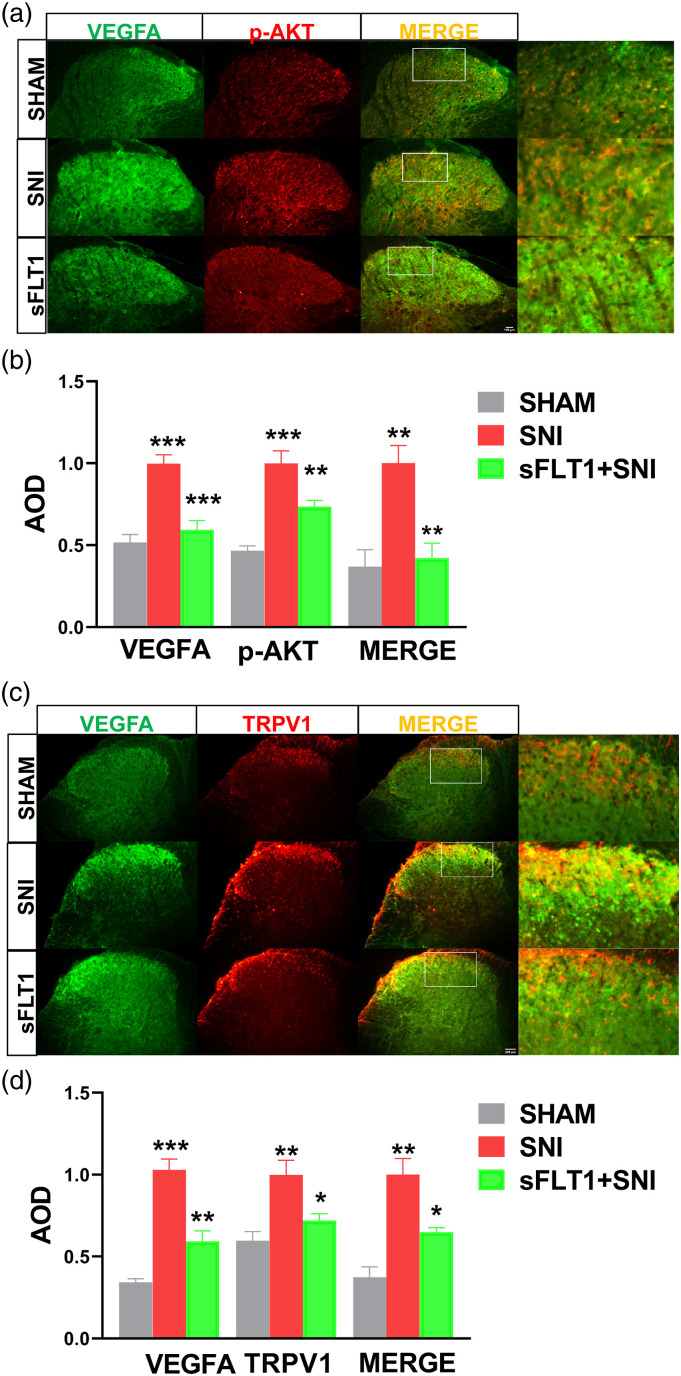
The VEGFA was mainly expressed in the superficial dorsal horn (laminae I-II) of the spinal cord in Sham group mice (Figure 4(a)). After spare nerve injury, the VEGFA robustly increased and expressed in laminae I-IV in the SNI group compared to the sham group mice. Meanwhile, the levels of p-AKT and TRPV1 in the laminae of the spinal cord were similarly enhanced to that of VEGFA expression (Figures 4(a)–(d)). Since p-AKT/TRPV1 are downstream of VEGFA, we inferred that VEGFA participates in neuropathic pain may through the p-AKT/TRPV1 pathway, inhibiting VEGFA would be an effective target for relieving pain perception.
Figure 4.
Immunofluorescence analyses the expression of VEGFA, p-AKT, and TRPV1 in the spinal cord. (a–d). VEGFA (green), p-AKT (red), and TRPV1(red) are mainly expressed in the superficial dorsal horn region (laminae I-II) of the spinal cord. The expression of VEGFA, p-AKT, and TRPV1 was robustly increased in SNI mice, but sFlt1 inhibited this increase (green, b and d). The average optical densities (AODs) in the images were quantified by ImageJ. The data are shown as the mean ± SEM (n = 3). The results were analyzed by One-way ANOVA and by multiple comparisons. ⁎ p < .05, ⁎⁎ p < .01, and ⁎⁎⁎ p < .001. TRPV1, transient receptor potential vanilloid type 1.
Targeting VEGFA ameliorated mechanical hypersensitivity and neuroinflammation in SNI model
Our results presented above showed that VEGFA is involved in pain perception and in mechanical hyperalgesia. Therefore, we next aimed to investigate the effect of blocking VEGFA on pain conditions. sFlt1, a high-affinity protein for VEGFA, is considered a suitable VEGFA antagonist. Whereas rpVEGFA is an ideal agonist of VEGFA. In this study, we designed an experiment to observe the effect of sFlt1 on SNI-induced NP and the neutralizing effect of the rpVEGFA on sFlt1 in the context of SNI. As shown in Figure 2(a), there was no significant difference in the basal mechanical withdrawal thresholds among the groups. The mice were treated with sFlt1, sFlt1+rpVEGFA, or saline daily according to the experimental design after the SNI surgery. The results showed that the mechanical pain threshold of SNI group was reduced significantly compared to that of the Sham group, whereas sFlt1 ameliorated the mechanical hypersensitivity significantly from day 3 to day 14, when compared to SNI group, the difference was very significant (Figure 2(a)); however, the thermal hypersensitivity was not change for sFlt1 treatment after SNI (Figure 2(b)).
In addition, the paw withdrawal thermal latency of mice treated with rpVEGFA was decreased significantly compared to that of mice in the other groups (Figure 2(b)). As showed in the first part, we inferred that rpVEGFA induces thermal hyperalgesia. Intriguingly, the mice administered rpVEGFA showed a decreased mechanical threshold compared with those of the other groups (p < .05) on the first day of SNI surgery (Figure 2(a)), which may due to the concurrent effects of rpVEGFA and nerve injury. The expression of VEGFA and VEGFR2 in the spinal cord was decreased after sFlt1 treatment; however, treatment with rpVEGFA reversed the sFlt1-induced VEGFA and VEGFR2 decreasing (Figures 3(a) and (b)).
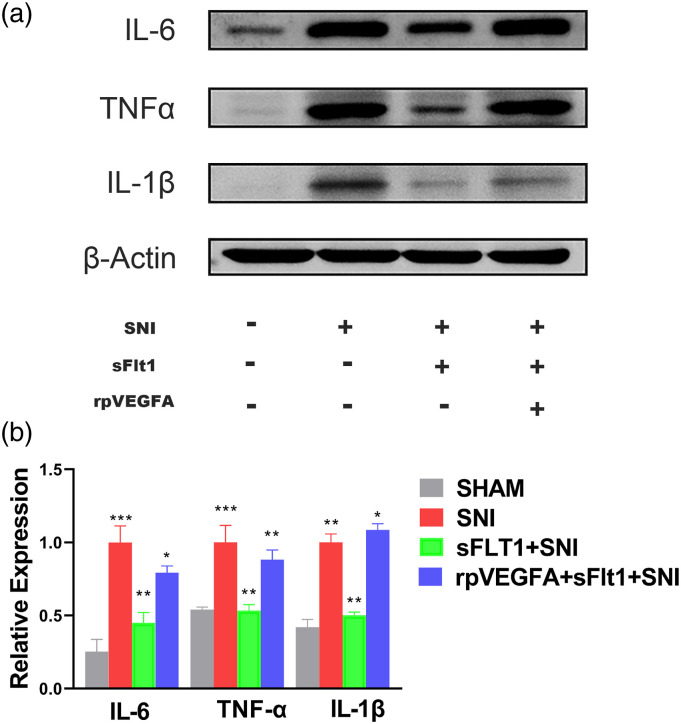
Neuroinflammation plays a vital role in NP, and pain perception is typically associated with the severity of neuroinflammation.50,51 Therefore, we detected inflammatory biomarkers in the spinal cord to evaluate neuroinflammation after nerve injury. Consistent with the nociceptive behavioral tests, the expression of IL-6, IL-1β, and TNF-α in the spinal dorsal horn (SDH) were remarkably upregulated in the SNI model, and this upregulation was partially reversed by sFlt1 treatment (Figures 5(a) and (b)). However, the VEGFA agonist, rpVEGFA blocked the sFlt1 induced downregulation of IL-6, IL-1β, and TNF-α. These results indicate that VEGFA and VEGFA-induced inflammation are involved in NP and that VEGFA may as a target for neuropathic pain therapy.
Figure 5.
Differential expression of inflammatory biomarkers in each group. (a–b) IL-6, TNF-α, and IL-1β in the spinal cord were detected by WB. The expressions of inflammatory biomarkers showed a similar tendency as SNI-induced hyperalgesia in each group. After SNI surgery, the IL-6, TNF-α, and IL-1β expression were significantly increased (red bar, b), sFlt1 inhibited the expression of IL-6, TNF-α, and IL-1β (green bar, b), and rpVEGFA inhibited the sFIt1-induced downregulation of the inflammatory biomarkers (blue bar, b). The data are shown as the mean ± SEM (n = 3). The results were analyzed by One-way analysis of variance ANOVA and by multiple comparisons. ⁎ p < .05, ⁎⁎ p < .01, and ⁎⁎⁎ p < .001.
sFlt1 inhibits Akt phosphorylation and TRPV1 expression rather than p38 pathway to reverse SNI-induced hyperalgesia and neuroinflammation
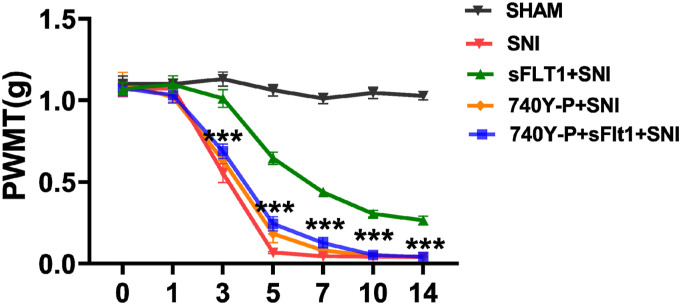
Research has shown that AKT is required for spinal central sensitization in nerve injury-induced NP.40 We herein investigate whether AKT plays a role in the VEGFA-evoked inflammatory response, which may relate to central sensitization. Therefore, the molecules downstream of VEGFA in the spinal cord were detected to elucidate the underlying mechanism. As shown in Figures 3(c) and (d), the p-AKT expression was significantly decreased in the sFlt1 treated SNI mice when compared to the SNI group, which indicate that sFlt1 treatment may inhibit the activation of the PI3K/AKT pathway. However, sFlt1 treatment did not affect the p38-MAPK pathway, as the level of p-p38 was not significantly different in sFlt1 treated SNI group from that in the SNI group (Figures 3(c) and (d)). Therefore, VEGFA-induced hyperalgesia may develop via the PI3K/p-AKT pathway after SNI. To validate our hypothesis, sFit1-treated SNI mice were administered with 740 Y-P, an agonist of the PI3K/AKT pathway.52 The nociceptive behavioral tests showed that the pain-relieving effect of sFlt1 was reversed by 740 Y-P (Figure 6). These results confirmed that VEGFA-induced neuropathic pain occurs via the modulation of AKT phosphorylation.
Figure 6.
The AKT agonist 740Y-P inhibited the effect of sFlt1. The PWMT of the 740 Y-P- and sFlt1-treated mice at different time points (n = 6). The paw withdrawal threshold was significantly reduced on 3rd to 14th day after SNI (red curve) when compared to sham group, sFlt1 ameliorate the reduced PWMT from 5th to 14th day (green curve); 740Y-P reversed the sFlt1 improved PWMT (blue curve).The data are shown as the mean ± SEM (n = 3). The results were analyzed by One-way analysis of variance ANOVA and by multiple comparisons, 740 Y-P versus sFlt1. ⁎ p < .05, ⁎⁎ p < .01, and ⁎⁎⁎ p < .001.
Transient receptor potential vanilloid type 1, a noxious sensor, is sensitive to inflammatory mediators released during injury or infection53 and is commonly known to be involved in NP.54,55 In previous investigations on the role of AKT in neuropathic pain, TRPV1 was determined to be a vital downstream molecule of AKT.25,37 In our study, the TRPV1 protein expression trend in each group was similar to those of p-AKT and VEGFA (Figure 4), consistent with the nociceptive behavioral results and the expression of inflammatory biomarkers, such as IL-6, IL-1β, and TNF-α, in each group. In addition, we performed double staining of VEGFA/p-AKT and VEGFA/TRPV1 in the spinal cord to elucidate crosslinks between VEGFA and the both signaling molecules. The co-expression of VEGFA with p-AKT or TRPV1 was increased in the SNI group, but downregulated their co-expression by sFlt1 (Figures 4(a)–(d)). Together with above results, our data proved that the crosslink of VEGFA/p-AKT and VEGFA/TRPV1 was increased in SNI mice and that sFlt1 inhibited these associations. These results demonstrated that VEGFA facilitates spare nerve injury-induced neuropathic pain through the Akt/TRPV1 pathway activation.
Discussion
This study extends our understanding of direct effect of VEGFA on pain perception and the mechanism of blocking VEGFA relieves neuropathic pain in a SNI mouse model. Our results reveal that VEGFA induces hyperalgesia is through upregulating inflammatory cytokines in the spinal cord and that VEGFA facilitates pain perception in both the peripheral and central nervous systems. From our behavior, pharmacological, molecule biological and immunohistochemistry research, we found that blocking VEGFA ameliorated mechanical hypersensitivity and neuroinflammation in the SNI model, and VEGFA potentially mediated pain sensation and nerve inflammation via the VEGFR2/AKT/TRPV1 pathway. Moreover, VEGFA and p-AKT or TRPV1 were co-expressed in the spinal cord after SNI. Additionally, the effect of blocking VEGFA was reversed by rpVEGFA and by an AKT agonist. Our results demonstrated that VEGFA, the downstream AKT pathway, and inflammatory cytokines are involved in SNI-induced neuropathic pain. These data imply that blocking VEGFA relieves SNI-induced neuropathic pain is via the AKT/TRPV1 signaling cascade.
In the peripheral nervous system, VEGFA affects sensory neurons by inducing sensory neuron axonal outgrowth and survival.56 Active VEGFA directly sensitizes C nociceptors to induce hyperalgesia.38 In the central nervous system, VEGFA-induced hyperalgesia is tightly associated with neuroinflammation due to the critical role of VEGFA in inflammation.57 It is widely accepted that neuroinflammation is implicated in neuropathic pain pathology, and VEGF signal transduction is tightly associated with inflammation. In our study, we demonstrated the direct effect of VEGFA on pain perception and in which involved in SNI-induced hyperalgesia and the inflammatory response after nerve injury. Because VEGFA is the most vital mediator of angiogenesis, its roles in biological processes have been well characterized.58 Multiple studies demonstrated its crucial role in cancer, in inflammation and in pain,25,28 and others have reviewed the roles of VEGFA in pain-associated diseases. Among that, accumulating evidence indicates that VEGFA plays a significant role in neuropathic pain.25,31 Although these studies demonstrated that VEGFA involved in NP, and inhibiting VEGFA can relieve the animal pain behavior, but the different mechanisms by which neuropathic pain is induced are still worthy of exploration.59,60
We utilized sFlt1 to explore the role of VEGFA in SNI-induced hyperalgesia. sFlt1 is an ideal VEGFA antagonist since it is a natural protein that has a high affinity for VEGFA. Although, sFlt1 can bind with VEGFA, VEGFB, and PLGF (Placental growth factor),61 we designed the sFlt1 to antagonist the rpVEGFA function in SNI model mice. In the SNI model, the expression of VEGFR2 was dramatically increased, but there was no significant change in VEGFR1 expression. This result is consistent with other studies on VEGFRs, which showing that VEGFA biologically functions mainly through VEGFR2.27,62 In our study, administration of sFlt1 to SNI mice partially ameliorated the increased expression of VEGFA and VEGFR2, and the expression levels of the inflammatory cytokines IL-1β, IL-6, and TNF-α decreased, followed by the increased mechanical pain threshold. Our research indicates that the inflammatory response plays an essential role in SNI-induced neuropathic pain, the VEGFA/VEGFR2 may be a candidate for neuropathic pain therapy. It is known that the VEGFA family exerts biological effects through the receptor tyrosine kinases VEGFR1 and VEGFR2.63,64 The expression of VEGFR2 was dramatically increased and inhibited by blocking VEGFA in SNI model. In contrast, there was no significant change in VEGFR1 expression compared to that of the sham group. Although the affinity of VEGFR1 for VEGFA is much higher than that of VEGFR2, however, it demonstrated that in our SNI-induced neuropathic pain, the VEGFA executes function is via VEGFR2. Previous studies have demonstrated that VEGFA is involved in angiogenesis, permeability, and inflammation through VEGFR2.24,65 Our results are consistent with these reports.
In addition, in our experiment, sFlt1 treatment consistently relieved mechanical hyperalgesia and reduced inflammation in the SNI model. An increasing number of recent studies have reported that inflammation plays an essential role in chronic pain, especially in neuropathic pain, which extends our previous knowledge about how inflammation is related to NP. It is reported that spinal IL-1β expression contributes to central sensitization and inflammatory pain hypersensitivity via the transcriptional upregulation of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2).66 Neutralization of TNF-α relieved the thermal hyperalgesia and mechanical allodynia induced by nerve injury.67 IL-6 knockout mice had significantly less mechanical allodynia after nerve injury than control mice.68 I.t. injection of TNF, IL-1β, or IL-6 elicited rapid pain hypersensitivity in naive animals.69 Above researches demonstrated the immune inflammatory cytokines involved in development of neuropathic pain. In our research, we also observed increased levels of the inflammatory cytokines IL-6, IL-1β, and TNF-α in the SNI model and VEGFA-induced hyperalgesia. However, sFlt1 treatment can relieve the mechanical hyperalgesia and reduce the inflammation after spared nerve injury. These studies all stressed the role of inflammation cytokines of TNF, IL-1β, or IL-6 in NP. Furthermore, we found that the effect of sFlt1 can be abolished when the SNI mice were treated by rpVEGFA. Therefore, VEGFA is a feasible target for neuropathic pain.
Some typical pathways, such as the p38-MAPK and AKT signaling pathways, are downstream of VEGFA,70 and considerable researches have demonstrated that p38-MAPK and AKT signaling are involved in neuropathic pain. It is reported that p38-MAPK in microglia of the spinal cord is activated, and the PI3K/AKT pathway is required for central sensitization in NP.40,41 In our experiment, the p38-MAPK pathway was not affected by the blocking of VEGFA in SNI, but AKT was involved in VEGFA-mediated NP. TRPV1, a member of the transient receptor potential cation channel subfamily, is also considered a downstream molecule of the PI3K/AKT pathway and participates in the transmission of pain71,72; this conclusion was also confirmed in our study. The immunostaining results showed that VEGFA was co-expressed with p-AKT and TRPV1 in laminae I and II of the SDH, respectively. This expression was also confirmed by the previous investigations.60,73,74 Therefore, we conclude that TRPV1 is associated with VEGFA-mediated pain transmission. Blocking VEGFA can ameliorate SNI-induced hyperalgesia and neuroinflammation.
In summary, our study demonstrated that VEGFA directly affects pain perception; it not only participates in SNI-induced hyperalgesia but also in nerve injury-induced neuroinflammation. VEGFA may be a new therapeutic target for neuropathic pain in which functions via the p-AKT/TRPV1 pathway.
Acknowledgements
All the experiments were performed in accordance with the guidelines of the National Health Commission and were approved by the Animal Studies Committee at Guangzhou Medical University.
Footnotes
Author contributions: The author contributions were as follows: L.W. and Z. P. designed the experiments. P.Z., Y.F., S.T.H., and T.Y. performed the behavioral, immunohistochemistry, and molecular biology experiments. P.Z. performed the molecular analysis. Y.F. contributed to the project. P.Z. and L.W. wrote the manuscript, and L.W. revised the writing
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by the National Natural Science Foundation of China (No. 81771182) to Li Wan and by the High-level University Construction Promotion Project of Guangzhou Medical University (2017-No.106-9).
ORCID iDs
Yang Tang https://orcid.org/0000-0003-1622-3199
References
- 1.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ 2014; 348: f7656–f7656. DOI: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 2.Feldman EL, Nave K-A, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017; 93(6): 1296–1313. DOI: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. Neuropathic pain. Nat Rev Dis Primers 2017; 3: 17002. DOI: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommer C, Leinders M, Üçeyler N. Inflammation in the pathophysiology of neuropathic pain. Pain 2018; 159(3): 595–602. DOI: 10.1097/j.pain.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 5.Segal JP, Tresidder KA, Bhatt C, Gilron I, Ghasemlou N. Circadian control of pain and neuroinflammation. J Neurosci Res 2018; 96(6): 1002–1020. DOI: 10.1002/jnr.24150. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Zhang Y-Q, Qadri YJ, Serhan CN, Ji R-R. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018; 100(6): 1292–1311. DOI: 10.1016/j.neuron.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis A, Bennett DLH. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 2013; 111(1): 26–37. DOI: 10.1093/bja/aet128. [DOI] [PubMed] [Google Scholar]
- 8.McMahon SB, Cafferty WBJ, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol 2005; 192(2): 444–462. DOI: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 2009; 194: 417–449. DOI: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie W, Strong JA, Zhang J-M. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience 2009; 160(4): 847–857. DOI: 10.1016/j.neuroscience.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z-C, Li L-H, Bian C, Yang L, Lv N, Zhang Y-Q. Involvement of NF-κB and the CX3CR1 signaling network in mechanical allodynia induced by tetanic sciatic stimulation. Neurosci Bull 2018; 34(1): 64–73. DOI: 10.1007/s12264-017-0149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Chi L, Kuebler WM, Goldenberg NM. Perivascular inflammation in pulmonary arterial hypertension. Cells 2020; 9(11): E2338. DOI: 10.3390/cells9112338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walters ET. Nociceptors as chronic drivers of pain and hyperreflexia after spinal cord injury: an adaptive-maladaptive hyperfunctional state hypothesis. Front Physiol 2012; 3: 309. DOI: 10.3389/fphys.2012.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 2000; 4(3): 247–257. DOI: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- 15.önig C, Morch E, Eitner A, Moller C, Turnquist B, Schaible H-G, Ebersberger A. Involvement of spinal IL-6 trans-signaling in the induction of hyperexcitability of deep dorsal horn neurons by spinal tumor necrosis factor-alpha. J Neurosci 2016; 36(38): 9782–9791. DOI: 10.1523/JNEUROSCI.4159-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji R-R, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018; 129(2): 343–366. DOI: 10.1097/ALN.0000000000002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki Y, Zhang L, Cheng J-K, Ji R-R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008; 28(20): 5189–5194. DOI: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Chavis JA, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha and retinoid X receptor agonists inhibit inflammatory responses of astrocytes. J Neuro 2006; 176(1–2): 95–105. DOI: 10.1016/j.jneuroim.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y-J, Ji R-R. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010; 7(4): 482–493. DOI: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji R-R, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain 2013; 154(Suppl 1): S10–S28. DOI: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M. SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci 2010; 30(16): 5754–5766. DOI: 10.1523/JNEUROSCI.5007-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim G, Wang S, Zhang Y, Tian Y, Mao J. Spinal leptin contributes to the pathogenesis of neuropathic pain in rodents. J Clin Invest 2009; 119(2): 295–304. DOI: 10.1172/JCI36785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Mi W-L, Li Q, Zhang M-T, Han P, Hu S, Mao-Ying Q-L, Wang Y-Q. Spinal IL-33/ST2 signaling contributes to neuropathic pain via neuronal CaMKII-CREB and astroglial JAK2-STAT3 cascades in mice. Anesthesiology 2015; 123(5): 1154–1169. DOI: 10.1097/ALN.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 24.Ved N, Da Vitoria Lobo ME, Bestall SM, Vidueira C, Beazley-Long N, Ballmer-Hofer K, Hirashima M, Bates DO, Donaldson LF, Hulse RP. Diabetes-induced microvascular complications at the level of the spinal cord: a contributing factor in diabetic neuropathic pain. J Physiol (Lond) 2018; 596(16): 3675–3693. DOI: 10.1113/JP275067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019; 176(6): 1248–1264. DOI: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brkovic A, Sirois MG. Vascular permeability induced by VEGF family members in vivo: role of endogenous PAF and NO synthesis. J Cell Biochem 2007; 100(3): 727–737. DOI: 10.1002/jcb.21124. [DOI] [PubMed] [Google Scholar]
- 27.Peach CJ, Mignone VW, Arruda MA, Alcobia D, Hill S, Kilpatrick L, Woolard J. Molecular pharmacology of VEGF-A isoforms: binding and signalling at VEGFR2. Int J Mol Sci 2018; 19(4): 1264. DOI: 10.3390/ijms19041264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frezzetti D, Gallo M, Maiello MR, D’Alessio A, Esposito C, Chicchinelli N, Normanno N, De Luca A. VEGF as a potential target in lung cancer. Expert Opin Ther Targets 2017; 21(10): 959–966. DOI: 10.1080/14728222.2017.1371137. [DOI] [PubMed] [Google Scholar]
- 29.Shibuya M. VEGF-VEGFR System as a target for suppressing inflammation and other diseases. Endocr Metab Immune Disord Drug Targets 2015; 15(2): 135–144. DOI: 10.2174/1871530315666150316121956. [DOI] [PubMed] [Google Scholar]
- 30.Semerano L, Duvallet E, Belmellat N, Marival N, Schall N, Monteil M, Grouard-Vogel G, Bernier E, Lecouvey M, Hlawaty H, Muller S, Boissier M-C, Assier E. Targeting VEGF-A with a vaccine decreases inflammation and joint destruction in experimental arthritis. Angiogenesis 2016; 19(1): 39–52. DOI: 10.1007/s10456-015-9487-0. [DOI] [PubMed] [Google Scholar]
- 31.Jais A, Solas M, Backes H, Chaurasia B, Kleinridders A, Theurich S, Mauer J, Steculorum SM, Hampel B, Goldau J, Alber J, Förster CY, Eming SA, Schwaninger M, Ferrara N, Karsenty G, Brüning JC. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell 2016; 165(4): 882–895. DOI: 10.1016/j.cell.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 32.Hulse RP, Drake RAR, Bates DO, Donaldson LF. The control of alternative splicing by SRSF1 in myelinated afferents contributes to the development of neuropathic pain. Neurobiol Dis 2016; 96: 186–200. DOI: 10.1016/j.nbd.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee GW, Son JY, Lee AR, Ju JS, Bae YC, Ahn DK. Central VEGF-A pathway plays a key role in the development of trigeminal neuropathic pain in rats. Mol Pain 2019; 15: 174480691987260. DOI: 10.1177/1744806919872602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie X, Ma L, Xi K, Zhang W, Fan D. MicroRNA-183 suppresses neuropathic pain and Expression of AMPA receptors by targeting mTOR/VEGF signaling pathway. Cell Physiol Biochem 2017; 41(1): 181–192. DOI: 10.1159/000455987. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Li G, Xu H, Tang X, Gao Y, Xu C, Liu S, Xie J, Tu G, Peng H, Qiu S, Liang S. Effects of anti-rVEGF on the expression of VEGF receptor-2 and P2X(2/3) receptors of the spinal dorsal horn in neuropathic pain rats. Brain Res Bull 2012; 87(2–3): 227–233. DOI: 10.1016/j.brainresbull.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Tooke K, Girard B, Vizzard MA. Functional effects of blocking VEGF/VEGFR2 signaling in the rat urinary bladder in acute and chronic CYP-induced cystitis. Am J Physiol Ren Physiol 2019; 317(7): F43–F51. DOI: 10.1152/ajprenal.00083.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hulse RP, Beazley-Long N, Hua J, Kennedy H, Prager J, Bevan H, Qiu Y, Fernandes ES, Gammons MV, Ballmer-Hofer K, Gittenberger de Groot AC, Churchill AJ, Harper SJ, Brain SD, Bates DO, Donaldson LF. Regulation of alternative VEGF-A mRNA splicing is a therapeutic target for analgesia. Neurobiol Dis 2014; 71: 245–259. DOI: 10.1016/j.nbd.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010; 116(5): 829–840. DOI: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karaman S, Leppänen V-M, Alitalo K. Vascular endothelial growth factor signaling in development and disease. Development 2018; 145(14). DOI: 10.1242/dev.151019. [DOI] [PubMed] [Google Scholar]
- 40.Liu W, Lv Y, Ren F. PI3K/Akt pathway is required for spinal central sensitization in neuropathic pain. Cell Mol Neurobiol 2018; 38(3): 747–755. DOI: 10.1007/s10571-017-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen Y-R, Suter MR, Ji R-R, Yeh G-C, Wu Y-S, Wang K-C, Kohno T, Sun W-Z, Wang C-C. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology 2009; 110(1): 155–165. DOI: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Suo M, Yu G, Zhang M. Antinociceptive and anti-inflammatory effects of cryptotanshinone through PI3K/Akt signaling pathway in a rat model of neuropathic pain. Chem Biol Interact 2019; 305: 127–133. DOI: 10.1016/j.cbi.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32(1): 77–88. DOI: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 44.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87(2): 149–158. DOI: 10.1016/s0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 45.Li N-Q, Tang Y, Huang S-T, Liu X-T, Zeng L-P, Li H, Wan L. Modulation of NR1 receptor by CaMKIIα plays an important role in chronic itch development in mice. Brain Res Bull 2020; 158: 66–76. DOI: 10.1016/j.brainresbull.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Gorris MAJ, Halilovic A, Rabold K, van Duffelen A, Wickramasinghe IN, Verweij D, Wortel IMN, Textor JC, de Vries IJM, Figdor CG. Eight-color multiplex immunohistochemistry for simultaneous detection of multiple immune checkpoint molecules within the tumor microenvironment. J Immunol 2018; 200(1): 347–354. DOI: 10.4049/jimmunol.1701262. [DOI] [PubMed] [Google Scholar]
- 47.Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, Mao Y, Wang L, Xie W, Zhong H, Zhang N, Luo M-H, Tao W, Wang H, Li J, Li J, Qiu B-S, Zhou J-N, Li X, Xu H, Wang K, Zhang X, Liu Y, Richter-Levin G, Xu L, Zhang Z. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci 2019; 22(10): 1649–1658. DOI: 10.1038/s41593-019-0468-2. [DOI] [PubMed] [Google Scholar]
- 48.Kiguchi N, Kobayashi Y, Kadowaki Y, Fukazawa Y, Saika F, Kishioka S. Vascular endothelial growth factor signaling in injured nerves underlies peripheral sensitization in neuropathic pain. J Neurochem 2014; 129(1): 169–178. DOI: 10.1111/jnc.12614. [DOI] [PubMed] [Google Scholar]
- 49.Selvaraj D, Gangadharan V, Michalski CW, Kurejova M, Stösser S, Srivastava K, Schweizerhof M, Waltenberger J, Ferrara N, Heppenstall P, Shibuya M, Augustin HG, Kuner R. A functional role for VEGFR1 expressed in peripheral sensory neurons in cancer pain. Cancer Cell 2015; 27(6): 780–796. DOI: 10.1016/j.ccell.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji R-R, Xu Z-Z, Gao Y-J. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014; 13(7): 533–548. DOI: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji R-R, Xu Z-Z, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosciences 2011; 34(11): 599–609. DOI: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin F, Yang Y, Wei S, Huang X, Peng Z, Ke X, Zeng Z, Song Y. Hydrogen Sulfide Protects Against High Glucose-Induced Human Umbilical Vein Endothelial Cell Injury Through Activating PI3K/Akt/eNOS Pathway - PubMed. Drug Des Devel Ther 2021; 14: 621–623. DOI: 10.2147/DDDT.S242521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basso L, Altier C. Transient Receptor Potential Channels in neuropathic pain. Curr Opin Pharmacol 2017; 32: 9–15. DOI: 10.1016/j.coph.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Dai Y. TRPs and pain. Semin Immunopathol 2016; 38(3): 277–291. DOI: 10.1007/s00281-015-0526-0. [DOI] [PubMed] [Google Scholar]
- 55.Moore C, Gupta R, Jordt S-E, Chen Y, Liedtke WB. Regulation of pain and itch by TRP channels. Neurosci Bull 2018; 34(1): 120–142. DOI: 10.1007/s12264-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hulse RP. Role of VEGF-A in chronic pain. Oncotarget 2017; 8(7): 10775–10776. DOI: 10.18632/oncotarget.14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Llorián-Salvador M, González-Rodríguez S. Painful Understanding of VEGF. Front Pharmacol 2018; 9: 1267. DOI: 10.3389/fphar.2018.01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011; 473(7347): 298–307. DOI: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z, Deng M, Huang J, Wu J, Li Z, Xing M, Wang J, Guo Q, Zou W. Microglial Annexin A3 downregulation alleviates bone cancer-induced pain via inhibiting the Hif-1α/VEGF signaling pathway. Pain 2020; 18: 2750–2762. Published online June. [DOI] [PubMed] [Google Scholar]
- 60.Lee GW, Son JY, Lee AR, Ju JS, Bae YC, Ahn DK. Central VEGF-A pathway plays a key role in the development of trigeminal neuropathic pain in rats. Mol Pain 2019; 15: 174480691987260. DOI: 10.1177/1744806919872602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shim JW, Madsen JR. VEGF Signaling in neurological disorders. Int J Mol Sci 2018; 19(1): 275. DOI: 10.3390/ijms19010275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sadremomtaz A, Mansouri K, Alemzadeh G, Safa M, Rastaghi AE, Asghari SM. Dual blockade of VEGFR1 and VEGFR2 by a novel peptide abrogates VEGF-driven angiogenesis, tumor growth, and metastasis through PI3K/AKT and MAPK/ERK1/2 pathway. Biochim Biophys Acta Gen Subj 2018; 1862(12): 2688–2700. DOI: 10.1016/j.bbagen.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, Im H-J. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J Bone Miner Res 2016; 31(5): 911–924. DOI: 10.1002/jbmr.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu S, Shi C, Anbazhagan AN, Das V, Arora V, Kc R, Li X, O‐Sullivan I, Wijnen A, Chintharlapalli S, Gott‐Velis G, Richard R, Mwale F, Shibuya M, Min S, Im HJ. Absence of VEGFR-1/Flt-1 signaling pathway in mice results in insensitivity to discogenic low back pain in an established disc injury mouse model. J Cell Physiol 2020; 235(6): 5305–5317. DOI: 10.1002/jcp.29416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9(6): 669–676. DOI: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 66.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001; 410(6827): 471–475. DOI: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 67.Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol 1998; 151(1): 138–142. DOI: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- 68.Ramer MS, Murphy PG, Richardson PM, Bisby MA. Spinal nerve lesion-induced mechanoallodynia and adrenergic sprouting in sensory ganglia are attenuated in interleukin-6 knockout mice. Pain 1998; 78(2): 115–121. DOI: 10.1016/S0304-3959(98)00121-3. [DOI] [PubMed] [Google Scholar]
- 69.Kawasaki Y, Zhang L, Cheng J-K, Ji R-R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. Comparative Study 2008; 28: 5189–5194. https://pubmed.ncbi.nlm.nih.gov/18480275/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu T, Lv Z, Chen Q, Guo M, Wang X, Huang F. Vascular endothelial growth factor over-expressed mesenchymal stem cells-conditioned media ameliorate palmitate-induced diabetic endothelial dysfunction through PI-3K/AKT/m-TOR/eNOS and p38/MAPK signaling pathway. Biomed Pharmacother 2018; 106: 491–498. DOI: 10.1016/j.biopha.2018.06.129. [DOI] [PubMed] [Google Scholar]
- 71.Adamek P, Heles M, Palecek J. Mechanical allodynia and enhanced responses to capsaicin are mediated by PI3K in a paclitaxel model of peripheral neuropathy. Neuropharmacology 2019; 146: 163–174. DOI: 10.1016/j.neuropharm.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 72.Shen S, Al-Thumairy HW, Hashmi F, Qiao L-Y. Regulation of transient receptor potential cation channel subfamily V1 protein synthesis by the phosphoinositide 3-kinase/Akt pathway in colonic hypersensitivity. Exp Neurol 2017; 295: 104–115. DOI: 10.1016/j.expneurol.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu J-T, Tu H-Y, Xin W-J, Liu X-G, Zhang G-H, Zhai C-H. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol 2007; 206(2): 269–279. DOI: 10.1016/j.expneurol.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 74.Cui Y-Y, Xu H, Wu H-H, Qi J, Shi J, Li Y-Q. Spatio-temporal expression and functional involvement of transient receptor potential vanilloid 1 in diabetic mechanical allodynia in rats. PLoS One 2014; 9(7): e102052. DOI: 10.1371/journal.pone.0102052. [DOI] [PMC free article] [PubMed] [Google Scholar]