Abstract
Background
Leptospirosis, a common zoonotic infection in developing countries, usually progresses to severe conditions and poor outcomes when not detected early. Microscopic agglutination test (MAT) and culture are available but are not accessible in all areas and are usually confined to specialized laboratories. There are several available immunochromatographic test kits (ICT) that offer ease of use, access, and affordability, but diagnostic accuracy is not yet well established. In this paper, we aim to review published literature on the use of ICTs for the detection of leptospirosis and evaluate their diagnostic efficiency.
Materials and methods
We systematically searched multiple databases (PubMed, Cochrane Library, and Google Scholar), including gray literature sources for published research articles as of April 13, 2022, on the diagnosis of acute leptospirosis using ICT. We assessed the methodological quality of each article using the revised QUADAS-2.
Results
From a total of 41 articles, 30 (73.2%) were identified as potentially relevant after reviewing the title and abstract and eliminating duplicate articles; then, 22 (53.7%) articles were included after scrutinizing and applying the inclusion/exclusion criteria to the full text. Almost all test kits detect IgM antibodies against the Leptospira species except for one which used IgG as a marker for diagnosis of acute leptospirosis. A wide range of sensitivity (15.8%–100.0%) and specificity (37.3%–100.0%) were recorded. Lipopolysaccharide (LPS)-specific Immunochromatographic Lateral Flow Assay presented the highest sensitivity (∼93–100%) and specificity (∼99.19–100%).
Conclusion
Rapid diagnosis of acute leptospirosis is highly warranted; however, available test kits present a wide range of diagnostic accuracy. We found that LPS-specific ICT kit has the highest diagnostic efficiency; however, our analysis was limited by the included studies’ heterogeneity in design and reporting; thus, we recommend standardization in the conduct and reporting of diagnostic accuracy of test kits as it is vital to evaluate the reliability of the test kit.
Keywords: Acute human leptospirosis, Immunochromatographic test, Leptospira, Leptospirosis
Acute human leptospirosis; Immunochromatographic test; Leptospira; Leptospirosis.
1. Introduction
Leptospirosis is a globally widespread zoonotic infection caused by Leptospira interrogans, with more than 250 recognized serovars [1]. Globally, around half a million cases are reported annually, with deaths exceeding 10% of the total prevalence [2]. It is acquired through contact with the host's mucosal surfaces with soil or water contaminated with the urine of infected mammals such as rodents [3]. The clinical manifestations of leptospirosis are too generalized, non-specific, and hard to differentiate from other infections such as dengue and malaria. Moreover, this infection affects several organs such as kidneys, lungs, liver, and heart [4, 5]. The majority of the cases progress to severe conditions and poor outcomes due to the lack of available rapid, sensitive, and specific tests to accurately detect the infection in its early stages [6, 7, 8].
The early detection of leptospirosis serves as a gateway to effectively manage the infection and control its spread [9]. However, diagnosis remains a challenge because the current tests available are expensive, time-consuming, require technical expertise and/or sophisticated equipment, and are not available in most leptospirosis-endemic areas [10]. For example, isolation of the etiologic agent of leptospirosis by culture requires a month of incubation which is too long to use for diagnosis and treatment [11]. Detection of antigens via immunohistochemical techniques and genes by polymerase chain reaction (PCR) would not be suitable for routine laboratory testing due to several limitations and low sensitivity [12]. Moreover, the microscopic agglutination test (MAT), which is often used as the reference test, requires high level of technical expertise and accurate timing of sample collection. It also uses live pathogenic Leptospira species, which pose an increased risk of infection to laboratory technicians [13, 14]. Because of these challenges in diagnosis, in most clinical settings, physicians only depend on the clinical features of the patient to make a probable diagnosis of leptospirosis [15]. This limitation leads to poor identification of the disease, and several cases are either undiagnosed and/or misdiagnosed, leading to an unclear magnitude of the infection.
Current research now focuses on developing affordable, reliable, and easy-to-use point-of-care tests that can rapidly detect acute leptospirosis to improve its diagnosis. Several immunochromatographic tests (ICT) have been developed to detect either the antibodies against Leptospira organisms [16, 17] or biomarkers that signify the presence of the organism [18, 19]. Although the use of this principle in other infections has shown promising results [20], its accuracy in detecting leptospirosis is not yet well established. Thus, this study aims to review published literatures regarding rapid ICT to detect acute human leptospirosis and summarize the sensitivity and specificity, as well as the positive predictive value (PPV) and negative predictive value (NPV), of the assays to verify their diagnostic accuracy.
2. Materials and methods
This systematic review was conducted in accordance with the Preferred Reporting of Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21]. MEDLINE using PubMed, Cochrane Library, and Google Scholar as well as gray literature sources were thoroughly searched for published research articles as of April 13, 2022, regarding the diagnosis of acute leptospirosis using ICT. A combination of search terms such as “acute human leptospirosis”, “diagnosis”, and “immunochromatography assay” were used to carry out the literature search and article selection. The articles were selected based on their titles and abstracts. References of the retrieved articles were also screened to identify additional eligible articles. Lastly, the full text of each article was then scrutinized for inclusion in this review.
Articles were included if: (1) they determined the diagnostic accuracy of an ICT for the rapid diagnosis of acute human leptospirosis; (2) employed a reference method, either microagglutination test (MAT), Leptospira species isolation via culture, polymerase chain reaction (PCR), immunohistochemistry (IHC), IgM enzyme-linked immunosorbent assay (ELISA), or combinations of these tests to confirm the presence of leptospirosis; and (3) written in English.
The reviewers assessed the methodological quality of each article using the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) [22]. The study applicability and risk of bias of each article were evaluated in terms of different domains such as patient selection, index test, reference method, and flow and timing. If the article lacks information in the manuscript, the reviewers scored it as “unclear.” Studies that have “high” or “unclear” judgment in one or more domains were excluded in this review.
3. Results
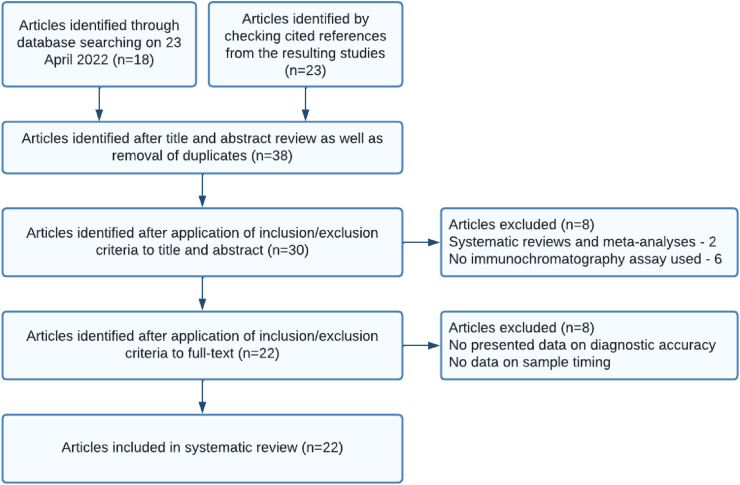
A total of 41 scholarly articles were searched from different search engines. Out of these articles, 30 (73.2%) were identified as potentially relevant after reviewing the title and abstract and eliminating duplicate articles. We determined 22 (53.7%) articles to be included in the review after scrutinizing and applying the inclusion/exclusion criteria to the full text (Figure 1).
Figure 1.
Flow diagram of the study selection process.
Included studies were published from 1999 through 2021. All of the studies evaluated immunochromatographic tests to detect acute human leptospirosis. Twelve studies employed a cross-sectional design [23, 24, 25, 26, 29, 30, 31, 35, 36, 38, 39, 41], eight are case-control studies [28, 32, 33, 34, 40, 42, 43, 44], and two studies are experimental [16, 37]. Evaluations were performed among participants from Laos [23, 38], Thailand [16, 24, 33, 39, 42], Sri Lanka [25, 31, 36], Malaysia [26, 28, 32, 34], Slovenia [29], United States [30, 33, 39, 40], Palau [33], India [35, 37, 39, 43, 44], Barbados [39], Kenya [39], New Zealand [39], Philippines [39], Puerto Rico [39], Suriname [39], The Netherlands [39, 40, 41], Russia [39], Seychelles [39], and Indonesia [40]. The sample matrix used in the studies for the ICT kits was serum and was collected in the first to second week after the onset of fever. All studies evaluated the diagnostic accuracy of commercially-available ICT kits except for two studies which developed and evaluated a new test kit for diagnosis of leptospirosis [16, 37]. Ten studies evaluated the diagnostic accuracy of Leptocheck-WB (Zephyr Biomedicals, India) diagnostic kit [24, 25, 26, 29, 31, 32, 36, 41, 43, 44], while there were two studies which evaluated ImmuneMed Leptospira IgM Duo (ImmunMed Inc., Republic of Korea) [26, 34], Leptotek lateral flow (Organon-Teknika, The Netherlands) [38, 41], and LEPTO Dipstick (Organon-Teknika, Ltd., The Netherlands) [30, 39]. A summary of the studies and the ICT kits they evaluated are summarized in Table 1.
Table 1.
Summary of studies included in the review.
| Study first author, number | Reference number | Year of Publication | Study Locale | Reference Test | Case Definition | ICT Kit Evaluated | Sample Size | Prevalence (%) |
|---|---|---|---|---|---|---|---|---|
| Dittrich et al. | [23] | 2018 | Laos | MAT, qPCR, and culture | MAT titer ≥1:400 | "Test-it” (Life Assay, South Africa) | 695 | 5.6 |
| Four-fold rise in titer in paired samples | ||||||||
| Positive culture | "Dual Path Platform (DPP)” (Chembio, Medford, NY) | |||||||
| Positive Leptospira DNA | ||||||||
| Dinhuzen et al. | [24] | 2021 | Thailand | MAT, qPCR, and culture | MAT titer ≥1:400 | "Medical Science Public Health” (Department of Medical Sciences, Ministry of Public Health, Thailand); "Leptocheck-WB” (Zephyr Biomedicals, India); "TRUSTLine” (Athenese-Dx, India) | 99 | 56.6 |
| Four-fold rise in titer in paired samples | ||||||||
| Positive culture | ||||||||
| Positive Leptospira DNA | ||||||||
| Doungchawee et al. | [16] | 2017 | Thailand | MAT and Culture | Positive culture | LEPKit | 168 | 47.6 |
| MAT titer ≥1:400 in single sample | ||||||||
| Seroconversion from negative to a titer of ≥1:100 | ||||||||
| Four-fold rise in titer in paired samples | ||||||||
| Bandara et al. | [25] | 2016 | Sri Lanka | MAT and PCR | Positive Leptospira DNA | "Leptocheck-WB” (Zephyr Biomedicals, India | 75 | 62.9 |
| MAT titer ≥1:400 in single sample | ||||||||
| Alia et al. | [26] | 2019 | Malaysia | MAT, culture, qPCR, and IHC | MAT titer ≥1:400 in single sample | "Leptocheck-WB” (Zephyr Biomedicals, India); "ImmuneMed Leptospira IgM Duo (ImmunMed Inc., Republic of Korea) | P = 50 | P = 38.0 |
| Paired sample with four-fold or greater rise | ||||||||
| Positive Leptospira DNA | R = 71.9 | |||||||
| Positive culture | R = 135 | |||||||
| Positive IHC in tissue samples | ||||||||
| Chang et al. | [28] | 2014 | Malaysia | MAT, and PCR | MAT titer ≥1:400 in single sample | “VISITEC®-LEPTO” (Omega Diagnostics, Scotland, UK) | Case = 113 | N/A |
| Positive Leptospira DNA | Control = 70 | |||||||
| Podgoršek et al. | [29] | 2015 | Slovenia | MAT | MAT titer ≥1:100 in single sample | "Leptocheck-WB” (Zephyr Biomedicals, India) | 590 | 2.2 |
| Effler et al. | [30] | 2002 | Hawaii | MAT and Culture | Positive culture | “LEPTO Dipstick” (Organon-Teknika, Ltd., The Netherlands) | 236 | 22.0 |
| Four-fold rise in titer in paired samples | ||||||||
| Niloofa et al. | [31] | 2015 | Sri Lanka | MAT | MAT titer ≥1:400 in single sample | "Leptocheck-WB” (Zephyr Biomedicals, India) | 888 | 39.8 |
| Seroconversion from negative to a titer of ≥1:100 | ||||||||
| Four-fold rise in titer in paired samples | ||||||||
| Rao et al. | [32] | 2019 | Malaysia | MAT | MAT titer ≥1:400 in single sample | "Leptocheck-WB” (Zephyr Biomedicals, India) | 142 | 46.5 |
| Four-fold rise in titer in paired samples | ||||||||
| Bajani et al. | [33] | 2003 | Thailand, Palau, and United States | Culture, IHC, and MAT | Positive culture | LDS Kit (Royal Tropical Institute, The Netherlands) | Case = 133; Control = 642 | N/A |
| Positive IHC in tissue samples | ||||||||
| Four-fold rise in titer in paired samples | ||||||||
| Amran et al. | [34] | 2018 | Malaysia | MAT | MAT titer ≥1:400 in single sample | "ImmuneMed Leptospira IgM Duo (ImmunMed Inc., Republic of Korea) | 197 | 47.2 |
| Four-fold rise in titer in paired samples | ||||||||
| Sehgal et al. | [35] | 2003 | India | Culture and MAT | Positive culture | Lepto Lateral Flow; Lepto Dipstick | 117 | 59.8 |
| Seroconversion from negative to a titer of ≥1:100 | ||||||||
| Four-fold rise in titer in paired samples | ||||||||
| MAT titer ≥1:400 in single sample | ||||||||
| Eugene et al.b | [36] | 2015 | Sri Lanka | MAT | MAT titer ≥1:400 in single sample | "Leptocheck-WB” (Zephyr Biomedicals, India) | 83 | 48.1 |
| Vanithamani et al. | [37] | 2015 | India | MAT, IgM ELISA, and Culture | Positive culture | LPS-specific Immunochromatographic Lateral Flow Assay | Case = 120; Control = 295 | N/A |
| Seroconversion from negative to a titer of ≥1:160 | ||||||||
| Four-fold rise in titer in paired samples | ||||||||
| Titer of ≥1:160 in IgM ELISA | ||||||||
| Blacksell et al. | [38] | 2006 | Laos | MAT | Four-fold rise in titer in paired samples | “Leptotek lateral flow” (Organon-Teknika, The Netherlands) | 186 | 12.4 |
| MAT titer ≥1:400 in single sample | ||||||||
| Smits et al., 1 | [39] | 1999 | Barbados | Culture and MAT | Positive culture | Lepto Dipstick | 134 | 32.8 |
| Four-fold rise in titer in paired samples | ||||||||
| MAT titer ≥1:800 in single sample | ||||||||
| India | MAT | MAT titer ≥1:80 in single sample | 163 | 38.7 | ||||
| Kenya | MAT | MAT titer ≥1:320 in single sample | ND | |||||
| New Zealand | Culture and MAT | Positive culture | 144 | 23.6 | ||||
| Four-fold rise in titer in paired samples | ||||||||
| MAT titer ≥1:400 in single sample | ||||||||
| Philippines | MAT | MAT titer ≥1:400 in single sample | 71 | 74.6 | ||||
| Puerto Rico | MAT | Four-fold rise in titer in paired samples | 104 | 6.7 | ||||
| MAT titer ≥1:400 in single sample | ||||||||
| Surinam (Study 1) | MAT | MAT titer ≥1:320 in single sample | 186 | 27.4 | ||||
| Surinam (Study 2) | MAT | MAT titer ≥1:320 in single sample | 70 | 62.9 | ||||
| The Netherlands | Culture and MAT | Positive culture | 428 | 4.0 | ||||
| MAT titer ≥1:160 in single sample | ||||||||
| Four-fold rise in titer in paired samples with a minimum titer of 1:160 for the 2nd sample | ||||||||
| Thailand | MAT | MAT titer ≥1:320 in single sample | 127 | 13.4 | ||||
| Four-fold rise in titer in paired samples with a minimum titer of 1:320 for the 2nd sample | ||||||||
| Hawaii | Culture and MAT | Positive culture | 201 | 16.9 | ||||
| Four-fold rise in titer in paired samples with a minimum titer of 1:200 for the 2nd sample | ||||||||
| Russia | MAT and slide agglutination test | MAT titer ≥1:100 in single sample | 87 | 52.9 | ||||
| Positive slide agglutination test | ||||||||
| Seychelles | MAT | Four-fold rise in titer in paired samples with a minimum titer of 1:100 for the 2nd sample | 118 | 63.6 | ||||
| Smits et al., 2 | [40] | 2001 | Hawaii, Indonesia, The Netherlands, Seychelles | MAT | Four-fold rise in titer in paired samples | LEPTO Lateral-flow Assay | Case = 135; Control = 285 | N/A |
| MAT titer ≥1:160 in single sample | ||||||||
| Goris et al. | [41] | 2013 | The Netherlands | MAT, IgM ELISA and Culture | Positive culture | “LEPTOTek Lateral-flow Assay” (Organon Teknika B.V. Boxtel, The Netherlands); "Leptocheck-WB” (Zephyr Biomedicals, India) | 5144 | 7.1 |
| Four-fold rise in titer in paired samples (at least 2 days apart) in MAT or IgM ELISA | ||||||||
| Titer ≥1:160 in single sample in MAT or IgM ELISA | ||||||||
| Silpasakorn et al. | [42] | 2011 | Thailand | MAT and Culture | Positive culture | SD Leptospira ICT (Standard Diagnostics Inc, Korea) | Case = 89 | N/A |
| Four-fold rise in titer in paired samples | Control = 72 | |||||||
| Panwala et al., 1 | [43] | 2015 | India | MAT | MAT titer ≥1:100 in single sample | "Leptocheck-WB” (Zephyr Biomedicals, India) | 100 | 28.0 |
| Panwala et al., 2 | [44] | 2011 | India | Culture, MAT and IgM ELISA | Positive culture | "Leptocheck-WB” (Zephyr Biomedicals, India) | Case = 130; Control = 310 | N/A |
| Seroconversion from negative to a titer of ≥1:100 using MAT or IgM ELISA | ||||||||
| Four-fold rise in titer in paired samples using IgM ELISA or MAT | ||||||||
| Titer of >1:100 in IgM ELISA and >200 in MAT | ||||||||
P—Prospective data; R—Retrospective data; N/A—Not applicable; ND—No data.
A wide range of sensitivity and specificity were recorded among the test kits evaluated in the studies included, as shown in Table 2. Among the evaluated ICT kits, the lipopolysaccharide (LPS)-specific Immunochromatographic Lateral Flow Assay developed by Vanithamani et al. presented the highest sensitivity and specificity at an estimate of 93.0–100.0% and 99.2–100.0%, respectively [37]. In terms of PPV and NPV, the LPS-specific Immunochromatographic Lateral Flow Assay developed by Vanithamani et al. also recorded the highest PPV (85.0–100.0%) [37]. Moreover, the Lepto Dipstick evaluated in the study of Smits et al. in Puerto Rico and Surinam has a 100.0% NPV.
Table 2.
Summary of diagnostic efficiencies of immunochromatographic tests evaluated in the included studies.
| Studies | Immunochromatographic Test Kits | % Sensitivity (95% CI) | % Specificity (95% CI) | % PPV (95% CI) | % NPV (95% CI) |
|---|---|---|---|---|---|
| Dittrich et al. (Reader 1) | "Test-it” (Life Assay, South Africa) | 71.0 (41.9–91.6) | 64.8 (59.8–69.3) | ND | |
| "Dual Path Platform (DPP)” (Chembio, Medford, NY) | 35.0 (15.4–59.2) | 62.1 (57.7–66.4) | |||
| Dittrich et al. (Reader 2) | "Test-it” (Life Assay, South Africa) | 62.5 (24.5–91.5) | 69.5 (63.2–75.4) | ||
| "Dual Path Platform (DPP)” (Chembio, Medford, NY) | 60.0 (14.7–94.7) | 54.5 (44.2–64.4) | |||
| Dittrich et al. (Reader 3) | "Test-it” (Life Assay, South Africa) | 80.0 (44.4–97.5) | 48.5 (41.3–55.7) | ||
| "Dual Path Platform (DPP)” (Chembio, Medford, NY) | 42.1% (20.3–66.5) | 58.7% (54.5–63.9) | |||
| Dinhuzen et al.d | "Medical Science Public Health” (Department of Medical Sciences, Ministry of Public Health, Thailand) | 60.7 (46.8–73.5) | 65.1 (49.1–79.0) | 69.4 (58.9–78.21) | 56.0 (46.2–65.3) |
| "Leptocheck-WB” (Zephyr Biomedicals, India) | 75.0 (61.36–85.6) | 53.5 (37.7–68.8) | 67.7 (59.6–75.0) | 62.2 (49.1–73.7) | |
| "TRUSTLine” (Athenese-Dx, India) | 33.9 (21.8–47.8) | 88.4 (74.9–96.1) | 79.2 (60.7–90.4) | 50.7 (45.3–56.1) | |
| Doungchawee et al. | LEPKit | 97.4 (90.1–99.5) | 94.5 (87.1–98.0) | 93.8 (85.4–97.7) | 97.7 (91.3–99.6) |
| Bandara et al.a | "Leptocheck-WB” (Zephyr Biomedicals, India) | 99.1 (91.4–100.0) | 68.1 (45.6–94.3) | 84.1 (64.0–97.9) | 97.9 (78.6–100.0) |
| Alia et al. (Prospective data)e | "Leptocheck-WB” (Zephyr Biomedicals, India) | 47.4 (24.5–71.1) | 80.7 (62.5–92.6) | 60.0 (38.8–78.0) | 71.4 (61.2–79.8) |
| "ImmuneMed Leptospira IgM Duo (ImmunMed Inc., Republic of Korea) | 15.8 (3.34–39.6) | 90.3 (74.3–98.0) | 50.0 (18.3–81.7) | 63.6 (58.3–68.7) | |
| Alia et al. (Retrospective data)e | "Leptocheck-WB” (Zephyr Biomedicals, India) | 90.7 (83.1–95.7) | 76.3 (59.8–88.6) | 90.7 (84.3–94.6) | 76.3 (62.8–86.0) |
| "ImmuneMed Leptospira IgM Duo (ImmunMed Inc., Republic of Korea) | 40.2 (30.4–50.7) | 89.5 (75.2–97.1) | 90.7 (78.9–96.2) | 37.0 (32.5–41.6) | |
| Chang et al.e,g | VISITECT®-LEPTO (Omega Diagnostics, Scotland, UK) | 24.1 (13.9–37.2) | 94.3 (86.0–98.4) | 77.8 (54.9–91.0) | 60.0 (56.2–63.7) |
| Podgoršek et al.b | "Leptocheck-WB” (Zephyr Biomedicals, India) | 92.3 (64.0–99.8) | 95.4 (93.9–97.3) | 33.3 (24.7–43.3) | 99.8 (98.8–100.0) |
| Effler et al.f | “LEPTO Dipstick” (Organon-Teknika, Ltd., The Netherlands) | 34.0 (23.0–48.0) | 96.0 (93.0–98.0) | 65.0 (45.0–80.0) | 88.0 (84.0–91.0) |
| Niloofa et al.a | "Leptocheck-WB” (Zephyr Biomedicals, India) | 87.4 (83.0–91.3) | 82.9 (79.1–86.1) | 77.8 (72.9–82.4) | 90.5 (86.6–93.5) |
| Rao et al.d | "Leptocheck-WB” (Zephyr Biomedicals, India) | 66.7 (54.0–77.8) | 79.0 (68.1–87.5) | 73.3 (63.3–81.5) | 73.2 (65.5–79.6) |
| Bajani et al.a | LDS Kit (Royal Tropical Institute, The Netherlands) | 94.8 (90.8–98.6) | 89.4 (87.0–91.7) | ND | |
| Amran et al.d | "ImmuneMed Leptospira IgM Duo (ImmunMed Inc., Republic of Korea) | 73.1 (62.9–81.8) | 90.4 (83.0–95.3) | 87.2 (78.8–92.6) | 79.0 (72.8–84.1) |
| Sehgal et al.d | Lepto Lateral Flow | 52.9 (40.6–64.9) | 93.6 (82.5–98.7) | 92.5 (80.1–97.4) | 57.1 (50.7–63.3) |
| Lepto Dipstick | 48.6 (36.4–60.8) | 85.1 (71.7–93.8) | 82.9 (70.2–90.9) | 52.6 (46.2–59.0) | |
| Eugene et al.a | "Leptocheck-WB” (Zephyr Biomedicals, India) | 95.0 (79.3–100.0) | 76.4 (60.8–93.2) | 76.5 (56.2–94.3) | 95.1 (75.9–100.0) |
| Vanithamani et al. | LPS-specific Immunochromatographic Lateral Flow Assay | ∼93.0–100.0 | ∼99.2–100.0 | 85.0–100.0 | >90.0 |
| Blacksell et al. | “Leptotek lateral flow” (Organon-Teknika, The Netherlands) | 70.0 (34.8–93.3) | 75.0 (62.1–85.3) | 31.8 (13.9–54.9) | 93.8 (82.8–98.7) |
| Smits et al., 1 | |||||
| Barbados | Lepto Dipstick | 81.0 (65.0–91.0) | 98.9 (93.0–100.0) | ND | |
| India | 81.1 (64.0–91.0) | 99.0 (94.0–100.0) | |||
| Kenya | ND | 99.4 (96.0–100.0) | |||
| New Zealand | 40.9 (21.0–63.0) | 88.1 (80.0–93.0) | |||
| Philippines | 92.5 (81.0–98.0) | 88.9 (64.0–98.0) | |||
| Puerto Rico | 85.7 (42.0–99.0) | 97.1 (91.0–99.0) | |||
| Surinam (Study 1) | 88.2 (74.0–95.0) | 80.0 (72.0–86.0) | |||
| Surinam (Study 2) | 94.1 (83.0–99.0) | 86.2 (76.0–96.0) | |||
| The Netherlands | 62.5 (26.0–90.0) | 93.2 (83.0–98.0) | |||
| Thailand | 40.4 (18.0–67.0) | 96.4 (91.0–99.0) | |||
| Hawaii | 50.0 (34.0–66.0) | 92.3 (87.0–96.0) | |||
| Russia | 65.2 (43.0–83.0) | 87.0 (65.0–97.0) | |||
| Seychelles | 35.1 (25.0–47.0) | 93.0 (80.0–98.0) | |||
| Smits et al., 2h | LEPTO Lateral-flow Assay | 65.9 | 93.3 | 93.7 | 68.3 |
| Goris et al.c | “LEPTOTek Lateral-flow Assay” (Organon Teknika B.V. Boxtel, The Netherlands) | 69.0 (59.0–77.0) | 96.0 (94.0–97.0) | 56.5 (49.4–63.3) | 97.3 (96.5–98.0) |
| "Leptocheck-WB” (Zephyr Biomedicals, India) | 55.0 (47.0–62.0) | 98.0 (97.0–98.0) | 64.1 (57.2–70.5) | 96.8 (96.3–97.2) | |
| Silpasakorn et al. | SD Leptospira ICT (Standard Diagnostics Inc, Korea) | 18.0 (11.3–27.3) | 98.6 (91.8–99.9) | 94.1 (71.1–99.9) | 49.3 (41.3–57.4) |
| Panwala et al., 1h | "Leptocheck-WB” (Zephyr Biomedicals, India) | 84.8 | 37.3 | 40.0 | 83.3 |
| Panwala et al., 2h | "Leptocheck-WB” (Zephyr Biomedicals, India) | 98.4 | 87.0 | 87.0 | 98.4 |
ND—No data or incomplete data.
PPV—Positive predictive value.
NPV—Negative predictive value.
CI—Confidence interval.
aused Bayesian latent class analyses in assumption that all tests evaluated are imperfect.
bsensitivity, specificity, PPV, and NPV were computed by the reviewers based on given data.
cPPV and NPV were computed by the reviewers based on given data.
d95% CI were computed by the reviewers based on given data.
e95% CI of sensitivity and specificity, PPV, and NPV were computed by the reviewers based on given data.
fprobable cases were excluded.
gdata computed with inconclusive results considered as positive.
hlacks data for computation of 95% CI.
Almost all test kits detect IgM antibodies against the Leptospira species except for one which used IgG as a marker for diagnosis of acute leptospirosis [42]. All studies used MAT as a reference test for the diagnosis of leptospirosis. In addition to MAT some studies employed additional reference tests such as culture [16, 23, 24, 26, 30, 33, 35, 37, 40, 41, 42, 44], PCR [23, 24, 25, 26], IHC [26, 33], and IgM ELISA [37, 41, 44].
4. Discussion
Several cases of leptospirosis are often unconfirmed because of lack of clinical suspicion, improper timing of sample collection, lack of laboratory tests to diagnose the infection, or a combination of these factors. A rapid diagnostic test that can accurately detect leptospirosis during its acute phase is vital for both the clinician and the patient to provide timely management and control of the disease. In this review, we systematically collated published articles that evaluated the diagnostic accuracy of ICT kits for the detection of acute human leptospirosis.
An ideal diagnostic kit should have high sensitivity and specificity during the acute phase of infection, relatively cheap, easy to use and interpret, stable even in extreme conditions, and able to provide quick results [45]. Various kits that employ the principle of immunochromatography for the detection of leptospirosis are available on the market. These kits offer simplicity and convenience in performance since they do not require sophisticated equipment and can give results within minutes. However, this review found lack of consensus in the diagnostic accuracy of the test kits evaluated in the different studies. Their sensitivity varied from an estimate of 15.8%–∼93.0–100.0% [26, 37] and the estimated specificity varied from 37.3% to ∼99.2–100% [37, 43]. In addition, manufacturers’ published diagnostic performance of "Leptocheck-WB” (Zephyr Biomedicals, India) test kit is at 95.0% sensitivity and 95.7% specificity [46] but evaluation from different studies show lower sensitivity [24, 26, 29, 31, 32, 41, 43] and specificity [24, 25, 26, 31, 32, 36, 43, 44]. This variability of results appears to be related to multiple factors such as the study design used in assessing the sensitivity and specificity of the test kits, duration of illness, Leptospira serovars that predominantly infect the population, reference tests used in the study, and production of the test kits.
Timing in sample collection for diagnosis of leptospirosis is highly critical. In this review, ICT kits evaluated in the included studies employed IgM antibody detection except for SD Leptospira ICT (Standard Diagnostics Inc, Korea) in the study of Silpasakorn et al. [42], which detects IgG antibodies against Leptospira antigen. Antibodies against Leptospira usually develop within the first two weeks of infection and peak in the third or fourth week. IgM antibodies typically become detectable within the first week or as early as the third- or fourth day post-onset [47]. However, there are instances wherein IgM antibodies may not develop at a detectable level, and IgG becomes the first to be elevated [48]. Hence, the samples of these patients may present a negative result in IgM-based ICT kits. Furthermore, studies have shown that IgM antibodies against Leptospira can persist in the bloodstream for years leading to false-positive results [49, 50].
Differences in the infecting Leptospira serovars have been found also to affect the diagnostic accuracy of ICT kits [27]. There is no conclusive reason on how differences in the serovars can influence test accuracy. One explanation could be because some serovars boost host antibody production more effectively than others or because some induce more severe disease, triggering stronger humoral immune responses [41]. In addition, antibodies may exhibit specificity on serovars of Leptospira, hence, affecting the accuracy of the test kits, especially if the antigen used do not have broad reactivity to antibodies [28]. In relation to this, Leptospira serovars are widely diverse in terms of geographical distribution, which causes variation in the diagnostic accuracy of the ICT kits in different geographical locations [41]. Therefore, it is imperative to perform local evaluation and validation of the ICT kits before implementation.
Variations in the production of test kits may also cause disparities in their diagnostic accuracy. For example, the antigens used to capture the antibody of interest differs per manufacturer. Test kits such as the Leptocheck-WB (Zephyr Biomedicals, India), LEPTO Dipstick (Organon-Teknika, Ltd., The Netherlands), and Leptotek lateral flow (Organon-Teknika, The Netherlands) are incorporated with crude antigen derived from Leptospira biflexa, serovar Patoc, strain Patoc I [40, 51, 52] while the Test-it (Life Assay, South Africa) test kit is incorporated with Leptospira interrogans serovar Copenhageni strain Wijnberg [53]. The antigen incorporated in the test kit developed by Doungchawee et al. are combinations of different serovars of Leptospira [16], while Vanithamani et al. utilized lipopolysaccharide extract from 12 different strains of Leptospira [37]. Moreover, differences in the amounts of antigen applied in the test band or different quantities of conjugate used in the kits may affect the intensity of staining of the band resulting to a subjective positivity score from the reader [41].
The choice of reference tests to confirm the diagnosis of leptospirosis may also affect the results. According to the World Health Organization (WHO), the standard requirements to diagnose leptospirosis are (i) isolation of Leptospira species from samples, (ii) a positive PCR, (iii) and a four-fold increase of antibody titers in paired sera using MAT [54]. All studies included in this review used MAT as the reference test. However, it is inherently flawed, hard to standardize, and incapable of differentiating current, recent, or past infections [12, 55]. Moreover, different cutoff points were used in defining leptospirosis cases in each study. For example, some studies considered a MAT titer of ≥1:400 in single sample to be considered positive [16, 23, 24, 25, 26, 28, 31, 32, 34, 35, 36, 38, 39] while others considered a MAT titer of ≥1:100 [29,39,43], and ≥1:160 [40,41]. Also, the validity of utilizing MAT as an immunological gold standard for evaluating diagnostic kits is being questioned [14]. Aside from MAT, some studies used PCR [23, 24, 25, 26, 28], culture [16, 23, 24, 26, 30, 33, 35, 37, 39, 41, 42, 44], immunohistochemistry [26, 33], and IgM ELISA [37, 41, 44] to define cases of leptospirosis which causes misclassifications and biased estimations of the diagnostic accuracy of the test kits being evaluated.
This review points out that the evidence bases for estimating the diagnostic accuracy of ICT kits is limited and at risk of biases because of the heterogeneity in the design and identification of leptospirosis cases used in each study. Further studies should be done using a reference standard with a sensitivity and specificity of almost 100% or statistical analyses that cope with the absence of a perfect reference test. A standard cutoff value must also be used, or combinations of different reference tests to establish cases are highly suggested to avoid bias.
5. Conclusion
Leptospirosis is a life-threatening infection that can be easily managed if detected early. However, laboratory tests to confirm diagnosis of leptospirosis are expensive, time-consuming, and would require sophisticated equipment. Immunochromatographic test kits are available in the market with the promise of ease of use and quick results. However, this review shows that ICT kits have a wide range of diagnostic accuracy which greatly depends on proper timing of sample collection, antigens used in the test kit, and predominant serovars infecting the population. Hence, it is vital to evaluate and test the validity of the test kit in the local setting prior to its use. In addition, we found heterogeneity in the study design and confirmatory tests used in identifying cases of leptospirosis which limited our analysis; thus, standardization is necessary to assess the diagnostic accuracy of ICT kits.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Assoc. Prof. Kulachart Jangpatarapongsa, PhD and Prof. Edilberto Manahan, PhD for serving as consultants in our study of leptospirosis.
References
- 1.Adler B., Lo M., Seemann T., Murray G.L. Veterinary Microbiology; 2011. Pathogenesis of Leptospirosis: the Influence of Genomics. [DOI] [PubMed] [Google Scholar]
- 2.WHO . World Heal Organ; 2011. Report of the Second Meeting of the Leptospirosis Burden Epidemiology Reference Group. [Google Scholar]
- 3.Picardeau M. Virulence of the zoonotic agent of leptospirosis: still terra incognita? Nat. Rev. Microbiol. 2017 doi: 10.1038/nrmicro.2017.5. [DOI] [PubMed] [Google Scholar]
- 4.Izurieta R., Galwankar S., Clem A. Leptospirosis: the “mysterious” mimic. J. Emergencies, Trauma, Shock. 2008 doi: 10.4103/0974-2700.40573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hin H.S., Ramalingam R., Chunn K.Y., Ahmad N., Ab Rahman J., Mohamed M.S. Case report: fatal co-infection-melioidosis and leptospirosis. Am. J. Trop. Med. Hyg. 2012 doi: 10.4269/ajtmh.2012.12-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajapakse S. Diagnosing and predicting outcome in leptospirosis: the need for clinically relevant basic sciences research. Ceylon Med. J. 2014 doi: 10.4038/cmj.v59i4.7861. [DOI] [PubMed] [Google Scholar]
- 7.Alian S., Davoudi A., Najafi N., Ghasemian R., Ahangarkani F., Hamdi Z. Clinical and laboratory manifestation and outcome of icterohemorrhagic leptospirosis patients in Northern Iran. Med. J. Islam. Repub. Iran. 2015 [PMC free article] [PubMed] [Google Scholar]
- 8.Ghasemian R., Shokri M., Makhlough A., Suraki-Azad M.A. The course and outcome of renal failure due to human leptospirosis referred to a hospital in North of Iran; A follow-up study. Casp J Intern Med. 2016 [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H.Y., Yen T.H., Lin C.Y., Chen Y.C., Pan M.J., Lee C.H., et al. Early identification of leptospirosis as an ignored cause of multiple organ dysfunction syndrome. Shock. 2012 doi: 10.1097/SHK.0b013e3182594ad7. [DOI] [PubMed] [Google Scholar]
- 10.Rajapakse S., Rodrigo C., Handunnetti S.M., Fernando D.D. Current immunological and molecular tools for leptospirosis: diagnostics, vaccine design, and biomarkers for predicting severity. Ann. Clin. Microbiol. Antimicrob. 2015 doi: 10.1186/s12941-014-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picardeau M. Medecine et Maladies Infectieuses; 2013. Diagnosis and Epidemiology of Leptospirosis. [DOI] [PubMed] [Google Scholar]
- 12.Musso D., La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J. Microbiol. Immunol. Infect. 2013 doi: 10.1016/j.jmii.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Sugunan A.P., Natarajaseenivasan K., Vijayachari P., Sehgal S.C. Percutaneous exposure resulting in laboratory-acquired leptospirosis - a case report. J. Med. Microbiol. 2004 doi: 10.1099/jmm.0.45735-0. [DOI] [PubMed] [Google Scholar]
- 14.Limmathurotsakul D., Turner E.L., Wuthiekanun V., Thaipadungpanit J., Suputtamongkol Y., Chierakul W., et al. Fool’s gold: why imperfect reference tests are undermining the evaluation of novel diagnostics: a reevaluation of 5 diagnostic tests for leptospirosis. Clin. Infect. Dis. 2012 doi: 10.1093/cid/cis403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jose L.R., Sumana M.N. Utilization of faine ’ s criteria for the diagnosis of leptospirosis. IOSR J. Dent. Med. Sci. 2016;15(3):28–30. [Google Scholar]
- 16.Doungchawee G., Sutdan D., Niwatayakul K., Inwisai T., Sitthipunya A., Boonsathorn N., et al. Development and evaluation of an immunochromatographic assay to detect serum anti-leptospiral lipopolysaccharide IgM in acute leptospirosis. Sci. Rep. 2017 doi: 10.1038/s41598-017-02654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goarant C., Bourhy P., D’Ortenzio E., Dartevelle S., Mauron C., Soupé-Gilbert M.E., et al. Sensitivity and specificity of a new vertical flow rapid diagnostic test for the serodiagnosis of human leptospirosis. PLoS Neglected Trop. Dis. 2013 doi: 10.1371/journal.pntd.0002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chirathaworn C., Janwitthayanan W., Sereemaspun A., Lertpocasombat K., Rungpanich U., Ekpo P., et al. Development of an immunochromatographic test with anti-LipL32-coupled gold nanoparticles for leptospira detection. New Microbiol. 2014 [PubMed] [Google Scholar]
- 19.Widiyanti D., Koizumi N., Fukui T., Muslich L.T., Segawa T., Villanueva S.Y.A.M., et al. Development of immunochromatography-based methods for detection of leptospiral lipopolysaccharide antigen in urine. Clin. Vaccine Immunol. 2013 doi: 10.1128/CVI.00756-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller E., Sikes H.D. Addressing barriers to the development and adoption of rapid diagnostic tests in global health. Nanobiomedicine. 2015 doi: 10.5772/61114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Rev Esp Nutr Humana y Diet; 2016. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiting P.F., Rutjes A.W.S., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., et al. Quadas-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011 doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 23.Dittrich S., Boutthasavong L., Keokhamhoung D., Phuklia W., Craig S.B., Tulsiani S.M., et al. A prospective hospital study to evaluate the diagnostic accuracy of rapid diagnostic tests for the early detection of leptospirosis in Laos. Am. J. Trop. Med. Hyg. 2018 doi: 10.4269/ajtmh.17-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinhuzen J., Limothai U., Tachaboon S., Krairojananan P., Laosatiankit B., Boonprasong S., et al. A prospective study to evaluate the accuracy of rapid diagnostic tests for diagnosis of human leptospirosis: result from Thai-LEPTO AKI study. PLoS Neglected Trop. Dis. 2021 doi: 10.1371/journal.pntd.0009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandara K., Gunasekara C., Weerasekera M., Ranasinghe N., Hapugoda M., Marasinghe C., et al. Comparison of three rapid diagnostic assays for diagnosis of leptospirosis in a resource poor setting. World J. Pharmaceut. Res. 2016;5(7):1771–1780. [Google Scholar]
- 26.Alia S.N., Joseph N., Philip N., Azhari N.N., Garba B., Masri S.N., et al. Diagnostic accuracy of rapid diagnostic tests for the early detection of leptospirosis. J Infect Public Health [Internet] 2019;12(2):263–269. doi: 10.1016/j.jiph.2018.10.137. Available from: [DOI] [PubMed] [Google Scholar]
- 27.McBride A.J.A., Santos B.L., Queiroz A., Santos A.C., Hartskeerl R.A., Reis M.G., et al. Evaluation of four whole-cell Leptospira-based serological tests for diagnosis of urban leptospirosis. Clin. Vaccine Immunol. 2007 doi: 10.1128/CVI.00217-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang C.H., Riazi M., Yunus M.H., Osman S., Noordin R. Limited diagnostic value of two commercial rapid tests for acute leptospirosis detection in Malaysia. Diagn. Microbiol. Infect. Dis. 2014 doi: 10.1016/j.diagmicrobio.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Podgoršek D., Cerar T., Logar M., Lešničar G., Remec T., Baklan Z., et al. Wien Klin Wochenschr; 2015. Evaluation of the Immunochromatographic (Leptocheck) Test for Detection of Specific Antibodies against Leptospires. [DOI] [PubMed] [Google Scholar]
- 30.Effler P.V., Bogard A.K., Domen H.Y., Katz A.R., Higa H.Y., Sasaki D.M. Evaluation of eight rapid screening tests for acute leptospirosis in Hawaii. J. Clin. Microbiol. 2002;40(4):1464–1469. doi: 10.1128/JCM.40.4.1464-1469.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niloofa R., Fernando N., De Silva N.L., Karunanayake L., Wickramasinghe H., Dikmadugoda N., et al. Diagnosis of leptospirosis: comparison between microscopic agglutination test, IgM-ELISA and IgM rapid immunochromatography test. PLoS One. 2015;10(6):1–12. doi: 10.1371/journal.pone.0129236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao M., Amran F., Aqilla N. Evaluation of a rapid kit for detection of IgM against leptospira in human. Can. J. Infect Dis. Med. Microbiol. 2019;2019 doi: 10.1155/2019/5763595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajani M.D., Ashford D.A., Bragg S.L., Woods C.W., Aye T., Spiegel R.A., et al. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J. Clin. Microbiol. 2003;41(2):803–809. doi: 10.1128/JCM.41.2.803-809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amran F., Liow Y.L., Noor Halim N.A. Evaluation of a commercial immuno-chromatographic assay kit for rapid detection of IgM antibodies against Leptospira antigen in human serum. J. Kor. Med. Sci. 2018;33(17):1–6. doi: 10.3346/jkms.2018.33.e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sehgal S.C., Vijayachari P., Sugunan A.P., Umapathi T. Field application of Lepto lateral flow for rapid diagnosis of leptospirosis. J. Med. Microbiol. 2003;52(10):897–901. doi: 10.1099/jmm.0.05064-0. [DOI] [PubMed] [Google Scholar]
- 36.Eugene E.J., Handunnetti S.M., Wickramasinghe S.A., Kalugalage T.L., Rodrigo C., Wickremesinghe H., et al. Evaluation of two immunodiagnostic tests for early rapid diagnosis of leptospirosis in Sri Lanka: a preliminary study. BMC Infect Dis [Internet] 2015;15(1):1–5. doi: 10.1186/s12879-015-1080-z. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanithamani S., Shanmughapriya S., Narayanan R., Raja V., Kanagavel M., Sivasankari K., et al. Lipopolysaccharide specific immunochromatography based lateral flow assay for serogroup specific diagnosis of leptospirosis in India. PLoS One. 2015;10(9):1–13. doi: 10.1371/journal.pone.0137130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blacksell S.D., Smythe L., Phetsouvanh R., Dohnt M., Hartskeerl R., Symonds M., et al. Limited diagnostic capacities of two commercial assays for the detection of Leptospira immunoglobulin M antibodies in Laos. Clin. Vaccine Immunol. 2006;13(10):1166–1169. doi: 10.1128/CVI.00219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smits H.L., Ananyina Y.V., Chereshsky A., Dancel L., Lai-A-Fat R.F.M., Chee H.D., et al. International multicenter evaluation of the clinical utility of a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human serum specimens. J. Clin. Microbiol. 1999;37(9):2904–2909. doi: 10.1128/jcm.37.9.2904-2909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smits H.L., Eapen C.K., Sugathan S., Kuriakose M., Gasem M.H., Yersin C., et al. Lateral-flow assay for rapid serodiagnosis of human leptospirosis. Clin. Diagn. Lab. Immunol. 2001;8(1):166–169. doi: 10.1128/CDLI.8.1.166-169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goris M.G.A., Leeflang M.M.G., Loden M., Wagenaar J.F.P., Klatser P.R., Hartskeerl R.A., et al. Prospective evaluation of three rapid diagnostic tests for diagnosis of human leptospirosis. PLoS Neglected Trop. Dis. 2013;7(7) doi: 10.1371/journal.pntd.0002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silpasakorn S., Waywa D., Hoontrakul S., Suttinont C., Losuwanaluk K., Suputtamongkol Y. Performance of Leptospira immunoglobulin M ELISA and rapid immunoglobulin G immunochromatographic assays for the diagnosis of leptospirosis. J. Med. Assoc. Thai. 2011;94(17):203–206. [PubMed] [Google Scholar]
- 43.Panwala T., Rajdev S., Mulla S. To evaluate the different rapid screening tests for diagnosis of leptospirosis. J. Clin. Diagn. Res. 2015;9(2):DC21–D24. doi: 10.7860/JCDR/2015/11188.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panwala T., Mulla S., Patel P. Seroprevalence of leptospirosis in South Gujarat Region by evaluating the two rapid commercial diagnostic kits against the MAT test for detection of antibodies to Leptospira interrogans. Natl J Community Med. 2011;2(1):14–18. [Google Scholar]
- 45.Banoo S., Bell D., Bossuyt P., Herring A., Mabey D., Poole F., et al. Evaluation of diagnostic tests for infectious diseases: general principles. Nat. Rev. Microbiol. 2010 [PubMed] [Google Scholar]
- 46.Zephyr Biomedicals Quick Reference Guide: Leptocheck ® WB Rapid test for the detection of antibodies to Leptospira [Internet] http://www.tulipgroup.com/Zephyr_New/product_range.htm#infe
- 47.Picardeau M., Bertherat E., Jancloes M., Skouloudis A.N., Durski K., Hartskeerl R.A. Diagnostic Microbiology and Infectious Disease; 2014. Rapid Tests for Diagnosis of Leptospirosis: Current Tools and Emerging Technologies. [DOI] [PubMed] [Google Scholar]
- 48.Bhatia M., Umapathy B., Navaneeth B. An evaluation of dark field microscopy, culture and commercial serological kits in the diagnosis of leptospirosis. Indian J. Med. Microbiol [Internet] 2015;33(3):416–421. doi: 10.4103/0255-0857.158570. Available from: [DOI] [PubMed] [Google Scholar]
- 49.Budihal S.V., Perwez K. Leptospirosis diagnosis: competancy of various laboratory tests. J. Clin. Diagn. Res. 2014 doi: 10.7860/JCDR/2014/6593.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Orry W., Arahou M., Hassikou R., Mennane Z. A review of laboratory diagnosis and treatment of leptospirosis. Int. J. Pharm. Pharmaceut. Sci. 2016 [Google Scholar]
- 51.Gussenhoven G.C., Van Der Hoorn M.A.W.G., Goris M.G.A., Terpstra W.J., Hartskeerl R.A., Mol B.W., et al. LEPTO dipstick, a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human sera. J. Clin. Microbiol. 1997 doi: 10.1128/jcm.35.1.92-97.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eapen C.K., Sugathan S., Kuriakose M., Abdoel T., Smits H.L. Evaluation of the clinical utility of a rapid blood test for human leptospirosis. Diagn. Microbiol. Infect. Dis. 2002 doi: 10.1016/s0732-8893(01)00361-3. [DOI] [PubMed] [Google Scholar]
- 53.Gloor C.I., Schweighauser A., Francey T., Rodriguez-Campos S., Vidondo B., Bigler B., et al. Diagnostic value of two commercial chromatographic “patient-side” tests in the diagnosis of acute canine leptospirosis. J. Small Anim. Pract. 2017 doi: 10.1111/jsap.12628. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organisation . Who; Geneva: 2010. Report of the First Meeting of the Leptospirosis Burden Epidemiology Reference Group. [Google Scholar]
- 55.Soo Z.M.P., Khan N.A., Siddiqui R. Leptospirosis: increasing importance in developing countries. Acta Trop. 2020 doi: 10.1016/j.actatropica.2019.105183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.