Abstract
The study in vivo assessed the effect of pinostrobin on the histology, immunohistochemistry, and biochemical parameters of thioacetamide (TAA) induced liver cirrhosis in Sprague Dawley rats. The rats were noticeably gavaged with two doses of pinostrobin (30 mg/kg and 60 mg/kg) with TAA and exhibited a substantial decrease in the liver index and hepatocyte propagation with much minor cell injury. These groups meaningfully down-regulated the proliferation of cellular nucleus antigen (PCNA) and alpha-smooth muscle actin (α-SMA). The liver homogenate displayed augmented antioxidant enzymes, superoxide dismutase (SOD) and catalase (CAT) activities escorted with reducing in malondialdehyde (MDA) level. The serum level of bilirubin, total protein, albumin, and liver enzymes (ALP, ALT, and AST) returned to normal and was similar to that of normal control and silymarin with TAA-treated groups. pinostrobin-fed groups also decreased the level of Tumor necrosis factor-alpha (TNF-α), Interleukin-6 (IL-6), and increased the level of Interleukin-10 (IL-10). Acute toxicity with a higher dose of 500 mg/kg of pinostrobin did not manifest any toxicological signs in rats. The hepatoprotective effect of pinostrobin could be due to potentially inhibited the progression of liver cirrhosis, down-regulation of PCNA and α-SMA proliferation, prevented oxidation of hepatocytes, improved SOD and CAT enzymes, condensed MDA, repairs of liver biomarkers, reduced cellular inflammation and modulation of inflammatory cytokines.
Keywords: Pinostrobin, Liver cirrhosis, TAA, Histology, PCNA, TNF-α, IL-6, IL-10, Liver function tests
1. Introduction
Pinostrobin is a phytochemical compound found naturally in numerous medicinal plants, including Lauraceae, Zingiberaceae, Fabaceae, and Polygonaceae (Dzoyem et al., 2017, Vasas et al., 2020). Pinostrobin is known to have a spectrum of pharmacological effects (Patel et al., 2016), together with antiulcer (Sun et al., 2020), antiplatelet, anti-mutagenic (Zhang et al., 2017), radical scavenging or anti-oxidant, anti-inflammatory, anti-cancer (Jaudan et al., 2018, Sopanaporn et al., 2020, Jones and Gehler, 2020, Sun et al., 2020), antimicrobial (Hernández Tasco et al., 2020), anti-parasitic (Vechi et al., 2020) and gastric curative action (Kanchanapiboon et al., 2020). Although the liver is a highly vital organ for cleansing, liver diseases can be the greatest health complication (Sivakrishnan and Pharm, 2019). Cirrhosis, hepatocellular carcinoma, viral hepatitis, and alcoholic hepatitis are the most common liver diseases, all of which are closely accompanied by jaundice (Rodniem et al., 2018). Numerous medicinal plants are known to protect the liver against the hepatotoxic effects of TAA in experimental animals. These effects are frequently revised in the scientific literature (Amin et al., 2013, Kadir et al., 2014, Azab and Albasha, 2018, Salama et al., 2018, Mousa et al., 2019, Khalil et al., 2021, Shareef et al., 2022, Al-Medhtiy et al., 2022). The most widespread hepatoprotection agent is silymarin, which is an herbal substance extracted from seeds of the Silybum marinum plant (Gillessen and Schmidt, 2020). The latter is used broadly as a therapeutic additive to reduce liver disease symptoms such as hepatitis, fatty acid infiltration, and cirrhosis resulting from toxic chemical and alcohol effects (Gillessen and Schmidt, 2020). Several researchers have used silymarin as a reference therapy for hepatoprotection against TAA hepatotoxicity (Rouhollahi et al., 2015, Bagherniya et al., 2018, Yang et al., 2019, Abood et al., 2020, Singh et al., 2020).
TAA increases oxidative stress and attracts free radicals, which causes damage to proteins, lipids, and DNA (da Silva et al., 2021, Elnfarawy et al., 2021). TAA makes hepatic cells impaired after its breakdown into sulphene and sulphone, which is caused by a hazardous path that includes Bio-transformation involving the CYP4502E1 enzyme (Correia and Kwon, 2020). Several studies by different co-researchers evidenced TAA has been used in the early stages of liver fibrosis (Rouhollahi et al., 2015, El-Baz et al., 2019, Urrutia-Hernández et al., 2019, Abood et al., 2020, Jantararussamee et al., 2021). The effectiveness of pinostrobin since traditional rights necessity verified to aid progress novel medicines functioning in contradiction of liver syndromes. However, the hepatoprotective activity of pinostrobin has not been reported previously in an experimental animal study. This study aims to assess the hepatoprotective action of pinostrobin on TAA-induced liver injuries in rats.
2. Material and methods
2.1. Thioacetamide
TAA was obtained from Sigma-Aldrich, Switzerland, and then liquefied in 10 % Tween 20 and mixed well until completely dissolved. At that time, 200 mg/kg body mass was inserted i.p rat three times weekly for 8 weeks. TAA-induced changes in biological morphology structures comparable to human liver cirrhosis (Kadir et al., 2013).
2.2. Silymarin
Silymarin is a reference drug (International Laboratory, USA) used in research as standard medicine. Silymarin was melted in 10 % Tween 20 and then gavaged to rats in a dose of 50 mg/kg (Alkiyumi et al., 2012, Amin et al., 2012).
2.3. Pinostrobin
Pinostrobin was purchased from Sigma-Aldrich Chemical Co., (USA). Pinostrobin was dissolved in 10 % Tween 20 and given to rats in doses of 30 and 60 mg/kg (5 mL/kg) (Abdulaziz Bardi et al., 2013).
2.4. Acute toxicity study and experimental animals
Thirty-six (18 males and 18 females) healthy Sprague Dawley rats (6–7 weeks old, weigh between 180 and 200 g) were acquired from the Experimental Animal House, Cihan University-Erbil. The rats were given standard rat pellets diet and tap water ad libitum and located in separate cages with a wide-mesh wire bottom to prevent coprophagia. The rats were kept in cages for one week for adaptation. The acute toxicity study was used to determine the safety of pinostrobin. The rats were allocated similarly into 3 groups; vehicle (10 % Tween 20, 5 mL/kg), 250 mg/kg, and 500 mg/kg of the pinostrobin (5 mL/kg). Before the dosing, the rats were fasted overnight (food but not water). Food was withdrawn for a further 3 to 4 h after dosing. The animals were observed for 24–48 h after the administration of the pinostrobin for the beginning of clinical or toxicological signs. Mortality, if any, was reported over 2 weeks. Then, the animals were sacrificed by giving an overdose of xylazine and ketamine anesthesia on the 15th day. Blood samples were collected by intracardiac puncture and serum was separated for biochemical parameters analysis. Histological and serum biochemical parameters were determined following standard methods (Gwaram et al., 2016, Salga et al., 2017).
2.5. Experimental animals for hepatoprotective activity
Sprague Dawley rats were obtained from the Animal House Unit Department of Medical Microbiology, Cihan University-Erbil. Rats weigh between 180 and 200 g were housed individually via wide-mesh wire bottoms to avoid coprophagy throughout the experimental time, at 25˚± 2˚C temperature, approximate moisture 55–65 %, and 12 h’ exposure to light/dark rotation. All the rats were fed on tape water and a standard pellet. The experiment was planned and approved by the Ethics Commission for Animal Research. Human care for whole experimental animals was applied and followed the Guide for Maintenance and usage of laboratory Animals which was produced by the National College of Knowledge and issued through the National Institute of health. Thirty healthy adult male Sprague Dawley rats were arbitrarily alienated into five groups with six rats respectively. Rat’s treatment protocol was determined following the method of (Alkiyumi et al., 2012, Bardi et al., 2014) with a few modifications; Group 1 (normal), which was treated with distilled water (5 mL/kg) i.p. injection for thrice a week, and 10 % Tween 20 (5 mL/kg) via oral administration every day for two months. Group 2 (hepatotoxic) inoculated i.p. (200 mg/kg) of TAA three times a week and daily oral administrated by 10 % Tween 20 (5 mL/kg) for two months. Group 3 (reference drug) was given TAA (200 mg/kg) i.p. injection three times weekly, followed by regular administration of Silymarin (50 mg/kg) for 2 months. Groups 4 and 5 received TAA (200 mg/kg) i.p injection thrice/week for 2 months, and daily oral administration of pinostrobin with 30 mg/kg, (group 4) and 60 mg/kg (group 5) for 2 months, respectively. After the last treatment at the end of the experimental time (two months), all animals were fated for 24 h and then processed for general anesthesia using ketamine and xylazine 30 mg/kg (100 mg/mL), 3 mg/kg (100 mg/mL) (Farghadani et al., 2019). Blood is withdrawn from intracardial puncture and stored in a gel-activated tube for liver functions test (Alshawsh et al., 2011, Omar et al., 2017).
2.6. Biochemical parameters (liver function test)
Blood in clot-activator tubes was separated by centrifugation for 15 min at 2500 rpm. A spectrophotometer is used to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, and total protein in addition to albumin. Biochemical parameters were assessed in the Erbil Hospital Laboratory (Farghadani et al., 2019, Salga et al., 2017).
2.7. Macroscopic appearance of liver
A liver assessment was done by opening the rat’s abdominal and thoracic cavities. The livers showed significant macroscopic proof of pathological changes. Also, other organs showed pathological gross lesions but were excluded from the current study. All livers were separately washed in cold saline and checked for any gross pathological abnormalities by taking microscopic images.
2.8. Histopathology of liver tissue
2.8.1. Liver tissue staining
Liver samples were washed in cold saline, cut 2 cm cubic, and fixed in 10 % phosphate-buffered formalin. Leica, Germany tissue processor machine was used to process the specimens. and embedded in paraffin. Five µm thickness slices are routinely stained by Hematoxylin &Eosin respectively (Farghadani et al., 2019, Mahmood et al., 2007), and the Masson trichrome stain was used to stain the collagen fibres (Abood et al., 2015). A Nikon microscope was used to evaluate the liver slides for histopathological change and characteristic areas were photographed.
2.8.2. Immunohistochemistry (PCNA) and (α-SMA)
Poly-l-lysine-treated glass slides were used for proliferating cell nuclear antigen (PCNA), and α-smooth muscle actin (α-SMA) staining methods, as previously described in detail (Bardi et al., 2014, Kadir et al., 2014). The propagation directory of PCNA-stained liver slices was determined by counting the proportion of labeled cells per 1000 liver cells, and the number of mitotic cells was expressed as the mitotic index (Abdulaziz Bardi et al., 2013).
2.9. Liver tissue homogenates for endogenous (CAT, SOD) enzymes and oxidative stress (MDA)
Neutral ice-cold phosphate buffer saline 10 % (w/v) was used to wash rats’ livers. Teflon homogenizer was used to homogenize liver samples (all steps done on ice), then at 4500 rpm centrifugation for 15 min at 4˚C cell debris was detached. The supernatant was collected to verify antioxidant activity via superoxide dismutase (SOD) and catalase (CAT) analysis kits (Cayman Chemical Company, USA) (Omar et al., 2017). Malondialdehyde (MDA) is a marker of cellular oxidative stress. MDA, assay kit was used to assess the levels of a thiobarbituric acid reactive substance (TBARS, Cayman Company).
2.10. Assessment of inflammatory cytokines
Valuation of TNF-α, IL-6, and IL-10 in liver tissue homogenate was accomplished utilizing a marketable ELISA kit from Cusabio Biotech Co., China. Briefly, the liver homogenate was centrifuged at 3000 g for 15 min and the supernatant was used to estimate cytokine levels using a commercial ELISA kit. The assessment was achieved according to the manufacturer’s procedure stated in Rat TNF-α ELISA Kit (109331), Rat IL-6 ELISA Kit (84597), and Rat IL-10 ELISA Kit (84236). Cytokine concentrations were designed using standard purified recombinant cytokines.
2.11. Statistical analysis of data
Data analyses were shown as mean ± standard error of the mean (SEM). One-way ANOVA with Tukey post hoc assessment was performed using SPSS software (version 24). The p-values statistical meaning at p < 0.05.
3. Results
3.1. Acute toxicity study
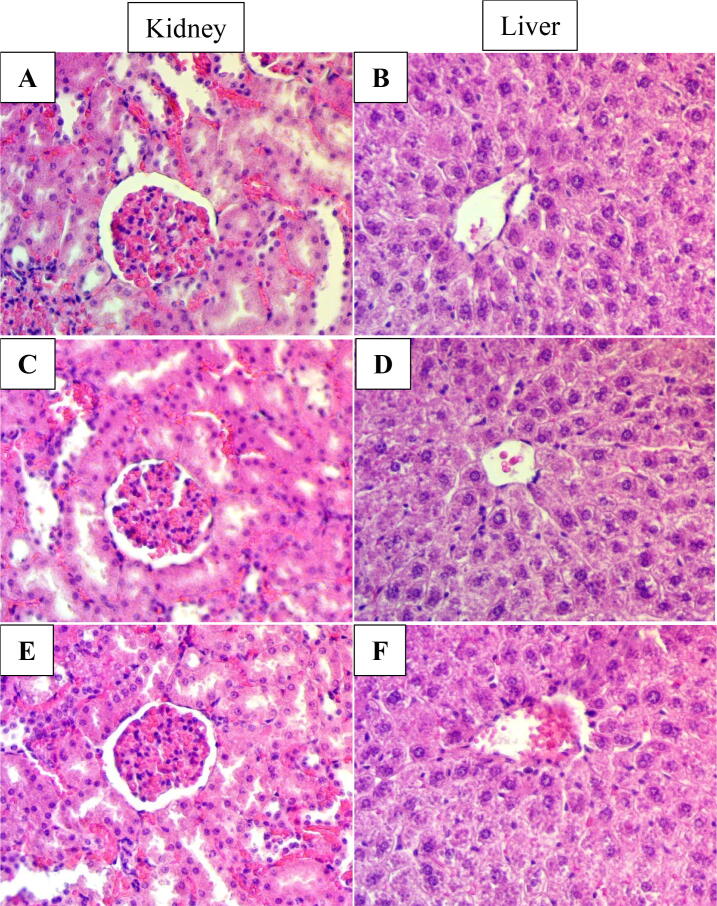
The acute toxicity test did not show any signs of toxicity. There were no histological signs of hepatic and renal toxicity. Moreover, the blood biochemistry parameters examination appeared normal (Fig. 1) (data was not shown and available at request).
Fig. 1.
Histological sections of liver and kidney in acute toxicity test. Rats were treated with a 5 mL/kg vehicle (10 % Tween 20) (A and B). Rats were treated with 250 mg/kg (5 mL/kg) pinostrobin (C and D). Rats were treated with 500 g/kg (5 mL/kg) pinostrobin (E and F). No significant changes in the structures of livers and kidneys between the treated and control groups (hematoxylin and eosin stain 40x).
3.2. Liver biochemical markers
The hepatotoxic effect of TAA was significantly increased (p < 0.001) ALT, ALP, total bilirubin, and AST levels indicating liver damage (Table 1). Moreover, the TAA group showed significant decreases (p < 0.001, mean ± SE) in total protein and albumin compared with the normal control group, demonstrating acute hepatocellular injury. Pinostrobin and silymarin-treated groups significantly dropped (p < 0.001, mean ± SE) enzyme levels of ALT, ALP, total bilirubin, and AST. Furthermore, total protein and total albumin values were elevated (p < 0.001) in pinostrobin and silymarin treatments in comparison with the TAA control group. Hence, pinostrobin revoked the hepatotoxic effect of TAA via reinstating typical liver activities. Pinostrobin effectively prevented TAA-induced hepatotoxicity at a dosage of 30 mg/kg, whereas slightly affected it at a dosage of 60 mg/kg.
Table 1.
The effects of pinostrobin on liver biochemical parameters in rats with TAA-induced hepatotoxicity.
| Groups | ALP (IU/L) | ALT (IU/L) | AST (IU/L) | T. Bilirubin (µM/L) | T. Protein (g/L) | T. Albumin (g/L) |
|---|---|---|---|---|---|---|
| Normal Control | 70.3 ± 0.6 | 30.4 ± 0.6 | 63.9 ± 0.7 | 1.2 ± 0.01 | 73.2 ± 0.7 | 32.9 ± 0.5 |
| TAA + 10 % Tween 20 | 193 ± 1.1* | 130.3 ± 0.5* | 172.5 ± 0.5* | 5.1 ± 0.07* | 45.8 ± 0.6* | 13.2 ± 0.2* |
| Silymarin + TAA (50 mg/kg) | 62.4 ± 0.6# | 28.1 ± 0.9# | 62.5 ± 0.6# | 1.4 ± 0.01# | 68.1 ± 0.7# | 29.4 ± 0.6# |
| TAA + pinostrobin (30 mg/kg) | 58.2 ± 0.8# | 22.3 ± 0.6# | 55.4 ± 0.5# | 1.9 ± 0.03# | 60 ± 0.8# | 22.1 ± 0.6# |
| TAA + pinostrobin (60 mg/kg) | 54.3 ± 0.5 # | 25 ± 0.4# | 58.7 ± 0.7# | 1.7 ± 0.05# | 64.8 ± 0.4# | 25.6 ± 0.6# |
The effects of pinostrobin or silymarin on serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) activities, as well as total bilirubin, albumin, and protein levels. The data are presented as mean ± SE (n = 6 per group). Significant difference from the normal control group at *p < 0.001, Significant difference from the TAA control group at #p < 0.001.
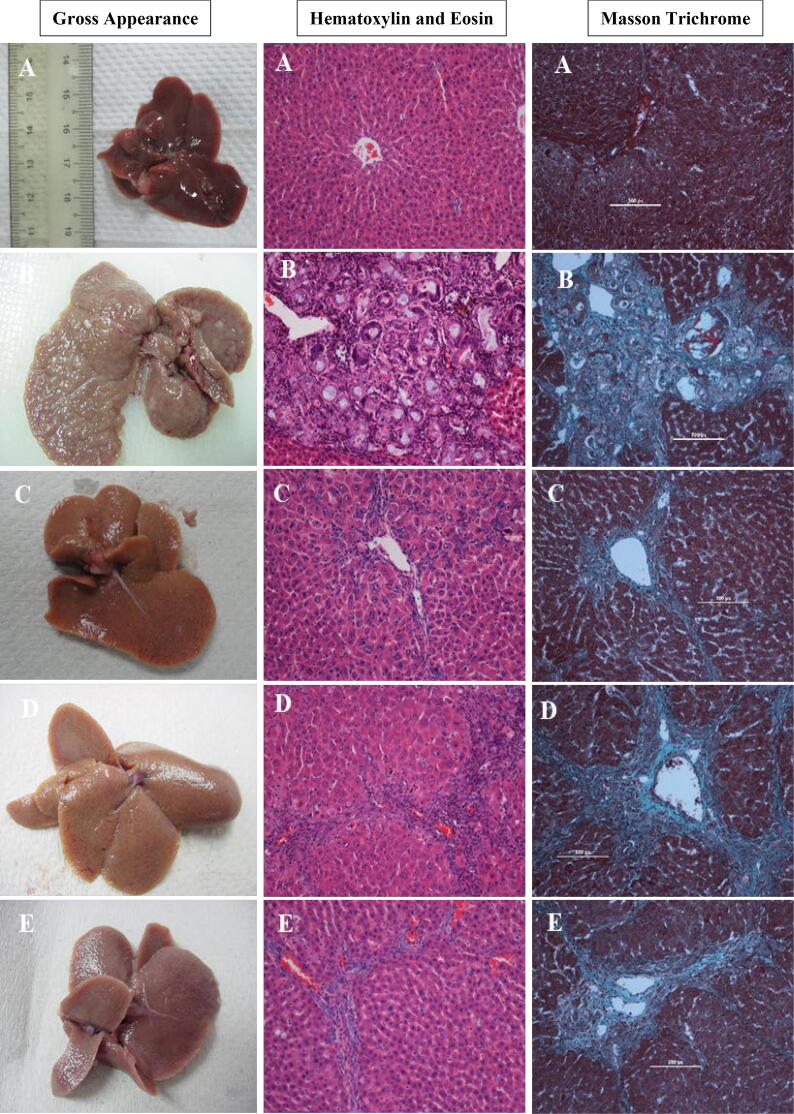
3.3. Gross appearance of liver
The morphological changes in the liver in all groups (Fig. 2) were evaluated and showed that the normal control group liver had a smooth surface with regular lobs (Fig. 2, A). TAA-induced hepatotoxicity group liver showed an irregular surface with many macros and micro nodules (Fig. 2, B). The TAA + silymarin-treated group had a smooth surface that was similar to the control group (Fig. 2, C). TAA + pinostrobin 30 mg/kg and TAA + pinostrobin 60 mg/kg groups exhibited liver smooth surface and closely maintain the liver’s normal architectural structure and shape (Fig. 2, D, E).
Fig. 2.
Histopathological examination of liver tissue sections. Hematoxylin and Eosin and Masson Trichrome stains are presenting histopathological sections and Gross appearance of the liver from (A) the normal group, (B) the TAA group, (C) the silymarin group, (D) Pinostrobin low dose group, (E) Pinostrobin high dose group. Stained liver sections were examined under a Nikon microscope (Y-THS, Japan). 20x magnification.
3.4. Histopathological examination of hepatocyte sections
Histopathological changes in liver sections stained with hematoxylin and eosin are shown in Fig. 2. Liver slides of the normal group display typical hepatocytes architecture, preserved cytoplasm, and distinguished nucleus and nucleolus with distinct regular plates of liver cells separated by sinusoidal capillaries and central vein (Fig. 2, A). Sections from the TAA group showed irregular hepatocyte architecture resulting from the presence of reforming nodules. Moreover, the liver section was divided via fibrous septa stretching from the central vein to the portal area. Hepatocytes presented severe damage, necrosis and extensive propagation of the bile duct, congested central vein, fatty changes, and granulocytes and monocytes which are presented surround the central vein due to the inflammation (Fig. 2, B). Silymarin + TAA, low and high doses of pinostrobin + TAA groups illustrated relative protection from hepatocyte disruptions induced by TAA. The hepatic cellular compositions showed a reduced amount of damage with a slight fibrotic septum. Insignificant penetration of lymphocytes was observed in these liver section groups. Moreover, the histopathological sections demonstrated remarkable regenerative parenchymal nodules, which are boarded with fibrous tissue as well as noteworthy growth in the cells-fat storing, bile ducts, and Kupffer cells (Fig. 2, C-E).
The liver tissues were stained with Masson's trichrome to assess tissue fibrosis. Collagen deposition was not detected in the normal control liver section (Fig. 2, A). TAA group was shown bile duct regeneration with notable dense fiber septa and increased collagen fiber accumulation around a congested central vein, which is referred to as severe fibrosis in the hepatic tissue (Fig. 2, B). The silymarin, 30 mg/kg, and 60 mg/kg of pinostrobin groups illustrated a reduction in the number of fibrous septa and regeneration nodules. In addition, the collagen fibers in all these three groups were observed to be homologous, which indicated the hepatoprotection activity of pinostrobin extract (Fig. 2, C-E).
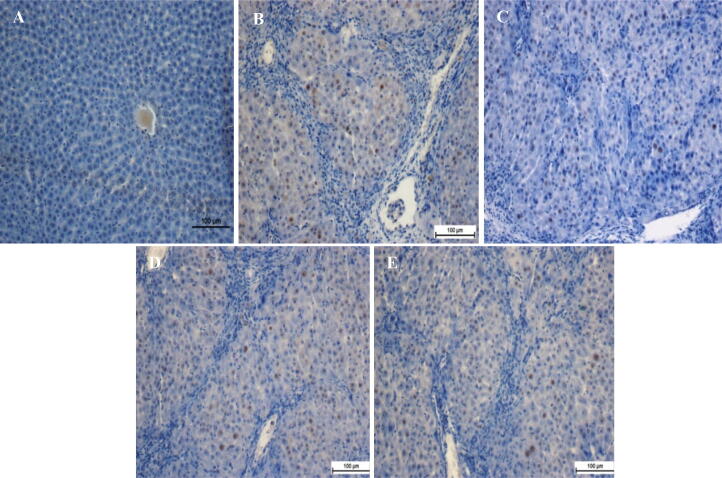
3.5. Immunohistochemical staining of liver sections
The effect of pinostrobin on hepatocyte proliferation after TAA-induced liver injury was observed through immunohistochemical analysis of PCNA appearance in the liver parenchyma using an anti-PCNA antibody (Fig. 3). Hepatocytes in the normal control group showed no PCNA staining, indicating that no cell renewal was taking place. In comparison, hepatocytes from the TAA control group had upregulated PCNA appearance and an increased mitotic directory, showing proliferation to restore the severe liver tissue impairment caused by TAA.
Fig. 3.
Immunostaining analysis of liver tissue sections. (A) Normal group, (B) TAA group, (C) Silymarin group, (D) Pinostrobin low dose group, and (E) Pinostrobin high dose group. Stained liver sections were examined under a Nikon microscope (Y-THS, Japan). 20X magnification.
Liver tissues treated with 30 mg/kg pinostrobin, 60 mg/kg pinostrobin, or silymarin had condensed hepatocyte renewal compared to the TAA control group, as designated by abridged PCNA countenance and a significant decrease of the mitotic index. by condensed PCNA appearance and a substantial discount of the mitotic index. Pinostrobin had an excellent outcome on PCNA labeling and mitotic index in a dose-dependent method.
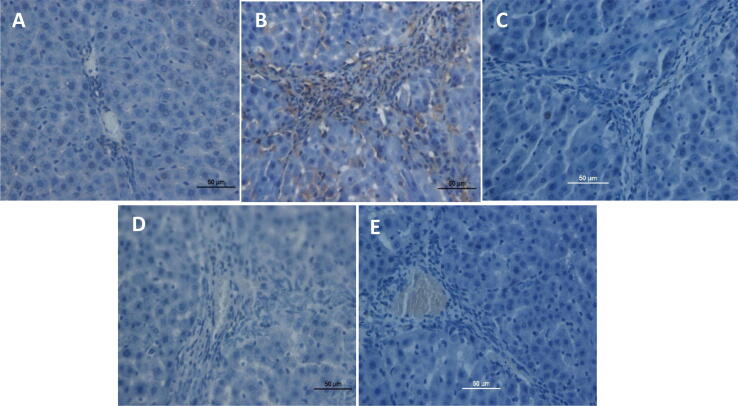
TAA-induced liver injury and the importance of pinostrobin were examined by immunohistochemical staining of α-SMA expression in the liver parenchyma utilizing specific antibodies. Down-regulation of α-SMA staining in the normal control group is indicative of the absence of cell regeneration (Fig. 4). On the contrary, the TAA-treated hepatotoxic control group had an outstanding α-SMA appearance signifying up-regulation of these proteins with a higher level of hepatocyte fibrosis. TAA-treated hepatotoxic control group elevated the mitotic figure index significantly suggesting proliferation to the regeneration of widespread hepatic damages induced by TAA. Rat-fed 60 mg/kg pinostrobin had reduced hepatic cell revitalization in comparison to the TAA-treated hepatotoxic control group, as indicated by α-SMA appearance and important lessening of the mitotic index. These results were comparatively similar to that of the silymarin-treated hepatoprotective group. Pinostrobin hepatoprotective treated groups were similar and resisted hepatocyte fibrosis by down-regulating α-SMA expressions. Whereas the 30 mg /kg pinostrobin hepatoprotective group exhibited mild to moderate expressions of α-SMA within the hepatocytes with a significant decrease in mitotic figure index but not analogous to the silymarin-treated hepatoprotective group. These results suggest that pinostrobin hepatoprotective treated groups had an estimable hepatoprotective effect by inhibiting fibrosis of hepatocytes and ameliorating propagation.
Fig. 4.
Alpha-smooth muscle actin (α-SMA) in the liver. (A) Normal group, (B) TAA group, (C) Silymarin group, (D) Pinostrobin low dose group, and (E) Pinostrobin high dose group. Stained liver sections were examined under a Nikon microscope with 40x magnification.
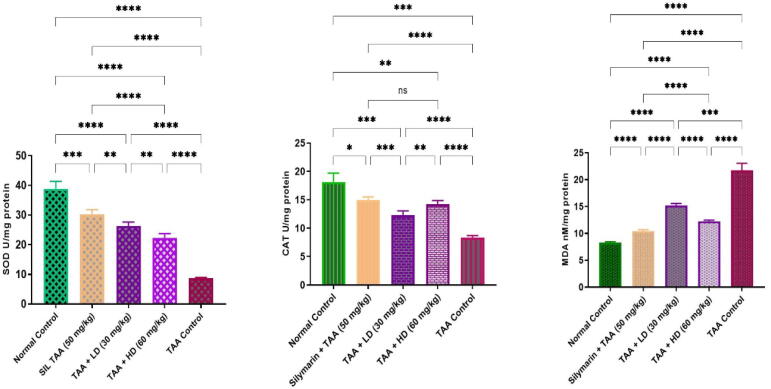
3.6. Effects of pinostrobin on endogenous antioxidant enzymes in TAA-induced liver cirrhosis in rats
The hepatotoxic group revealed significantly lower SOD and CAT activities, in comparison to the normal group (Fig. 5). Experimental groups fed pinostrobin exhibited significantly restored depletion of SOD and CAT levels to normal values (Fig. 5). The MDA levels were significantly lower in rats treated with pinostrobin when compared to the hepatotoxic control group. The Normal control and silymarin-treated rats showed non-significant changes in their SOD, CAT, and MDA profiles.
Fig. 5.
Effects of pinostrobin on antioxidant enzyme activities (SOD and CAT) and MDA level in the liver. Data are expressed as mean ± SEM. Means among groups (n = 6 rate/group) show a significant difference. ns, non-significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001, ****, p < 0.0001.
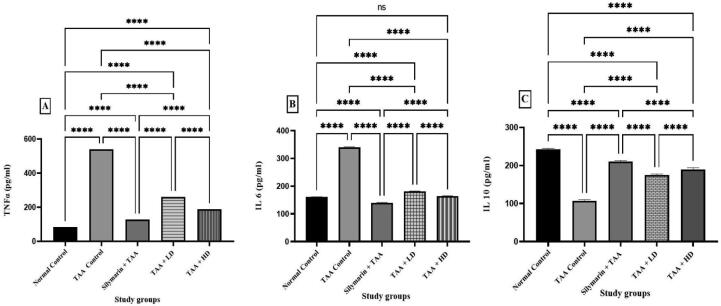
3.7. Effect of pinostrobin on TNF-α, IL-6, and IL-10 in TAA-induced liver cirrhosis in rats
Pinostrobin showed an immune-modulatory influence on liver tissue homogenate by dropping the level of TNF-α and IL-6 and increased in the level of IL-10 (Fig. 6).
Fig. 6.
Effect of pinostrobin on TNF-α, IL- 6, and IL-10 on TAA-induced liver cirrhosis in rats. Data are expressed as mean ± SEM. Means among groups (n = 6 rate/group) show a significant difference. ns, non-significant; ****, p < 0.0001.
4. Discussion
The present study was started with an oral acute toxicity trial of pinostrobin on investigational rats, the result revealed safety with no morbidity and death during the whole experimental period even at higher concentrations “i.e., 500 mg/kg of pinostrobin”. Constantly, various investigations by several academics using different medicinal plant extracts or their active ingredients showed safe, and no sign of toxic effect was stated (Salama et al., 2012, Al-Wajeeh et al., 2016, Farghadani et al., 2019, Saremi et al., 2020).
In the present study, the hepatotoxic group was related to a visible increase in activities of liver markers in blood circulation such as ALP, ALT, AST, and bilirubin levels. Similarly, numerous academics reported an increase in liver function markers (Salama et al., 2013, Bardi et al., 2014, Azab and Albasha, 2018). The increase in liver function biomarkers imitates hepatocellular dysfunction. With the consistency of the results of the current study increase in liver markers activities and bilirubin levels in the hepatotoxic group were previously reported by several researchers (Alkiyumi et al., 2012, Salama et al., 2018, Bradosty et al., 2021, Shareef et al., 2022, Al-Medhtiy et al., 2022). These values were meaningfully reduced to near-normal levels after feeding with pinostrobin. With the consistency of our findings, several coworkers used various plant extracts to show reduced liver function enzyme activities and bilirubin levels, which have been previously reported elsewhere (Salama et al., 2018, Abood et al., 2020, Sinaga et al., 2021, Shareef et al., 2022, Al-Medhtiy et al., 2022).
The hepatoprotective achievement may be due to its effect against cell leakage and injury of hepatocyte covering. TAA is specified to burden with RNA initiative from the nucleus to the cytoplasm, starting exterior injury which results in a rising statement of serum liver pointers (Alshawsh et al., 2011, Chen et al., 2018).
In the current study, total protein and albumin quantities in serum were reduced in the TAA control group. Though, silymarin or pinostrobin feeding groups bring back these values to a closely normal level. With the agreement of the results of our investigation enormous numbers of scientists displayed that rats gavaged silymarin or various plant extracts brought the albumin and protein to almost normal levels (Abood et al., 2020, Bradosty et al., 2021, Chavan et al., 2021, Al-Medhtiy et al., 2022) reported that ethanolic leave extracts of Garuga pinnata can serve as promising herbal medicine for the treatment of both acute and chronic hepatotoxicity due to the presence of flavonoids.
Outcomes of the existing research showed a decline in collagen deposition in pinostrobin-fed groups in tissue sections stained with Masson's trichrome dye. Similar to the findings of the present investigation, other researchers used a variety of plant extracts to support the finding that collagen fibers were reduced when compared to the TAA control group (Abdulaziz Bardi et al., 2013, Kadir et al., 2014, El-Baz et al., 2019, Abood et al., 2020).
Histopathological (H & E staining) and Masson’s Trichrome staining, and immunostaining displayed the repressing effect of feeding with pinostrobin, which could be owing to its capability to prevent hepatocyte propagation, as designated by down-regulation of PCNA staining. Similarly, exposure that green tea potentially inhibited the progression of liver cirrhosis, and down-regulation of PCNA proliferation (Shareef et al., 2022). The results of the existing study exhibited that the normal liver group or silymarin-treated collections demonstrated down-regulation of PCNA, suggesting the absence of cell regeneration. Up-regulation of PCNA countenance hepatocytes was observed in a hepatotoxic set, exemplifying comprehensive construction, and imaginable exertion to the reconstruction of tissue impairment (El-Lakkany et al., 2019, El-maadawy et al., 2021). Otherwise, rats fed with silymarin or pinostrobin dramatically reduced cell proliferation PCNA stain. In scientific literature, huge numbers of remedial plants with hepatoprotective potential have been noticeable by other investigators (Giribabu et al., 2018, Marzouki et al., 2019, Keshk et al., 2019, Abd Eldaim et al., 2021).
In TAA treated hepatotoxic group, TAA formed reactive-oxygen-species (ROS) producing activation of hepatic satellite cells (HSC) which is the main source of extracellular matrix (ECM) manufacture in chronic liver cirrhosis and up-regulation of α-SMA. Stimulation of HSC is accompanied by cell propagation and upgrading of ECM construction, the appearance of α-SMA to myofibroblasts (Kadir et al., 2014). The marks of our research accessible pinostrobin feeding down-regulated appearance of α-SMA compared to the hepatotoxic group which exhibited noticeable up-regulation of α-SMA. Pinostrobin significantly prevents HSC activation by avoiding the creation of ROS. Several types of research by various researchers found the down-regulation of α-SMA in TAA-induced liver cirrhosis (Yang et al., 2019, Ujiie et al., 2020).
In the present study, SOD, and CAT, in liver tissues homogenate significantly decline in the hepatotoxic group as compared to the normal group. Both enzymes become flagged by free radicals resulting in liver weakening (Gowifel et al., 2020). Meanwhile, pinostrobin expressively elevated the concentration of serum CAT and SOD by the self-protective liver from the injurious influence of free radicals compared to the TAA control group. Matching outcomes have been described formerly by uncountable researchers (Salama et al., 2018, Shareef et al., 2022, Al-Medhtiy et al., 2022). MDA as a lipid peroxidation marker is a usual injurious process (El-Mihi et al., 2017, Gowifel et al., 2020). MDA levels elevated in tissue improved lipid peroxidation (Hajrezaie et al., 2015). Rise MDA initiates damages and tragedy of antioxidant protection to block the expansion of additional free radicals (Zheng et al., 2018). The existing search exhibited TAA yield increase in MDA quantity has been promisingly reduced by pinostrobin feeding. Parallel results have been previously reported by various academics elsewhere (Salama et al., 2018, Zaidi and Mahboob, 2020, Bradosty et al., 2021). A drop in hepatic SOD and CAT activities in the hepatotoxic group might explain elevated MDA.TAA created liver fibrosis in rats. Nonetheless, a rat gavaged with pinostrobin could dramatically accelerate the recovery of the liver injuries suggestively preventing the impact of TAA intoxication. These results are likewise consistent with former studies stated by abundant inventors using diverse medicinal plants (Rouhollahi et al., 2015, Salama et al., 2018, Said et al., 2019, Abood et al., 2020). Shareef et al. showed that green tea potentially inhibited the progression of liver cirrhosis, prevented oxidation of hepatocytes, recovered SOD and CAT enzymes, condensed MDA, and reduced cellular inflammation (Shareef et al., 2022).
TAA induces an inflammatory response that initiates a dynamic chain of immune responses associated with the release of vast amounts of inflammatory cytokines such as TNF-α and IL-6, which in turn produce an increased quantity of ROS (Wei et al., 2003). TNF-α, being a major proinflammatory cytokine produced by macrophages, attracts neutrophils to liver injury (Martin and Wallace, 2006, Kishimoto, 2005). IL-6 is another important proinflammatory cytokine shown to mediate immune response and acute inflammation. IL-6 activates granulocytes and agranulocytes, which in turn trigger a stress response in injured tissue (Mei et al., 2012, Sabat et al., 2010) suggesting that IL-10 can suppress inflammatory response and inhibit TNF-α production. Previous reports showed that TAA was able to increase proinflammatory cytokines and decrease anti-inflammatory cytokines in liver tissue (Jadaun et al., 2019, El-Lakkany et al., 2019). Our results were in agreement with these observations, where exposure to TAA showed elevated TNF-α and IL-6 and a decrease in IL-10 levels, compared to normal controls. However, pretreatment inhibited the depletion of IL-10 and elevation of TNF-α and IL-6 levels, which shows its anti-inflammatory effect on TAA-induced liver cirrhosis in the rat.
5. Conclusion
The current study found that pinostrobin had a significant hepatoprotective effect in reducing TAA toxicity in rats, as evidenced by biochemical liver parameters, endogenous enzymes, histology, and immunohistochemistry. Pinostrobin intensely raises the CAT & SOD activities, with a significant reduction of hepatic MDA. The hepatoprotective effect of pinostrobin could be attributed to its ability to inhibit hepatocyte multiplication, reduce oxidative stress and lipid peroxidation, down-regulate PCNA, and α-SMA, possess antioxidant and free radical scavenger properties, and modulating proinflammatory cytokine.
6. Ethics announcement
-
1.
The current experiment was authorized through the conscience team for animal investigation, Faculty of Science, Cihan University-Erbil, and Ethic No. ERB, 115, 11/03/2019. All animals for the duration of trials, obtained human attention by principles set forth by the “Director for the Maintenance and Use of research laboratory Animals” which was organized by the Nationwide School of Sciences has issued by the National Institution of healthiness.
-
2.
This manuscript has not been published in whole or in part elsewhere.
-
3.
The manuscript is not currently being considered for publication in another journal.
-
4.
All authors have read and approved the manuscript.
CRediT authorship contribution statement
Suhayla Hamad Shareef: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. Morteta H. Al-Medhtiy: Investigation, Writing – review & editing. Ahmed S. Al Rashdi: Investigation, Funding acquisition. Peshawa Y. Aziz: Visualization, Writing – review & editing. Mahmood A. Abdulla: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the oil and gas company at sea Hawk drilling and services for supporting this work [Grant Code: 07/ 2021].
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Suhayla H. Shareef, Email: suhayla.shareef@su.edu.krd, suhayla.shareef@cihanuniversity.edu.iq.
Morteta H. Al-Medhtiy, Email: mortetah.mohamed@uokufa.edu.iq.
Ahmed S. Al Rashdi, Email: ahmed.alrashdi2@moh.gov.om.
Peshawa Y. Aziz, Email: peshawa.aziz@spu.edu.iq.
Mahmood A. Abdulla, Email: mahmood.ameen@cihanuniversity.edu.iq.
References
- ABD ELDAIM, M. A., TOUSSON, E., EL SAYED, I. E. T., ABD ELMAKSOUD, A. Z. & AHMED, A. A. 2021. Ameliorative effects of 9-diaminoacridine derivative against Ehrlich ascites carcinoma–induced hepatorenal injury in mice. Environmental Science and Pollution Research, 28, 21835-21850. [DOI] [PubMed]
- ABDULAZIZ BARDI, D., HALABI, M. F., ABDULLAH, N. A., ROUHOLLAHI, E., HAJREZAIE, M. & ABDULLA, M. A. 2013. In vivo evaluation of ethanolic extract of Zingiber officinale rhizomes for its protective effect against liver cirrhosis. BioMed Research International, 2013. [DOI] [PMC free article] [PubMed]
- Abood W.N., Al-Henhena N.A., Abood A.N., Al-Obaidi M.M.J., Ismail S., Abdulla M.A., Al batran R. The wound-healing potential of the fruit extract of Phaleria macrocarpa. Bosn. J. Basic Med. Sci. 2015;15:25. doi: 10.17305/bjbms.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abood W.N., Bradosty S.W., Shaikh F.K., Salehen N.A., Farghadani R., Agha N.F.S., Al-Medhtiy M.H., Kamil T.D.A., Agha A.S., Abdulla M.A. Garcinia mangostana peel extracts exhibit hepatoprotective activity against thioacetamide-induced liver cirrhosis in rats. J. Funct. Foods. 2020;74 [Google Scholar]
- Alkiyumi S.S., Abdullah M.A., Alrashdi A.S., Salama S.M., Abdelwahab S.I., Hadi A.H.A. Ipomoea aquatica extract shows protective action against thioacetamide-induced hepatotoxicity. Molecules. 2012;17:6146–6155. doi: 10.3390/molecules17056146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Medhtiy M.H., Jabbar A.A., Shareef S.H., Ibrahim I.A.A., Alzahrani A.R., Abdulla M.A. Histopathological evaluation of Annona muricata in TAA-induced liver injury in rats. Processes. 2022;10:1613. [Google Scholar]
- ALSHAWSH, M. A., ABDULLA, M. A., ISMAIL, S. & AMIN, Z. A. 2011. Hepatoprotective effects of Orthosiphon stamineus extract on thioacetamide-induced liver cirrhosis in rats. Evidence-based complementary and alternative medicine, 2011. [DOI] [PMC free article] [PubMed]
- Al-Wajeeh N.S., Hajerezaie M., Noor S.M., Halabi M.F., Al-Henhena N., Azizan A.H.S., Kamran S., Hassandarvish P., Shwter A.N., Ali H.M. The gastro protective effects of Cibotium barometz hair on ethanol-induced gastric ulcer in Sprague-Dawley rats. BMC Vet. Res. 2016;13:1–12. doi: 10.1186/s12917-017-0949-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Amin Z.A., Alshawsh M.A., Kassim M., Ali H.M., Abdulla M.A. Gene expression profiling reveals underlying molecular mechanism of hepatoprotective effect of Phyllanthus niruri on thioacetamide-induced hepatotoxicity in Sprague Dawley rats. BMC Complement. Altern. Med. 2013;13:1–10. doi: 10.1186/1472-6882-13-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMIN, Z. A., BILGEN, M., ALSHAWSH, M. A., ALI, H. M., HADI, A. H. A. & ABDULLA, M. A. 2012. Protective role of Phyllanthus niruri extract against thioacetamide-induced liver cirrhosis in rat model. Evidence-based complementary and alternative medicine, 2012. [DOI] [PMC free article] [PubMed]
- Azab A.E., Albasha M.O. Hepatoprotective effect of some medicinal plants and herbs against hepatic disorders induced by hepatotoxic agents. J. Biotechnol. Bioeng. 2018;2:8–23. [Google Scholar]
- Bagherniya M., Nobili V., Blesso C.N., Sahebkar A. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: a clinical review. Pharmacol. Res. 2018;130:213–240. doi: 10.1016/j.phrs.2017.12.020. [DOI] [PubMed] [Google Scholar]
- Bardi D.A., Halabi M.F., Hassandarvish P., Rouhollahi E., Paydar M., Moghadamtousi S.Z., Al-Wajeeh N.S., Ablat A., Abdullah N.A., Abdulla M.A. Andrographis paniculata leaf extract prevents thioacetamide-induced liver cirrhosis in rats. PLoS One. 2014;9:e109424. doi: 10.1371/journal.pone.0109424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADOSTY, S. W., HAMAD, S. W., AGHA, N. F. S., SHAIKH, F. K., QADIR NANAKALI, N. M., AZIZ, P. Y., SALEHEN, N. A., SUZERGOZ, F. & ABDULLA, M. A. 2021. In vivo hepatoprotective effect of Morinda elliptica stem extract against liver fibrosis induced by thioacetamide. Environmental toxicology, 36, 2404-2413. [DOI] [PubMed]
- Chavan S., Dias R., Magdum C. Garuga pinnata attenuates oxidative stress and liver damage in chemically induced hepatotoxicity in rats. Egypt. J. Basic Appl. Sci. 2021;8:235–251. [Google Scholar]
- Chen X., Zhang J., Yi R., Mu J., Zhao X., Yang Z. Hepatoprotective effects of Lactobacillus on carbon tetrachloride-induced acute liver injury in mice. Int. J. Mol. Sci. 2018;19:2212. doi: 10.3390/ijms19082212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia M.A., Kwon D. Why hepatic CYP2E1-elevation by itself is insufficient for inciting NAFLD/NASH: inferences from two genetic knockout mouse models. Biology. 2020;9:419. doi: 10.3390/biology9120419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva B.S., Paulino A.M.B., Taffarel M., Borba I.G., Telles L.O., Lima V.V., Aguiar D.H., Dias M.C., Nascimento A.F., Sinhorin V.D.G. High sucrose diet attenuates oxidative stress, inflammation and liver injury in thioacetamide-induced liver cirrhosis. Life Sci. 2021;267 doi: 10.1016/j.lfs.2020.118944. [DOI] [PubMed] [Google Scholar]
- Dzoyem J.P., Nkuete A.H., Ngameni B., Eloff J.N. Anti-inflammatory and anticholinesterase activity of six flavonoids isolated from Polygonum and Dorstenia species. Arch. Pharm. Res. 2017;40:1129–1134. doi: 10.1007/s12272-015-0612-9. [DOI] [PubMed] [Google Scholar]
- El-Baz F.K., Salama A., Salama R.A.A. Therapeutic Effect of Dunaliella salina Microalgae on Thioacetamide- (TAA-) Induced Hepatic Liver Fibrosis in Rats: Role of TGF- β and MMP9. Biomed Res. Int. 2019;2019:1–9. [Google Scholar]
- El-Lakkany N.M., El-Maadawy W.H., El-Din S.H.S., Saleh S., Safar M.M., Ezzat S.M., Mohamed S.H., Botros S.S., Demerdash Z., Hammam O.A. Antifibrotic effects of gallic acid on hepatic stellate cells: In vitro and in vivo mechanistic study. J. Tradit. Complement. Med. 2019;9:45–53. doi: 10.1016/j.jtcme.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-MAADAWY, W. H., SEIF EL-DIN, S., EZZAT, S. M., HAMMAM, O., SAFAR, M., SALEH, S. & EL-LAKKANY, N. 2021. Rutin Ameliorates Hepatic Fibrosis via Targeting Hepatic Stellate Cells’ Activation, Proliferation and Apoptosis. Journal of Herbs, Spices & Medicinal Plants, 1-20.
- El-Mihi K.A., Kenawy H.I., El-Karef A., Elsherbiny N.M., Eissa L.A. Naringin attenuates thioacetamide-induced liver fibrosis in rats through modulation of the PI3K/Akt pathway. Life Sci. 2017;187:50–57. doi: 10.1016/j.lfs.2017.08.019. [DOI] [PubMed] [Google Scholar]
- Elnfarawy A.A., Nashy A.E., Abozaid A.M., Komber I.F., Elweshahy R.H., Abdelrahman R.S. Vinpocetine attenuates thioacetamide-induced liver fibrosis in rats. Hum. Exp. Toxicol. 2021;40:355–368. doi: 10.1177/0960327120947453. [DOI] [PubMed] [Google Scholar]
- Farghadani R., Seifaddinipour M., Rajarajeswaran J., Abdulla M.A., Mohd Hashim N.B., Khaing S.L., Salehen N.B. In vivo acute toxicity evaluation and in vitro molecular mechanism study of antiproliferative activity of a novel indole Schiff base β-diiminato manganeseIII complex in hormone-dependent and triple negative breast cancer cells. PeerJ. 2019;7:e7686. doi: 10.7717/peerj.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillessen A., Schmidt H.-H.-J. Silymarin as supportive treatment in liver diseases: a narrative review. Adv. Ther. 2020;37:1279–1301. doi: 10.1007/s12325-020-01251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giribabu N., Karim K., Kilari E.K., Kassim N.M., Salleh N. Anti-inflammatory, antiapoptotic and pro-proliferative effects of Vitis vinifera seed ethanolic extract in the liver of streptozotocin-nicotinamide-induced type 2 diabetes in male rats. Can. J. Diabetes. 2018;42:138–149. doi: 10.1016/j.jcjd.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Gowifel A.M.H., Khalil M.G., Nada S.A., Kenawy S.A., Ahmed K.A., Salama M.M., Safar M.M. Combination of pomegranate extract and Curcumin ameliorates thioacetamide-induced liver fibrosis in rats: impact on TGF-β/Smad3 and NF-κB signaling pathways. Toxicol. Mech. Methods. 2020;30(8):620–633. doi: 10.1080/15376516.2020.1801926. [DOI] [PubMed] [Google Scholar]
- Gwaram N.S., Musalam L., Ali H.M., Abdulla M.A., Shaker S.A. Synthesis, spectral characterization and biological activity of Zn (II) complex with 2′-[1-(2-hydroxyphenyl) ethylidene] benzenesulfanohydrazide. Arab. J. Chem. 2016;9:S1197–S1207. [Google Scholar]
- Hajrezaie M., Salehen N., Karimian H., Zahedifard M., Shams K., Batran R.A., Majid N.A., Khalifa S.A., Ali H.M., El-Seedi H. Biochanin a gastroprotective effects in ethanol-induced gastric mucosal ulceration in rats. PLoS One. 2015;10:e0121529. doi: 10.1371/journal.pone.0121529. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- HERNÁNDEZ TASCO, A. J., RAMÍREZ RUEDA, R. Y., ALVAREZ, C. J., SARTORI, F. T., SACILOTTO, A. C. B., ITO, I. Y., VICHNEWSKI, W. & SALVADOR, M. J. 2020. Antibacterial and antifungal properties of crude extracts and isolated compounds from Lychnophora markgravii. Natural product research, 34, 863-867. [DOI] [PubMed]
- Jadaun A., Sharma S., Verma R., Dixit A. Pinostrobin inhibits proliferation and induces apoptosis in cancer stem-like cells through a reactive oxygen species-dependent mechanism. RSC Adv. 2019;9:12097–12109. doi: 10.1039/c8ra08380k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantararussamee C., Rodniem S., Taweechotipatr M., Showpittapornchai U., Pradidarcheep W. Hepatoprotective effect of probiotic lactic acid bacteria on thioacetamide-induced liver fibrosis in rats. Probiotics Antimicrob. Proteins. 2021;13:40–50. doi: 10.1007/s12602-020-09663-6. [DOI] [PubMed] [Google Scholar]
- Jaudan A., Sharma S., Malek S.N.A., Dixit A., Hsieh Y.-H. Induction of apoptosis by pinostrobin in human cervical cancer cells: Possible mechanism of action. PLoS One. 2018;13(2):e0191523. doi: 10.1371/journal.pone.0191523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES, A. A. & GEHLER, S. 2020. Acacetin and pinostrobin inhibit malignant breast epithelial cell adhesion and focal adhesion formation to attenuate cell migration. Integrative Cancer Therapies, 19, 1534735420918945. [DOI] [PMC free article] [PubMed]
- KADIR, F. A., KASSIM, N. M., ABDULLA, M. A. & YEHYE, W. A. 2013. Hepatoprotective role of ethanolic extract of Vitex negundo in thioacetamide-induced liver fibrosis in male rats. Evidence-Based Complementary and Alternative Medicine, 2013. [DOI] [PMC free article] [PubMed]
- KADIR, F. A., KASSIM, N. M., ABDULLA, M. A., KAMALIDEHGHAN, B., AHMADIPOUR, F. & YEHYE, W. A. 2014. PASS-predicted hepatoprotective activity of Caesalpinia sappan in thioacetamide-induced liver fibrosis in rats. The Scientific World Journal, 2014. [DOI] [PMC free article] [PubMed] [Retracted]
- Kanchanapiboon J., Kongsa U., Pattamadilok D., Kamponchaidet S., Wachisunthon D., Poonsatha S., Tuntoaw S. Boesenbergia rotunda extract inhibits Candida albicans biofilm formation by pinostrobin and pinocembrin. J. Ethnopharmacol. 2020;261 doi: 10.1016/j.jep.2020.113193. [DOI] [PubMed] [Google Scholar]
- Keshk W.A., Soliman N.A., Ali D.A., Elseady W.S. Mechanistic evaluation of AMPK/SIRT1/FXR signaling axis, inflammation, and redox status in thioacetamide-induced liver cirrhosis: The role of Cichorium intybus linn (chicory)-supplemented diet. J. Food Biochem. 2019;43:e12938. doi: 10.1111/jfbc.12938. [DOI] [PubMed] [Google Scholar]
- Khalil H.M.A., Eliwa H.A., El-Shiekh R.A., Al-Mokaddem A.K., Hassan M., Tawfek A.M., El-Maadawy W.H. Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways. J. Ethnopharmacol. 2021;277:114141. doi: 10.1016/j.jep.2021.114141. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: from basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- Mahmood A., Sidik K., Fouad H. Prevention of ethanol-induced gastric mucosal injury by Ocimum basilicum seed extract in rats. ASM Sci. J. 2007;1:1–6. [Google Scholar]
- Martin G.R., Wallace J.L. Gastrointestinal inflammation: a central component of mucosal defense and repair. Exp. Biol. Med. 2006;231:130–137. doi: 10.1177/153537020623100202. [DOI] [PubMed] [Google Scholar]
- Marzouki R., Brahmia A., Bondock S., Keshk S.M., Zid M.F., Al-Sehemi A.G., Koschella A., Heinze T. Mercerization effect on structure and electrical properties of cellulose: development of a novel fast Na-ionic conductor. Carbohydr. Polym. 2019;221:29–36. doi: 10.1016/j.carbpol.2019.05.083. [DOI] [PubMed] [Google Scholar]
- Mei X., Xu D., Xu S., Zheng Y., Xu S. Novel role of Zn (II)–curcumin in enhancing cell proliferation and adjusting proinflammatory cytokine-mediated oxidative damage of ethanol-induced acute gastric ulcers. Chem. Biol. Interact. 2012;197(1):31–39. doi: 10.1016/j.cbi.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Mousa A.A., El-Gansh H.A.I., Eldaim M.A.A., Mohamed M.-A.-E.-G., Morsi A.H., el Sabagh H.S. Protective effect of Moringa oleifera leaves ethanolic extract against thioacetamide-induced hepatotoxicity in rats via modulation of cellular antioxidant, apoptotic and inflammatory markers. Environ. Sci. Pollut. Res. 2019;26:32488–32504. doi: 10.1007/s11356-019-06368-4. [DOI] [PubMed] [Google Scholar]
- Omar H., Nordin N., Hassandarvish P., Hajrezaie M., Azizan A.H.S., Fadaeinasab M., Majid N.A., Abdulla M.A., Hashim N.M., Ali H.M. Methanol leaf extract of Actinodaphne sesquipedalis (Lauraceae) enhances gastric defense against ethanol-induced ulcer in rats. Drug Des. Devel. Ther. 2017;11:1353. doi: 10.2147/DDDT.S120564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N.K., Jaiswal G., Bhutani K.K. A review on biological sources, chemistry and pharmacological activities of pinostrobin. Nat. Prod. Res. 2016;30:2017–2027. doi: 10.1080/14786419.2015.1107556. [DOI] [PubMed] [Google Scholar]
- Rodniem S., Tiyao V., Nilbu-Nga C., Poonkhum R., Pongmayteegul S., Pradidarcheep W. Protective effect of alpha-mangostin on thioacetamide-induced liver fibrosis in rats as revealed by morpho-functional analysis. Histol. Histopathol. 2018;34:419–430. doi: 10.14670/HH-18-052. [DOI] [PubMed] [Google Scholar]
- Rouhollahi E., Moghadamtousi S.Z., Hajiaghaalipour F., Zahedifard M., Tayeby F., Awang K., Abdulla M.A., Mohamed Z. Curcuma purpurascens BI. rhizome accelerates rat excisional wound healing: involvement of Hsp70/Bax proteins, antioxidant defense, and angiogenesis activity. Drug Des. Devel. Ther. 2015;9:5805. doi: 10.2147/DDDT.S88196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabat R., Grütz G., Warszawska K., Kirsch S., Witte E., Wolk K., Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Said A.M., Waheed R.M., Khalifa O.A. Protective role of rosemary ethanolic extract on thioacetamide induced hepatic encephalopathy: Biochemical and molecular studies. Aust. J. Basic Appl. Sci. 2019;13:1–6. [Google Scholar]
- Salama S.M., Abdulla M.A., Alrashdi A.S., Ismail S., Alkiyumi S.S., Golbabapour S. Hepatoprotective effect of ethanolic extract of Curcuma longa on thioacetamide induced liver cirrhosis in rats. BMC Complement. Altern. Med. 2013;13:1–17. doi: 10.1186/1472-6882-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALAMA, S. M., BILGEN, M., AL RASHDI, A. S. & ABDULLA, M. A. 2012. Efficacy of Boesenbergia rotunda treatment against thioacetamide-induced liver cirrhosis in a rat model. Evidence-based complementary and alternative medicine, 2012. [DOI] [PMC free article] [PubMed]
- Salama S.M., Ibrahim I.A.A., Shahzad N., Al-Ghamdi S., Ayoub N., Alrashdi A.S., Abdulla M.A., Salehen N.A., Bilgen M. Hepatoprotectivity of Panduratin A against liver damage: In vivo demonstration with a rat model of cirrhosis induced by thioacetamide. APMIS. 2018;126:710–721. doi: 10.1111/apm.12878. [DOI] [PubMed] [Google Scholar]
- Salga M.S., Ali H.M., Abdulla M.A., Abdelwahab S.I., Elhassantaha M.M., Yagoub U. Synthesis and gastroprotective activities of some zinc (II) complexes derived from (E)-2-(1-(2-(piperazin-1-yl) ethylimino) ethyl) phenol and (E)-4-(1-(2-(piperazin-1-yl) ethylimino) ethyl) benzene-1, 3-diol Schiff bases against aspirin induced ulceration. Arab. J. Chem. 2017;10:S1578–S1589. [Google Scholar]
- Saremi K., Rad S.K., Khalilzadeh M., Hussaini J., Majid N.A. In vivo acute toxicity and anti-gastric evaluation of a novel dichloro Schiff base: bax and HSP70 alteration. Acta Biochim. Biophy. Sin. 2020;52:26–37. doi: 10.1093/abbs/gmz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shareef S.H., Ibrahim I.A.A., Alzahrani A.R., Al-Medhtiy M.H., Abdulla M.A. Hepatoprotective effects of methanolic extract of green tea against Thioacetamide-Induced liver injury in Sprague Dawley rats. Saudi J. Biol. Sci. 2022;29:564–573. doi: 10.1016/j.sjbs.2021.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinaga E., Fitrayadi A., Asrori A., Rahayu S.E., Suprihatin S., Prasasty V.D. Hepatoprotective effect of Pandanus odoratissimus seed extracts on paracetamol-induced rats. Pharm. Biol. 2021;59:31–39. doi: 10.1080/13880209.2020.1865408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Kumar A., Verma R.K., Shukla R. Silymarin encapsulated nanoliquid crystals for improved activity against beta amyloid induced cytotoxicity. Int. J. Biol. Macromol. 2020;149:1198–1206. doi: 10.1016/j.ijbiomac.2020.02.041. [DOI] [PubMed] [Google Scholar]
- Sivakrishnan S., Pharm M. Liver diseases—an overview. World J. Pharm. Pharm. Sci. 2019;8:1385–1395. [Google Scholar]
- Sopanaporn J., Suksawatamnuay S., Sardikin A., Lengwittaya R., Chavasiri W., Miyakawa T., Yompakdee C. Pinostrobin suppresses the Ca2+-signal-dependent growth arrest in yeast by inhibiting the Swe1-mediated G2 cell-cycle regulation. FEMS Yeast Res. 2020;20:foaa026. doi: 10.1093/femsyr/foaa026. [DOI] [PubMed] [Google Scholar]
- Sun X., Liu X., Chen S. The pharmacokinetics, tissue distribution, metabolism, and excretion of pinostrobin in rats: ultra-high-performance liquid chromatography coupled with linear trap quadrupole orbitrap mass spectrometry studies. Front. Pharmacol. 2020;11:1903. doi: 10.3389/fphar.2020.574638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujiie N., Nakano T., Yamada M., Sato C., Nakanishi C., Fujishima F., Ito K., Shindo T., Shimokawa H., Kamei T. Low-energy extracorporeal shock wave therapy for a model of liver cirrhosis ameliorates liver fibrosis and liver function. Sci. Rep. 2020;10:1–7. doi: 10.1038/s41598-020-58369-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URRUTIA-HERNÁNDEZ, T. A., SANTOS-LÓPEZ, J. A., BENEDÍ, J., SÁNCHEZ-MUNIZ, F. J., VELÁZQUEZ-GONZÁLEZ, C., DE LA O-ARCINIEGA, M., JARAMILLO-MORALES, O. A. & BAUTISTA, M. 2019. Antioxidant and hepatoprotective effects of croton hypoleucus extract in an induced-necrosis model in rats. Molecules, 24, 2533. [DOI] [PMC free article] [PubMed]
- Vasas A., Lajter I., Kúsz N., Forgó P., Jakab G., Fazakas C., Wilhelm I., Krizbai I.A., Hohmann J. Flavonoid, stilbene and diarylheptanoid constituents of Persicaria maculosa Gray and cytotoxic activity of the isolated compounds. Fitoterapia. 2020;145 doi: 10.1016/j.fitote.2020.104610. [DOI] [PubMed] [Google Scholar]
- Vechi G., Tenfen A., Capusiri E.S., Gimenez A., Cechinel-Filho V. Antiparasitic activity of two Brazilian plants: Eugenia mattosii and Marlierea eugeniopsoides. Nat. Prod. Res. 2020:1–5. doi: 10.1080/14786419.2020.1739676. [DOI] [PubMed] [Google Scholar]
- Wei X., Heywood G., di Girolamo N., Thomas P. Nicorandil inhibits the release of TNF-α from a lymphocyte cell line and peripheral blood lymphocytes. Int. Immunopharmacol. 2003;3:1581–1588. doi: 10.1016/S1567-5769(03)00176-0. [DOI] [PubMed] [Google Scholar]
- Yang H.Y., Kim K.S., Lee Y.H., Park J.H., Kim J.-H., Lee S.-Y., Kim Y.-M., Kim I.S., Kacew S., Lee B.M. Dendropanax morbifera ameliorates thioacetamide-induced hepatic fibrosis via TGF-β1/Smads pathways. Int. J. Biol. Sci. 2019;15:800. doi: 10.7150/ijbs.30356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi S.N.F., Mahboob T. Hepatoprotective role of curcumin in rat liver cirrhosis. Pak. J. Pharm. Sci. 2020;33:1519–1526. [PubMed] [Google Scholar]
- ZHANG, Y.-X., YANG, T.-T., XIA, L., ZHANG, W.-F., WANG, J.-F. & WU, Y.-P. 2017. Inhibitory effect of Propolis on platelet aggregation in vitro. Journal of healthcare engineering, 2017. [DOI] [PMC free article] [PubMed]
- Zheng L., Yin L., Xu L., Qi Y., Li H., Xu Y., Han X., Liu K., Peng J. Protective effect of dioscin against thioacetamide-induced acute liver injury via FXR/AMPK signaling pathway in vivo. Biomed. Pharmacother. 2018;97:481–488. doi: 10.1016/j.biopha.2017.10.153. [DOI] [PubMed] [Google Scholar]