Abstract
Maternal and collateral donors were associated with a higher incidence of graft-versus-host disease (GvHD) after haploidentical hematopoietic stem cell transplantation (haplo-HSCT). A more effective regimen for GvHD prophylaxis after haplo-HSCT with maternal/collateral donors needed to be explored. A retrospective study was performed on 62 patients after haploidentical peripheral blood stem cell transplantation (haplo-PBSCT) with maternal/collateral donors, which included 35 patients with low-dose antithymocyte globulin (ATG) plus low-dose posttransplant cyclophosphamide-based (low-dose ATG/PTCy-based) and 27 with ATG-based regimens for GvHD prophylaxis. The 180-day cumulative incidences (CIs) of grades II-IV and III-IV acute GvHD (aGvHD) were 17.7% and 6.8% in low-dose ATG/PTCy-based group, which were significantly lower than that in ATG-based group (55.4% and 31.9%) (P = 0.003 for grade II-IV and P = 0.007 for III-IV aGvHD). In low-dose ATG/PTCy-based group, the 1-year overall survival (OS) and relapse-free survival (RFS) were 80.0%and 80.4%, which were higher than that in ATG-based group with OS of 59.4% and RFS of 62.0%. In multivariate analysis, the low-dose ATG/PTCy-based regimen significantly reduced the risk of grade II-IV (HR = 0.357; P = 0.049) and grade III-IV aGvHD (HR = 0.190; P = 0.046) as an independent risk factor. The results suggested that the low-dose ATG/PTCy-based regimen could effectively prevent the occurrence of aGvHD after haplo-PBSCT with maternal/collateral donors compared with the ATG-based regimen.
Keywords: graft-versus-host disease (GvHD), maternal/collateral related donors, low-dose antithymocyte globulin (ATG), low-dose posttransplant cyclophosphamide (PTCy), haploidentical peripheral blood stem cell transplantation (haplo-PBSCT)
Introduction
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has been historically associated with poor outcomes, owing to the high rates of graft-versus-host disease (GvHD)1. Along with the wider application of antithymocyte globulin (ATG)-based or post-transplant cyclophosphamide (PTCy)-based regimens for GvHD prophylaxis, the cumulative incidence (CI) of GvHD could be significantly reduced and the survival of patients undergoing T cell replete (TCR) haplo-HSCT could be improved2–5. However, these regimens could not effectively prevent the occurrence of GvHD after haplo-HSCT with maternal or collateral related donors. Because maternal or collateral related donors were associated with a higher incidence of GvHD and worse survival compared with direct family donors, maternal or collateral related donors were the last choices within some family related donors for haplo-HSCT6–9. The exact causes of maternal/collateral donor increasing the risk of GvHD with maternal or collateral related donors were uncertain, but it may reflect the incompatibility of human leukocyte antigen (HLA) matching between donors and recipients and the immunity to minor histocompatibility antigen encoded genes (mHAgs) on the Y chromosome (H-Y)10,11. PTCy was effective to prevent GvHD through killing active T cells and enhancing quick Treg reconstitution12. However, there was no conclusive research to prove that PTCy was effective to prevent GvHD with maternal/collateral donors. Therefore, we sought to explore a more effective regimen for GvHD prophylaxis after haplo-HSCT with maternal or collateral related donors.
A novel regimen for GvHD prophylaxis in haploidentical peripheral blood stem cell transplantation (haplo-PBSCT), which was composed of low-dose ATG plus low-dose PTCy combined with cyclosporine (CsA) and mycophenolate mofetil (MMF) (low-dose ATG/PTCy-based), has been developed at our center. Our previous results showed that this new regimen could significantly reduce the CIs of acute GvHD (aGvHD) without influencing the effects of graft versus leukemia (GVL) after haploidentical and unrelated PBSCT13–15. To explore the efficacy of the low-dose ATG/PTCy-based regimen for GvHD prophylaxis in haplo-PBSCT with maternal and collateral donors, we retrospectively analyzed the outcomes of haplo-PBSCT with maternal land collateral related donors in our center. The results suggested that this regimen could effectively prevent the occurrence of GvHD after haplo-PBSCT with maternal and collateral related donors.
Patients and Methods
Patients, Donors, and Graft Sources
Sixty-two patients with hematological malignancies were enrolled into this retrospective study. All the patients underwent haplo-PBSCT with maternal or collateral related donors from March 2016 to January 2022. Collateral donors were defined as the non-immediate family relatives (uncle/aunt, cousin). A total 153 patients underwent haplo-PBSCT with non-maternal/collateral family donors in the same period. HLA haplo-matched was defined as at least three loci mismatched between family donor and recipient at HLA-A, -B, -C, -DRB1, and -DQB1loci16. Donor-specific antibody (DSA) was defined as previous reference17. Haploidentical donor selection was performed according the reference18. The rationale of donor selection was that (1) DSA negative; otherwise desensitization for recipients if there were no alternative donors; (2) male, younger; (3) children > sibling > father > mother/collateral relatives; a younger collateral donor was preferred if a direct family donor was older than 50 years old; (4) ABO compatibility between donor and recipient; (5) matched serum CMV status between donors and recipients. Patients with positive DSA (median fluorescence intensity, 5000 < MFI < 10,000) were desensitized with Rituximab (375 mg/m2) on day −7 plus high-dose gamma globulin (0.4 g/kg/d for 5 days) on days −5 to −1. The peripheral blood stem cell (PBSC) grafts for all patients were mobilized with granulocyte colony-stimulating factor (G-CSF, 7.5–10 μg/kg/d) for 5 days. The target value for CD34+ cells was a minimum of 8 × 106/kg of recipient’s weight. A single unrelated cord blood (UCB) was given to some patients as the third-party cells co-infused with PBSC grafts. This study had ethical approval from the local ethical committees and conducted in accordance with the Declaration of Helsinki. All patient data originated from clinical trials with mandatory written informed consent.
Conditioning Regimens
Reduced-intensity conditioning (RIC) regimen was given to patients above 55 years old (≥55 years) or HSCT comorbidity index above 2 (HCT-CI > 2), while myeloablative conditioning (MAC) regimens were designed for patients below 55 years old (<55 years) or HCT-CI ≦ 2. In the present study, all patients received MAC regimens in this study. The MAC regimen for myeloid malignancies was composed of busulphan (Bu, 3.2 mg/kg/d for 4 days), cytarabine (Ara-C, 1–2 g/m2/d for 5 days), and fludarabine (Flu, 30 mg/m2/d for 5 days). The RIC regimen included Bu (3.2 mg/kg/d for 2 days), Flu, Ara-C, and total body irradiation (TBI, 3Gy on day −1). The doses and schedules of Flu and Ara-C were the same as that in the MAC regimen. For lymphoid malignancies, all patients received MAC regimens including Bu (3.2mg/kg/d for 4 days) combined with cyclophosphamide (CTX, 50 mg/kg/d for 2 days) and etoposide (VP16, 10 mg/kg/d for 2 days) or TBI (total 10 Gy for 5 times) combined with the same doses of CTX and VP16 as above13,19.
GvHD Prophylaxis
Low-dose ATG/PTCy-based and ATG-based regimens were used in the present study. All patients received ATG-based regimen for GvHD prophylaxis before 2017, while ATG- and low-dose ATG/PTCy-based regimens were prescribed to patients according to physician’s decision and patient’s choice after 2017. In the ATG-based regimen, total dose of ATG (10 mg/kg) was given at a dose of 2.5 mg/kg/d from day −4 to −1 and PTCy (CTX 50 mg/kg/d for 1 day on day +3), cyclosporine (CsA), and mycophenolate mofetil (MMF)13. The ATG-based regimen included ATG, CsA, short-term methotrexate (MTX), and MMF. In the low-dose ATG/PTCy-based regimen, low-dose ATG was given at a dose of 2.5 mg/kg/d for 2 days on day −2 to −1. Intravenous MTX (10 mg/m2/d) was delivered on days + 1, + 3, and + 6 after graft infusion. CsA was given from day +4 in low-dose ATG/PTCy regimen and day −5 in ATG-based regimen, respectively, with 2 mg/kg/d with a nadir of 200 to 300 ng/ml and tapered from day +90 to day +180. MMF was given for 30 days after transplantation with a dose of 15 mg/kg/d three times a day (maximum dose of 3g/d) if aGvHD does not occur13. MMF started from day +4 in low-dose ATG/PTCy-based regimen and day +1 in ATG-based regimen, respectively.
Engraftment, Chimerism Monitoring, and GvHD Evaluation
Neutrophil engraftment time was the day when the absolute neutrophil count (ANC) ≥ 0.5 × 109/L for 3 consecutive days after transplantation without G-CSF. Platelet engraftment was defined as the first day of 7 consecutive days with platelet counts of >20 × 109/L without platelet transfusion20. Complete remission (CR) and hematologic and molecular relapse were defined by European Society for Blood and Marrow Transplantation (EBMT) criteria21. Quantitative chimerism monitoring was performed as previous references22–24. The diagnosis and classification of acute GvHD (aGvHD) and chronic GvHD (cGvHD) were performed as described inreferences25,26. Revised disease risk index (R-DRI) was defined as previous references27,28.
Supportive Care
All patients received G-CSF since day +5 until neutrophil recovery. Levofloxacin and acyclovir were given from the beginning of conditioning to neutrophil recovery. Posaconazole was given from the day of conditioning until 1 month after engraftment13. Cytomegalovirus (CMV) DNA in serum and Epstein–Barr virus (EBV) in whole blood was weekly monitored by polymerase chain reaction (PCR) until day 100. When >1,000 copies of CMV/ml of serum and >5000 copies of EBV/ml of the whole blood were detected, the preemptive therapy was performed with ganciclovir or foscarnet sodium for CMV and anti-CD20 monoclonal antibody for EBV reactivation13.
Definitions
Overall survival (OS) was defined as the time from the first day of transplantation to death as a result of any cause. Relapse-free survival (RFS) was measured from the date when the relapse or death whichever occurred first. Cumulative incidence of relapse (CIR) was defined from the first day of transplantation or the date of getting CR after transplantation until relapse. Non-relapse mortality (NRM) was defined as death as a result of any cause other than relapse. CI of GvHD was defined from the first day of transplantation to aGvHD or cGvHD. Only patients with successful neutrophil engraftment were evaluated for aGvHD and cGvHD.
Statistical Analysis
The Kaplan–Meier outcome curves for the OS and RFS were used for all 62 patients. CI functions were used to assess NRM, relapse, aGvHD, and cGvHD. For multivariate analysis, univariate analysis was performed at first using the log-rank test to identify prognostic factors. Second, factors with lower P value (P < 0.5) in univariate analysis were enrolled into multivariate analysis. Finally, the Cox proportional hazards regression model was constructed to assess previously defined risk factors in multivariate analysis. All statistical tests were two-sided and P value < 0.05 was considered significant. The statistical analyses were performed using IBM SPSS 17.0 statistical software (IBM, North Harbour, Portsmouth, UK).
Results
Patient Characteristics
Sixty-two patients undergoing maternal or collateral donor transplantation were diagnosed with hematological malignancies including myeloid malignancies (61.3%), lymphoid malignancies (33.9%), and mixed lineage leukemia (MAL, 4.8%). Myeloid malignancies included acute myeloid leukemia (AML, 53.2%) and myelodysplasia (MDS, 8.1%), while lymphoid malignancies mainly included acute lymphoblastic leukemia (ALL) accounting for 19.4% of total patients. Thirty-five patients received low-dose ATG/PTCy-based regimen, while 27 received ATG-based regimen for prevention of GvHD. In the whole cohort, the median age was 30 (range: 15–54) years old. In the low-dose ATG/PTCy-based group, the median age was 32 years old, which was older than that of 28 in the ATG-based group (P = 0.061). At the time of transplantation, 54 patients were in CR while 8 in active disease. Maternal donors accounted for 48.1%, while collateral donors accounted for 51.6% (P = 0.329). The ratio of maternal to collateral related donor in the low-dose ATG/PTCy-based group was similar with that in the ATG-based group (P = 0.429). DSA was performed in all patients. DSA was positive for nine patients (14.5%), including three in the ATG-based group and six in the low-dose ATG/PTCy-based group, respectively (P = 0.512).The median follow-up time for all patients was 347.5 days (range: 1–957) and for patients in the ATG-based group (248 days [range: 1–957]) was similar with that in the low-dose ATG/PTCy-based group (363 days [range: 52–946]; P = 0.121). The median follow-up time for all survivors was 377.5 days (range: 71–957) while for survivors in ATG-based group was 323.5 days (range: 71–957) and in low-dose ATG/PTCy group was 396 days (range: 119–946), respectively. The other characteristics were similar between two groups (Table 1).
Table 1.
Patient and Donor Characteristics.
| Characteristics | Total (n = 62) | ATG (n = 27) | ATG/PTCy (n = 35) | P value |
|---|---|---|---|---|
| Age (median [range], year) | 30 (15–54) | 28 (16–52) | 32 (15–54) | 0.061 |
| Gender (male/female) | 40/22 | 20/7 | 20/15 | 0.173 |
| Disease type | 0.103 | |||
| Myeloid malignancies | 38 (61.3%) | 21 (77.8%) | 17 (48.6%) | |
| Lymphoid malignancies | 21 (33.9%) | 4 (14.8%) | 17 (48.6%) | |
| MAL | 3 (4.8%) | 2 (7.4%) | 1 (2.8%) | |
| ECOG (median, [range]) | 0 (0–4) | 0 (0–2) | 0 (0–4) | 0.094 |
| HCT-CI (median, [range]) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0.783 |
| R-DRI | 0.672 | |||
| Low risk | 6 (9.7%) | 1 (3.7%) | 5 (14.3%) | |
| Intermediate risk | 25 (40.3%) | 13 (48.1%) | 12 (34.3%) | |
| High risk | 31 (50%) | 13 (48.1%) | 18 (51.4%) | |
| Disease status | 0.699 | |||
| CR | 54 (87.1%) | 23 (85.2%) | 31 (88.6%) | |
| NR | 8 (12.9%) | 4 (14.8%) | 4 (11.4%) | |
| HLA-matching | 0.473 | |||
| 5/10 | 39 (62.9%) | 15 (55.6%) | 24 (68.6%) | |
| 6/10 | 10 (16.1%) | 6 (22.2%) | 4 (11.4%) | |
| 7/10 | 13 (21.0%) | 6 (22.2%) | 7 (20.0%) | |
| Donor source | 0.329 | |||
| Mother | 30 (48.4%) | 15 (55.6%) | 15 (42.9%) | |
| Collateral relatives | 32 (51.6%) | 12 (44.4%) | 20 (57.1%) | |
| Donor gender (male/female) | 19/43 | 5/22 | 14/21 | 0.071 |
| Donor age (median, [range], year) | 40 (24–64) | 40 (27–64) | 38 (24–56) | 0.314 |
| Blood type matching | 0.993 | |||
| Matched | 23 (37.1%) | 10 (37.0%) | 13 (37.1%) | |
| Mismatched | 39 (62.9%) | 17 (63.0%) | 22 (62.9%) | |
| PBSC graft | ||||
| MNC (median, [range], ×108/kg) | 16.55 (7.1–40.14) | 14.24 (7.1–40.14) | 17.05 (9.78–39.74) | 0.727 |
| CD34+cells (median, [range], ×106/kg) | 10.48 (2.12–46.96) | 10.38 (2.12–46.96) | 10.62 (3.48–25.56) | 0.979 |
| CD3+cells (median, [range], ×108/kg) | 2.96 (1.57–15.07) | 2.73(1.7–9.6) | 3.08 (1.57–15.07) | 0.551 |
| UCB | ||||
| Nucleated cells (median, [range], ×107/kg) | 2.35 (0.39–3.39) | 2.01 (1.13–2.97) | 2.41 (0.39–3.39) | 0.462 |
| CD34+cells (median, [range], ×104/kg) | 6.10 (1.13–14.30) | 6.6 (1.48–13.00) | 5.50 (1.13–14.30) | 0.838 |
| Follow-up time (median [range], days) | 347.5 (1–957) | 248 (1–957) | 363 (52–946) | 0.121 |
ATG: antithymocyte globulin; PTCy: posttransplant cyclophosphamide; MAL: mixed lineage leukemia; ECOG: Eastern Cooperative Oncology Group score standard; HCT-CI: Hematopoietic Cell Transplantation Comorbidity Index; R-DRI: revised disease risk index; CR: complete response; NR: non-remission; HLA: human leukocyte antigen; PBSC: peripheral blood stem cell; MNC: mononuclear cell; UCB: unrelated cord blood.
Engraftment
All 62 patients received G-CSF mobilized PBSC grafts with mononuclear cells (MNCs) 16.55 (7.1–40.14) ×108/kg, CD34+ cells 10.48 (2.12–46.96) ×106/kg, and CD3+ cells 2.96 (1.57–15.07) ×108/kg. Eight patients (29.6%) in the ATG group and 12 patients (34.3%) in the low-dose ATG/PTCy group received a single UCB (P = 0.703).The median number of MNCs, CD34+ cells, CD3+ cells in PBSC grafts and nucleated cells, and CD34+ cells of UCB were similar between two groups (Table 1). The median time of neutrophil engraftment were 11.5 days (range: 10–20) and 12 days (range: 10–21) in the ATG-based and low-dose ATG/PTCy-based groups, respectively (P = 0.706). The median time of platelet engraftment were similar between the ATG-based (13.5 days, range: 10–25) and low-dose ATG/PTCy-based groups (13 days, range: 10–33) (P = 0.622). One patient in the ATG-based group died of septic shock on day +1 before engraftment. 60 to 61 (98.4%) evaluable patients achieved full donor chimerism at 30 days after transplantation. The engraftment ratio of evaluable patients between the low-dose ATG/PTCy-based and ATG-based groups were similar (97.1% vs 100%, P = 0.384).
Immune Reconstitution
The median lymphocyte counts (CD3+, CD4+, and CD8+) during the first-year posttransplant was shown in Fig. S1. There was no significant difference between the ATG-based and low-dose ATG/PTCy-based group for immune reconstitution (CD3+: P = 0.426; CD4+: P = 0.997; CD8+: P = 0.586) (Fig. S1a–c). In the ATG-based group, median CD3+, CD4+, and CD8+ were 1,025 (447–7,314), 178 (27–305.1) and 896 (378–5,382)/μl on day +120, respectively (Fig. S1a–c), while in the low-dose ATG/PTCy-based group, the median CD3+, CD4+, and CD8+ were 949 (52–4,169), 209.9 (5–618), and 654.5 (24–3,584)/μl day +120 (Fig. S1a–c).
GvHD
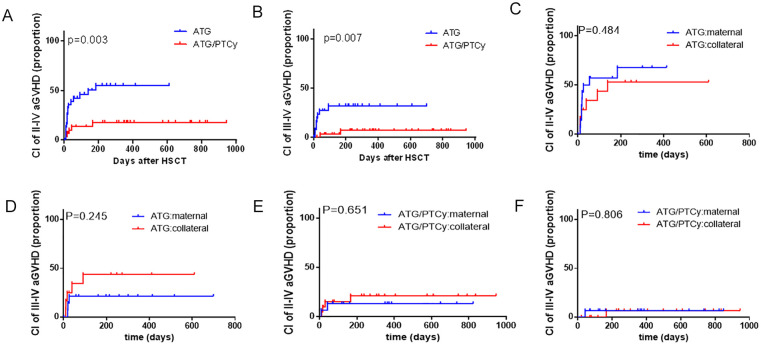
For patients with maternal/collateral related donors, the 180-day CIs of grade I-IV aGvHD were 65.1% (95% CI 48.4%–77.7%) and 40.0% (95% CI 19.8%–59.5%) in the ATG-based and low-dose ATG/PTCy-based groups, respectively (P = 0.084). The 180-day CI of grade II-IV aGvHD was significantly reduced in the low-dose ATG/PTCy-based group (17.7% [95% CI 1.51%–48.9%]) as compared with that in the ATG-based group (55.4% [95% CI 36.8%–70.5%]) (Fig. 1A, P = 0.003). The CI of grade III-IV aGvHD in the low-dose ATG/PTCy-based group (6.8% [95% CI 0.0%–51.7%]) was also significantly lower than that in the ATG-based group (31.9% [95% CI 9.9%–56.7%]) (Fig. 1B, P = 0.007). The CIs of aGvHD were similar between maternal and collateral related donors with whether ATG-based regimen (Fig. 1C, P = 0.484 for grade II-IV aGvHD; Fig. 1D, P = 0.245 for grade III-IV aGvHD) or low-dose ATG/PTCy-based regimen (Fig. 1E, P = 0.651 for grade II-IV aGvHD; Fig. 1F, P = 0.806 for grade III-IV aGvHD).
Figure 1.
Cumulative incidences (CIs) of acute graft-versus-host disease (aGvHD) with maternal/collateral donors. (A) CIs of grade II-IV aGvHD between the ATG-based and low-dose ATG/PTCy-based groups. (B) CIs of grade III-IV aGvHD between the ATG-based and low-dose ATG/PTCy-based groups. (C) CIs of grade II-IV aGvHD between maternal and collateral donors in the ATG-based group. (D) CIs of grade III-IV aGvHD between maternal and collateral donors in the ATG-based group. (E) CIs of grade II-IV aGvHD between maternal and collateral donors in the low-dose ATG/PTCy-based group. (F) CIs of grade III-IV aGvHD between maternal and collateral donors in the low-dose ATG/PTCy-based group. aGvHD: acute graft-versus-host disease; ATG: antithymocyte globulin; PTCy: posttransplant cyclophosphamide.
For patients with ATG-based regimen, the CI of grade II-IV aGvHD was significantly higher with maternal/collateral donors (55.4% [95% CI 36.8%–70.5%]) than that with direct family donors (except mother) (24.1% [95% CI 8.9%–43.4%]) (Fig. S2a, P = 0.002). The CI of grade III-IV aGvHD with maternal/collateral donors was also significantly higher than that with direct family donors (except mother) (31.9% [95% CI 9.9%–56.7%] vs 10.8% [95% CI 0.7%–37.3%], P = 0.013) (Fig. S2b).
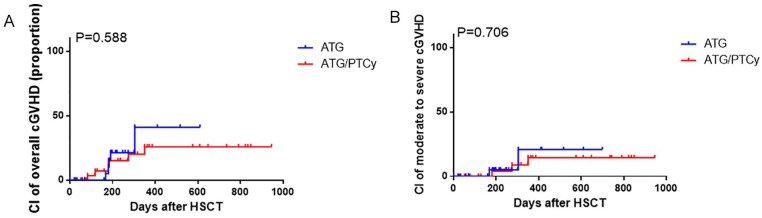
For patients with maternal/collateral related donors, the 1-year CIs of overall cGvHD were 41.0% (95% CI 5.8%–75.7%) in the ATG-based group and 25.8% (95% CI 4.5%–55.4%) in the low-dose ATG/PTCy-based group, respectively (Fig. 2A, P = 0.588). For moderate-to-severe cGvHD, the 1-year CI of 14.5% (95% CI 0.2%–54.3%) in the low-dose ATG/PTCy-based group was similar with that of 20.8% (95% CI 0.1%–72.7%) in the ATG-based group (Fig. 2B, P = 0.706).
Figure 2.
CIs of cGvHD with maternal/collateral donors. (A) CIs of overall cGvHD. (B) CIs of moderate-to-severe cGvHD. aGvHD: acute graft-versus-host disease; ATG: antithymocyte globulin; CI: cumulative incidences; HSCT: hematopoietic stem cell transplantation; PTCy: posttransplant cyclophosphamide.
Infectious Complications
The CIs of CMV viremia within 1 year after transplantation were 66.7% and 51.4% in the ATG-based group and the low-dose ATG/PTCy-based group, respectively (P = 0.235). Twenty-two patients suffered from Epstein–Barr virus (EBV) viremia (35.5%), including 12 in the ATG-based group and 10 in the low-dose ATG/PTCy-based group (P = 0.201). No significant differences were found for infectious pneumonia between the ATG-based and low-dose ATG/PTCy-based groups. Patients in the ATG-based group developed more bacterial or viral enteritis than that in the low-dose ATG/PTCy-based group (P = 0.040) (Table 2).
Table 2.
Infectious Complications.
| Total (n = 62) | ATG (n = 27) | ATG + PTCY (n = 35) | P value | |
|---|---|---|---|---|
| CMV viremia | 36 (58.1%) | 18(66.7%) | 18 (51.4%) | 0.235 |
| CMV disease | 8 (12.9%) | 4(14.8%) | 4 (11.4%) | 0.699 |
| EBV viremia | 22(35.5%) | 12 (44.4%) | 10(28.6%) | 0.201 |
| Hemorrhagic cystitis BK virus related | 16(25.8%) | 9(33.3%) | 7(20%) | 0.241 |
| Pneumonia | ||||
| Bacterial | 10 (16.1%) | 4 (14.8%) | 6 (17.1%) | 0.809 |
| Virus | 6 (9.7%) | 3 (11.1%) | 3 (8.6%) | 0.742 |
| Fungal | 2 (3.2%) | 1 (3.7%) | 1 (2.9%) | 0.855 |
| Pneumocystis carinii pneumonia | 1 (1.6%) | 0 | 1 (2.9%) | 0.384 |
| TB | 1 (1.6%) | 1 (3.7%) | 0 | 0.258 |
| Undefined pneumonia | 1(1.6%) | 1 (3.7%) | 0 | 0.258 |
| Bacterial/viral intestinal infection | 17(27.4%) | 11 (40.7%) | 6(17.1%) | 0.040 |
ATG: antithymocyte globulin; CMV: cytomegalovirus; EBV: Epstein–Barr virus; PTCy: posttransplant cyclophosphamide; TB: tuberculosis.
NRM, Relapse, and Survival
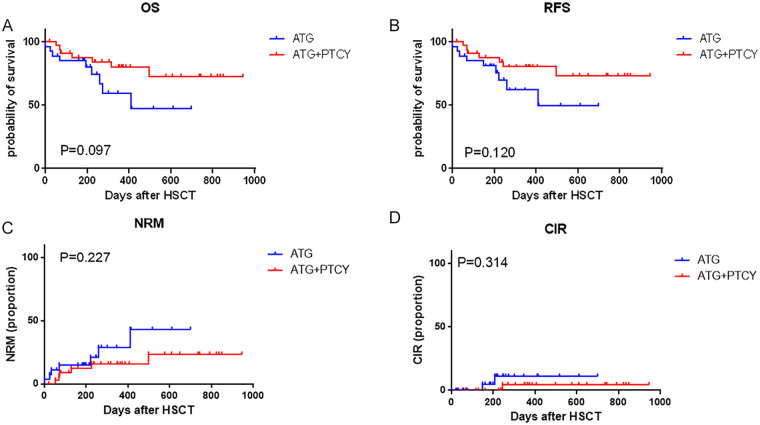
Fourteen patients died of non-relapse causes including seven in the ATG-based group and seven in the low-dose ATG/PTCy-based group. The 1-year NRM were 28.9% (95% CI 5.1%–59.6%) in the ATG-based group and 15.8% (95% CI 1.2%–46.6%) in the low-dose ATG/PTCy-based group, respectively (P = 0.227, Fig. 3C). In the ATG-based group, six patients died of infection and one died from cerebral hemorrhage. In the low-dose ATG/PTCy-based group, three patients died from cerebral hemorrhage, three died of infection, and one died from aGvHD.
Figure 3.
Clinical outcomes of haplo-PBSCT with maternal/collateral donors between the ATG-based and low-dose ATG/PTCy-based groups. (A) Probability of overall survival (OS). (B) Probability of relapse-free survival (RFS). (C) Non-relapse mortality (NRM). (D) Cumulative incidences (CIs) of relapse. ATG: antithymocyte globulin; CIR: Cumulative incidence of relapse; HSCT: hematopoietic stem cell transplantation; NRM: non-relapse mortality; PBSCT: peripheral blood stem cell transplantation; PTCy: posttransplant cyclophosphamide; RFS: relapse-free survival.
All eight patients in active disease status at transplantation achieved CR after transplantation. In the whole cohort, three cases relapsed with a median relapse time of 207 days (range: 148–244 days), including two in the ATG-based group and one in the low-dose ATG/PTCy-based group. Two cases were diagnosed with AML and the other with T cell lymphoblastic lymphoma. All the three cases were in CR status at transplantation and soon died after relapse. The 1-year CIR were 11.1% (95% CI 0.01%–58.4%) in the ATG-based group and 4.5% (95% CI 0.0%–64.7%) in low-dose ATG/PTCy-based group, respectively. (P = 0.314, Fig. 3D).
The 1-year overall survival (OS) and relapse-free survival (RFS) were 72.1% (95% CI 56.8%–82.8%) and 73.3% (95% CI 56.4%–85.3%) for all patients, respectively. In the low-dose ATG/PTCy-based group, the 1-year OS and RFS were 80.0% (95% CI 60.4%–90.6%) and 80.4% (95% CI 61.2%–90.7%), which were higher than that in the ATG-based group with OS of 59.4% (95% CI 32.8%–78.4%) (P = 0.097, Fig. 3A) and RFS of 62.0% (95% CI 36.1%–79.9%) (P = 0.120, Fig. 3B).
Univariate and Multivariate Analysis
The univariate analysis for GvHD, OS, RFS, NRM, and CIR were shown in Table S2–S7. The multivariate analysis (Table 3) revealed that the low-dose ATG/PTCy-based regimen significantly reduced the risk of grade II-IV (HR = 0.357, 95% CI 0.128–0.996; P = 0.049) and grade III-IV aGvHD (HR = 0.190, 95% CI 0.037–0.972; P = 0.046) as an independent risk factor. Higher score of Eastern Cooperative Oncology Group score standard (ECOG), higher index of revised disease risk index (R-DRI), and grade III-IV aGvHD were significantly associated with worse OS, RFS, and NRM. No significant prognostic factors for relapse were identified in univariate analysis (Table S6) because of only three cases relapsed.
Table 3.
Multivariate Analysis for aGvHD, OS, RFS, and NRM.
| Outcomes | Multivariate analysis | ||
|---|---|---|---|
| Hazard ratio | 95% CI | P value | |
| Grade II-IV aGvHD | |||
| ATG/PTCy vs ATG | 0.357 | 0.128–0.996 | 0.049 |
| Grade III-IV aGvHD | |||
| ATG/PTCy vs ATG | 0.190 | 0.037–0.972 | 0.046 |
| OS | |||
| ECOG | 6.376 | 1.908–21.310 | 0.003 |
| R-DRI | |||
| High risk vs Low/intermediate risk | 7.498 | 1.448–38.828 | 0.016 |
| Grade III-IV aGvHD vs grade 0-I aGvHD | 12.492 | 1.261–123.746 | 0.031 |
| RFS | |||
| ECOG | 5.393 | 2.323–12.518 | <0.001 |
| R-DRI | |||
| High risk vs Low/intermediate risk | 6.738 | 1.643–27.641 | 0.008 |
| Grade III-IV aGvHD vs grade 0-I aGvHD | 14.132 | 1.422–140.442 | 0.024 |
| NRM | |||
| ECOG | 15.664 | 1.904–128.839 | 0.010 |
| R-DRI | |||
| High risk vs Low/intermediate risk | 12.826 | 1.174–140.162 | 0.037 |
| Grade III-IV aGvHD vs grade 0-I aGvHD | 60.148 | 1.678–2156.401 | 0.025 |
aGvHD: acute graft-versus-host disease; OS: overall survival; RFS: relapse-free survival: NRM: non-relapse mortality; ATG: antithymocyte globulin; PTCy: posttransplant cyclophosphamid; ECOG: Eastern Cooperative Oncology Group score standard; R-DRI: revised disease risk index.
Discussion
In the present study, the data showed that the low-dose ATG/PTCy-based regimen significantly decreased the CIs of grade II-IV and grade III-IV aGvHD from maternal /collateral donors as compared with ATG-based regimen and did not exacerbate the risk of relapse. The results suggested that the low-dose ATG/PTCy-based regimen could significantly mitigate the high risk of GvHD from maternal/collateral donor transplantation.
Compared with other ATG-based or PTCy-based regimens for haploidentical transplantation from maternal/collateraldonors6–8,29, our low-dose ATG/PTCy-based regimen could more efficiently lower the CIs of grade II-IV (17.7%) and grade III-IV aGvHD (6.8%). Wang et al.30 reported that ATG-based regimen (ATG 10 mg/kg) combined with low-dose PTCy (29 mg/kg) could significantly decreased the CIs of acute and chronic GvHD and NRM with maternal/collateral donors, while the 1-year CIs of relapse and overall survival were similar with our results. The CIs of grade II-IV aGvHD, overall and moderate-to-severe cGvHD from the present study were similar with that from Wang’s study (grade II-IV:17.7% vs 26%, overall cGvHD: 25.8% vs 30%, moderate-to-severe cGvHD: 14.5% vs 17%, respectively). The dosage of ATG in our regimen was only half of that in Wang’s study. The larger dosage of ATG might cause more deep immunosuppression and later immune reconstitution, which could result in higher incidences of virus reactivation and infections13,14.
Our data were consistent with the results from previous reports about the higher incidences of grade II-IV aGvHD with ATG-based regimen for maternal/collateral donor transplantation6,8. According to a study with 990 patients who received a haplo-SCT with PTCy, the 100-day CIs of grade II-IV and III-IV aGvHD were 22% and 6% for the whole population. In this study, parent donors, particularly mothers, are associated with worse progression (PFS) and GvHD-relapse-freesurvival6. However, other studies found that maternal donors were not associated with poorer outcomes31,32. Some studies did not show that the collateral donors could increase the risk of GvHD. Ye et al.33 reported that in TCR haplo-HSCT with ATG-based regimen for GvHD prophylaxis, the incidences of grade II-IV aGvHD had no differences between first-degree related donors (including mother donors) and non-first-degree related donors. But there were more male donors in non-first-degree related donors (NFDs) than that in first-degree related donors (FDs) (NFD, 82% vs FD, 61%; P = 0.04), which would decrease the incidences of GvHD with NFDs because female donor to male recipient was an important risk factor for GvHD34. Elmariah et al.35 reported that in TCR haploidentical bone marrow transplantation (haplo-BMT) from NFDs (collateral donors of 85%, grandchildren donors of 15%) with PTCy-based regimen for GvHD prophylaxis, the incidence of grade II-IV was only 24% within 180 days after transplantation and only one patient experienced grade III-IV. But non-myeloablative pre-conditioning (NAC) regimen and bone marrow graft were used in the study. NAC regimen and bone marrow graft were protective factors for aGvHD36, which might lower the incidence of aGvHD in the study. These results further suggested that the low-dose ATG/PTCy-based regimen could mitigate the risk of GvHD after haplo-PBSCT with maternal/collateral donors.
When GvHD was well prevented, relapse after haplo-HSCT might be one of the most important concerns. Several clinical trials had proved that the addition of ATG to classical GvHD prophylaxis regimens did not affect the risk of relapse37,38. Although high-dose PTCy might be associated with a higher rate of relapse3, the EBMT group confirmed that standard-dose PTCy did not increase relapse risk39,40. In the present study, there were no significant differences of 1-year CIR between ATG-based and low-dose ATG/PTCy-based group (11.1% vs 4.5%, P = 0.314). No significant risk factors were found for relapse; because there were only three patients (3/62) relapsed after transplantation. Compared with ATG-based regimen, the low-dose ATG/PTCy-based regimen did not increase the relapse risk after haplo-PBSCT with maternal/collateral donors.
In our study, the 1-year OS and RFS were higher in the low-dose ATG/PTCy-based group compared with the ATG-based group for patients with maternal/collateral donor transplantation (OS:80.0% vs 59.4%, P = 0.097; RFS:80.4% vs 62.0%, P = 0.120, respectively). The 1-year RFS were greater than OS because all relapsed patients died. Previous research suggested that the survival of transplantation with maternal or collateral donors was worse than that with direct family donors in haplo-PBSCT, owing to the higher probability of GvHD and NRM9. For children with acute leukemia, avoiding of mother donors might improve the outcomes of Haplo-HSCT41. Grade III-IV aGvHD and generalized cGvHD were reported to be the most important factors affecting the survival and were the major causes of NRM42. In our study, the 1-year NRM was relatively higher in the ATG-based group compared with that in the low-dose ATG/PTCy-based group, while there was no statistical difference (28.9% vs 15.8%, P = 0.227). In the low-dose ATG/PTCy-based group, three patients died of infection, and one died from aGvHD. Compared with the ATG-based prophylaxis, the PTCy-based regimen was with lower virus infection in the haplo-SCT43. Our results showed that CMV and EBV reactivation were relatively lower in the low-dose ATG/PTCy-based group than that in the ATG-based group (CMV: 51.4% vs 66.7%, EBV: 28.6% vs 44.4%). Nevertheless, the ATG-based group was with significantly higher bacterial/viral Intestinal infection than the low-dose ATG/PTCy-based group (40.7% vs 17.1%, P = 0.040). Therefore, the low-dose ATG/PTCy-based regimens could decrease the CIs of aGvHD, further to improve the prognosis of patients after haplo-PBSCT with maternal or collateral donors.
In conclusion, compared with the ATG-based regimen, the low-dose ATG/PTCy-based regimen for GvHD prophylaxis could obviously decrease the incidences of grade II-IV and III-IV aGvHD after haplo-SCT with maternal/collateral donors, further to improve the prognosis without increasing the relapse risk. However, there were several limitations in this study, such as the limited number of samples and the shorter follow-up time. Therefore, we should go further studies with larger sample size and longer follow-up time to confirm the superior efficacies of the low-dose ATG/PTCy regimen after haplo-PBSCT with maternal /collateral donors.
Supplemental Material
Supplemental material, sj-docx-1-cll-10.1177_09636897221139103 for Low-Dose Anti-Thymocyte Globulin Plus Low-Dose Posttransplant Cyclophosphamide as an Effective Regimen for Prophylaxis of Graft Versus Host Disease After Haploidentical Peripheral Blood Stem Cell Transplantation With Maternal/Collateral Related Donors by Ting Li, Qiaomei He, Jun Yang, Yu Cai, Chongmei Huang, Xiaowei Xu, Huiying Qiu, Jiahua Niu, Kun Zhou, Yin Zhang, Xinxin Xia, Yu Wei, Chang Shen, Xueying Ding, Yin Tong, Liping Wan and Xianmin Song in Cell Transplantation
Supplemental material, sj-tif-2-cll-10.1177_09636897221139103 for Low-Dose Anti-Thymocyte Globulin Plus Low-Dose Posttransplant Cyclophosphamide as an Effective Regimen for Prophylaxis of Graft Versus Host Disease After Haploidentical Peripheral Blood Stem Cell Transplantation With Maternal/Collateral Related Donors by Ting Li, Qiaomei He, Jun Yang, Yu Cai, Chongmei Huang, Xiaowei Xu, Huiying Qiu, Jiahua Niu, Kun Zhou, Yin Zhang, Xinxin Xia, Yu Wei, Chang Shen, Xueying Ding, Yin Tong, Liping Wan and Xianmin Song in Cell Transplantation
Supplemental material, sj-tif-3-cll-10.1177_09636897221139103 for Low-Dose Anti-Thymocyte Globulin Plus Low-Dose Posttransplant Cyclophosphamide as an Effective Regimen for Prophylaxis of Graft Versus Host Disease After Haploidentical Peripheral Blood Stem Cell Transplantation With Maternal/Collateral Related Donors by Ting Li, Qiaomei He, Jun Yang, Yu Cai, Chongmei Huang, Xiaowei Xu, Huiying Qiu, Jiahua Niu, Kun Zhou, Yin Zhang, Xinxin Xia, Yu Wei, Chang Shen, Xueying Ding, Yin Tong, Liping Wan and Xianmin Song in Cell Transplantation
Footnotes
Author Contributions: This study was conceived and designed by Song X; LiTand He Q analyzed and interpreted the data and wrote the manuscript; Yang J, Cai Y, Huang C, Xu X, Qiu H, Niu J, Zhou K, Zhang Y, Xia X, Wei Y, Shen C, Ding X, Tong Y, and Wan L took care of patients in clinical practice. All authors read and approved the final manuscript.
Ethical Approval: This study was approved by the Ethics Committee of the coordinating institution of Shanghai General Hospital (Code: 2022KY023)
Statement of Human and Animal Rights: This study was approved by the Ethics Committee of the coordinating institution of Shanghai General Hospital (Code: 2022KY023). Informed consent was waived because we used data sets provided by Shanghai General Hospital.
Statement of Informed Consent: Informed consent was waived because we used data sets provided by Shanghai General Hospital.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by a 3-year development project from Shanghai Shen Kang Hospital Development Center (SHDC2020CR1012B, 16CR1010A for Xianmin Song, SHDC12018X09 for Jun Yang); Clinical Research Innovation Plan of Shanghai General Hospital (CTCCR-2018BP03 for Jun Yang); Medical Guidance Project of Science and Technology Commission of Shanghai Municipality (18411968400 for Jun Yang); Clinical Research Special General Project of Shanghai Municipal Health and Family Planning Commission (201840043for Jun Yang); and National Clinical Research Center for Hematologic Disease (2020ZKPC02 for Xianmin Song).
ORCID iDs: Liping Wan  https://orcid.org/0000-0001-6868-0258
https://orcid.org/0000-0001-6868-0258
Xianmin Song  https://orcid.org/0000-0003-0637-5324
https://orcid.org/0000-0003-0637-5324
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical bloodor marrow transplantation. Nat Rev Clin Oncol. 2016;13:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apperley J, Niederwieser D, Huang XJ, Nagler A, Fuchs E, Szer J, Kodera Y. Haploidentical hematopoietic stem cell transplantation: a global overview comparing Asia, the European Union, and the United States. Biol Blood Marrow Transplant. 2016;22:23–26. [DOI] [PubMed] [Google Scholar]
- 3. Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, et al. HLA haploidentical bone marrow transplantation for hematologic malignancies usingnonmyeloablative conditioning and high-dose, post transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCurdy SR, Luznik L. Post-transplantation cyclophosphamide for chimerism-based tolerance. Bone Marrow Transplant. 2019;54(Suppl 2):769–74. [DOI] [PubMed] [Google Scholar]
- 5. Lv M, Chang YJ, Huang XJ. Update of the “Beijing Protocol” haplo-identical hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54(Suppl 2):703–707. [DOI] [PubMed] [Google Scholar]
- 6. Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH, Chen H, Han W, Chen Y-H, Wang F-R, Wang J-Z, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124:843–50. [DOI] [PubMed] [Google Scholar]
- 7. Mariotti J, Raiola AM, Evangelista A, Carella AM, Martino M, Patriarca F, Risitano A, Bramanti S, Busca A, Giaccone L, Brunello L, et al. Impact of donor age and kinship on clinical outcomes after T-cell-replete haploidentical transplantation with PT-Cy. Blood Adv. 2020;4:3900–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mo X-D, Zhang Y-Y, Zhang X-H, Xu L-P, Wang Y, Yan C-H, Chen H, Chen Y-H, Chang Y-J, Liu K-Y, Huang X-J. The role of collateral related donors in haploidentical hematopoietic stem cell transplantation. Science Bulletin. 2018;63:1376–82. [DOI] [PubMed] [Google Scholar]
- 9. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, Lai Y-R, Liu D-H, Liu Q-F, Liu T, Ren H-Y, et al. The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol. 2021;14:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Randolph SSB, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donorscontribute to a selective graft-versus-leukemiaeffect in male recipients of HLA-matched, relatedhematopoietic stem cell transplants. Blood. 2004;103:347–52. [DOI] [PubMed] [Google Scholar]
- 11. Vogt MH, van den Muijsenberg JW, Goulmy E, Spierings E, Kluck P, Kester MG, van Soest RA, Drijfhout JW, Willemze R, Frederik Falkenburg JH. The DBY gene codes for an HLA-DQ5-restricted human male-specific minor histocompatibility antigen involved in graft-versus-host disease. Blood. 2002;99:3027–32. [DOI] [PubMed] [Google Scholar]
- 12. Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Post-transplantation cyclophosphamide prevents graft-versus-hostdisease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest. 2019;130:2357–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Jiang J, Cai Y, Li S, Wan L, Zhu J, Liu H, Shao S, Bai H, Wang C, Song X. Low-dose antithymocyte globulin plus low-dose posttransplant cyclophosphamideas graft-versus-host disease prophylaxis in haploidentical peripheral blood stem cell transplantation combined withunrelated cord blood for patients with hematologic malignancies: a prospective, phase II study. Bone Marrow Transplant. 2019;54:1049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu X, Yang J, Cai Y, Li S, Niu J, Zhou K, Jiang Y, Xu X, Shen C, Huang C, Qiu H, et al. Low dose antithymocyteglobulin with low dose posttransplant cyclophosphamide (low dose ATG/PTCy) can reduce the risk of graft versus-host disease as compared with standard-dose anti-thymocyteglobulin in haploidentical peripheral hematopoietic stem celltransplantation combined with unrelated cord blood. Bone Marrow Transplant. 2021;56:705–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun X, Yang J, Cai Y, Wan L, Huang C, Qiu H, Tong Y, Xu X, Zhou K, Ding X, Song X. Low-dose antithymocyte globulin plus low-dose posttransplant cyclophosphamide combined with cyclosporine and mycophenolate mofetil for prevention of graft-versus-host disease after HLA-matched unrelated donor peripheral blood stem cell transplantation. Bone Marrow Transplant. 2021;56(10):2423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, Rhubart P, Cowan K, Piantados S, Fuchs EJ. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using post transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–86. [DOI] [PubMed] [Google Scholar]
- 17. Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, Fujioka T, Tamaki H, Ikegame K, Okada M, Soma T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47(4):508–15. [DOI] [PubMed] [Google Scholar]
- 18. Chang YJ, Luznik L, Fuchs EJ, Huang XJ. How do we choose the best donor for T-cell-replete, HLA-haploidentical transplantation? J Hematol Oncol. 2016;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperley J, Slavin S, Pasquini M, Sandmaier BM, Barrett J, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinelli G, Trabetti E, Farabegoli P, Testoni N, Bandini G, Motta MR, Vittone A, Terragna C, Pignatti PF, Tura S. Early detection of bone marrow engraftment by amplification of hypervariable DNA regions. Haematologica. 1997;82(2):156–60. [PubMed] [Google Scholar]
- 21. Bacigalupo A. Hematopoietic stem cell transplants after reducedintensity conditioning regimen (RI-HSCT): report of a workshop of the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2000;25(8):803–805. [DOI] [PubMed] [Google Scholar]
- 22. Jiang Y, Wan LP, Qin YW, Wang XR, Yan SK, Xie KC, Wang C. Chimerism status is correlated to acute graft-versus-host disease after allogeneic stem cell transplantation. Int J Hematol. 2014;99(3):323–28. [DOI] [PubMed] [Google Scholar]
- 23. Qin YW, Jiang Y, Wang XR, Wan LP, Yan SK, Wang C. Comparison of STR-PCR and FISH value for monitoring chimerism after sex-mismatched allogeneic hematopoietic stem cell transplantation. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;17:1016–20. [PubMed] [Google Scholar]
- 24. El-Cheikh J, Vazquez A, Crocchiolo R, Furst S, Calmels B, Castagna L, Lemarie C, Granata A, Ladaique P, Oudin C, Faucher C, et al. Acute GVHD is a strong predictor of full donor CD3+ T cell chimerism after reduced intensity conditioning allogeneic stem cell transplantation. Am J Hematol. 2012;87(12):1074–78. [DOI] [PubMed] [Google Scholar]
- 25. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–28. [PubMed] [Google Scholar]
- 26. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, et al. National Institutes of Health consensus developmentproject on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP, Ritz J, Sorror ML, Lee SJ, Joachim Deeg H, Storer BE, et al. A disease riskindex for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Chen H, Chen J, Han M, Hu J, Jiong H, Huang H, Lai Y, Liu D, Liu Q, Liu T, et al. The consensus on themonitoring, treatment, and prevention of leukemia relapse after allogeneic chematopoietic stem cell transplantation in China. Cancer Lett. 2018;438:63–75. [DOI] [PubMed] [Google Scholar]
- 29. Xu LP, Wang SQ, Ma YR, Gao SJ, Cheng YF, Zhang YY, Mo W-J, Mo X-D, Zhang Y-P, Yan C-H, Chen Y-H, et al. Who is the best haploidentical donor for acquired severe aplastic anemia? Experience from a multicenter study. J Hematol Oncol. 2019;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Wu DP, Liu QF, Xu LP, Liu KY, Zhang XH, Yu W-J, Xu Y, Huang F, Huang X-J. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol. 2019;12:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Rood JJ, Loberiza FR, Jr, Zhang MJ, Oudshoorn M, Claas F, Cairo MS, Champlin RE, Peter Gale R, Ringdén O, Hows JM, Horowitz MH. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease afterbone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–77. [DOI] [PubMed] [Google Scholar]
- 32. Ichinohe T, Uchiyama T, Shimazaki C, Matsuo K, Tamaki S, Hino M, Watanabe A, Hamaguchi M, Adachi S, Gondo H, Uoshima N, et al. ; Japanese Collaborative Study Group for NIMA-Complementary Haploidentical Stem Cell Transplantation. Feasibility of HLA-haploidentical hematopoietic stem cell transplantation between noninherited maternal antigen (NIMA)-mismatched family memberslinked with long-term fetomaternalmicrochimerism. Blood. 2004;104:3821–28. [DOI] [PubMed] [Google Scholar]
- 33. Ye Y, Wang M, Malard F, Shi J, Lu Y, Ouyang G, Lan J, Tan Y, Zhao Y, Yu J, Lai X, et al. Comparison of non-first-degree related donors and first-degree related donors in haploidentical HSCT: a multi-centre retrospective analysis. Bone Marrow Transplant. 2021;56(10):2567–74. [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Wu DP, Liu QF, Xu LP, Liu KY, Zhang XH, Xu Y, Huang F, Huang XJ. Donor and recipient age, gender and ABO incompatibility regardless of donor source: validated criteria for donor selection for haematopoietic transplants. Leukemia. 2018;32(2):492–98. [DOI] [PubMed] [Google Scholar]
- 35. Elmariah H, Kasamon YL, Zahurak M, Macfarlane KW, Tucker N, Rosner GL, Bolaños-Meade J, Fuchs EJ, Wagner-Johnston N, Swinnen LJ, Huff CA, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide using non-first-degree related donors. Biol Blood Marrow Transplant. 2018;24(5):1099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C, Ciurea SO, Fasan O, Gaballa S, Hamadani M, Munshi P, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chang YJ, Wu DP, Lai YR, Liu QF, Sun YQ, Hu J, Hu Y, Zhou J-F, Li J, Wang S-Q, Li W, et al. Antithymocyte globulin for matched sibling donor transplantation in patients with hematologic malignancies: a multicenter, open-label, randomized controlled study. J Clin Oncol. 2020;38:3367–76. [DOI] [PubMed] [Google Scholar]
- 38. Kröger N, Solano C, Bonifazi F. Antilymphocyte globulin forchronic graft-versus-host disease. N Engl J Med. 2016;374:1894–95. [DOI] [PubMed] [Google Scholar]
- 39. Santoro N, Ruggeri A, Labopin M, Bacigalupo A, Ciceri F, Gülbaş Z, Huang H, Afanasyev B, Arcese W, Wu D, Koc Y, et al. Unmanipulated haploidentical stem cell transplantation in adults with acute lymphoblastic leukemia: a study on behalf of the Acute Leukemia Working Party of the EBMT. J Hematol Oncol. 2017;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rocha V, Arcuri LJ, Seber A, Colturato V, Zecchin VG, Kuwahara C, Nichele S, Gouveia R, Fernandes JF, Macedo AV, Tavares R, et al. Impact of mother donor, peripheral blood stem cells and measurable residual disease on outcomes after haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide in children with acute leukaemia. Bone Marrow Transplant. 2021;56(12):3042–48. [DOI] [PubMed] [Google Scholar]
- 41. Robinson TM, O’Donnell PV, Fuchs EJ, Luznik L. Haploidentical bone marrow and stem cell transplantation: experience with post transplantation cyclophosphamide. Semin Hematol. 2016;53(2):90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, Thomas ED, Hansen JA. Effect of HLAincompatibility on graft-versus -host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29:79–91. [DOI] [PubMed] [Google Scholar]
- 43. Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, Zoellner AK, Bücklein V, Reibke R, Mumm F, Rieger CT, et al. Virus infection in HLA-haploidentical hematopoietic stem cell transplantation: incidence in the context of immune recovery in two different transplantation settings. Ann Hematol. 2015;94(10):1677–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cll-10.1177_09636897221139103 for Low-Dose Anti-Thymocyte Globulin Plus Low-Dose Posttransplant Cyclophosphamide as an Effective Regimen for Prophylaxis of Graft Versus Host Disease After Haploidentical Peripheral Blood Stem Cell Transplantation With Maternal/Collateral Related Donors by Ting Li, Qiaomei He, Jun Yang, Yu Cai, Chongmei Huang, Xiaowei Xu, Huiying Qiu, Jiahua Niu, Kun Zhou, Yin Zhang, Xinxin Xia, Yu Wei, Chang Shen, Xueying Ding, Yin Tong, Liping Wan and Xianmin Song in Cell Transplantation
Supplemental material, sj-tif-2-cll-10.1177_09636897221139103 for Low-Dose Anti-Thymocyte Globulin Plus Low-Dose Posttransplant Cyclophosphamide as an Effective Regimen for Prophylaxis of Graft Versus Host Disease After Haploidentical Peripheral Blood Stem Cell Transplantation With Maternal/Collateral Related Donors by Ting Li, Qiaomei He, Jun Yang, Yu Cai, Chongmei Huang, Xiaowei Xu, Huiying Qiu, Jiahua Niu, Kun Zhou, Yin Zhang, Xinxin Xia, Yu Wei, Chang Shen, Xueying Ding, Yin Tong, Liping Wan and Xianmin Song in Cell Transplantation
Supplemental material, sj-tif-3-cll-10.1177_09636897221139103 for Low-Dose Anti-Thymocyte Globulin Plus Low-Dose Posttransplant Cyclophosphamide as an Effective Regimen for Prophylaxis of Graft Versus Host Disease After Haploidentical Peripheral Blood Stem Cell Transplantation With Maternal/Collateral Related Donors by Ting Li, Qiaomei He, Jun Yang, Yu Cai, Chongmei Huang, Xiaowei Xu, Huiying Qiu, Jiahua Niu, Kun Zhou, Yin Zhang, Xinxin Xia, Yu Wei, Chang Shen, Xueying Ding, Yin Tong, Liping Wan and Xianmin Song in Cell Transplantation