Abstract
Background
Flow-diverting (FD) stents, with or without coiling, are a mainstay in endovascular treatment of intracranial aneurysms (IAs). One observed complication from flow diverter stent (FDS) insertion has been in-stent stenosis. Though previously studied in the short-term period, the long-term history of this complication has yet to be described.
Methods
We performed a retrospective cohort study of consecutive IAs treated with Pipeline Embolization Device (PED), with or without coiling, at our centre between September 2014 and December 2018 that had at least one digital subtraction angiogram (DSA) during follow-up. In-stent stenosis was measured from DSA images, and associated patient and procedural characteristics were analysed.
Results
94 patients treated with PED for IA were identified. On initial DSA during follow-up, 52 patients (55.3%) had in-stent stenosis within the PED. Of these 52 patients, 17 had a second DSA during follow-up. In this 2nd DSA, improvement and/or stable in-stent stenosis was seen 16 patients (94.1%). One patient in this group had worsening in-stent stenosis had a vertebrobasilar junction FD stent. Of the patients without in-stent stenosis on initial DSA, 15 had a second DSA during follow-up. Only one of these patients (6.7%) had new appearance of in-stent stenosis (measuring 5%). Multivariate analysis found statin use to be predictive of in-stent stenosis (p = 0.020, Odds ratio = 0.279 and 95% confidence interval = 0.095–0.821).
Conclusions
In-stent stenosis after FDS placement was seen in 53.2% of cases, which had between 1–50% of stenosis. 82.4% had resolution/improvement of their stenosis. Statin use was protective of in-stent stenosis.
Keywords: angiography, intracranial aneurysm, flow diverter stent, in-stent stenosis
Introduction
Flow diverters (FD) have dramatically changed the treatment paradigm of intracranial aneurysms (IAs), while traditionally managed with surgical or coil-based endovascular techniques,
FD offer an endovascular option to treat wide neck and large/giant aneurysms effectively with low complication rates.1,2 In a survey of members of the Society of NeuroInterventional Surgery, Society of Vascular and Interventional Neurology, and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Combined Cerebrovascular Section, 51% of neurointerventionalists indicated FD as the preferred treatment of wide-necked Internal Carotid Artery (ICA) aneurysms. 3
In the U.S. the Pipeline Embolization Device (PED; Medtronic, Irvine, California, USA) remains the most commonly used FD for the treatment of IAs. Multiple prospective studies, registries, and retrospective analyses have demonstrated high occlusion rates with low morbidity and mortality for a wide range of aneurysms, including small, medium, large/giant unruptured aneurysms, distal, blister and ruptured aneurysms.4–8 Analyses of complications associated with the use of FD often focus on the rates of ischemic and hemorrhagic events, mortality, technical failures such poor device opening, migration or removal, and need for additional treatments.9,10 In-stent stenosis within FD is an angiographic phenomenon that is poorly understood and rarely described in the literature. Mühl-Benninghaus et al. in a study of 18 patients treated with FD observed about 30% in-stent stenosis in all 18 patients on short-term follow-up, which improved to 12% on long-term follow-up without any associated neurologic complications. 11 A retrospective study of 36 patients treated with SILK FD by Essbaiheen et al. reported a 44% incidence of in-stent stenosis. Sixteen of these patients had early angiographic evidence of in-stent stenosis while 11 patients had complete resolution of this stenosis at long-term follow up with no further de novo in-stent stenosis formation, and no clinical sequalae. 12 Wang et al. described the largest cohort of in-stent stenosis; of 118 patients evaluated by digital subtraction angiogram (DSA) 6–12 months from implantation of PED, 6 had in-stent stenosis (5%). 13 Factors associated with this mainly transient imaging finding remain unknown. The goal of our investigation was to analyse this phenomenon by looking at incidence and interval progression of this angiographic finding, its associated clinical outcomes, and identify possible demographic, morphological, clinical, or procedural elements associated with in-stent stenosis.
Materials and methods
Patient selection and data collection
This study was approved by the local institutional review board at our centre for retrospective data collection and review. Consecutive cases of IAs treated with FD between September 2014 and December 2018 were included in this study if they had at least DSA during follow-up. Aneurysm treatment modality was based on operator preference, choice, and number of devices. For elective cases, a typical antiplatelet regimen consisted of dual-antiplatelet therapy for the first six months (aspirin 325mg once-per-day and clopidgrel 75mg once-per-day), followed by long-term use of a single agent (aspirin 81mg) though with appropriate changes to ticagrelor/prasugrel or less frequent clopidogrel dosing based on platelets reactivity unit (PRU) as described by Kim, et al. 14 In all participating centres, patients were tested for antiplatelet drug response using VerifyNow (Accumetrics, San Diego, California, USA) before PED implantation. PRU goals were between 60 and 200. A variety of treatment approaches including the use of IIb/IIIa agents, and different loading doses of antiplatelet agents, were utilized in cases of ruptured aneurysms. All ruptured aneurysms were treated within 24 h from initial evaluation. Most of the ruptured aneurysm cases were initially coiled with adequate aneurysm dome protection, except for five cases of blister-like aneurysms that were treated with a FD. Of those ruptured aneurysms that were initially coiled, the FD device was subsequently placed 2 weeks after initial treatment.
Electronic medical records were reviewed for demographic information including age, sex, and past medical history. Past medical history included history of hypertension, diabetes, dyslipidemia, coronary artery disease, atrial fibrillation, smoking status, prior cerebrovascular accident, and chronic kidney disease. This information was collected by International Classification of Diseases, Tenth Revision codes. As well, medication history was reviewed for a history of statin therapy. Associated procedural complications, mortality at 30 days, and final follow-up information was obtained.
The DSA images were analysed by an independent operator who was blinded to treatment outcomes. Aneurysm location, presence of residual aneurysm opacification on follow up imaging using the Raymon-Roy Occlusion Classification, 15 and the degree of in-stent stenosis were collected (Figure 1). In-stent stenosis was obtained on the lateral projection for anterior circulation aneurysms and anterior-posterior (AP) projection for posterior circulation aneurysms. Percent of in-stent stenosis was defined as (1-[estimated diameter of the most stenotic segment with digital subtraction divided by the estimated diameter of stent measured on without digital subtraction]) x100.
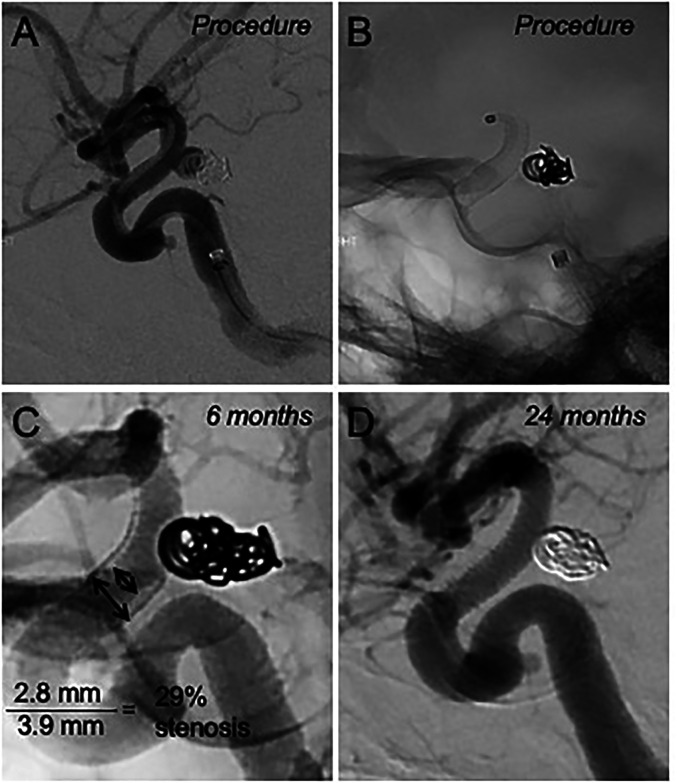
Figure 1.
A case example of in-stent stenosis. A. Digital subtraction angiography (DSA) and B. single fluoroscopy image, lateral projections, ICA occlusion. 4.0 × 16 mm PED was used to treat recurrence of a previously coiled P-comm aneurysm. C. Follow up DSA performed 6 months after the initial procedure. Unsubtracted view, lateral projection, showing 29% in-stent stenosis. The patient was started on a high dose statin. D. Repeat DSA at 24 months from the index procedure, lateral view, demonstrating interval resolution of stenosis.
Statistical analysis
Data entry and statistical analysis were performed using IBM SPSS Statistics Version 25 (IBM Corp., Armonk, NY). A p-value of less than 0.05 was considered to indicate statistical significance. Differences in continuous variables were compared using Student’s t-tests or Welch’s t-tests. Differences in categorical variables were compared using Chi-square tests. Stepwise logistic regression models were built in an attempt to model the odds of in-stent thrombosis and find predictive factors.
Results
Demographic variables and results of FDS treatment
A total of 94 patients had IA treated with PED. Of these, all had at least one diagnostic follow-up DSA, therefore no patients were excluded by this criterion. Demographic and clinical information is summarized in Table 1. The median age was 59 years (interquartile range, 48.5–66.5 years). 76 of 94 patients were females (80.9%). There were 65 unruptured aneurysms (69.1%) and 29 ruptured aneurysms (30.1%) in our cohort. The location of aneurysm was more often in the anterior circulation (90.4%). The initial follow-up DSA was obtained a median six months after treatment and showed complete aneurysm occlusion in 22 patients (78.6%) treated with PED + coils and in 51 patients (77.3%) treated with PED alone. The overall rate of residual aneurysm filling was 22.3% at six months. Of those with a second follow-up imaging modality, it was performed a median of 20 months from treatment, and consisted of DSA in 32% and magnetic resonance angiography (MRA) in 68% of cases, respectively. All 21 patients with residual aneurysm seen at initial follow-up DSA had a second DSA; only 8 patients (8.5%) remained with residual aneurysm. At the end of follow-up, the overall aneurysm occlusion rate was 91.5%; these 8 patients (8.5%) were retreated with a second PED.
Table 1.
Demographic information on the study population.
| No in-stent stenosis (N = 42) (%) |
In-stent stenosis (N = 52) (%) | p-value | |
|---|---|---|---|
| Age at PED Placement; Mean ± 2SE | 61.0 ± 3.15 | 54.7 ± 3.49 | 0.011* |
| Sex | 0.583 | ||
| Female | 35 (83.3) | 41 (78.8) | |
| Male | 7 (16.7) | 11 (21.2) | |
| Comorbidities | |||
| Diabetes | 6 (14.3) | 4 (7.7) | 0.334 |
| Hypertension | 19 (45.2) | 28 (53.8) | 0.407 |
| Hyperlipidemia | 17 (40.5) | 9 (17.3) | 0.013* |
| Statin Use | 20 (47.6) | 9 (17.3) | 0.002* |
| Chronic Kidney Disease | 0 (0.0) | 1 (1.9) | 1.00 |
| Coronary Artery Disease | 1 (2.4) | 4 (7.7) | 0.376 |
| Prior Cerebrovascular Accident | 16 (38.1) | 18 (34.6) | 0.727 |
| Atrial Fibrillation | 1 (2.4) | 0 (0.0) | 0.447 |
| Smoking Status | 0.455 | ||
| Current | 17 (40.5) | 27 (51.9) | |
| Former | 10 (23.8) | 8 (15.4) | |
| History of SAH | 0.024* | ||
| None | 34 (81.0) | 31 (59.6) | |
| Acute SAH (0–2 weeks) | 0 (0.0) | 6 (11.5) | |
| 2 + Weeks Ago | 8 (19.0) | 15 (28.8) | |
| Aneurysm Location | 0.623 | ||
| Cervical ICA | 1 (2.4) | 0 (0.0) | |
| Petrous ICA | 0 (0.0) | 1 (1.9) | |
| Cavernous ICA | 4 (9.5) | 5 (9.6) | |
| Paraclinoid ICA | 2 (4.8) | 3 (5.8) | |
| Supraclinoid ICA | 26 (61.9) | 27 (51.9) | |
| Superior Hypophyseal Artery | 0 (0.0) | 3 (5.8) | |
| Posterior Communicating Artery | 6 (14.3) | 4 (7.7) | |
| Terminal ICA | 0 (0.0) | 1 (1.9) | |
| Middle Cerebral Artery | 1 (2.4) | 1 (1.9) | |
| Intradural Vertebral Artery | 0 (0.0) | 4 (7.7) | |
| Basilar Artery | 1 (2.4) | 2 (3.8) | |
| Posterior Cerebral Artery | 1 (2.4) | 1 (1.9) | |
| Anterior circulation | 2 (4.8) | 7 (13.5) | 0.181 |
| Multiple aneurysms | 13 (31.0) | 12 (23.1) | 0.390 |
| Number of pipelines | 0.759 | ||
| 1 | 35 (83.3) | 44 (84.6) | |
| 2 | 6 (14.3) | 8 (15.4) | |
| 3 | 1 (2.4) | 0 (0.0) | |
| Coils used in embolization | 10 (23.8) | 18 (34.6) | 0.255 |
| Pipeline length | 22.4 ± 2.89 | 21.3 ± 2.42 | 0.554 |
| Pipeline diameter | 3.76 ± 0.259 | 3.89 ± 0.157 | 0.361 |
*Indicates significance at the p < 0.05 level.
PED: Pipeline Embolization Device; SE: Standard Error; SAH: Subarachnoid Hemorrhage; ICA: Internal Carotid Artery.
In-stent stenosis comparisons
At the time of initial DSA during follow-up, in-stent stenosis was noted in 52 patients (55.3%) and measured 17% on average. Of the 52 patients with in-stent stenosis on initial DSA, 17 had a second DSA during follow-up. In this 2nd DSA, improvement and/or stable in-stent stenosis was seen 16 patients (94.1%). One patient of the 32 now had in-stent stenosis that was not present initially and, in this patient, the stenotic area continued to worsen until the stent eventually occluded by an unclear mechanism. This patient ultimately succumbed from a stroke secondary to this occlusion 13 months after device placement. This was our only mortality (1.1% of cases).
One other patient who had a 2nd DSA was found to have developed mild (∼5%) in-stent stenosis though no stenosis was seen in their 1st DSA. Sixty-three patients had an MRA at the end of follow-up. 40 of these patients initially had in-stent stenosis – all had patency of their stent on follow-up MRA. Of the 23 patients without in-stent stenosis, 22 continued to show patency (one patient without in-stent stenosis originally was found to have an asymptomatic occlusion of their flow diverter stent (FDS) during follow-up).
Univariate analysis found that older age, history of hyperlipidemia, history of statin use, and lack of acute Subarachnoid Hemorrhage (SAH) were all protective of in-stent stenosis (Table 1). In multivariate logistic regression, the only variable found to be predictive of in-stent stenosis was statin use (p = 0.020, Odds ratio = 0.279 and 95% confidence interval = 0.095–0.821), indicating that it was protective for in-stent stenosis.
Complications
A total of five complications (5.3%) and one death (1.1%) as above described occurred in our cohort. Complications consisted of two patients with transient amaurosis fugax, two with asymptomatic FDS occlusion, and one with a cavernous fistula formation after FDS placement. No statistical difference was seen in comparison between the in-stent and no in-stent groups.
Discussion
Currently, information about in-stent stenosis post-FDS placement in the literature is sparse. The incidence of in-stent stenosis, from mild to severe, has been reported in the wide range of 7% to 93.4% in Pipeline FDS devices.16–20 In-stent stenosis is typically seen at the six-month angiographic follow-up at which time the degree of stenosis of the majority of the reported cases is reported as mild to moderate.18–20 In our cohort, in-stent stenosis was seen in 53 patients (56.4%) in the 1st DSA during follow-up (median of 6 months post-procedure) and 96.2% of the cases were between 1–50% degree of stenosis. The historically reported wide range of incidence could be due to heterogeneity between the studied population and the fact that it might be under-reported.
Long-term clinically significant predictors for in-stent stenosis of FDS have previously been evaluated.12,18–22 Bescke et al. reported one of the highest prevalence of in-stent stenosis in their cohort with 93.4% of the patients having mild stenosis. 17 John et al. and Ravindran et al. observed that 40 to 50% of the patients in their cohorts had in-stent stenosis resolution in their long-term angiographic follow-up, and in both cohorts all patients remained asymptomatic from in-stent stenosis.19,20 In our study, of those with in-stent stenosis that had a 2nd DSA follow-up, 82.4% (14/17) improved or resolved completely, two had stable in-stent stenosis and one as aforementioned had symptomatic complete occlusion. A representative patient with initial in-stent stenosis that resolved can be seen in Figure 1.
Chalouhi et al. reported that lack of aspirin therapy and an internal carotid location were strong independent factors for developing in-stent stenosis. 18 In our statistical analysis, aneurysm location was not associated with development of in-stent stenosis and all patients were maintained on dual antiplatelets medications (aspirin 325mg with clopidogrel 75mg) for at least six months. This difference may be because in Chalouhi et al.’s study, the incidence of in-stent stenosis is substantially lower compared to our cohort, underpowering its statistical analyses. 18 Also, all of our patients were homogenous in respect to antiplatelet medication regimens which may account for these differences. Furthermore, the only variable found to be predictive and protective of in-stent stenosis in our study was statin use. This finding has strong support because of the important role of inflammation in the pathogenesis of in-stent stenosis, and the potential anti-inflammatory effects of statin therapy in intracranial atherosclerotic diseases.23,24
Presently, there is limited evidence in the neurointerventional literature to establish guidelines for the management of in-stent stenosis. In the cardiac literature for percutaneous coronary artery revascularization, the incidence of in-stent stenosis is reported to be about 22–26%, having a clinical impact for major cardiac events.25,26 Patel et al. described the evidence of how the inflammatory cascade of growth factors and cellular proliferation in endothelial remodeling led to intimal hyperplasia post stent placement. 27 Consequently, most coronary artery stent studies have focus on drug eluting stents with immunosuppressive properties for prevention of neointimal hyperplasia.26,28 The in-stent stenosis phenomenon post-FDS placement is not limited to the PED.12,21,22 Although most studies have shown that in-stent stenosis is a benign finding, it is necessary to be cautious with this presumed conclusion because most of the evidence for in-stent stenosis after FDS placement are from retrospective studies and not prospective studies or randomized controlled trials.
Strengths and limitations
Our study has several important limitations. First, this was a retrospective study from a single centre. This resulted in a modest sample size limiting our statistical power and also limiting the generalizability of our results. It is possible that because of this limitation, we were unable to derive additional associations between in-stent stenosis and potentially associated factors. Also, a 2nd DSA during follow-up was only available for 34% of patients in our cohort.
Conclusions
Overall, this study showed that in-stent stenosis post Pipeline FDS placement is a common angiographic finding and was seen in about 53.2% of cases, a majority of which had between 1–50% of stenosis. We observed that in those patients with in-stent stenosis and a 2nd DSA during follow-up, 82.4% had resolution and improvement of their stenosis. Statin use was protective of the development of in-stent stenosis. Further studies are warranted to analyse the cost and efficacy of adding a statin for the prevention of in-stent stenosis.
Acknowledgements
None
Authors disclosures: M. Mokin: Grant National Institutes of Health (NIH) R21NS109575. Consultant; Medtronic, Cerenovus. Stock options: BrainQ, Serenity medical, Synchron, Endostream.
Other Authors: None
Author contributions: GFM, EP, IP, ZR, WG, MM– study concept and design. GFM, EP & MM wrote the manuscript. GFM and EP – statistical analysis. All authors edited the manuscript and approved the final version.
Data sharing statement: Data are available upon reasonable request
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Gabriel Flores-Milan https://orcid.org/0000-0001-8327-0126
Elliot Pressman https://orcid.org/0000-0002-5160-802X
References
- 1.Chancellor B, Raz E, Shapiro M, et al. Flow diversion for intracranial aneurysm treatment: trials involving flow diverters and long-term outcomes. Neurosurgery 2020; 86: S36–S45. 2019/12/16. [DOI] [PubMed] [Google Scholar]
- 2.Vakharia K, Munich SA, Waqas M, et al. Treatment of anterior circulation aneurysms in the internal carotid artery with flow diverters. Neurosurgery 2020; 86: S55–S63. 2019/12/16. [DOI] [PubMed] [Google Scholar]
- 3.Fargen KM, Soriano-Baron HE, Rushing JT, et al. A survey of intracranial aneurysm treatment practices among United States physicians. J Neurointerv Surg 2018; 10: 44–49. 2017/02/12. [DOI] [PubMed] [Google Scholar]
- 4.Hanel RA, Kallmes DF, Lopes DK, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the PREMIER study 1 year results. J Neurointerv Surg 2020; 12: 62–66. 2019/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Primiani CT, Ren Z, Kan P, et al. A2, M2, P2 aneurysms and beyond: results of treatment with pipeline embolization device in 65 patients. J Neurointerv Surg 2019; 11: 903–907. 2019/01/25. [DOI] [PubMed] [Google Scholar]
- 6.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015; 36: 108–115. 2014/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokin M, Chinea A, Primiani CT, et al. Treatment of blood blister aneurysms of the internal carotid artery with flow diversion. J Neurointerv Surg 2018; 10: 1074–1078. 2018/02/27. [DOI] [PubMed] [Google Scholar]
- 8.Becske T, Potts MB, Shapiro M, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg 2017; 127: 81–88. 2016/10/16. [DOI] [PubMed] [Google Scholar]
- 9.Zhou G, Su M, Yin YL, et al. Complications associated with the use of flow-diverting devices for cerebral aneurysms: a systematic review and meta-analysis. Neurosurg Focus 2017; 42: E17. 2017/06/02. [DOI] [PubMed] [Google Scholar]
- 10.Briganti F, Leone G, Marseglia M, et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J 2015; 28: 365–375. 2015/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhl-Benninghaus R, Haussmann A, Simgen A, et al. Transient in-stent stenosis: a common finding after flow diverter implantation. J Neurointerv Surg 2019; 11: 196–199. 2018/07/05. [DOI] [PubMed] [Google Scholar]
- 12.Essbaiheen F, AlQahtani H, Almansoori TM, et al. Transient in-stent stenosis at mid-term angiographic follow-up in patients treated with SILK flow diverter stents: incidence, clinical significance and long-term follow-up. J Neurointerv Surg 2019; 11: 166–170. 2018/09/09. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Richard SA, Jiao H, et al. Institutional experience of in-stent stenosis after pipeline flow diverter implantation: a retrospective analysis of 6 isolated cases out of 118 patients. Medicine (Baltimore) 2021; 100: e25149. 2021/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KS, Fraser JF, Grupke S, et al. Management of antiplatelet therapy in patients undergoing neuroendovascular procedures. J Neurosurg 2018; 129: 890–905. 2017/12/02. [DOI] [PubMed] [Google Scholar]
- 15.Mascitelli JR, Moyle H, Oermann EK, et al. An update to the raymond-Roy occlusion classification of intracranial aneurysms treated with coil embolization. J Neurointerv Surg 2015; 7: 496–502. 2014/06/06. [DOI] [PubMed] [Google Scholar]
- 16.Luo B, Kang H, Zhang H, et al. Pipeline Embolization device for intracranial aneurysms in a large Chinese cohort: factors related to aneurysm occlusion. Ther Adv Neurol Disord 2020; 13: 1756286420967828. 2020/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becske T, Brinjikji W, Potts MB, et al. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery 2017; 80: 40–48. 2017/04/01. [DOI] [PubMed] [Google Scholar]
- 18.Chalouhi N, Polifka A, Daou B, et al. In-pipeline stenosis: incidence, predictors, and clinical outcomes. Neurosurgery 2015; 77: 875–879; discussion 879. 2015/07/23. [DOI] [PubMed] [Google Scholar]
- 19.John S, Bain MD, Hui FK, et al. Long-term follow-up of in-stent stenosis after pipeline flow diversion treatment of intracranial aneurysms. Neurosurgery 2016; 78: 862–867. 2015/11/26. [DOI] [PubMed] [Google Scholar]
- 20.Ravindran K, Salem MM, Enriquez-Marulanda A, et al. Quantitative assessment of in-stent stenosis after pipeline embolization device treatment of intracranial aneurysms: a single-institution series and systematic review. World Neurosurg 2018; 120: e1031–e1040. 2018/09/12. [DOI] [PubMed] [Google Scholar]
- 21.Cohen JE, Gomori JM, Moscovici S, et al. Delayed complications after flow-diverter stenting: reactive in-stent stenosis and creeping stents. J Clin Neurosci 2014; 21: 1116–1122. 2014/02/15. [DOI] [PubMed] [Google Scholar]
- 22.Pérez M A, Bhogal P, Henkes E, et al. In-stent stenosis after p64 flow diverter treatment. Clin Neuroradiol 2018; 28: 563–568. 2017/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah PK. Inflammation, neointimal hyperplasia, and restenosis: as the leukocytes roll, the arteries thicken. Circulation 2003; 107: 2175–2177. 2003/05/07. [DOI] [PubMed] [Google Scholar]
- 24.Kim HJ, Kim EK, Kwon SU, et al. Effect of statin on progression of symptomatic intracranial atherosclerosis. Can J Neurol Sci 2012; 39: 801–806. 2012/10/09. [DOI] [PubMed] [Google Scholar]
- 25.Moussa I, Reimers B, Moses J, et al. Long-term angiographic and clinical outcome of patients undergoing multivessel coronary stenting. Circulation 1997; 96: 3873–3879. 1997/12/24. [DOI] [PubMed] [Google Scholar]
- 26.Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002; 346: 1773–1780. 2002/06/07. [DOI] [PubMed] [Google Scholar]
- 27.Patel SD, Waltham M, Wadoodi A, et al. The role of endothelial cells and their progenitors in intimal hyperplasia. Ther Adv Cardiovasc Dis 2010; 4: 129–141. 2010/03/05. [DOI] [PubMed] [Google Scholar]
- 28.Alfonso F, Byrne RA, Rivero F, et al. Current treatment of in-stent restenosis. J Am Coll Cardiol 2014; 63: 2659–2673. 2014/03/19. [DOI] [PubMed] [Google Scholar]