Abstract
Background and Aim
Distal cerebral circulation aneurysms (DCCAs) remain treatment challenges for neurointervention. The off-label use of the pipeline embolization device (PED) for these aneurysms remains controversial. This study aimed to evaluate the safety and efficacy of PED for DCCAs in a multicenter cohort of patients.
Methods
Between March 2016 and June 2021, we retrospectively analyzed the neurointerventional data on the clinical and radiological records of all patients undergoing PED treatment of DCCAs at three medical centers.
Results
A total of 53 consecutive patients with 53 DCCAs were treated with PED. The mean aneurysm size was 12.3 ± 5.7 mm. In total, 75.4% (40/53) were fusiform and 24.5% (13/53) were saccular. Of these, 17.0% (9/53) were recurrent aneurysms that were previously treated with endovascular or microsurgical approaches. The technical success rate was 100%, among which 81.1% (43/53) procedures were completed with a single PED, and the rest (10/53, 18.8%) required telescoping with two devices. Angiographic follow-up data were available for 51 patients, with a median follow-up time of 12 months. At the latest follow-up, 46/51 (90.2%) aneurysms showed complete obliteration, and 4/51 (7.8%) showed reduced filling. Periprocedural complications such as hemorrhage were observed in two patients with MCA aneurysms (3.8%, 2/53), and ischemic events occurred in six patients (11.3%, 6/53). The overall mortality and morbidity rates were 7% (4/53).
Conclusions
PED is a viable option for treating DCCAs, especially for recurrent aneurysms. Coverage of bifurcation branches and perforator may increase the risk of complications.
Keywords: Embolization, cerebral aneurysm, endovascular, flow diversion, pipeline embolization device
Introduction
The Pipeline Embolization Device (PED; Covidien, Irvine, CA, USA) is a flow-diverting stent approved for treating unruptured wide-necked small, medium, large or giant aneurysms from the petrous ICA to the ICA terminus. 1 Recently, off-label use of the PED has been extended to almost all types of cerebral aneurysms, including distal cerebral circulation aneurysms (DCCAs), defined as aneurysms at or beyond the M1 middle cerebral artery, P1 posterior cerebral artery, and A1 anterior cerebral artery. 2 DCCAs account for 5%–13% of all intracranial aneurysms, 3 and aneurysms at this location remain a challenge for both microsurgical and traditional endovascular therapeutic approaches.4,5
The promising performance of the PED against anatomically complex abnormalities offers a new treatment option for refractory lesions. 6 The luminal reconstruction ability and avoidance of PED in jailing a microcatheter to coil the aneurysmal sac further justifies its use. 7 However, the application of the PED in these settings has particular concerns, such as the narrow parent artery diameter, interference with the previously inserted device, and the mismatch in the distal-proximal artery diameters, which complicate the deployment of the PED and may hamper the flow diversion effect of the stent. Therefore, we reviewed cases from a multicenter cohort of patients to explore the actual effect of the PED on these groups of aneurysms.
Methods
Patient selection
The institutional review board at each participating center approved the retrospective data collection and review for this study. A consecutive series of patients with DCCAs who underwent PED treatment at three Chinese centers between March 2016 and November 2020 were included in this study. At these centers, flow diversion and other traditional endovascular therapeutic approaches were discussed in a multidisciplinary process each case. The indications for flow-diverting endovascular therapy rather than standard coiling, clipping, or trapping with bypass were based on patient demographics, symptoms, and aneurysm characteristics. For aneurysms with jet blood flowing into the lumen or with a diameter ≥ 15 mm, we usually choose to pack the aneurysmal sac loosely, because coils can change the hemodynamics of the lumen of the aneurysm and accelerate the formation of thrombus. Data regarding the patients' clinical and radiological data were retrospectively collected.
Endovascular procedures
The patients were premedicated with aspirin (100 mg/day) and clopidogrel (75 mg/day) for 7 days before the intervention. Although thromboelastography was used to discriminate hyporesponders to clopidogrel, the test was not conducted for all patients in our series. The required inhibition rates were 30%–80% for adenosine diphosphate and >70% for arachidonic acid. All procedures were performed under general anesthesia with full heparinization. A tri-axial system with a 6-Fr introducer sheath, intermediate catheter, and appropriate microcatheter was adopted to deploy the PED. Additional coiling was performed using a pre-jailed catheter. If the patients had no complications, aspirin was discontinued at 12 months and clopidogrel was discontinued at 6 months post-procedure.
Clinical and imaging follow-Up
Modified Rankin scores (mRS) were evaluated preoperatively and at the last follow-up. All patients were followed up with digital subtraction angiography (DSA) or computed tomographic angiography. The occlusion status was evaluated according to the O’Kelly Marotta (OKM) grading scale, which was developed specifically for the angiographic assessment of aneurysms treated with flow-diverting stents. 8
Statistical analysis
Data are presented as the mean ± standard deviation for continuous variables with a normal distribution and the median and interquartile range (IQR) for data without a normal distribution. The Wald test was used for univariate analysis to test whether explanatory covariates are predictive of the following dependent variables: periprocedural complications and complete occlusion. Predictive factors in univariate analysis (p < 0.05) were entered into a multivariate logistic regression analysis. Statistical significance was set at ≤ 0.05. Statistical analysis was performed using R, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Population
The characteristics of each included patients are listed in Supplemental Table 1 and summarized in Table 1. A total of 53 consecutive patients (24 women, 29 men; mean age, 53 years; range, 16–69 years) with 53 DCCAs were included in this retrospective analysis. MCA aneurysms were the most commonly treated lesions (38/53, 71.6%). ACA, PCA, and AcomA aneurysm account for 13.2%, 9.4%, and 5.6% in our series, respectively. The mean maximal aneurysm diameter was 12.3 ± 5.7 mm. In total, 75.4% (40/53) were fusiform and 24.5% (13/53) were saccular. Nine patients had recurrent aneurysms, four of which were previously treated with coil embolization, four with clip reconstruction, and one with SAC. Most of the patients (24/53, 45.3%) showed symptoms such as headaches and dizziness, and the other patients (29/41, 54.7%) in our series presented with asymptomatic incidental aneurysms.
Table 1.
Baseline clinical and radiographic data of 53 DCCA patients treated with the PED.
| Female sex - n (%) | 29 (54.7) |
| Age - median (range) | 53 (16-69) |
| Clinical history - n (%) | |
| Symptomatic | 24 (45.3) |
| Incidental | 29 (54.7) |
| Aneurysm location - n (%) | |
| ACA | 7 (13.2) |
| MCA | 38 (71.6) |
| PCA | 5 (9.4) |
| AcomA | 3 (5.6) |
| Maximal aneurysm diameter (mean ± SD) mm | 12.3 ± 5.7 |
| <5 mm, yes - n (%) | 4 (7.5) |
| 5-14.9 mm, yes - n (%) | 38 (71.6) |
| 15-24.5 mm, yes - n (%) | 9 (16.9) |
| ≥25 mm, yes - n (%) | 2 (3.7) |
| Previously treated - n (%) | 9 (17.0) |
| Endovascular - n (%) | 4 (7.5) |
| Microsurgical clipping - n (%) | 4 (7.5) |
| Both - n (%) | 1 (1.8) |
| Pretreatment mRS - n (%) | |
| Good (mRS = 0-2) | 52 (98.2) |
| Poor (mRS = 3-5) | 1 (1.8) |
| Proximal Parent Vessel Diameter (mean ± SD) mm | 2.5 (0.5) |
| Distal Parent Vessel Diameter (mean ± SD) mm | 2.3 (0.4) |
| Average parent vessel diameter (mean ± SD) mm | 2.4 (0.4) |
ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; AcomA, anterior communication artery; PED, Pipeline Embolization Device; mRS, modified Rankin Scale;.
Procedural results
PED was successfully performed in all 53 patients. Treatment with a single PED was performed in 43 patients (81.2%). Two devices were used for the patients in our series. Adjunctive coil placement was performed in 16 cases (30.1%). Our series demonstrated an average diameter of 2.5 mm proximal to the aneurysm and 2.3 mm distally. The average diameter of the proximal and distal parent vessels was 2.4 mm. The procedural results and clinical follow-up outcomes are summarized in Table 2.
Table 2.
Endovascular treatment and outcomes.
| Treatment modality- n (%) | |
| Adjunctive coils place | 16 (30.1) |
| Multiple PED placed | 10 (18.8) |
| Follow-up in months (Median; IQR) | 12 (6.0-12.0) |
| Latest imaging follow-up data available - n (%) | 51 (96.3) |
| Occlusion status at last follow-up - n (%) | |
| Completely occluded | 46 (90.2) |
| Near completely occluded with neck remnant | 4 (7.8) |
| Incompletely occluded | 1 (2.0) |
| Latest clinical follow-up data available - n (%) | 53 (100.0) |
| Thromboembolic complications - n (%) | 6 (11.3) |
| Hemorrhagic complications - n (%) | 2 (3.8) |
| Clinical follow-up - n (%) | |
| Good (mRS = 0-2) | 49 (92.5) |
| Poor (mRS = 3-6) | 4 (7.5) |
| Mortality - n (%) | 2 (4.9) |
| Overall morbidity - n (%) | 4 (7.5) |
PED, pipeline embolization device; mRS: modified Rankin Scale.
Angiographic follow-Up
Angiographic follow-up data were available for 51 patients (median follow-up time, 12 months; IQR, 6–12 months). Overall, 90.2% (46/51) and 7.8% (4/51) of aneurysms showed complete obliteration with OKM scales D and C, respectively. One patient showed aneurysm persistence after flow diversion in the six-month follow-up with OKM scale B (#49, Figure 1 A-D). One patient had parent artery occlusion and one had in-stent stenosis at follow-up (#6 Figure 2 A-C; #28 Figure 2 D-F). Among the nine patients with recurrent aneurysms, eight underwent angiographic follow-up, and all showed satisfactory obliteration.
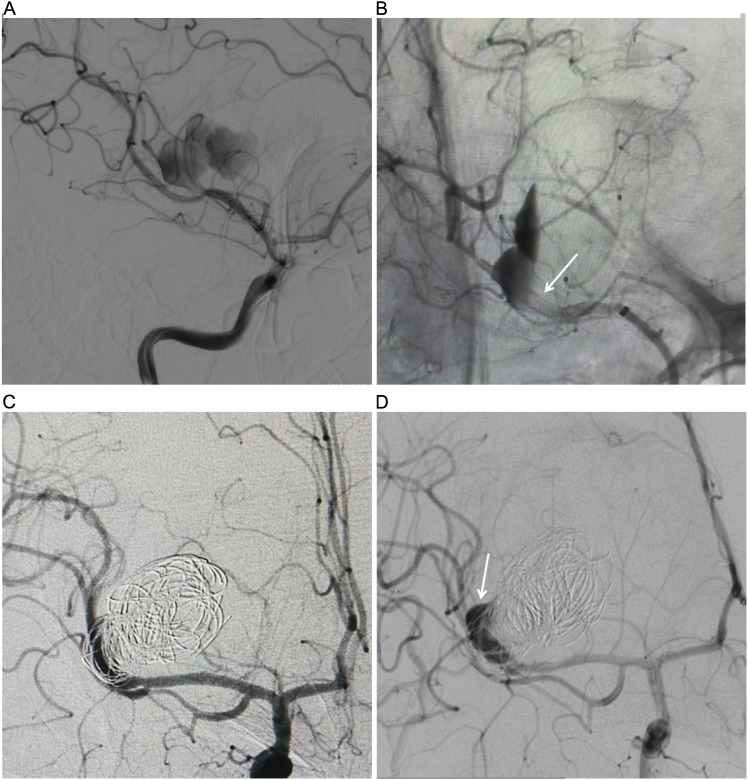
Figure 1.
(A) DSA showing the right MCA M2 dissecting aneurysm. (B) DSA in working position view immediately after flow diversion shows diminished filling of the right MCA dissecting aneurysms. The expanded Pipeline flow diversion device in the MCA M1 to M2 segment (arrow). (C) DSA in AP view immediately after PED-assisted coil embolization shows diminished filling of the aneurysm. (D) Six-month DSA follow-up shows persistence after flow diversion at the aneurysm nack (arrow).
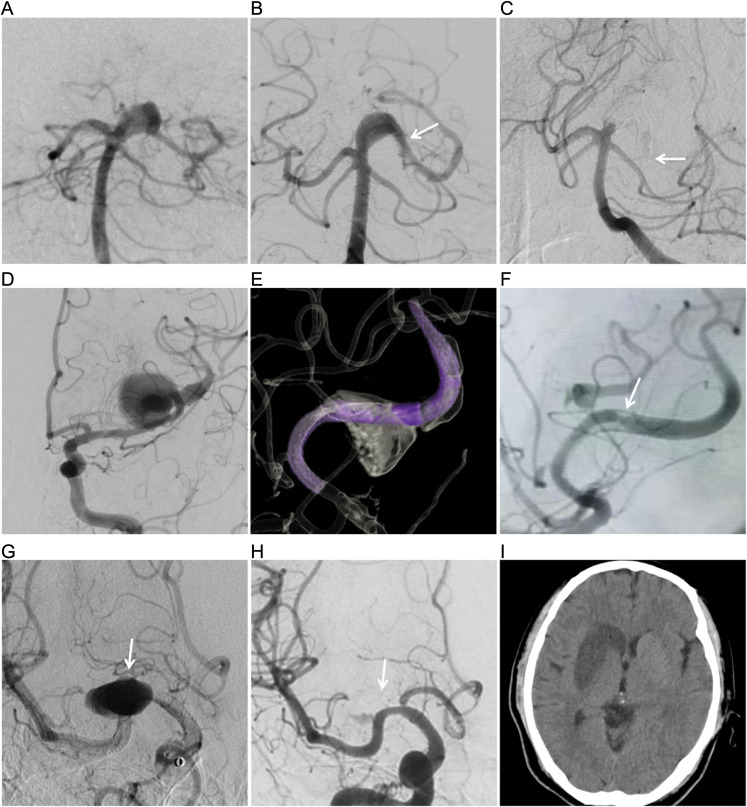
Figure 2.
(A) digital subtraction angiogram (DSA) showing the posterior cerebral artery (PCA) aneurysm. (B) DSA in working position views immediately after flow diversion shows diminished filling of the right PCA dissecting aneurysms. The arrow indicates the distal end of the PED. (C) Six-month DSA follow-up shows parent artery occlusion and contraction distal to the PED. The arrow indicates the distal end of the PED. (D) DSA shows the middle cerebral artery (MCA) dissecting aneurysm. (E) Two PED were partially overlapped in the parent vessel of left MCA dissecting aneurysm. (F) Six-month DSA follow-up shows complete occlusion of the aneurysm and 50% in-stent stenosis (arrow). (G) DSA in AP views immediately after flow diversion shows diminished filling of the aneurysm and the regular filling of lenticulostriate artery (arrow). (H) DSA in AP views 18 h after flow diversion showing disappeared aneurysm and internal lenticulostriate artery (arrow). (I) CT showing new infarct in the right temporal island and basal ganglia three days after the procedure.
Clinical and complications outcomes
Periprocedural complications and clinical outcomes are summarized in Table 3. Six patients experienced periprocedural complications associated with cerebral infarction. The first patient (# 10, Figure 3 A-D) with ACA A1 aneurysm experienced a Heubner's perforator-territory stroke with an infarct in the left basal ganglia and centrum semiovale, presented with mixed aphasia and right limb movement disorder. Symptoms slightly improved with intravenous administration of tirofiban, but the remaining symptoms improved with mRS 3 at the six-month follow-up. The second patient with a right MCA M1 aneurysm presented with aphasia, left central facial paralysis, and left limb hemiplegia. DSA showed diminished internal lenticulostriate artery and CT showing new infarct in the right temporal island and basal ganglia 3 days after the procedure (#23, Figure 2 G-I). The patient was discharged with central facial paralysis and severe hemiparesis (mRS score, 4). The third (#8) and fourth patient (#41) with MCA M1 aneurysm presented with aphasia and hemiparesis. Their symptoms improved with intravenous application of tirofiban with an mRS of 1. The fifth patient (#33) showed transient hemiparesis 24 h after treatment. IIb/IIIa inhibitor and urinary kallindinogenase were used to treat acute cerebral infarction. The patient was discharged with slight neurologic deterioration and an mRS score of 1 at 15 months. The sixth patient (#38), treated with aspirin and clopidogrel, developed severe right hemiparesis due to acute parent artery thrombosis 17 h after treatment. The artery was completely recanalized after systemic tirofiban injection. The patient was discharged with mild right limb weakness and an mRS score of 1 at six months. Two hemorrhagic complications were noted: one patient experienced SAH and ipsilateral intraparenchymal hemorrhagic events and died despite active rescue (#13, Figure 4), and one patient (#24) experienced delayed SAH 10 days after the PED treatment and died in the local hospital. Clinical follow-up data were available for all 53 patients; 92.5% of patients (49/53) had a good clinical outcome (mRS 0-2) at the last follow-up. Overall, the median clinical follow-up was 12 months (range, 4–24 months). Four patients (9.8%) presented with poor neurological outcomes (mRS 3-6) at follow-up.
Table 3.
Periprocedural complications.
| Aneurysm description | Treatment | Description of complication | Management, outcome |
|---|---|---|---|
| # 8. Fusiform MCA M1, previously clipped | PED | Perforator-territory ischemic events, presented aphasia and paroxysmal right limb weakness, mRS 3 | Symptoms improved with intravenous application of tirofiban, mRS 0 at 12 months |
| # 10. Fusiform ACA A2, incidental |
PED + Coils | Perforator-territory ischemic events with infarct in the left basal ganglia and centrum semiovale, presented aphasia and right limb movement disorder, mRS 4 | Symptoms improved with intravenous application of tirofiban, mRS 3 at 6 months |
| # 13. Fusiform MCA M1, symptomatic | PED | Postoperative SAH and ipsilateral intraparenchymal hemorrhage | Decompressive craniectomy, hematoma evacuation. Patient died in 6 days post-procedure. |
| # 23. Fusiform MCA M1, incidental | PED | Perforator-territory ischemic events with infarct in the right temporal island and basal ganglia, presented aphasia, left central facial paralysis, and left limb hemiplegia, mRS 4 | Symptoms slightly improved after administration of IIb/IIIa inhibitor, mRS 3 at 11 months |
| # 24. Fusiform MCA M2, previously clipped | PED | Developed SAH 10 days post-procedure | Died in the emergency department of the local hospital. |
| # 33. Fusiform MCA M2, TIA | PED*2 | Transient hemiparesis 24 h after treatment, mRS 2 | Symptoms improved after after administration of IIb/IIIa inhibitor and Urinary Kallindinogenase, mRS 1 at 15 months |
| # 38. Saccular ACA A1, incidental |
PED + Coils | Postoperative in-stent thrombosis. Clinical deterioration 17 h post-procedure presented aphasia and right limb movement disorder, mRS 4 | Symptoms improved with intravenous application of tirofiban, mRS 1 at 6 months |
| # 41. Fusiform MCA M1, incidental | PED | Perforator-territory ischemic events, presented aphasia and left limb movement disorder, mRS 3 | Symptoms improved with intravenous application of tirofiban, mRS 1 at 10 months |
ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; AcomA, anterior communication artery; mRS, modified Rankin Score; TIA, transient ischemic attacks; PED, pipeline embolization device.
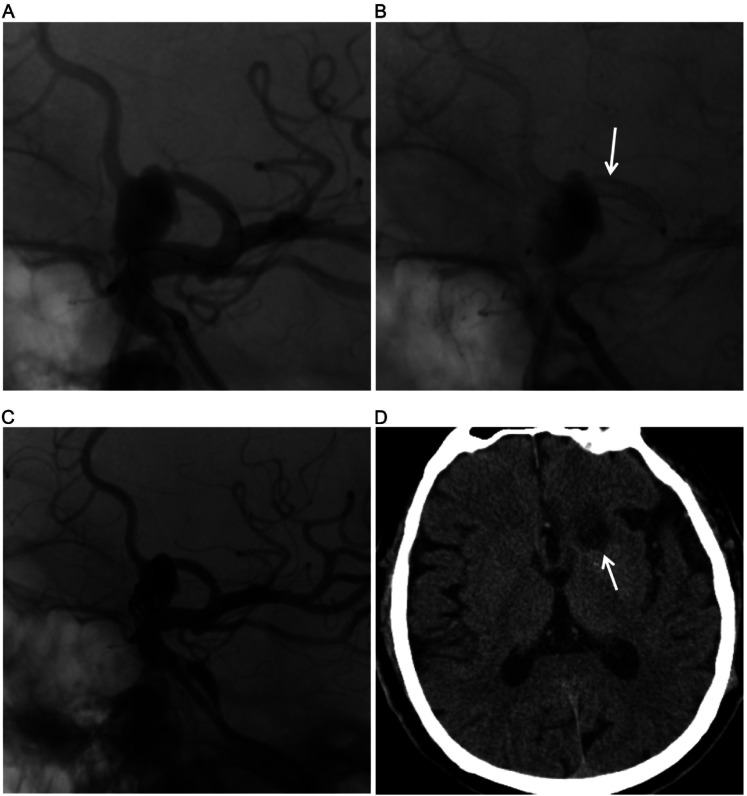
Figure 3.
(A) DSA showing the anterior cerebral artery (ACA) aneurysm. (B) DSA in working position views immediately after flow diversion shows diminished filling of ACA aneurysms (arrow). (C) Loose packing of the aneurysm sac after PED release. (D) CT shows a new infarct in the left basal ganglia and centrum semiovale (arrow).
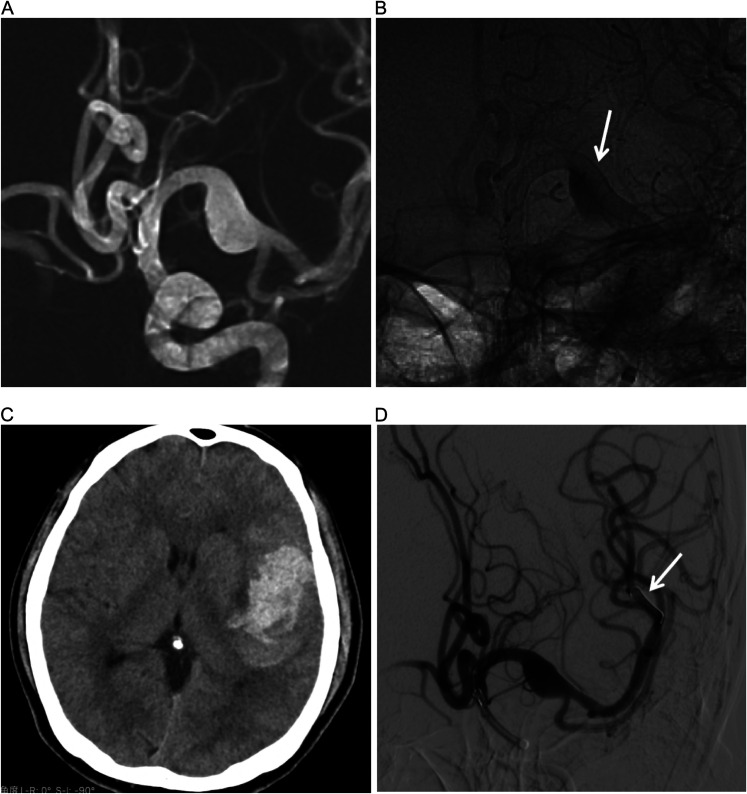
Figure 4.
(A) DSA fluoroscopic roadmap image showing a right MCA M1 dissecting aneurysm. (B) DSA in working position views immediately after flow diversion showing diminished filling of aneurysms (arrow). (C) CT showing hemorrhage in the left temporal lobe. (D) The micro delivery wire damaged the small distal artery during the release of the stent (arrow).
Factors related to aneurysm occlusion and periprocedural complications
The following factors were tested as predictors of achieving complete occlusion and periprocedural complications: age, aneurysm size, presentation, aneurysm location, previous treatment, adjunctive coil placement, multiple stent placement, and average parent vessel diameter. Univariate Wald's test analysis for complete occlusion showed aneurysm size (OR, 0.83; 95% CI, 0.71–0.98; p = 0.026) and average parent vessel diameter (OR, 0.05; 95% CI, 0–0.66; p = 0.023) were statistically significant for complete occlusion. In multivariate analysis, no factor was statistically significant. In the univariate analysis for complications, no factors were statistically significant (Table 4).
Table 4.
Univariate and multivariate analysis of predicting factors for aneurysm occlusion and periprocedural complications.
| Univariate P Value |
Univariate OR (95%CI) |
Multivariate P Value | Multivariate OR (95%CI) |
|
|---|---|---|---|---|
| Independent variables for complete occlusion | ||||
| Patient age | 0.934 | 0.997 (0.94, 1.06) | ||
| Aneurysm size | 0.026 | 0.83 (0.71, 0.98) | 0.080 | 0.83 (0.71, 0.98) |
| Presentation | 0.810 | 1.26 (0.19, 8.26) | ||
| Aneurysm location (ACA vs others) | 0.995 | 14,826,768.30 (0, Inf) | ||
| Aneurysm location (MCA vs others) | 0.630 | 0.57 (0.06, 5.58) | ||
| Aneurysm location (PCA vs others) | 0.996 | 13,767,713.40 (0, Inf) | ||
| Aneurysm location (AcomA vs others) | 0.200 | 0.18 (0.01, 2.47) | ||
| Previous treatment (Yes vs No) | 0.994 | 15,216,946.50 (0, Inf) | ||
| Adjunctive coils placed (Yes vs No) | 0.570 | 1.94 (0.20, 18.85) | ||
| Multiple stents placed (Yes vs No) | 0.897 | 1.12 (0.21, 5.88) | ||
| Average parent vessel diameter | 0.023 | 0.05 (0, 0.66) | 0.061 | 0.05 (0, 0.66) |
| Independent variables for complications | ||||
| Patient age | 0.132 | 1.06 (0.98, 1.15) | ||
| Aneurysm size | 0.557 | 1.04 (0.92, 1.18) | ||
| Presentation | 0.086 | 0.22 (0.04, 1.24) | ||
| Aneurysm location (ACA vs others) | 0.299 | 2.67 (0.42, 16.97) | ||
| Aneurysm location (MCA vs others) | 0.822 | 1.22 (0.22, 6.84)) | ||
| Aneurysm location (PCA vs others) | 0.994 | 0 (0, Inf) | ||
| Aneurysm location (AcomA vs others) | 0.994 | 0 (0, Inf) | ||
| Previous treatment (Yes vs No) | 0.517 | 1.81 (0.30, 10.86) | ||
| Adjunctive coils placed (Yes vs No) | 0.729 | 0.74 (0.13, 4.12) | ||
| Multiple stents placed (Yes vs No) | 0.145 | 0.21 (0.02, 1.72) | ||
| Average parent vessel diameter | 0.096 | 5.15 (0.75, 35.37) | ||
ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; AcomA, anterior communication artery; mRS, modified Rankin Scale; Inf, Infinite;.
Discussion
The off-label use of the PED in distal cerebral circulation aneurysms has been controversial. Our study demonstrated a promising performance of 90.2% complete occlusion rate, 98% near-complete occlusion rate, and 92.5% had a good clinical outcome (modified Rankin Scale score of 0–2). Our results are comparable to the results of a meta-analysis of distal anterior circulation aneurysms, with a median follow-up of 12.0 months, by Cagnazzo et al. 9 Extensive studies have shown a high complete occlusion rate in patients with DCCAs after off-label use of the PED.10,11 Atallah et al. 12 retrospectively reviewed 23 patients with 23 DCCAs treated with PED, and at last follow-up, 78.3% of patients manifested with complete occlusion, while 95% had a good clinical outcome. Bender et al. 13 reviewed 67 patients with DCCAs treated with the PED and reported an 88% complete occlusion rate at 6 months, reporting that almost 94% of patients had a good clinical outcome. Similarly, Primiani et al. 14 reported 83% complete aneurysm occlusion, and 95% of patients with good clinical outcomes after treating 65 aneurysms starting on or beyond the A2, M2, and P2 segments with the PED.
With respect to the predictors of aneurysm occlusion, Cagnazzo et al. 9 demonstrated different occlusion rates depending on the artery covered, while the MCA location was an independent factor of incomplete occlusion. Similarly, of the five aneurysms that were not completely occluded in our series, four were located in the MCA. The diameter of the MCA M1 segment was relatively larger than that of the distal artery, which may explain why a smaller average parent artery diameter was associated with a higher complete occlusion rate in our univariate analysis. Previous flow diversion studies have also shown a decrease in the complete occlusion rate with increasing aneurysm size,15,16 which our study's results also showed.
Ischemic complications
Our study showed higher periprocedural complication rates of 15.1% (8/53) in reports of DCCAs with ischemic complications, with a 7.5% (4/53) morbidity rate. Of the six patients who presented with immediate postprocedural cerebral infarction complications, most of their symptoms improved at discharge or clinical follow-up, with an mRS score of 0 to 1.
Our study, which included 38 MCA M1 aneurysms, demonstrated a perforator-territory ischemia rate of 7.9% (3/38 patients) after lenticulostriate vessel coverage in the M1 segments. Kathryn et al. 17 reported a similar rate of 9.6% (5/52 patients) after the coverage of MCA M1 segments by flow diverters, but none of them had radiographic infarcts in the lenticulostriate territory. Some studies also focused on covered perforator vessels of aneurysms on the circle of Willis treated by flow diverter and showed a 17.6% (3/17 patients) rate of temporary ischemic complication. 18 Branching vessels and perforators arising from aneurysms, such as the medial and lateral lenticulostriate arteries, are abundant at the level of the A1-segment and the M1 segment of the ACA and MCA, respectively, thereby increasing the risk of perforator stroke when they are covered with PED. 19 Regarding non-perforator areas, Primiani et al. 14 analyzed 65 patients with A2, M2, P2, and distal aneurysms treated with PED and found an overall complication rate of 7.7%, which was significantly lower than that in our study. Furthermore, only one patient (1.5%) with an M2 aneurysm showed ischemic stroke with slow filling of the side branch that resolved after administration of a IIb/IIIa inhibitor. Most of the aneurysms included in our study were located in non-perforator areas, which may explain the relatively low complication rate. Asymptomatic occlusion of covered cortical branches seems universal, yet ischemic complications are preferably linked to lenticulostriate territory occlusions.
Regarding potential ischemic complications for ACA A1 segments aneurysms, we have to consider the perforating medial lenticulostriate vessels. We had one ACA A1 aneurysm in which the recurrent artery of Heubner (RAH) was jailed, and the patient (#10) experienced permanent symptomatic perforator-territory ischemia. Although some studies have indicated that the diameter of the RAH approximates to the ophthalmic artery and anterior choroidal artery, these vessels can be safely jailed when the PED is used in patients with distal ICA aneurysms.20,21 PED used in ACA and AcomA aneurysms may also induce perforator occlusion, especially at the A1-A2 junction. 22 This fact highlights the importance of being aware that ischemic complications can occur at any time during the endovascular procedure, especially in the M1 and A1 segments of the MCA.
The risk of in-stent stenosis (acute or chronic) must be considered when using PED in DCCAs. Ravindran et al. 23 reported a 7.1% rate of chronic in-stent stenosis after reviewing 162 intracranial aneurysms, and all these patients remained asymptomatic. Two patients (3.9%, 2/51) had asymptomatic in-stent stenosis. This is even lower than the 5%–10% risk in the general PED population and the 4.8% chronic in-stent stenosis rate reported by Cagnazzo et al.9,24 One patient had parent artery occlusion, and the other had 50% in-stent stenosis. The distal end of the PED stent showed a slight contraction in the follow-up DSA, similar to the mouth of the fish (#6, Figure 2 A-C). Therefore, we hypothesized that this type of contraction mechanism might be attributed to significant vascular diameter changes in the distal and proximal parts of the parent vessel. The stent cannot be fully opened at the relatively narrow distal end, which increases the risk of in-stent stenosis and artery occlusion. Selecting a proper PED size is essential to ensure adequate flow diversion and limit the risk of ischemic complications. This phenomenon is common in the basilar and posterior cerebral arteries because the significant change in vascular diameter from the basilar artery to the posterior cerebral artery makes it challenging to completely open the distal end of the PED. To resolve this problem, we suggest using two PEDs of different sizes to treat fusiform or dissecting aneurysms with a wide aneurysm sac neck. However, multiple stents increase the metal coverage, which mitigates FD while also increasing the risk of in-stent stenosis. 25 Therefore, it is imperative to recognize the native anatomy of the distal vessel to select the appropriate size of PED.
Hemorrhagic complications
In their research, Brinjikji et al. reported that the incidence of delayed aneurysmal SAH after PED was approximately 4%. 11 Several studies have also reported low hemorrhagic complication rates in PED for intracranial aneurysms.26,27 Two patients in our series experienced a delayed postoperative intraparenchymal hemorrhage. One of them (#13), with a fusiform aneurysm of the MCA M1 segment, had a sudden severe headache 9 h after surgery, and the CT showed hemorrhage in the left temporal lobe. The patient died within 5 days of PED placement. We postulate that hematoma complications might have arisen because the micro delivery wire may have damaged the small distal artery during the release of the stent, causing late rupture of the small distal artery (Figure 4 D). Rehman et al. 28 reported that the deployment of a braided stent requires more manipulation than a laser-cut device with “pull” and “push” techniques to expand the device entirely, which can also be traumatic to a weakened artery.
Another patient with a recurrent large saccular aneurysm of the right MCA M1 experienced fatal and delayed subarachnoid hemorrhage following flow diversion when defecating forcefully at home 10 days after the procedure. Some hemodynamic studies have attempted to explore the mechanism of delayed aneurysm rupture. Hassan et al. 29 found that a slow blood flow jet still exists inside the aneurysm at the end of the procedure. Cebral et al. 30 reported that PED placement could increase intra-aneurysmal pressure. Similarly, Li et al. 31 also found that the flow velocity of the aneurysm lumen was decreased in delayed ruptured aneurysms, while the pressure was increased. These factors might be related to delayed aneurysm rupture after treatment. Therefore, the elevation of intra-aneurysmal pressure and residual blood flow in the aneurysm lumen may explain the patient's delayed postoperative rupture. Appropriate management of perioperative blood pressure may be a practical method to prevent this phenomenon. 30 For some large or large aneurysms, combination treatment of PED placement and coil embolization of the aneurysm has been recommended to promote intraluminal thrombosis and promote the transition from an unstable thrombus to a stabilized, organized thrombus. 32
PED for recurrence of aneurysms
Flow diversion treatment has been expected to induce the progressive occlusion of recurrent aneurysms. Lin et al. 33 reviewed nine recurrent aneurysms that were subsequently retreated with PED and showed 83% complete aneurysm occlusion. Our study demonstrated comparable results, wherein 87.5% (7/8) of recurrent aneurysms achieved satisfactory results. Nine patients had recurrent aneurysms in our series, five of which were previously treated with coil embolization, four were treated with clip reconstruction, and one experienced both. Four patients underwent simple coil packing, which appeared to be narrow-necked dissecting aneurysms before the initial treatment. However, due to the lack of experience in the primary hospital, three patients received coil embolization during the initial treatment, which may cause aneurysm recurrence. Another patient (#25) underwent stent-assisted coiling. The reason for the recurrence of the aneurysm in this patient may be that the coil embolization was not dense. In these patients, the parent artery remained unobstructed after initial treatment. Therefore, all five patients received flow diversion (PED) treatment without additional coiling. One patient (# 4) with recurrent right MCA M1 dissecting aneurysm previously treated with primary coil embolization had been deployed with two PEDs of different sizes. Because of the moderate stenosis at the stent overlap point of two PEDs proximal to the parent artery, we used a balloon to expand the PED to make the stent fully apposed to the parent vessel wall.
Several studies have already evaluated the safety and efficacy of PED in the treatment of recurrent aneurysms, but those focusing on DCCAs are scant. Renowden et al. 34 reported a complication rate of 3% after recoiling of recurrent coiled aneurysms, while Ringer et al. 35 reported that the total risk of retreatment mortality was 1.28% per patient. These complication rates are similar and even lower with coiling than with PED, but in cases with multiple retreatments, the number of reinterventions required with each treatment may enhance the complication risk of conventional endovascular techniques. Henkes et al. reported that complete occlusion was achieved in only 46.9% of retreated aneurysms after the first recoiling attempt and 35.2% after the second retreatment. 36 Tahtinen et al. focused on the role of stent-assisted coil embolization in the management of recurrent aneurysms. After stent-assisted coiling of recurrent aneurysms, only 59% of aneurysms achieved complete occlusion, and 16% of patients needed additional endovascular treatment. 37 In Daou et al.'s 38 study of PED for previously coiled aneurysms, 25% of patients had two attempts at coiling before resorting to PED placement, which was the definitive and final treatment. Overall, higher recurrence rates were found with the recoiling of previously coiled aneurysms than PED treatment, which justifies PED implementation for the treatment of previously coiled aneurysms. FD via PED can be considered a management alternative for recurrent distal cerebral aneurysms.
Limitations
This study has some limitations, including those inherent to the retrospective observational series, such as the limited number of cases and the relatively short follow-up period. Moreover, our patient collective may not be homogeneous, as primary aneurysms and recurrent aneurysms were included. A prospective design can help control for patients’ baseline characteristics to better analyze the influencing factors of operational complications. Since the follow-up period was relatively short, it is possible that the overall occlusion rate could increase over time, and long-term complications may have been missed. We believe that DCCAs are different from aneurysms in a more proximal location with respect to their anatomy, tortuosity, and branching vessels. Therefore, extensive further studies with long-term follow-up are needed to confirm the safety and efficacy of the PED in DCCAs. Further research and discussion are essential regarding endovascular treatment modalities versus surgical procedures for the treatment of DCCAs. Finally, off-label usage of the PED requires more experience, thus the PED in DCCAs should be deployed only by experienced interventionists.
Conclusion
PED is a viable option for treating DCCAs, especially for recurrent aneurysms or those that are not amenable to traditional surgical or endovascular techniques. Coverage of bifurcation branches and perforator may increase the risk of complications.
Supplemental Material
Supplemental material, sj-docx-1-ine-10.1177_15910199211063703 for Pipeline for the treatment of distal cerebral circulation aneurysms: A multicenter study focusing on periprocedural Complications by Chao Ma, Haoyu Zhu, Shikai Liang, Fei Liang, Jintao Han, Zichang Jia, Yupeng Zhang and Chuhan Jiang in Interventional Neuroradiology
Footnotes
Authors' note: Chao Ma, Department of Neurosurgery, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China. Yupeng Zhang, Interventional Neuroradiology Center, Beijing Tiantan Hospital, Capital Medical University, Beijing, China.
Ethics approval: Each of the three centers’ institutional review board provided ethical approval for this study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributorship statement: Chao Ma acquired most of the data, analyzed and interpreted the data, and drafted the article. The other authors (HYZ, SKL, FL, JTH, ZCJ) participated in the interventional procedures as an assistant and helped to analyze the data. Chuhan Jiang and Yupeng Zhang participated in the interventional procedures as primary surgeons and made substantial contributions to the design of the work.
Data sharing statement: Data are available upon reasonable request
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of Beijing (grant number 7212007).
ORCID iD: Chuhan Jiang https://orcid.org/0000-0003-2560-8576
Supplemental material: Supplemental material for this article is available online.
References
- 1.Hanel RA, Kallmes DF, Lopes DK, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the PREMIER study 1 year results. J Neurointerv Surg 2020; 12: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel PD, Chalouhi N, Atallah E, et al. Off-label uses of the pipeline embolization device: a review of the literature. Neurosurg Focus 2017; 42: E4. [DOI] [PubMed] [Google Scholar]
- 3.Nossek E, Zumofen DW, Setton A, et al. Treatment of distal anterior cerebral artery aneurysms with the pipeline embolization device. J Clin Neurosci 2017; 35: 133–138. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Hernandez A, Zador Z, Rodriguez-Mena R, et al. Distal aneurysms of intracranial arteries: application of numerical nomenclature, predilection for cerebellar arteries, and results of surgical management. World Neurosurg 2013; 80: 103–112. [DOI] [PubMed] [Google Scholar]
- 5.Chalouhi N, Jabbour P, Starke RM, et al. Endovascular treatment of proximal and distal posterior inferior cerebellar artery aneurysms. J Neurosurg 2013; 118: 991–999. [DOI] [PubMed] [Google Scholar]
- 6.Saleme S, Iosif C, Ponomarjova S, et al. Flow-diverting stents for intracranial bifurcation aneurysm treatment. Neurosurgery 2014; 75: 623–631. quiz 31. [DOI] [PubMed] [Google Scholar]
- 7.Becske T, Potts MB, Shapiro M, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg 2017; 127: 81–88. [DOI] [PubMed] [Google Scholar]
- 8.Joshi MD, O’Kelly CJ, Krings T, et al. Observer variability of an angiographic grading scale used for the assessment of intracranial aneurysms treated with flow-diverting stents. AJNR Am J Neuroradiol 2013; 34: 1589–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagnazzo F, Perrini P, Dargazanli C, et al. Treatment of unruptured distal anterior circulation aneurysms with flow-diverter stents: a meta-analysis. AJNR Am J Neuroradiol 2019; 40: 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallmes DF, Brinjikji W, Boccardi E, et al. Aneurysm study of pipeline in an observational registry (ASPIRe). Interv Neurol 2016; 5: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 12.Atallah E, Saad H, Mouchtouris N, et al. Pipeline for distal cerebral circulation aneurysms. Neurosurgery 2019; 85: E477–E484. [DOI] [PubMed] [Google Scholar]
- 13.Bender MT, Zarrin DA, Campos JK, et al. Tiny pipes: 67 cases of flow diversion for aneurysms in distal vessels measuring less than 2.0 mm. World Neurosurg 2019; 127: e193–e201. [DOI] [PubMed] [Google Scholar]
- 14.Primiani CT, Ren Z, Kan P, et al. A2, M2, P2 aneurysms and beyond: results of treatment with pipeline embolization device in 65 patients. J Neurointerv Surg 2019; 11: 903–907. [DOI] [PubMed] [Google Scholar]
- 15.Park MS, Mazur MD, Moon K, et al. An outcomes-based grading scale for the evaluation of cerebral aneurysms treated with flow diversion. J Neurointerv Surg 2017; 9: 1060–1063. [DOI] [PubMed] [Google Scholar]
- 16.Bender MT, Colby GP, Lin LM, et al. Predictors of cerebral aneurysm persistence and occlusion after flow diversion: a single-institution series of 445 cases with angiographic follow-up. J Neurosurg 2018; 130: 259–267. [DOI] [PubMed] [Google Scholar]
- 17.Wagner KM, Srinivasan VM, Srivatsan A, et al. Outcomes after coverage of lenticulostriate vessels by flow diverters: a multicenter experience. J Neurosurg 2019; 132(2): 473–480. doi: 10.3171/2018.8.JNS18755 [DOI] [PubMed] [Google Scholar]
- 18.Gawlitza M, Januel AC, Tall P, et al. Flow diversion treatment of complex bifurcation aneurysms beyond the circle of Willis: a single-center series with special emphasis on covered cortical branches and perforating arteries. J Neurointerv Surg 2016; 8: 481–487. [DOI] [PubMed] [Google Scholar]
- 19.Lin N, Lanzino G, Lopes DK, et al. Treatment of distal anterior circulation aneurysms With the pipeline embolization device: a US multicenter experience. Neurosurgery 2016; 79: 14–22. [DOI] [PubMed] [Google Scholar]
- 20.Puffer RC, Kallmes DF, Cloft HJ, et al. Patency of the ophthalmic artery after flow diversion treatment of paraclinoid aneurysms. J Neurosurg 2012; 116: 892–896. [DOI] [PubMed] [Google Scholar]
- 21.Raz E, Shapiro M, Becske T, et al. Anterior choroidal artery patency and clinical follow-up after coverage with the pipeline embolization device. AJNR Am J Neuroradiol 2015; 36: 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Galdamez M, Romance A, Vega P, et al. Pipeline endovascular device for the treatment of intracranial aneurysms at the level of the circle of Willis and beyond: multicenter experience. J Neurointerv Surg 2015; 7: 816–823. [DOI] [PubMed] [Google Scholar]
- 23.Ravindran K, Salem MM, Enriquez-Marulanda A, et al. Quantitative assessment of In-stent stenosis after pipeline embolization device treatment of intracranial aneurysms: a single-institution series and systematic review. World Neurosurg 2018; 120: e1031–e1040. [DOI] [PubMed] [Google Scholar]
- 24.John S, Bain MD, Hui FK, et al. Long-term follow-up of In-stent stenosis after pipeline flow diversion treatment of intracranial aneurysms. Neurosurgery 2016; 78: 862–867. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro M, Raz E, Becske T, et al. Variable porosity of the pipeline embolization device in straight and curved vessels: a guide for optimal deployment strategy. AJNR Am J Neuroradiol 2014; 35: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dabus G, Grossberg JA, Cawley CM, et al. Treatment of complex anterior cerebral artery aneurysms with pipeline flow diversion: mid-term results. J Neurointerv Surg 2017; 9: 147–151. [DOI] [PubMed] [Google Scholar]
- 27.Clarencon F, Di Maria F, Gabrieli J, et al. Flow diverter stents for the treatment of anterior cerebral artery aneurysms: safety and effectiveness. Clin Neuroradiol 2017; 27: 51–56. [DOI] [PubMed] [Google Scholar]
- 28.Rehman AA, Turner RC, Wright S, et al. An autopsy report of basilar artery aneurysm flow diversion complicated by postoperative day 3 hemorrhage from vessel rupture. J Neurointerv Surg 2019; 11(5): e2. doi: 10.1136/bcr-2018-014511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan T, Ahmed YM, Hassan AA. The adverse effects of flow-diverter stent-like devices on the flow pattern of saccular intracranial aneurysm models: computational fluid dynamics study. Acta Neurochir (Wien 2011; 153(8): 1633–1640. [DOI] [PubMed] [Google Scholar]
- 30.Cebral JR, Mut F, Raschi M, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol 2011; 32: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Tian Z, Zhu W, et al. Hemodynamic analysis of postoperative rupture of unruptured intracranial aneurysms after placement of flow-diverting stents: a matched case-control study. AJNR Am J Neuroradiol 2019; 40: 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulcsar Z, Houdart E, Bonafe A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol 2011; 32: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin LM, Iyer RR, Bender MT, et al. Rescue treatment with pipeline embolization for postsurgical clipping recurrences of anterior communicating artery region aneurysms. Interv Neurol 2017; 6: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renowden SA, Koumellis P, Benes V, et al. Retreatment of previously embolized cerebral aneurysms: the risk of further coil embolization does not negate the advantage of the initial embolization. AJNR Am J Neuroradiol 2008; 29: 1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ringer AJ, Rodriguez-Mercado R, Veznedaroglu E, et al. Defining the risk of retreatment for aneurysm recurrence or residual after initial treatment by endovascular coiling: a multicenter study. Neurosurgery 2009; 65: 311–315. discussion 15. [DOI] [PubMed] [Google Scholar]
- 36.Henkes H, Fischer S, Liebig T, et al. Repeated endovascular coil occlusion in 350 of 2759 intracranial aneurysms: safety and effectiveness aspects. Neurosurgery 2006; 58: 224–232. [DOI] [PubMed] [Google Scholar]
- 37.Tahtinen OI, Manninen HI, Vanninen RL, et al. Stent-assisted embolization of recurrent or residual intracranial aneurysms. Neuroradiology 2013; 55: 1221–1231. [DOI] [PubMed] [Google Scholar]
- 38.Daou B, Starke RM, Chalouhi N, et al. The Use of the pipeline embolization device in the management of recurrent previously coiled cerebral aneurysms. Neurosurgery 2015; 77: 692–697. discission 97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ine-10.1177_15910199211063703 for Pipeline for the treatment of distal cerebral circulation aneurysms: A multicenter study focusing on periprocedural Complications by Chao Ma, Haoyu Zhu, Shikai Liang, Fei Liang, Jintao Han, Zichang Jia, Yupeng Zhang and Chuhan Jiang in Interventional Neuroradiology