Abstract
Background & Purpose
It has been hypothesized that circulating neutrophils have a direct correlation with the composition of emboli in acute ischemic stroke (AIS). The aim of this study is to evaluate the association between neutrophil-lymphocyte ratio (NLR) in peripheral blood and the expression of neutrophil extracellular traps (NETs) within stroke emboli.
Methods
Consecutive patients with acute ischemic stroke (AIS) due to large vessel occlusion (LVO) that underwent mechanical thrombectomy (MT) were included. Patients were divided into two groups based on NLR median value. Retrieved thrombi were histologically analyzed using Martius Scarlett Blue (MSB) for main thrombus components including red blood cells (RBCs), white blood cells (WBCs), fibrin and platelet. Immunohistochemistry staining for von Willebrand Factor (vWF) and anti-citrullinated H3 (H3Cit; NETs marker) was also performed.
Results
Samples from a total of 84 patients were included. The average percentage of RBCs, WBCs, fibrin, platelet, H3Cit, and vWF components in thrombi were 45.1%, 3.5%, 21.8%, 29.6%, 19.7% and 14.8% respectively. When stratifying by NLR group [low (≤3.94) versus high (>3.95)], high NLR group had significantly more WBCs (4.5%), fibrin (24.2%), H3Cit (22.7%) and vWF (17.1%) thrombus fractions compared to low NLR group. Additionally, RBC content (38.8%) was lower in the high NLR group.
Conclusions
NLR is correlated with the amounts of WBCs, fibrin, NETs and vWF within the thrombi retrieved from AIS patients due to LVO.
Keywords: acute ischemic stroke, endovascular therapy, neutrophil-lymphocyte ratio, neutrophil extracellular traps
Introduction
Many studies have shown the association of histological composition of emboli retrieved from acute ischemic stroke (AIS) patients with effectiveness of treatment and clinical outcome in these patients; both pharmacological and mechanical.1–4 Recent histological and immunohistochemistry studies confirmed the presence of neutrophil extracellular traps (NETs) as a major component of stroke emboli and a factor influencing resistance to fibrinolysis with alteplase. 5 The quantitative analysis of NETs has been correlated with the structure of embolus and the number of thrombectomy device passes necessary to achieve recanalization suggesting a role of neutrophils and acute inflammatory response during the formation of thrombus and the treatment of AIS.6–8 Given the fact that the presence of NETs is a factor influencing resistance to thrombolysis, identifying peripheral biomarkers of a NET rich thrombus could be important in the future for tailoring thrombolytic strategies, especially as novel agents targeting NETs become available. Neutrophil lymphocyte ratio (NLR) is a simple and easily obtainable peripheral blood biomarker and has been shown to be a useful measure to indirectly assess an underlying inflammatory process. In this study we tested the hypothesis that NLR is associated with the expression of NETs within thrombus.
Materials and methods
Study design
Consecutive patients with acute ischemic stroke (AIS) due to large vessel occlusion (LVO) that underwent mechanical thrombectomy (MT) from 2016 to 2021 were included. Institutional review board approval was obtained prior to the beginning of the study and waiver of consent was granted. Patients were included if they were >18 years of age, had retrieved thrombus available for histopathological analysis and had neutrophil and lymphocyte counts available from complete blood count results at the time of admission.
Data collection
For the purpose of this study, we collected data regarding patient demographics, patients’ stroke subtypes according to TOAST criteria (the classification of the Trial of Org 10172 in Acute Stroke Treatment), 9 location of vessel occlusion, thrombectomy approach (aspiration, stent-retriever or combination), number of thrombectomy passes, and final mTICI (modified Thrombolysis in Cerebral Infarction) score. 10 We also gathered neutrophil and lymphocyte counts from complete blood count (CBC) reports on admission for each patient. Neutrophil-lymphocyte ratio (NLR) was calculated by dividing neutrophil count by lymphocyte count on admission CBC.
Thrombi processing and histological characterization
Thrombus materials retrieved from each patient were immediately fixed in 10% phosphate buffered formalin. After standardized tissue processing and embedding in paraffin, thrombus sections were prepared in 3–5 µm thickness. The representative slides from each thrombus were stained with Martius Scarlet Blue (MSB) for calculating main thrombus composition including red blood cells (RBCs), white blood cells (WBCs), fibrin and platelet. Immunohistochemistry (IHC) staining was also performed using Leica 3 M autostainer for CitH3, a marker of neutrophil extracellular traps (NETs) activity. Stained slide for each staining were then scanned with Motic Easy scanner. Histological quantification was performed using Orbit Image Analysis Software (www. Orbit. bio) as per the standard operating procedure in our lab. Details of the methodology for Orbit image analysis has been previously described. 11
Martius scarlet blue staining
After deparaffinization of thrombus sections with xylene, slides were rehydrated through different concentration of ethanol and water. Slides were placed in Bouin's fluid at 56C for 1 h and then rinsed with tap water for 5 min. Subsequently the slides were placed for 10 min in filtered ammonium Celestine Blue solution, rinsed with running tap water for 5 min. The slides were then stained in Mayer's hematoxylin for 5 min and rinsed with warm tap water until the tissue turned to a blue colour. The slides were submerged in 95% alcohol for 1 min before staining for 8 min with fresh Martius Yellow solution. The slides were again rinsed with distilled water for 1 min before staining with fresh Crystal Scarlet solution (10 min). Slides were put in phosphotungstic acid solution for 7 min followed by a last staining with Methyl Blue for another 10 min. Ultimately the slides were rinsed in 1% aqueous acetic for 1 min, then dehydrated with absolute alcohol (two fast dips), cleared with xylene solution, and mounted in DPX. The sections selected to use for this staining were the most representative slides of the thrombi. With MSB staining, we were able to differentiate the main components of the thrombi such as red blood cells, white blood cells, fibrin, and platelets.
Immunohistochemistry
Immunohistochemical staining for NETs: CitH3 (anti-citrulinated H3, Abcam ab1791, 1/1000 dilution) and vWF (Dako anti-human von Willebrand Factor -M061601 clone F8/86, 1/200 dilution) was performed without Antigen retrieval (No Epitope retrieval 1 or 2 solutions were used). BOND Polymer Refine Red Detection kit was used for staining. With this staining kit, area of tissue stained with red or pink-red colour is considered positive. Primary antibody (CitH3 or vWF) incubation time was 15 min, followed by a 20 min incubation with a secondary antibody. Counterstaining of tissue using hematoxylin was performed for 5 min. Sections were then washed in warm soapy water to remove non-specific staining followed by rinsing in distilled water. Sections were then dehydrated in ethanol 100% and ethanol 95%, cleared in xylene, and mounted with DPX. Positive and negative controls were included for each run of staining.
Statistical analysis
Continuous data were presented as means with standard deviations (SD), and categorical data were presented as frequencies and percentages. Percentages of each thrombus component were considered continuous variable. Patients were divided into two groups based on NLR median value and were as follows: 1) low NLR: up to 3.94, 2) High NLR: 3.95 or higher. Chi-square test, and independent t-test were employed to evaluate the associations and differences between two groups. Statistical analyses were performed using SPSS 25.0 and p value of less than 0.05 indicated a statistically significant difference.
Results
Patient population
Samples from a total of 84 patients were included. Mean age was 69.3 (±12.13) years. Overall rate of successful recanalization (mTICI 2b-3) was 92.8% (78/84). Mean number of thrombectomy passes was 2.19 (±1.45). 51 patients were treated with aspiration technique followed by 23 patients with combination (stent-retriever + aspiration) and 5 patients with stent-retriever. A total of 39 (46.4%) patients received IV-tPA. In term of stroke etiology, 20 patients (23.8%) had a large artery atherosclerosis source (LAA), 40 (47.6%) a cardiac embolic (CE), 11 (13%) were cryptogenic and 13 patients (15.4%) had other causes (Table 1).
Table 1.
Baseline characteristics of patients.
| Variable | Low NLR (n = 42) | High NLR (n = 42) | P-value |
|---|---|---|---|
| Age, yrs | 67.95 ± 13.36 | 70.64 ± 10.75 | 0.31 |
| Diabetes, n (%) | 14 (33.3) | 15 (35.7) | 0.81 |
| NIHSS Score | 17 [10.5–23] | 17.5 [7.25–21.5] | 0.39 |
| Occlusion Site | 0.36 | ||
| ICA, n | 3 | 7 | |
| ICA Terminus, n | 5 | 7 | |
| M1, n | 25 | 20 | |
| M2, n | 9 | 7 | |
| A1, n | 1 | 1 | |
| Basilar, n | 3 | 1 | |
| P1, n | 0 | 1 | |
| IV-tPA | 0.24 | ||
| Yes, n (%) | 21 (50.0) | 18 (42.9) | |
| No, n (%) | 21 (50.0) | 24 (57.1) | |
| Etiology | 0.94 | ||
| LAA, n | 9 | 11 | |
| Cardioembolic, n | 20 | 20 | |
| Unknown, n | 6 | 5 | |
| Other | 7 | 6 | |
| Final TICI Score 2b-3 (n) | 41 | 37 | 0.2 |
| Number of passes required | 1 [1–3] | 2 [1–3] | 0.06 |
| Thrombectomy technique | 0.01 | ||
| Aspiration, n | 29 | 22 | |
| Stent retriever, n | 1 | 4 | |
| Combination, n | 7 | 16 | |
| Hemorrhagic conversion, n (%) | 9 (21.4) | 10 (23.8) | 0.21 |
Abbreviations: NIHSS: National Institutes of Health Stroke Scale, LAA: Large Artery Atherosclerosis, ICA: Internal Carotid Artery, IV-tPA: Intravenous Tissue Plasminogen Activator, TICI: Thrombolysis In Cerebral Infarction.
Continuous data are summarized as mean and standard deviation.
Association of NLR with thrombus composition
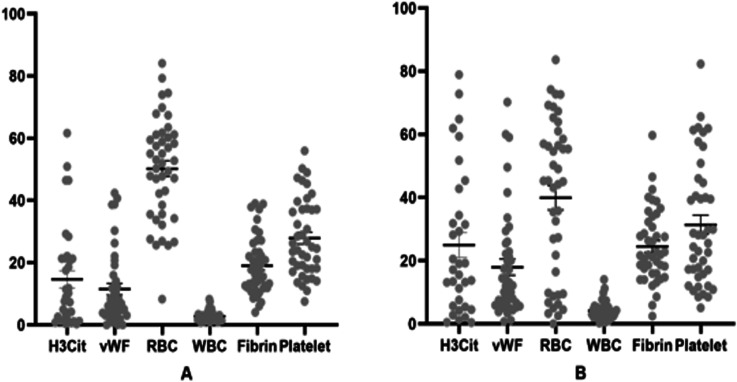
The overall average percentage of RBCs, WBCs, fibrin, platelet H3Cit, and vWF components in thrombi were 45.1%, 3.5%, 21.8%, 29.6%, 19.7% and 14.8% respectively (Figure 1). As shown in Table 2, when stratifying by NLR group (low NLR vs. high NLR), WBCs, fibrin, H3Cit and vWF thrombus fractions were significantly higher in the high NLR group (Figure 2). Additionally, RBC content was lower in the high NLR group (Table 2).
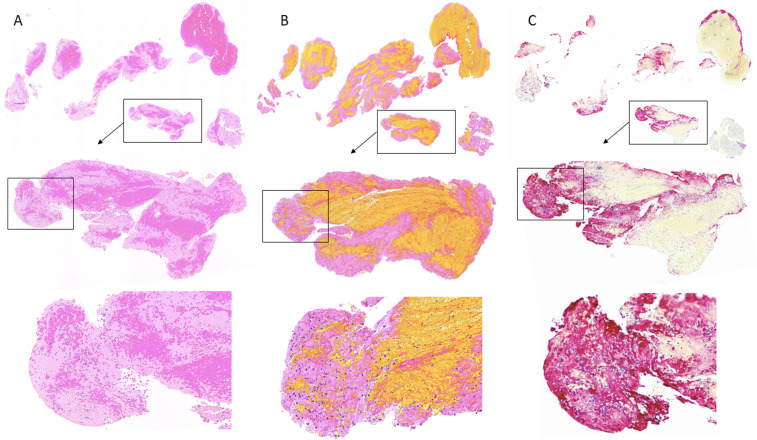
Figure 1.
Representative Thrombus showing high expression of CitH3 (C; red colour) in the area that has high number of neutrophils (A: H&E staining; B: MSB staining).
Table 2.
Comparison of thrombus composition between low and high NLR.
| NLR Group | P-value | ||
|---|---|---|---|
| Thrombus component (%) | Low | High | |
| H3Cit | 14.66 ± 16.24 | 22.79 ± 21.75 | 0.03 |
| VWF | 11.5 ± 11.87 | 17.13 ± 16.41 | 0.04 |
| Red Blood Cells | 50.21 ± 16.61 | 38.82 ± 25.23 | 0.02 |
| White Blood Cells | 2.8 ± 1.71 | 4.47 ± 2.81 | 0.008 |
| Fibrin | 19.07 ± 9.31 | 24.2 ± 10.63 | 0.02 |
| Platelets | 27.91 ± 12.4 | 32.49 ± 20.3 | 0.33 |
Abbreviations: NLR: Neutrophil-lymphocyte ratio, H3Cit: anti-citrulinated H3, VWF: Von Willebrand Factor.
Continuous data are summarized as mean and standard deviation.
Figure 2.
Scatter plot showing each component of thrombus for low NLR group (A) and high NLR group (B).
Association of NLR and NETs with etiology
There was no significant difference between emboli with cardio embolic and large artery atherosclerosis etiologies in terms of NLR (6.5 vs. 5.2, p = 0.5), RBC (42.4 vs. 43.4, p = 0.9), WBC (3.4 vs. 3.2, p = 0.8), fibrin (23.8 vs. 21.7, p = 0.7), platelet (30.4 vs. 31.7, p = 0.8), CitH3 (21.3 vs. 16.6, p = 0.5), vWF (12.4 vs. 18.3, p = 0.1).
Association of NLR and embolus composition with number of passes required for successful recanalization
Number of thrombectomy passes (single vs. multiple pass) required for successful recanalization (mTICI 2b or higher) was not associated with RBCs (43.4% vs. 46.2%, p = 0.56), WBCs (3.3% vs. 3.9%, p = 0.22), fibrin (22.2% vs. 20.8%, p = 0.54), platelet (30.5% vs. 29.7%, p = 0.84), and vWF (13.7% vs. 14.6%, p = 0.76) content of thrombi. CitH3 was higher in the thrombi of patients who require multiple passes for successful recanalization (12.2% vs. 23.66%, p = 0.01). NLR was also not associated with number of thrombectomy passes (5 .1 for single pass vs. 6 .2 multiple pass, p = 0 .4 1).
Discussion
In this study we demonstrated that a higher NLR is associated with higher NETs expression in emboli retrieved from patients suffering from LVO stroke. We also found that higher NLR is correlated with higher percentage of WBCs, fibrin and vWF as well as lower percentage of RBCs in thrombi. Our results also indicate that FPE is associated with the lower expression of NETs in emboli. These results are important because they suggest that a higher NLR could be used as a peripheral biomarker for a NETs-rich thrombus. As novel thrombolytic agents aimed at lysis of NETs within stroke emboli are further developed (i.e. DNAse), such easy to obtain laboratory values can help to identify a subset of patients who would most benefit from these novel thrombolytic agents.
Furthermore, our results suggest that systemic inflammation (i.e. a higher NLR) could be a major factor impacting the composition of stroke emboli. Lastly, the inverse association between NETs and FPE suggests that the presence of NETs within stroke emboli impact the physical properties of thrombi, rendering them harder to remove.
Thrombus- stabilizing feature of NETs suggests that DNase may increase the thrombolytic therapy effect by degrading NETs although it is unclear to what extent NETs degradation contributes to thrombolytic potential of DNase. 5 DNase has been shown to enhance tPA-mediated lysis of thrombi. Ducroux et al. reported that recombinant DNase 1 accelerates tPA-induced thrombolysis however DNase 1 alone was not effective. In another study, authors also reported that ex-vivo lysis of patient thrombi was more successful when combination of DNase 1 and tPA was used. 12
Deleterious effects of the acute inflammatory response have been subjects of research in recent years and the role of this natural response could play an important role during the development of AIS. 13 Neutrophil response and inflammatory mediators such as proinflammatory cytokines which include IL-1ß, IL-6, and TNFα during the occurrence of AIS could be counted as a protective measure and a paradoxically deleterious effect to the overall clinical outcome.14,15 Our results suggest that there is an association between systemic inflammation (as defined by a high NLR) and thrombus composition (higher NETs content).
First Pass Effect (FPE) is an important factor affecting patient clinical outcome and overall morbidity during recovery, the higher expression of NETs within the thrombus will reduce the probability of achieving FPE. 16 Previous studies have demonstrated that NETs are also correlated with higher amount of thrombectomy device passes to reach recanalization which will result in poorer clinical outcomes. This phenomenon is mainly due to the fact that NETs are the components of the fibrin rich thrombi that have a stiffer structure, and this makes thrombectomy procedures more difficult to perform, requiring more passes of the thrombectomy device. Ducroux et al. noted that thrombi with higher amounts of NETs may be correlated with reperfusion resistance during mechanical thrombectomy. 12 Also, Staessens et al. by studying the heterogenous thrombus composition noted the modification that fibrin suffers with the presence of NETs and its correlation with a higher number of device passes needed to achieve recanalization. 7
Our study also has limitations, including its single-center, retrospective cohort. Additionally, thrombi may not always have been removed en bloc during thrombectomy, and device manipulation during the procedure may cause thrombus fragmentation and subsequent embolization, affecting the composition of retrieved thrombi. Additionally, not all procedures were performed by the same operator. The experience and conditions to perform the procedures may change from one interventionist to the other which in turn can affect quality of thrombus retrieved.
Conclusion
NLR is correlated with the amounts of WBCs, fibrin, NETs and vWF within the thrombi retrieved from AIS patients due to LVO.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Neurological Disorders and Stroke (grant number RO1 NS105853).
ORCID iDs: Jorge Arturo Larco https://orcid.org/0000-0002-8259-2681
Mehdi Abbasi https://orcid.org/0000-0001-6978-2563
Yang Liu https://orcid.org/0000-0002-9132-0258
Daying Dai https://orcid.org/0000-0003-4051-6450
Ramanathan Kadirvel https://orcid.org/0000-0002-6786-9953
David F. Kallmes https://orcid.org/0000-0002-8495-0040
Waleed Brinjikji https://orcid.org/0000-0001-5271-5524
References
- 1.Brinjikji W, Duffy S, Burrows A, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg 2017; 9: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouwer PA, Brinjikji W, De Meyer SF. Clot pathophysiology: why Is It clinically important? Neuroimaging Clin N Am 2018; 28: 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Meyer SF, Andersson T, Baxter B, et al. Analyses of thrombi in acute ischemic stroke: a consensus statement on current knowledge and future directions. Int J Stroke 2017; 12: 606–614. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald S, Rossi R, Mereuta OM, et al. Per-pass analysis of acute ischemic stroke clots: impact of stroke etiology on extracted clot area and histological composition. J Neurointerv Surg 2021; 13: 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laridan E, Denorme F, Desender L, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol 2017; 82: 223–232. [DOI] [PubMed] [Google Scholar]
- 6.Essig F, Kollikowski AM, Pham M, et al. Immunohistological analysis of neutrophils and neutrophil extracellular traps in human thrombemboli causing acute ischemic stroke. Int J Mol Sci 2020; 21: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staessens S, De Meyer SF. Thrombus heterogeneity in ischemic stroke. Platelets 2020; 00: 1–9. [DOI] [PubMed] [Google Scholar]
- 8.Novotny J, Oberdieck P, Titova A, et al. Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology 2020; 94: e2346–e2360. [DOI] [PubMed] [Google Scholar]
- 9.Adams HP, jr, Bendixen BH, et al. d. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST trial of Org 10172 in acute stroke treatment. stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 10.Almekhlafi MA, Mishra S, Desai JA, et al. Not all “successful” angiographic reperfusion patients are an equal validation of a modified TICI scoring system. Interv Neuroradiol 2014; 20: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald S, Wang S, Dai D, et al. Orbit image analysis machine learning software can be used for the histological quantification of acute ischemic stroke blood clots. PloS one 2019; 14: e0225841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ducroux C, Di Meglio L, Loyau S, et al. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 2018; 49:754–757. [DOI] [PubMed]
- 13.Yu S, Arima H, Bertmar C, et al. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J Neurol Sci 2018; 387: 115–118. [DOI] [PubMed] [Google Scholar]
- 14.Rayasam A, Hsu M, Kijak JA, et al. Immune responses in stroke: how the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology 2018; 154: 363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malone K, Amu S, Moore AC, et al. The immune system and stroke: from current targets to future therapy. Immunol Cell Biol 2019; 97: 5–16. [DOI] [PubMed] [Google Scholar]
- 16.Abbasi M, Liu Y, Fitzgerald S, et al. Systematic review and meta-analysis of current rates of first pass effect by thrombectomy technique and associations with clinical outcomes. J Neurointerv Surgery 2021; 13: 212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]