Abstract
Patient serum opsonins against transferrin binding protein A+B (TbpA+B) complexes from two Neisseria meningitidis strains (K454 and B16B6, with 85- and 68-kDa TbpB, respectively) were quantified by a functional phagocytosis and oxidative burst assay. TbpA+B complexes adsorbed to fluorescent beads were opsonized with individual acute and convalescent sera from 40 patients infected by a variety of meningococcal strains. Flow cytometric quantitation of leukocyte phagocytosis products (PP) demonstrated that disease-induced serum opsonins recognized TbpA+B, and the highest anti-TbpA+B serum opsonic activities were found between admission to hospital and 6 weeks later. The PP values obtained with TbpA+B from strain B16B6 (PPB16B6) were higher than those obtained with TbpA+B from strain K454 (PPK454), with both acute and convalescent sera (P < 0.0001), and correlated positively with higher immunoglobulin G enzyme-linked immunosorbent assay titers against TbpA+B from strain B16B6 than from strain K454 (P < 0.001). In spite of considerable variations between individuals, significant correlations were found between the PPB16B6 and PPK454 values, and the PP values did not depend on the variability of the TbpB proteins of the disease-causing strains. Simultaneously measured oxidative burst activity correlated closely with the PP values. We conclude that highly cross-reactive anti-TbpA+B serum opsonins are produced during meningococcal disease. The anti-TbpA+B opsonic activities were not affected by the variability of the TbpB proteins of the disease-causing strains, which further adds to the evidence for the vaccine potential of meningococcal TbpA+B complexes.
Neisseria meningitidis infections continue to be a serious health problem worldwide. However, infection with one meningococcal strain in immunocompetent individuals seems to give life-long immunity to infection with homologous and heterologous serogroups, indicating that antibodies against subcapsular antigens may generate long-lasting and cross-protective immunity against meningococcal disease (3). Information regarding the immunogenicity of various outer membrane proteins during meningococcal infections is thus of interest in the complex process of determining the protective potential of future meningococcal vaccine components.
The relative contribution of bactericidal versus opsonic antibodies in the protection against meningococcal disease is not known. Whereas the majority of studies concerning the immune response following meningococcal disease and vaccination have focused on the role of human serum bactericidal activity against meningococci, some reports indicate that phagocytic elimination of meningococci is an important host defense mechanism (7, 26, 28, 30) which is stimulated by serum opsonins (complement and antibodies). Increasing serum opsonic activity against meningococci has been demonstrated during meningococcal disease (18, 19), and disease-induced opsonins have been shown to recognize meningococcal outer membrane components PorA and PorB (23).
There is considerable interest in the vaccine potential of N. meningitidis transferrin binding proteins (TbpA and TbpB, forming the TbpA+B complexes). Tbps are surface exposed on all meningococcal outer membranes in iron-restricted environments like mammalian tissue fluids. The TbpA+B complexes are involved in the uptake of iron from human transferrin (hTf) (16), which is necessary for bacterial survival and growth. Whereas TbpA is a highly conserved protein, two major families of TbpB molecules with high and low molecular masses have been identified (27). Antibodies to meningococcal TbpA+B complexes and to the isolated proteins have been detected in both patients and carriers (2, 12, 15, 20), and these antibodies have been shown to cross-react with Tbps isolated from different meningococcal strains (15). Tbps have also been shown to elicit bactericidal and protective antibodies in laboratory animals (4, 10, 24). No study has so far investigated the opsonic activity of anti-Tbp antibodies.
The aim of this study was to evaluate whether anti-Tbp serum opsonins are produced in response to meningococcal infections. Anti-TbpA+B opsonic activities were quantified in acute and convalescent sera from 40 patients infected by a variety of meningococcal strains, using TbpA+B from two different strains expressing high- and low-molecular-weight (MW) TbpBs in an antigen-specific opsonophagocytosis and oxidative burst assay (21, 22). The anti-TbpA+B opsonic activities were compared to anti-TbpA+B immunoglobulin G (IgG) responses, as evaluated by enzyme-linked immunosorbent assays (ELISAs).
MATERIALS AND METHODS
Patients and disease-causing strains.
Serum samples were obtained from 40 survivors (22 females and 18 males; ages, 14 to 58 years; median age, 18 years) of meningococcal disease within the first day of admission to Haukeland University Hospital, Bergen, Norway, between admission and 6 weeks (intermediate samples, available between days 3 and 24; median day, 15; n = 39 [Table 1]) and 6 weeks after admission.
TABLE 1.
Clinical characteristics of patients with meningococcal disease (n = 40) and characterization of associated strains
| Patient group and no.a | Meningococcal strain | Approx MW
(103)b
|
Disease categoryc | Preadmission symptoms (h) | Intermediate sampling day | |
|---|---|---|---|---|---|---|
| TbpA | TbpB | |||||
| I (n = 8) | ||||||
| 1 | B:15P1.7,16 | NT | NT | 2 | 12 | 15 |
| 2 | B:15P1.7,16 | 98 | 83 | 1 | 12 | 17 |
| 3 | B:15P1.7,16 | NT | NT | 1 | 18 | 16 |
| 4 (V−A+C[1]) | B:15P1.7,16 | NT | NT | 3 | 20 | 12 |
| 5 | B:15P1.7,16 | 95 | ND | 4 | 11 | 16 |
| 6 | B:15P1.7,16 | 98 | 81 | 1 | 25 | 10 |
| 7 | B:15P1.7,16 | NT | NT | 4 | 24 | 16 |
| 8 | B:15P1.7,16 | 98 | 83 | 2 | 10 | 17 |
| II (n = 7) | ||||||
| 9 | C:15P1.7,16 | 98 | 83 | 4 | 20 | |
| 10 | C:15P1.7,16 | 98 | 83 | 3 | 20 | 24 |
| 11 | C:15P1.7,16 | 95 | ND | 4 | 48 | 17 |
| 12 | C:15P1.7,16 | 98 | 83 | 1 | 16 | 8 |
| 13 | C:15P1.7,16 | 98 | 83 | 1 | 24 | 5 |
| 14 | C:15P1.7,16 | 98 | 81 | 2 | 16 | 7 |
| 15 | C:15P1.7,16 | NT | NT | 3 | 30 | 4 |
| III (n = 10) | ||||||
| 16 | B:15:P1.2 | 98 | 85 | 3 | 23 | 9 |
| 17 | B:15:P1.2,5 | 98 | 85 | 4 | 36 | 5 |
| 18 | B:15:P1.12,13 | NT | NT | 2 | 12 | 24 |
| 19 | B:15:P1.12,13 | NT | NT | 2 | 11 | 15 |
| 20 | B:15:P1.12 | 98 | 85 | 4 | 24 | 18 |
| 21 | B:15:P1.12 | NT | NT | 4 | 18 | 5 |
| 22 (V−B [3]) | B:15:P1.12 | 98 | 83 | 1 | 14 | 13 |
| 23 (V−B [3]) | B:15:P1.12 | 98 | 83 | 3 | 18 | 15 |
| 24 (V−B [3]) | B:15:P1.12 | NT | NT | 1 | 14 | 10 |
| 25 (V−B [1]) | B:15:P1.12 | NT | NT | 1 | 26 | 3 |
| IV (n = 6) | ||||||
| 26 | C:2a:P1.2 | 95 | 64 | 4 | 20 | 6 |
| 27 | C:2a:P1.2 | 98 | 68 | 2 | 11 | 13 |
| 28 | C:2a:P1.2 | 98 | 68 | 4 | 11 | 14 |
| 29 | C:2a:P1.2 | 98 | 85 | 2 | 28 | 4 |
| 30 (V−B [4] | C:2a:P1.2 | NT | NT | 2 | 20 | 15 |
| 31 (V−B [4] | C:2a:P1.2 | 98 | 68 | 2 | 10 | 17 |
| V (n = 9) | ||||||
| 32 | B:NT:P1.12 | 95 | 85 | 4 | 18 | 13 |
| 33 | B:NT:P1.16 | 98 | 85 | 1 | 24 | 18 |
| 34 | B:NT:P1.3 | NT | NT | 4 | 20 | 8 |
| 35 | B:NT:NT | 98 | 83 | 3 | 30 | 14 |
| 36 | B:4:NT | 98 | ND | 4 | 10 | 16 |
| 37 | B:4:P1.12 | 98 | 85 | 2 | 25 | 16 |
| 38 | B:8:P1.15 | 98 | 83 | 1 | 18 | 16 |
| 39 | B:19:P1.15 | 98 | 85 | 4 | 16 | 8 |
| 40 | Microscopyd | NT | NT | 3 | 11 | 14 |
V−A+C, immunized with meningococcal polysaccharide A+C vaccine; V−B, immunized with meningococcal serogroup B OMV vaccine. Numbers in brackets indicate years since vaccination.
ND, not determined, band not detected; NT, not tested.
1, meningitis; 2, septicemia with shock; 3, meningitis and septicemia with shock; 4, septicemia without septic shock and with or without meningitis.
Gram-negative diplococci in cerebrospinal fluid.
The patients were grouped according to the serogroup, serotype, and serosubtype patterns of the disease-causing strains (groups I to V [Table 1]). Also, the MWs of the TbpA and TbpB of 27 strains were determined by using a Western blot technique (Table 1). Briefly, the patient strains were grown overnight in Mueller-Hinton broth containing 10 μg of ethylenediamine di-o-hydroxyphenylacetic acid per ml. Bacteria were harvested by centrifugation and resuspended in phosphate-buffered saline (PBS)–2% (vol/vol) Elugent (Calbiochem, Nottingham, England) for 15 min before removal of the bacteria by centrifugation. The detergent extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 10% acrylamide gel, transferred to Immobilon P (Millipore Corporation, Bedford, Mass.), and probed with horseradish peroxidase (HRP)-conjugated hTf (Jackson ImmunoResearch Laboratories, West Grove, Pa.) for identification of TbpB or rabbit anti-recombinant TbpA serum followed by anti-rabbit IgG-HRP peroxidase conjugate.
Six patients were previously vaccinated with two doses of a meningococcal outer membrane vesicle (OMV) preparation (from N. meningitidis 44/76, B:15:P1.7,16) (14) (Table 1). The OMV vaccine did not contain Tbps (17), and the protective efficacy after two doses of this vaccine was estimated to be only 57% after a 29-month observation period (5). Patient 4 was immunized with the Meningococcal Polysaccharide A+C Vaccine (Pasteur-Merieux Serum and Vaccines, Lyon, France) 1 year prior to disease (Table 1).
Sera from five healthy students without previous meningococcal disease or vaccination and reaction mixtures without serum were included as controls. Control sera were also obtained from a patient with pneumococcal meningitis and a patient with varicella-zoster meningoencephalitis on admission to hospital and 2 and 6 weeks later.
All sera had normal complement activity as judged by the hemolytic activity of complement (50% hemolytic activity) and were stored in aliquots at −70°C until used.
Fluorochromes and buffers.
Polystyrene microspheres (Fluoresbrite Plain Microspheres PC Red; Polysciences Inc., Warrington, Pa.) and the oxidative burst indicators dihydrorhodamine 123 (DHR-123) and rhodamine 123 (R-123) (Molecular Probes, Eugene, Oreg.) were used (22, 29). Dulbecco's PBS (DPBS) was used with 5 × 10−3 M glucose and 5 mg of bovine serum albumin (BSA; Boehringer Mannheim GmbH, Mannheim, Germany) per ml (DPBS-GA) as well as 9 × 10−4 M CaCl2 · 2H2O and 5 × 10−4 M MgSO4 · H2O (21).
TbpA+B complexes.
TbpA+B complexes were prepared as described previously (2) from N. meningitidis K454 (B:15:P1.7,16 with TbpB of approximately 85 kDa) (15) and B16B6 (B:2a:P1.2 with TbpB of approximately 68 kDa) (27). These strains were chosen because the TbpBs are representative of the two families of high- and low-MW TbpB described by Rokbi et al. (27). The TbpA+B concentrations were determined by densitometry after SDS-PAGE, as evaluated against various concentrations of transferrin on the gels (6). The TbpA+B solution from strain K454 was concentrated from 535 to 800 μg/ml by a vacuum-operated filter unit (Immersible-CX units with agitator and low-binding ultrafilters with 10,000-MW cutoff; Millipore) to equal the concentration of the TbpA+B preparation from strain B16B6 prior to adsorption to polystyrene beads.
TbpA+B-coating of fluorescent beads.
Fluorescent polystyrene beads were coated with TbpA+B complexes from N. meningitidis K454 and B16B6. Briefly, 300 μl of fluorescent beads (4.55 × 1010 beads/ml) were washed twice in borate buffer (0.1 M boric acid [pH 8.5]) and incubated with 480 μg of TbpA+B complexes (600 μl of the 800-μg/ml solutions) with end-over-end rotation at room temperature (RT) overnight. Remaining sites on the bead surfaces were blocked with 2% (wt/vol) BSA in 0.1 M boric acid before suspension in storage buffer (21) and kept protected from daylight in aliquots at 4°C until used. The TbpA+B complexes retained the ability to bind hTf after adsorption to beads, as evaluated by binding of peroxidase-conjugated hTf (Jackson ImmunoResearch Laboratories).
Densitometry of SDS-polyacrylamide gels of TbpA+B solutions before and after incubation with beads (LKB UltraScan XL laser densitometer) demonstrated that TbpA+B had adsorbed to the beads, and the amount of adsorbed TbpA+B from strain K454 was 90% of that from strain B16B6. In addition, the differences in the amounts of TbpA+B bound to the beads were determined by binding of hTf. Briefly, doubling dilutions of TbpA+B-coated bead (TbpA+B-bead) suspensions (2.5 × 108 beads/ml) were incubated with peroxidase-conjugated hTf for 2.5 h, washed three times in sodium acetate-saline-Brij 35 buffer (120 M sodium acetate, 150 M NaCl, 0.05% [vol/vol] Brij 35), and exposed to 1,2-phenylenediamine dihydrochloride (orthophenylenediamine) in 0.1 M citric acid-phosphate buffer (pH 5.0). The reaction was stopped with 1.25 M H2SO4, and the optical densities of the supernatants were read at 492 nm (Titertek Multiscan MCC; Labsystems, Turku, Finland). The optical densities of supernatants from reaction mixtures with strain K454 TbpA+B-beads were about 90% (89 to 93%) of those obtained with corresponding amounts of strain B16B6 TbpA+B-beads (not shown).
Leukocytes.
As previously described, human leukocytes were separated and adjusted to 1.25 × 107 nonlymphocytes (polymorphonuclear leukocytes and monocytes, i.e., the potentially phagocytosing cells) per ml in DPBS-GA (21).
Phagocytosis and oxidative burst assays.
TbpA+B-beads and control beads coated with BSA were opsonized with patient and control sera and incubated with DHR-123 and leukocytes as previously described for other antigen-coated beads (21–23) except that the incubation time was extended to 15 min followed by flow cytometry (FCM) analysis (Coulter Epics XL-MCL flow cytometer; Coulter Corporation, Harpenden, England).
The green R-123 and the red bead fluorescence were collected in separate FCM channels, and electronic color compensations eliminated spectral overlaps between the fluorochromes. The FCM coincidence rate was repeatably 1 to 2%, and daily calibrations were performed (DNA-Check, EPICS Alignment Fluorospheres; Coulter) (22).
FCM parameters.
Nonlymphocytes were analyzed for associated R-123 and bead fluorescence (22). The percentage of phagocytosing nonlymphocytes was defined as the percentage of nonlymphocytes with associated bead fluorescence, and the mean number of beads per phagocytosing cell was calculated by dividing the mean bead fluorescence associated with nonlymphocytes by the fluorescence of single beads (21, 22). The phagocytosis product (PP) was defined as the percentage of phagocytosing nonlymphocytes multiplied by the mean number of beads per phagocytosing cell (23). The PP values were designated with the strain from which the TbpA+B complexes were isolated (PPK454 and PPB16B6, respectively). Oxidative burst activity was reflected by the mean nonlymphocyte R-123 fluorescence (23).
ELISAs.
Purified TbpA+B complexes (1 μg/ml in 0.05 M sodium carbonate buffer [pH 9.6], 100 μl per well) isolated from N. meningitidis K454 and B16B6 were coated onto 96-well plates (Maxisorb Immuno-plate; Nunc, Roskilde, Denmark) and incubated overnight at RT. Blocking was performed with 200 μl of PBS containing 0.01% (vol/vol) Tween 20 and 10% (vol/vol) newborn calf serum (blocking buffer) for 1 h at RT. Serial dilutions of test sera were prepared in blocking buffer in 96-well Serowell plates (Bibby Sterilin Ltd., Stone, Staffordshire, England), transferred to the coated plates, and incubated at RT for 2 h. After washing, 100 μl of an appropriate dilution of either biotin-conjugated anti-human pan-IgG (Stratech Scientific, Luton, England) or biotin-conjugated anti-human Ig subclass-specific antibodies (Sigma, Poole, Dorset, England) was added to each well and incubated for 1 h. The plates were washed, and 100 μl of an appropriate dilution of streptavidin-HRP conjugate (Pierce, Chester, Cheshire, England) was added to each well and incubated for 1 h at RT. Finally, after washing the plates, 100 μl of TMBlue substrate solution (Intergen, Milford, Mass.) was added to each well and incubated with shaking for 10 min at RT. The reaction was stopped with 50 μl of 2 M sulfuric acid per well, and absorbances were measured at 450 nm on a Titertek ELISA reader (MCC 340; Life Sciences International, Basingstoke, Hampshire, England). All plates contained duplicate rows of pooled 6-week-postinfection sera as a standard. ELISA titers were expressed as the reciprocal of serum dilutions required to obtain the midpoint of the standard dose-response curve, and titers on each plate were adjusted to the standard serum titer.
CLSM.
Confocal laser scanning microscopy (CLSM) (MRC 1000; Bio-Rad, Hemel Hempstead, England) was performed immediately after incubation of opsonized TbpA+B- and BSA-coated beads with leukocytes as described for the FCM assay (21–23).
Statistical methods.
The FCM results are presented as means of duplicate measurements. Nonparametric statistics (median and range) were employed. Wilcoxon's signed rank test was used to determine differences between data. A P value of <0.05 was considered statistically significant. Correlations were evaluated by Spearman's rank correlation coefficient.
RESULTS
Phagocytosis.
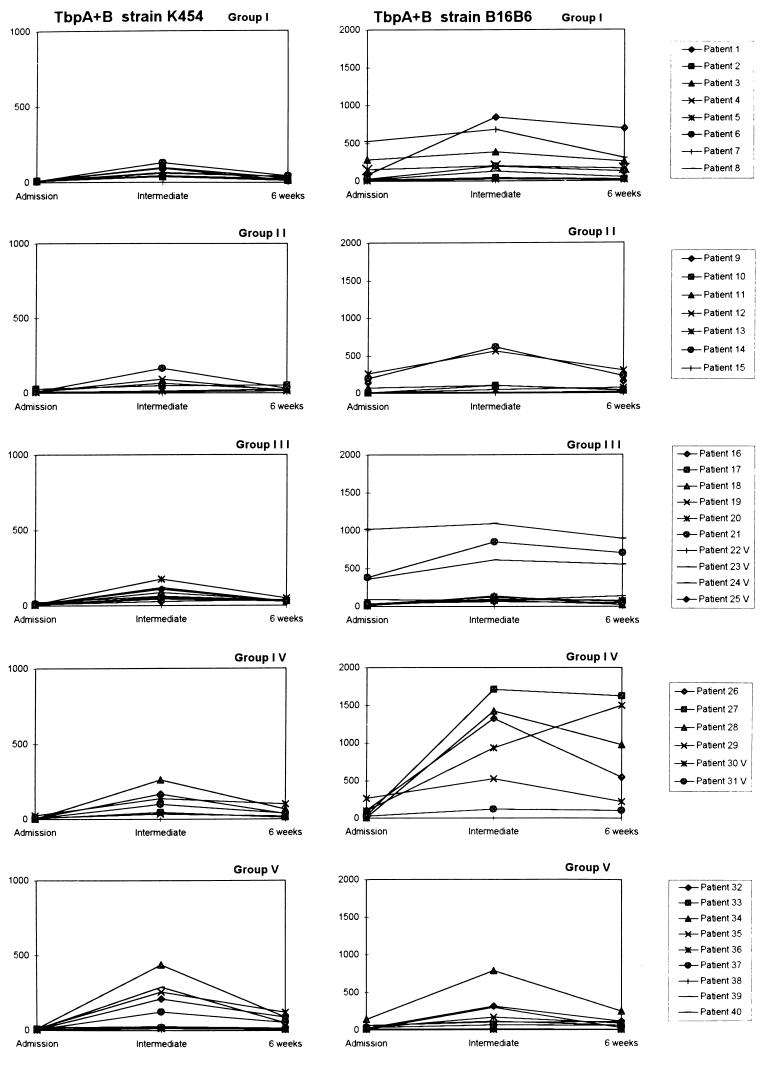
The amount of opsonins that recognized TbpA+B complexes increased in patient sera during the course of meningococcal disease, as reflected by enhanced phagocytosis of opsonized antigen-coated beads by human leukocytes (Fig. 1). Increases in patient serum opsonic activities were most frequently reflected in both the percentage of phagocytosing nonlymphocytes and the mean number of beads per cell, as indicated in the summary of FCM parameters obtained with TbpA+B-beads opsonized with sera from patients in groups I to V (Table 2). However, since both of these parameters are required to describe the total opsonophagocytosis, the product of these two parameters (PP) was used to present the anti-TbpA+B serum opsonic activities (PPK454 and PPB16B6, using beads with TbpA+B from strains K454 and B16B6, respectively) (Fig. 1).
FIG. 1.
Serum opsonic activities of individual patients in groups I to V (Table 1) against meningococcal TbpA+B complexes from meningococcal strains K454 and B16B6 during meningococcal disease as reflected by PPK454 and PPB16B6, respectively. Note different scales on the PPK454 and PPB16B6 ordinates. The antigen-specific opsonic activities were measured in sera obtained on admission to hospital (admission), between admission and 6 weeks later (intermediate), and 6 weeks after admission (6 weeks).
TABLE 2.
Phagocytosis and oxidative burst responses mediated by anti-TbpA+B serum opsonins in meningococcal disease patients
| Parameter and patient groupa | Median (range) for beads
coated with TbpA+B from strain:
|
|||||
|---|---|---|---|---|---|---|
| K454
|
B16B6
|
|||||
| Admission | Intermediateb | 6 wk | Admission | Intermediateb | 6 wk | |
| % Phagocytosing nonlymphocytesc | ||||||
| I | 6 (4–7) | 37 (24–57) | 19 (11–30) | 41 (7–94) | 53 (16–97) | 55 (12–97) |
| II | 6 (4–20) | 33 (6–65) | 18 (9–37) | 45 (4–87) | 52 (8–91) | 53 (11–90) |
| III | 6 (3–15) | 34 (18–65) | 22 (17–32) | 29 (9–98) | 42 (30–98) | 50 (14–98) |
| IV | 5 (4–13) | 47 (24–75) | 25 (9–45) | 41 (7–87) | 95 (39–97) | 92 (39–98) |
| V | 6 (4–12) | 58 (8–86) | 29 (6–56) | 25 (6–67) | 57 (6–93) | 35 (4–72) |
| Beads/phagocytosing nonlymphocyte (mean no.) | ||||||
| I | 1.3 (1.1–1.7) | 1.9 (1.4–2.3) | 1.4 (1.2–1.6) | 1.4 (1.2–5.6) | 3.9 (1.6–8.7) | 2.6 (1.3–7.1) |
| II | 1.4 (1.3–1.6) | 1.8 (1.3–2.5) | 1.4 (1.2–1.7) | 1.5 (1.0–3.0) | 2.1 (1.3–6.8) | 1.3 (1.3–3.4) |
| III | 1.4 (1.2–2.1) | 1.8 (1.5–2.7) | 1.4 (1.3–1.7) | 1.3 (1.2–10.3) | 2.6 (2.2–11.1) | 2.1 (1.5–9.1) |
| IV | 1.3 (1.1–1.8) | 2.5 (1.6–3.5) | 1.5 (1.2–2.3) | 1.4 (1.1–3.0) | 11.9 (3.0–17.6) | 8.4 (2.7–16.6) |
| V | 1.4 (1.0–1.6) | 2.1 (1.3–3.8) | 1.5 (1.3–2.2) | 1.2 (1.1–2.1) | 2.9 (1.1–8.5) | 1.8 (1.2–3.5) |
| PPd | ||||||
| I | 7 (7–12) | 63 (37–128) | 27 (13–42) | 54 (8–522) | 199 (27–843) | 144 (16–690) |
| II | 9 (6–26) | 57 (8–165) | 22 (14–52) | 69 (5–260) | 103 (10–614) | 71 (14–304) |
| III | 8 (6–22) | 58 (27–177) | 31 (27–48) | 35 (11–1,015) | 108 (64–1,094) | 76 (20–890) |
| IV | 6 (4–23) | 120 (36–262) | 37 (11–100) | 67 (8–265) | 1,130 (117–1,709) | 762 (103–1,623) |
| V | 8 (4–18) | 123 (13–437) | 51 (7–121) | 29 (7–141) | 112 (7–785) | 62 (5–249) |
| Oxidative burste | ||||||
| I | 0.2 (0.2–0.2) | 0.5 (0.3–1.1) | 0.3 (0.2–0.4) | 0.4 (0.2–2.1) | 1.6 (0.3–10.5) | 0.9 (0.2–7.4) |
| II | 0.2 (0.2–3.3) | 0.5 (0.2–0.7) | 0.2 (0.2–0.5) | 0.5 (0.2–5.6) | 0.9 (0.2–3.9) | 0.4 (0.2–1.4) |
| III | 0.2 (0.2–0.3) | 0.4 (0.2–0.7) | 0.3 (0.2–0.4) | 0.3 (0.3–13.0) | 0.7 (0.3–17.1) | 0.5 (0.2–13.7) |
| IV | 0.2 (0.2–0.3) | 0.7 (0.3–1.0) | 0.3 (0.2–0.7) | 0.4 (0.2–0.9) | 11.5 (0.7–24.3) | 7.4 (0.6–24.7) |
| V | 0.2 (0.2–0.3) | 0.5 (0.2–4.4) | 0.3 (0.2–0.8) | 0.2 (0.2–0.9) | 0.7 (0.2–10.1) | 0.4 (0.2–2.2) |
Allocation of patients into groups depending on serogroups, serotypes, and subtypes of meningococcal strains (Table 1).
Serum samples from 39 patients on days 3 to 24 (median, day 15) (Table 1).
Percent nonlymphocytes with associated bead fluorescence.
Percent phagocytosing nonlymphocytes multiplied by mean number of beads per phagocytosing cell.
Mean nonlymphocyte R-123 fluorescence.
The majority of patients had low anti-TbpA+B serum opsonic activities on admission to hospital (Fig. 1; Table 2). In all except one patient (patient 30), the highest anti-TbpA+B opsonic activities were measured in intermediate serum samples (n = 39; median, day 15; range, day 3 to 24; P < 0.0001 for both PPK454 and PPB16B6 values, comparing intermediate and admission sera). The PP values obtained with 6-week sera were lower than those obtained with intermediate sera (P < 0.0001 for both PPK454 and PPB16B6 values), but they were still significantly higher than PP values obtained with admission sera (P < 0.0001 for both PPK454 and PPB16B6 values).
The overall magnitudes of the PPB16B6 values were higher than the PPK454 values (P < 0.0001, using admission, intermediate, and 6-week sera). The median (range) PPK454 were 7 (4 to 26), 64 (8 to 437), and 30 (7 to 121), using patient admission, intermediate, and 6-week sera, respectively. The median (range) PPB16B6 were 34 (5 to 1015), 138 (7 to 1709), and 107 (5 to 1623), using admission, intermediate, and 6-week sera, respectively. However, significant correlations were found between the PPK454 and PPB16B6 values for admission (r = 0.36 [P < 0.05]) and intermediate (r = 0.55 [P < 0.01]) sera.
The patients were grouped according to the serogroups, serotypes, and serosubtypes of the disease-causing strains (Table 1). Considerable interindividual variations in the anti-TbpA+B opsonic activities were demonstrated in patient sera within the groups (Fig. 1; Table 2). The PPB16B6 values induced by intermediate sera from patients of group IV (C:2a:P1.2 disease) were higher than those obtained with intermediate sera from the other patients (P = 0.046) (Table 2). Apart from this, no significant differences in PP values were found between the patient groups.
Strain K454 expressed a 98-kDa TbpA and a 85-kDa (high-MW) TbpB, whereas strain B16B6 expressed a 98-kDa TbpA and a 68-kDa (low-MW) TbpB protein. The MWs of the TbpA and TbpB proteins of 27 of the disease-causing strains were also identified (Table 1). Twenty strains expressed high-MW TbpB proteins (81 to 85 kDa), four strains in patient group IV expressed low-MW TbpB proteins (64 to 68 kDa), whereas no TbpB bands were observed in three of the strains. Low but interindividually variable PPK454 values were observed using intermediate sera both from patients infected by strains with high- and low-MW TbpB. The highest PPB16B6 values were detected in three of the four intermediate sera from patients infected by strains expressing low-MW TbpB (Table 1; Fig. 1). However, no statistically significant differences in PPB16B6 values were observed between sera from patients infected by low- versus high-MW TbpB-expressing strains (P = 0.068).
Seven patients were previously vaccinated with either a complex serogroup B meningococcal outer membrane vesicle vaccine preparation without expressed Tbps (n = 6) or a serogroup A+C polysaccharide-based vaccine (n = 1) (Table 1). No significant differences were found between the vaccinee anti-TbpA+B opsonic activities compared with those of the other patients.
Sera from five healthy students induced a median PPK454 of 15 (range, 7 to 28) and a median PPB16B6 of 111 (range, 29 to 284). The anti-TbpA+B opsonic activities in the control patients did not change during pneumococcal meningitis and varicella-zoster meningoencephalitis.
Oxidative burst.
The phagocyte oxidative burst responses induced by antigen-specific opsonins were reflected by the intracellular oxidation of the nonfluorescent substrate DHR-123 by reactive oxygen metabolites to green-fluorescent R-123 (Table 2). The mean R-123 fluorescence after stimulation with opsonized TbpA+B-coated beads corresponded to the PP values obtained with admission sera (r = 0.41 and 0.92 [P < 0.01] for anti-K454 TbpA+B and anti-B16B6 TbpA+B activities, respectively), with intermediate sera (r = 0.90 and 0.99 [P < 0.01] for anti-K454 TbpA+B and anti-B16B6 TbpA+B activities, respectively), and with 6-week sera (r = 0.78 and 0.99 [P < 0.01] for anti-K454 TbpA+B and anti-B16B6 TbpA+B activities, respectively).
Sera from five healthy students induced median R-123 fluorescences of 0.20 (range, 0.18 to 0.27) and 0.24 (range, 0.21 to 1.52), using opsonized beads coated with TbpA+B from strains K454 and B16B6, respectively. R-123 fluorescences obtained with control patient sera did not change during pneumococcal meningitis and varicella-zoster meningoencephalitis (data not shown).
ELISA.
The medians and ranges of serum titers obtained with each antigen in intermediate samples are given in Table 3; the highest titers were observed with TbpA+B from strain B16B6. Greater than fivefold rises in titer during meningococcal disease were found against K454 TbpA+B in 40% of intermediate and 42.5% of 6-week sera and against B16B6 TbpA+B in 60% of intermediate sera and 45% of 6-week sera. Furthermore, 40% of sera showed a greater than fivefold rise in titer to Tbps isolated from both strains. Sera were also judged to have a positive response to each antigen when a titer greater than three times the dilution cutoff was obtained. Using this analysis, 67.5% of intermediate and 70% of 6-week sera were positive for K454 TbpA+B, while 82.5% of intermediate and 70% of 6-week sera were positive for B16B6 TbpA+B.
TABLE 3.
Meningococcal disease patient intermediate sera with anti-TbpA+B main and subclass IgG antibodies, as evaluated by ELISA
| Antibody | Median (range; no. of sera tested) IgG
titera with TbpA+B from strain:
|
|
|---|---|---|
| K454 | B16B6 | |
| IgG | 711 (35–10,781; 39) | 1,437 (54–15,683; 39) |
| IgG1b | 445 (22–5,203; 32) | 1,523 (72–17,615; 33) |
| IgG2 | 65 (65–3,256; 32) | 52 (52–2,694; 33) |
| IgG3b | 588 (53–7,342; 31) | 67 (31–3,583; 33) |
| IgG4b | 54 (54–68; 31) | 47 (47–67; 33) |
Reciprocal of serum dilution required to give 50% endpoint absorbance of the standard (pooled convalescent sera).
Assay performed only with sera with IgG titers above 150.
The titers of anti-TbpA+B IgG antibodies in the meningococcal disease patient sera were correlated to the anti-TbpA+B opsonic activities, as reflected by PPK454 values (r = 0.04 [nonsignificant], r = 0.33 [P < 0.05], and r = 0.5 [P < 0.01] between anti-K454 TbpA+B IgG and PPK454 values using admission, intermediate, and 6-week sera, respectively) and by PPB16B6 values (r = 0.24, 0.37, and 0.33 [P < 0.05] between anti-B16B6 TbpA+B IgG and PPB16B6 values using admission, intermediate, and 6-week sera, respectively). The anti-B16B6 TbpA+B IgG titers were significantly higher than the anti-K454 TbpA+B IgG titers in intermediate sera (P < 0.001), whereas no significant differences were found between the IgG titers against TbpA+B from the two strains in admission and 6-week sera.
Anti-TbpA+B IgG subclass analyses were performed in intermediate sera with anti-TbpA+B IgG titers above 150. The responses were mainly IgG1 and/or IgG3 subclasses (Table 3). Similar amounts of IgG1 and IgG3 were detected against TbpA+B from strain K454 (median titers of 445 and 588, respectively), whereas mainly IgG1 was found against TbpA+B from strain B16B6 (median titer of 1,523) (Table 3).
CLSM.
CLSM images confirmed that opsonized TbpA+B-beads were phagocytosed and that R-123 fluorescence was induced (not shown).
DISCUSSION
We have demonstrated for the first time that human serum opsonins produced during meningococcal disease recognize epitopes on meningococcal TbpA+B complexes. The antigen-specificity needed to perform this study was ensured by adsorption of isolated TbpA+B to polystyrene beads. Also, the antigens retained the ability to bind hTf after adsorption to the beads, indicating that their conformation was similar to that of native proteins. This provided a mimicry of bacteria with selected antigens surface-exposed and available for in vitro recognition and attachment of TbpA+B-specific serum opsonins.
Increased patient anti-TbpA+B serum opsonic activities were detected during disease. The overall magnitudes of the patient opsonic responses against TbpA+B from strain B16B6 were significantly higher than those observed against complexes from strain K454 (Fig. 1). This could in part be due to a 10% lower degree of adsorption of TbpA+B complexes from strain K454 to the beads than that of the B16B6 complexes. However, the IgG titers against B16B6 TbpA+B were higher than against K454 TbpA+B in the same intermediate sera, strongly indicating that the higher amount of antibodies detected against TbpA+B from strain B16B6 during disease were responsible for the higher opsonic activities against TbpA+B from strain B16B6 than from K454.
Even though considerable variations between individuals were observed in the anti-Tbp A+B serum opsonic activities, significant correlations were found between the PPK454 and PPB16B6 values, indicating human opsonic cross-reactivity against TbpA+B epitopes. Also, in spite of higher PPB16B6 in patient group IV, the PP values were found to be statistically independent of the MW of the TbpB of the disease-causing strains. Accordingly, the cross-reactive opsonins may be directed against the more conserved TbpA molecule or against epitopes present on both high- and low-MW TbpBs or epitopes formed by the TbpA+B complex. A previous study with rabbit sera and Western blot techniques demonstrated little cross-reaction between the higher- and lower-MW TbpB isotypes (27). Also, antibodies raised in rabbits to Tbps from B16B6 have been shown to be bactericidal, but only against strains with the same TbpB MW isotype (24). The immune responses to TbpA and TbpB have, however, been shown to vary considerably depending on the route of antigen administration, the immunological method used, and the species-specific immune reactivity in various murine versus human host species (2). Thus, conclusions regarding the immunogenicity of TbpA and TbpB and, accordingly, the usefulness of Tbps as meningococcal vaccine candidates depend on data on the human immune responses to Tbps. Antibodies to meningococcal TbpA+B complexes and to the isolated proteins have been detected in both patients and carriers (2, 12, 15, 20), and these antibodies have been shown to cross-react with Tbps isolated from different meningococcal strains (15). To further clarify the cross-reactiveness of the anti-Tbp patient opsonins observed in the present study, studies are planned to evaluate patient opsonic responses against separate TbpA and TbpB molecules.
The anti-TbpA+B IgG responses were primarily of subclasses IgG1 and IgG3. These are the most effective to trigger complement activation and bind to Fcγ receptors (1, 8, 31), which implies that the anti-TbpA+B opsonic activities primarily are due to disease-induced antigen-specific IgG1 and IgG3 antibodies. Whereas IgG1 was detected against TbpA+B from both strains, IgG3 was found almost entirely against TbpA+B from strain K454. The reason for this is not known.
Phagocytic uptake of bacteria without initiation of intracellular killing mechanisms has been suggested as a means by which meningococci invade the host (3, 13). Since both ingestion and oxidative killing are probably needed to mount an effective phagocytic clearance of invading bacteria, the effects of human anti-TbpA+B opsonins on both of these steps were evaluated in the present study. With this in vitro approach to reflect the antigen specificity of the serum opsonic activities of surviving patients, the results indicate that anti-TbpA+B antibodies generate functional serum opsonic responses.
Meningococcal Tbps are essential for the acquisition of iron from hTf and are expressed by all strains during growth in iron-restricted environments such as human tissue fluids. Accordingly, it is thought that Tbps are vital for meningococcal pathogenicity. The importance of Tbps for gonococcal pathogenicity has been demonstrated in a human challenge experiment, as a mutant lacking TbpA+B failed to cause disease (9). Murine antibodies raised against the TbpA+B complex have been shown to block the bacterial acquisition of iron from hTf (25). TbpA+B complexes have also been shown to elicit bactericidal antibodies following immunization of mice (4, 10, 24) and after vaccination of adult volunteers (11), and the present study demonstrates that human opsonic antibodies produced in response to meningococcal disease are directed against TbpA+B epitopes. The multifunctional nature of anti-Tbp antibodies suggests that the meningococcal TbpA+B complex may be a multipotent meningococcal vaccine candidate.
We conclude that human serum opsonins produced during meningococcal disease recognize meningococcal TbpA+B complexes and that both phagocytosis and intracellular oxidative burst activities are initiated by anti-TbpA+B opsonins. TbpA+B from strain B16B6 was the most immunogenic in both ELISA and functional phagocytic assays, but extensive opsonic cross-reactivity was demonstrated against TbpA+B from the two strains. Functional studies with separate TbpA and TbpB molecules as target antigens are planned to clarify the relative importance of the TbpA and TbpB components of the complex as mediators of serum opsonic responses during meningococcal disease.
ACKNOWLEDGMENTS
The serotyping of N. meningitidis strains was performed at the National Institutes of Public Health, in Oslo, Norway, and in Birmingham, England. The serum complement hemolytic activity was measured by the Department of Microbiology and Immunology, Haukeland University Hospital, Bergen, Norway. Steinar Sørnes (Institute of Medicine) and Eduardo Ramirez (FFS-Medical Research Center, University of Bergen) are thanked for assisting with FCM and CLSM analyses. The CLSM was provided by the FFS-Medical Research Center, University of Bergen.
The research performed at CAMR was funded by the United Kingdom Department of Health.
REFERENCES
- 1.Aase A, Michaelsen T E. Opsonophagocytosis activity induced by chimeric antibodies of the four human IgG subclasses with or without help from complement. Scand J Immunol. 1994;39:581–587. doi: 10.1111/j.1365-3083.1994.tb03416.x. [DOI] [PubMed] [Google Scholar]
- 2.Ala'Aldeen D A A, Stevenson P, Griffiths E, Gorringe A, Irons L I, Robinson A, Hyde S, Borriello S P. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect Immun. 1994;62:2984–2990. doi: 10.1128/iai.62.7.2984-2990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ala'Aldeen D A A, Griffiths E. Vaccines against meningococcal diseases. In: Ala'Aldeen D A A, Hormaeche C E, editors. Molecular and clinical aspects of bacterial vaccine development. Chichester, England: John Wiley & Sons Ltd.; 1995. pp. 1–39. [Google Scholar]
- 4.Ala'Aldeen D A A, Borriello S P. The meningococcal transferrin-binding proteins 1 and 2 are both surface exposed and generate bactericidal antibodies capable of killing homologous and heterologous strains. Vaccine. 1996;14:49–53. doi: 10.1016/0264-410x(95)00136-o. [DOI] [PubMed] [Google Scholar]
- 5.Bjune G, Høiby E A, Grønnesby J K, Arnesen Ø, Fredriksen J H, Halstensen A, Holten E, Lindbak A-K, Nøkleby H, Rosenqvist E, Solberg L K, Closs O, Eng J, Frøholm L O, Lystad A, Bakketeig L S, Hareide B. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 6.Boulton I C, Gorringe A R, Allison N, Robinson A, Gorinsky B, Joannou C L, Evans R W. Transferrin binding protein B isolated from Neisseria meningitidisdiscriminates between apo and diferrin human transferrin. Biochem J. 1998;334:269–273. doi: 10.1042/bj3340269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredius R G M, Derkx B H F, Fijen A P, de Wit T P M, de Haas M, Weening R S, van de Winkel J G J, Out T A. Fcγ receptor IIa (CD32) polymorphism in fulminant meningococcal shock in children. J Infect Dis. 1994;170:848–853. doi: 10.1093/infdis/170.4.848. [DOI] [PubMed] [Google Scholar]
- 8.Burton D R, Gregory L, Jefferis R. Aspects of the molecular structure of IgG subclasses. Monogr Allergy. 1986;50:510–516. [PubMed] [Google Scholar]
- 9.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 10.Danve B, Lissolo L, Mignon M, Dumas P, Colombani S, Schryvers A B, Quentin-Millet M-J. Transferrin-binding proteins isolated from Neisseria meningitidiselicit protective and bactericidal antibodies in laboratory animals. Vaccine. 1993;11:1214–1220. doi: 10.1016/0264-410x(93)90045-y. [DOI] [PubMed] [Google Scholar]
- 11.Danve B, Lissolo L, Guinet F, Boutry E, Speck D, Cadoz M, Nassif W, Quentin-Millet M J. Safety and immunogenicity of a Neisseria meningitidis groupB transferrin binding protein vaccine in adults. In: Nassif X, Quentin-Millet M-J, Taha M-K, editors. Abstracts of the Eleventh International Pathogenic Neisseria Conference, 1 to 6 November 1998, Nice, France. Paris, France: Editions E.D.K.; 1998. p. 53. [Google Scholar]
- 12.Ferreiros C M, Ferron L, Criado M T. In vivo human immune response to transferrin-binding protein 2 and other iron-regulated proteins of Neisseria meningitidis. FEMS Immun Med Microbiol. 1994;8:63–68. doi: 10.1111/j.1574-695X.1994.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 13.Figueroa J E, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredriksen J H, Rosenqvist E, Wedege E, Bryn K, Bjune G, Frøholm L O, Lindbak A K, Møgster B, Namork E, Rye U, Stabbetorp G, Winsnes R, Aase B, Closs O. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1991;14:67–80. [PubMed] [Google Scholar]
- 15.Gorringe A R, Borrow R, Fox A J, Robinson A. Human antibody response to meningococcal transferrin binding proteins: evidence for vaccine potential. Vaccine. 1995;13:1207–1212. doi: 10.1016/0264-410x(95)00055-6. [DOI] [PubMed] [Google Scholar]
- 16.Gray-Owen S D, Shryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths E, Sierra G, Holst J. Quality control of the Cuban and Norwegian serogroup B vaccines used in the Iceland study. In: Evans J S, Jost S E, Maiden M C J, Feavers I M, editors. Neisseria 94. 1994. pp. 437–438. Proceedings of the Ninth International Pathogenic Neisseria Conference, Winchester, United Kingdom. [Google Scholar]
- 18.Guttormsen H-K, Bjerknes R, Næss A, Lehmann V, Halstensen A, Sørnes S, Solberg C O. Cross-reacting serum opsonins in patients with meningococcal disease. Infect Immun. 1992;60:2777–2783. doi: 10.1128/iai.60.7.2777-2783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halstensen A, Sjursen H, Vollset S E, Frøholm L O, Næss A, Matre R, Solberg C O. Serum opsonins to serogroup B meningococci in meningococcal disease. Scand J Infect Dis. 1989;21:267–276. doi: 10.3109/00365548909035696. [DOI] [PubMed] [Google Scholar]
- 20.Johnson A S, Gorringe A R, Fox A J, Borrow R, Robinson A. Analysis of the human Ig isotype response to individual transferrin binding proteins A and B from Neisseria meningitidis. FEMS Immunol Med Microbiol. 1997;19:159–167. doi: 10.1111/j.1574-695X.1997.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann A K, Halstensen A, Holst J, Bassøe C-F. Functional assays for evaluation of serogroup B meningococcal structures as mediators of human opsonophagocytosis. J Immunol Methods. 1997;200:55–68. doi: 10.1016/s0022-1759(96)00185-8. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann A K, Halstensen A, Bassøe C-F. Flowcytometric quantitation of human opsonophagocytosis and oxidative burst responses to meningococcal antigens. Cytometry. 1998;33:406–413. doi: 10.1002/(sici)1097-0320(19981201)33:4<406::aid-cyto3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann A K, Halstensen A, Aaberge I S, Holst J, Michaelsen T E, Sørnes S, Wetzler L M, Guttormsen H-K. Human opsonins induced during meningococcal disease recognize outer membrane proteins PorA and PorB. Infect Immun. 1999;67:2552–2560. doi: 10.1128/iai.67.5.2552-2560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lissolo L, Maitre-Wilmotte G, Dumas P, Mignon M, Danve B, Quentin-Millet M-J. Evaluation of transferrin-binding protein 2 within the transferrin-binding protein complex as a potential antigen for future meningococcal vaccines. Infect Immun. 1995;63:884–890. doi: 10.1128/iai.63.3.884-890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pintor M, Ferron L, Gomez J A, Powell N B L, Ala'Aldeen D A A, Borriello S P, Criado M T, Ferreiros C M. Blocking of iron uptake from transferrin binding proteins in Neisseria meningitidis. Microb Pathog. 1996;20:127–139. doi: 10.1006/mpat.1996.0012. [DOI] [PubMed] [Google Scholar]
- 26.Raff H V, Devereux D, Shuford W, Abbot-Brown D, Maloney G. Human monoclonal antibody with protective activity for Escherichia coli K1 and Neisseria meningitidisgroup B infections. J Infect Dis. 1988;157:118–126. doi: 10.1093/infdis/157.1.118. [DOI] [PubMed] [Google Scholar]
- 27.Rokbi B, Mazarin V, Maitre-Wilmotte G, Quentin-Millet M-J. Identification of two major families of transferrin receptors among Neisseria meningitidisstrains based on antigenic and genomic features. FEMS Microbiol Lett. 1993;110:51–58. doi: 10.1111/j.1574-6968.1993.tb06294.x. [DOI] [PubMed] [Google Scholar]
- 28.Ross S C, Rosenthal P J, Berberich H M, Densen P. Killing of Neisseria meningitidisby human neutrophils: implications for normal and complement-deficient individuals. J Infect Dis. 1987;155:1266–1275. doi: 10.1093/infdis/155.6.1266. [DOI] [PubMed] [Google Scholar]
- 29.Rothe G, Oser A, Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil activity in neutrophil granulocytes. Naturwissenschaften. 1988;75:354–355. doi: 10.1007/BF00368326. [DOI] [PubMed] [Google Scholar]
- 30.Schlesinger M, Greenberg R, Levy J, Kaythy H, Levy R. Killing of meningococci by neutrophils: effect of vaccination on patients with complement deficiency. J Infect Dis. 1994;170:449–453. doi: 10.1093/infdis/170.2.449. [DOI] [PubMed] [Google Scholar]
- 31.Van de Winkel J G J, Capel P J A. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]