Abstract
Background:
It is unclear, whether the initial disease severity may help to predict which COVID-19 patients at risk of developing persistent symptoms.
Aim:
The aim of this study was to examine whether the initial disease severity affects the risk of persistent symptoms in post-acute COVID-19 syndrome and long COVID.
Methods:
A systematic search was conducted using PUBMED, Google Scholar, EMBASE, and ProQuest databases to identify eligible articles published after January 2020 up to and including 30 August 2021. Pooled odds ratio (OR) and confidence intervals (CIs) were calculated using random effects meta-analysis.
Findings:
After searching a total of 7733 articles, 20 relevant observational studies with a total of 7840 patients were selected for meta-analysis. The pooled OR for persistent dyspnea in COVID-19 survivors with a severe versus nonsevere initial disease was 2.17 [95%CI 1.62 to 2.90], and it was 1.33 [95%CI 0.75 to 2.33] for persistent cough, 1.30 [95%CI 1.06 to 1.58] for persistent fatigue, 1.02 [95%CI 0.73 to 1.40] for persistent anosmia, 1.22 [95%CI 0.69 to 2.16] for persistent chest pain, and 1.30 [95%CI 0.93 to 1.81] for persistent palpitation.
Conclusions:
Contrary to expectations, we did not observe an association between the initial COVID-19 disease severity and common persistent symptoms except for dyspnea and fatigue. In addition, it was found that being in the acute or prolonged post-COVID phase did not affect the risk of symptoms. Primary care providers should be alert to potential most prevalent persistent symptoms in all COVID-19 survivors, which are not limited to patients with critical–severe initial disease.
Key words: COVID-19, disease severity, persistent, symptoms
Introduction
Coronavirus disease 2019 (COVID-2019) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel betacoronavirus of the genus Coronavirus, the family Coronaviridea, and subfamily Orthocoronavirinea (Kim et al., 2021). According to the World Health Organization (WHO), there were more than 225 million confirmed COVID-19 cases including more than 4.6 million deaths globally as of 15 September 2021 (WHO, 2021). The clinical impact of COVID-19 infection ranges from mild disease to life-threatening ARDS (WHO, 2020).
Recent advances in the field of COVID-19 disease such as the widespread implementation of COVID-19 vaccines have led to an increase in the number of patients recovering from COVID-19 (Rossman et al., 2021). However, a substantial proportion of these will experience persistent and prolonged residual effects of COVID-19 disease after recovery from acute infection (Cabrera Martimbianco et al., 2021). As a result, a large body of literature has emerged around the problem of finding optimal strategies for this population, which is a new challenge for researchers.
There is increasing clarity in the literature on the prevalence of post-acute and long COVID symptoms in COVID-19 survivors (Cabrera Martimbianco et al., 2021; Sanchez-Ramirez et al., 2021). Nevertheless, there is less/no clarity on the factors that determine this risk, such as the initial disease severity (Huang et al., 2021b; Townsend et al., 2021). Answers to these question may help to guide healthcare professionals in improving early symptom recognition of post-acute and long COVID symptoms, timely intervention and provide a general consensus to avoid wasting scarce resources in health and social care.
Although there are several studies in the literature comparing the risks of the most prevalent persistent symptoms (dyspnea, fatigue, cough, chest pain, anosmia and palpitation) of post-acute and long COVID between the different disease severity levels, the results are inconsistent (Halpin et al., 2021; Sanchez-Ramirez et al., 2021). Therefore, the aim of the study was to estimate symptom-specific pooled associations of the initial disease severity with the risk of persistent symptoms in post-acute and/or long COVID by conducting a meta-analysis of the available literature.
Materials & methods
Protocol and registration
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement (Moher et al., 2009). The systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42021272990).
Eligibility criteria
Studies were included if they were peer-reviewed, observational (cohort and cross-sectional) studies written in English, included adult (age ≥ 18 years) COVID-19 patients (confirmed by nasopharyngeal swab or sputum RT-PCR), compared the prevalence of persistent symptoms between patient recovery after COVID-19 with severe and nonsevere initial disease in post-acute and/or long COVID, and provided the population statistics, which would allow calculation of the odds ratio (OR) of persistent symptoms according to disease severity.
Studies not published in a peer-reviewed journal, with no laboratory confirmation evidence of SARS-CoV-2 infection, with no comparison group regarding disease severity, with a follow-up time shorter than four weeks after symptom onset/admission or three weeks after discharge/recovery, including patients younger than 18 years, case reports, and those which did not include original data (meta-analysis, reviews, editorials, and opinions) were excluded.
Definitions used
The initial disease severity was defined according to WHO, Clinical Management of COVID-19: Interim Guidance (WHO, 2020). COVID-19 survivors with a mild/moderate initial disease were classified as nonsevere, while patients with a severe/critical initial disease were classified as severe initial disease. As these definitions differ between the included studies, how we decided on binary classification (severe and nonsevere) is detailed in Table 1. The definition of post-acute COVID-19 syndrome include any COVID-19 survivors who still had signs and symptoms of COVID-19 at 4 to 12 weeks after acute infection. Long COVID included any COVID-19 survivors with symptoms persisting beyond 12 weeks of the onset of acute infection (Nalbandian et al., 2021).
Table 1.
Main characteristics of the studies
| Study | Country | Setting | Sample Size | Age * | Females | ICU Admission | Obesity (BMI ≥ 30) | CS use | Follow-up | Initial disease severity (severe versus nonsevere) | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Garrigues et al., 2020 | France | Single center | 120 | 63.2 | 45 (37.5) | 24 (20.0) | NR | NR | 110 days, PA | ICU versus ward | 5 |
| De Graaf et al., 2021 | Netherlands | Single-center | 81 | 60.8 | 30 (37) | 34 (42) | NR | 3 (0.4) | 6 weeks, PD | ICU versus non-ICU | 5 |
| Halpin et al., 2021 | UK | Single-center | 100 | 66.6 | 46 (46.0) | 32 (32.0) | 24 (24.0) | NR | 4–8 weeks, PD | ICU versus. ward | 5 |
| Huang et al., 2021a | China | Single-center | 1733 | 57 | 836 (48) | 76 (4) | NR | 398 (23) | 6 months, PSO | **Scale 3–4 versus Scale 5–6 | 6 |
| Huang et al., 2021b | China | Cohort, single-center | 1276 | 59 | 595 (47) | 54 (4) | NR | 307 (24) | 12 months, PSO | **Scale 3–4 versus Scale 5–6 | 6 |
| Iqbal et al., 2021 | Pakistan | Single-center | 158 | 40.1 | 87 (55.1) | 13 (8.2) | NR | NR | 38.1 days, PR | Severe versus mild/moderate | 7 |
| Jacobson et al., 2021 | USA | Single-center | 118 | 43.3 | 55 (46.6) | 11 (9.3) | NR | 0 (0) | 3–4 months, PD | Hospitalized versus non-hospitalized | 4 |
| Lerum et al., 2021 | Norway | Prospective, multicenter | 103 | 59 | 49 (47.5) | 15 (15) | NR | NR | 3 months, PA | ICU versus non-ICU | 6 |
| Lombardo et al., 2021 | Italy | Cohort, multicenter | 303 | 53 | 165 (54) | 8 (2.6) | 49 (16) | 14 (4.6) | 12 months, PR | Hospitalized versus non-hospitalized | 5 |
| Lorenzo et al., 2020 | Italy | Prospective and retrospective single center | 185 | 57 | 62 (33.5) | 4 (2.2) | NR | NR | 23 days, PD | Hospitalized versus discharged from ED | 6 |
| Maestre-Muñiz et al., 2021 | Spain | Single-center | 766 | 65.1 | 378 (49.3) | NR | 139 (18.1) | NR | 1 year, PD | Hospitalized versus non-hospitalized | 6 |
| Meije et al., 2021 a,b | Spain | Prospective, single-center | 294/302 | 68.8 | 131 (43.4) | 27 (8.9) | 41 (13.6) | 125 (41.4) | 45 days/7 months, PD | PaO2/FiO2 > 300 versus PaO2/FiO2 ≤ 300 | 5 |
| Menges et al., 2021 | Switzerland | Prospective longitudinal cohort, single-center | 431 | 47 | 214 (49.7) | 10 (2.3) | NR | NR | 220 days, PD | Hospitalized versus non-hospitalized | 4 |
| Morin et al., 2021 | France | Prospective, single-center | 478 | 61 | 201 (42.1) | 142 (29.7) | 130/351 (37.0) | 24 (5.0) | 4 months, PD | Intubated versus non-intubated | 7 |
| Qin et al., 2021 | China, France, Russia | Prospective, multi-center | 647 | 58 | 362 (56) | NR | NR | NR | 3 months, PD | Severe versus nonsevere | 6 |
| Shendy et al., 2021 | Egypt | Single-center | 81 | 34 | 55 (67.9) | NR | 35 (43.2) | NR | 3–5 months PR | Hospitalized versus non-hospitalized | 7 |
| Sykes et al., 2021 | UK | Single-center | 134 | 59.6 | 46 (34.3) | 27 (20) | NR | NR | 113 days, PD | ICU versus ward | 5 |
| Voruz et al., 2021 | Swittzerland | Prospective, single-center | 45 | 56.9 | 19 (42.2) | 15 (33.3) | NR | NR | 6–9 months, PSO | Severe versus mild/moderate | 6 |
| Wang et al., 2020 | China | Prospective, single-center | 131 | 49 | 72 (54.9) | NR | NR | NR | 4 weeks, PD | Severe versus nonsevere | 4 |
| Zayet et al., 2021 | France | Retrospective observational cohort, single-center | 354 | 49.6 | 223 (63) | 5 (11.1) | NR | NR | 9 months, PSO | Hospitalized versus non-hospitalized | 5 |
ED = emergency department, PA = post-admission; PSO = post-symptom onset; PD = post-discharge; PR = post-recovery, NR = not reported, CS = corticosteroid, PaO2 = partial pressure arterial oxygen, FiO2 = fraction of inspired oxygen.
Median or mean ages (years).
Scale 3: not requiring supplemental oxygen; Scale 4: requiring supplemental oxygen; or Scale 5–6: requiring high-flow nasal cannula, noninvasive mechanical ventilation, or invasive mechanical ventilation.
Databases and search strategy
A systematic search was conducted using PUBMED, Google Scholar, EMBASE, and ProQuest databases to identify eligible articles published after January 2020 up to and including August 302 021. The search strategy was formulated using the terms: ‘dyspnea’, ‘cough’, ‘fatigue’, ‘anosmia’, ‘chest pain’, ‘palpitation’ in combination with ‘long’ or ‘persistent’ or ‘chronic’ or ‘prolonged’ or ‘survivor’ or ‘post-acute’ or ‘post-recovery’ or ‘post-discharge’ and ‘COVID’ (including ‘COVID-19’, ‘coronavirus disease 2019’, ‘2019-nCoV’, ‘SARS COV2’, ‘Covid 19’) not ‘children’.
Study selection and data extraction
Titles and abstracts were first screened independently by ED and TB based on the inclusion and exclusion criteria with any disagreement resolved by consensus. Next, the full text of the selected studies was independently screened by two authors (ED and TB) for inclusion eligibility. The results were checked and discussed by ED and TB to agree on the final decision, and any disagreement was again resolved by consensus.
From the included studies, data were extracted on the study characteristics (author, year, country of origin, study design, sample size, type [‘post-admission’ or ‘post-discharge’ or ‘post-recovery’ or ‘post-symptom onset’], and length of follow-up), patient characteristics (mean or median age, gender, definition of acute COVID-19 infection, history of corticosteroid use, obesity status, and intensive care unit requirement), and patient clinical features (dyspnea, cough, fatigue, anosmia, chest pain, and palpitation). All data and details on binary categories (severe versus nonsevere initial disease) were collected using specific data collection forms by two authors (ED and TB) independently, in duplicate with any disagreements resolved through discussion. Missing data were excluded from the analysis of that particular variable.
Bias risk assessment
Minimizing the potential for bias in a meta-analysis, such as sampling or measurement bias, can help avoid underestimating or overestimating the parameter of interest (Hoy et al., 2012). In this study, the quality assessment was made with the Newcastle–Ottawa Scale (NOS) (GA Wells et al., 2021). Studies were scored on overall quality ranging from 0 (minimum) to 9 (maximum) stars according to the assessment of the NOS scale criteria. Studies with a quality score ≤4 were categorized as low-quality studies, and those with a score of ≥5 were categorized as high-quality studies (Xie et al., 2021). Publication bias was evaluated by visual inspection of the asymmetry of the funnel plots and analyzed with Egger’s linear regression, and a P-value of <0.05 was considered statistically significant publication bias.
Statistical approach
In this study, the pooled effect (OR) refers to the risk of persistent symptoms in COVID-19 survivors with a severe compared to nonsevere initial disesase. The pooled OR and confidence intervals (CI) were calculated based on the Mantel–Haenszel (MH) and inverse variance (I) methods. Final results were given with the MH method as there were very minor differences between the MH and I methods for the present study.
Meta-analysis was conducted using the R v3.6.1 packages ‘meta v4.10-0’, ‘metafor v2.1-0’, and ‘PRISMAstatement v1.1.1’. All meta-analyses were performed using the random effects model. Meta-analysis was performed separately for each variable. To determine the heterogeneity between studies, I 2 statistic (I 2 >%75 significant heterogeneity), Cochran’s Q (Q) value and significance (significant P value indicates heterogeneity) were used. Meta-regression was performed to explore the potential variable (12-week period), contributing to heterogeneity. Forest plots were used to illustrate results from relevant articles.
Results
Study selection
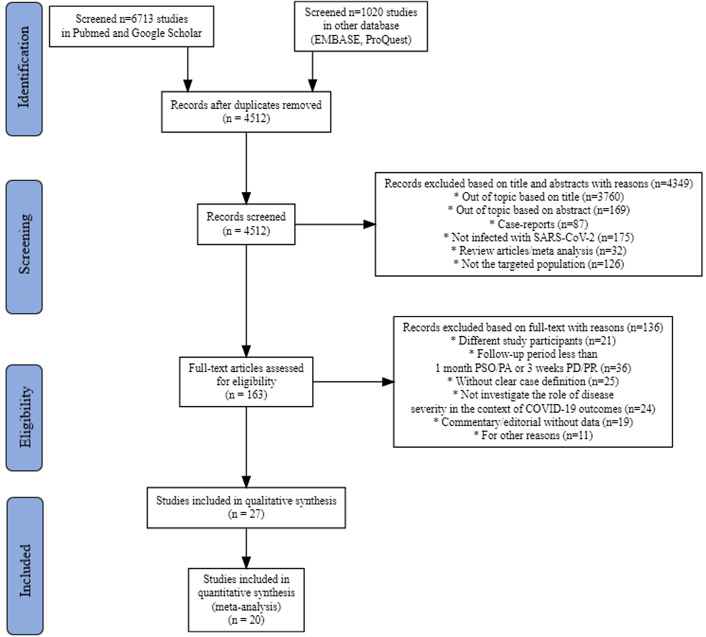
On 30 August 2021, a total of 7733 studies were identified in the initial search of the four databases with the predefined search terms. After removing duplications and evaluating according to elimination and eligibility criteria, a meta-analysis was conducted with 20 studies.(Garrigues et al., 2020; Wang et al., 2020; de Graaf et al., 2021; De Lorenzo et al., 2020; Halpin et al., 2021; Huang et al., 2021a, 2021b; Iqbal et al., 2021; Jacobson et al., 2021; Lerum et al., 2021; Lombardo et al., 2021; Maestre-Muñiz et al., 2021; Meije et al., 2021; Menges et al., 2021; Morin et al., 2021; Qin et al., 2021; Shendy et al., 2021; Sykes et al., 2021; Voruz et al., 2021; Zayet et al., 2021) The details of the study selection process are shown in a PRISMA flow chart (Figure 1).
Figure 1.
Flow diagram of the literature search
Characteristics of studies included and participants
The main characteristics of these 20 studies are shown in Table 1. Most of the studies (n = 17, 85%) were single-center cohort and observational studies with a sample size varying from 45 (Voruz et al., 2021) to 1733 (Huang et al., 2021a) patients. Six studies included patients who aged <50 years. The present systematic review and meta-analysis included data from COVID-19 survivors observed between January and December 2020. Most of (70%) the studies evaluated in this meta-analysis included COVID-19 survivors with symptoms persisting beyond 12 weeks of onset of acute infection (long COVID) (Table 2). The follow-up intervals ranged from 23 days to 12 months post-discharge.
Table 2.
Clinical characteristics of studies on persistent symptoms
| Author | Follow Period | Dyspnea | Cough | Fatigue | Anosmia | Chest pain | Palpitation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial disease severity (events/severe total, events/nonsevere total) | |||||||||||||
| Garrigues et al. | >12 week | 12/24 | 38/96 | 6/24 | 14/96 | 14/24 | 52/96 | 4/24 | 9/96 | – | – | – | – |
| Graaf et al. | ≤12 week | – | – | – | – | – | – | – | – | 8/34 | 7/47 | 5/34 | 7/47 |
| Halpin et al. | ≤12 week | 21/32 | 29/68 | – | – | 23/32 | 41/68 | – | – | – | – | – | – |
| Huang et al. (a) | >12 week | – | – | – | – | 95/117 | 943/1538 | 14/117 | 162/1538 | 10/117 | 65/1538 | 13/117 | 141/1538 |
| Huang et al. (b) | >12 week | – | – | – | – | 21/95 | 234/1185 | 6/95 | 51/1185 | 4/95 | 88/1185 | 7/95 | 110/1185 |
| Iqbal et al. | ≤12 week | 12/13 | 28/145 | 9/13 | 26/145 | 12/13 | 119/145 | 5/13 | 16/145 | 12/13 | 25/145 | – | – |
| Jacobson et al. | >12 week | 11/22 | 20/96 | 0/22 | 1/96 | 8/22 | 28/96 | 2/22 | 23/96 | 2/22 | 14/96 | 0/22 | 7/96 |
| Lerum et al. | ≤12 week | 5/15 | 32/88 | – | – | – | – | – | – | – | – | – | – |
| Lombardo et al. | >12 week | 70/189 | 40/114 | – | – | 101/189 | 57/114 | 49/189 | 35/114 | – | – | – | – |
| Lorenzo et al. | ≤12 week | 40/126 | 18/59 | – | – | – | – | – | – | – | – | – | – |
| Maestre et al. | >12 week | 240/445 | 104/321 | – | – | 162/445 | 110/321 | 98/445 | 95/321 | 29/445 | 33/321 | 44/445 | 24/321 |
| Meije et al. (a/b) | ≤12 week/ | 18/115 (a) | 28/179 (a) | 64/115 (a) | 93/179 (a) | 14/115 (a) | 33/179 (a) | 12/115 (a) | 18/179 (a) | – | – | ||
| >12 week | 13/115 (b) | 15/179 (b) | 8/115 (b) | 9/179 (b) | 36/115 (b) | 42/179 (b) | 9/115 (b) | 18/179 (b) | 3/115 (b) | 5/179 (b) | |||
| Menges et al. | >12 week | 37/81 | 59/350 | – | – | 38/81 | 195/350 | – | – | – | – | – | – |
| Morin et al. | >12 week | 25/73 | 53/405 | 5/73 | 16/405 | 24/73 | 110/405 | 6/73 | 19/405 | 9/73 | 25/405 | – | – |
| Qin et al. | >12 week | 30/248 | 26/399 | 18/248 | 20/399 | 46/248 | 41/399 | – | – | 3/248 | 3/399 | 34/248 | 29/399 |
| Shendy et al. | >12 week | – | – | – | – | 8/11 | 44/70 | – | – | – | – | – | – |
| Sykes et al. | >12 week | 19/27 | 60/107 | 5/27 | 40/107 | 9/27 | 44/107 | 0/27 | 13/107 | 1/27 | 23/107 | – | – |
| Voruz et al. | >12 week | – | – | – | – | 1/15 | 5/30 | 2/15 | 0/30 | – | – | – | – |
| Wang et al. | ≤12 week | 2/69 | 0/62 | 4/69 | 8/62 | 0/69 | 0/62 | – | – | 0/69 | 0/62 | 0/69 | 0/62 |
| Zayet et al. | >12 week | – | – | – | – | – | – | 36/121 | 64/233 | – | – | – | – |
The table shows the number of each persistent symptom in total severe and total nonsevere cases.
A total of 7840 patients were included. The studies included 1671 (46.8%) females and 4169 (53.2%) males, with mean/median age ranging from 34 (Shendy et al.) to 68.8 (Meije et al., 2021) years. The rate of ICU admission was reported in 16 studies as mean 8% (range, 42% (de Graaf et al., 2021) to 2.2% (De Lorenzo et al., 2020)) of reported patients, and four studies did not report this rate (Wang et al., 2020; Maestre-Muñiz et al., 2021; Qin et al., 2021; Shendy et al., 2021). Obesity (BMI ≥ 30) rates were reported in only six studies at mean 22%, while that for CS use was 20.3% according to available data from seven studies. Seven studies (Wang et al., 2020 Huang et al., 2021a, 2021b; Iqbal et al., 2021; Meije et al., 2021; Qin et al., 2021; Voruz et al., 2021) included nonhospitalized patients.
Pooled ORs of persistent symptoms among COVID-19 survivors
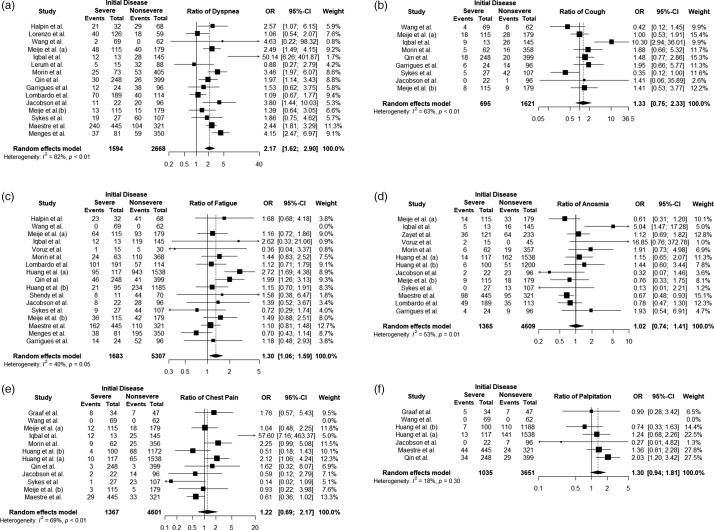
Persistent dyspnea
Fourteen studies including 4270 patients reported persistent dyspnea. Severe survivors of COVID-19 compared to nonsevere had a pooled OR of 2.17 [95%CI 1.62 to 2.90; P < 0.001] and a heterogeneity value of I 2 = 61.6%, Q = 36.48 (P = 0.009) (Figure 2a). While 46% of the studies (40.1% weight total) produced a nonsignificant OR for dyspnea, none of the studies did not pose a risk for persistent symptom in nonsevere cases (OR < 1). In addition, in the study of Iqbal et al., although its OR is high (50.14), its effect on pooled OR is 1.7%.
Figure 2.
Forest plots of ORs for all persistent symptoms
Persistent cough
Eight studies including 2382 patients reported persistent cough. Severe survivors of COVID-19 compared to nonsevere had a pooled OR of 1.33 [95%CI 0.75 to 2.33; P = 0.325] and heterogeneity value of I 2 = 62.9%, Q = 21.57 (P = 0.005) (Figure 2b). The OR CIs for the persistent symptom of cough in almost all studies (90.1 weight total) included a value of 1. Therefore, the pooled OR was found to be insignificant.
Persistent fatigue
Sixteen studies including 7117 patients reported persistent fatigue. Severe survivors of COVID-19 compared to nonsevere had a pooled OR of 1.30 [CI 1.06 to 1.58; P = 0.01] and heterogeneity value of I 2 = 40.5%, Q = 25.19 (P = 0.04) (Figure 2c). Five studies (Wang et al., 2020; Iqbal et al., 2021; Jacobson et al., 2021; Menges et al., 2021; Shendy et al., 2021) included patients who aged <50 years.
Persistent anosmia
Twelve studies including 6042 patients reported persistent anosmia. Severe survivors of COVID-19 compared to nonsevere had a pooled OR of 1.02 [CI 0.73 to 1.40; P = 0.921] and heterogeneity value of I 2 = 53.3%, Q = 25.70 (P = 0.011) (Figure 2d). Although a very high OR (16.85) was obtained from Voruz et al.’s study for the persistent symptom of anosmia, the CI of this study includes a value of 1. Only the study of Iqbal et al. gives significant OR, its weight is 5%.
Persistent chest pain
Eleven studies including 6118 patients reported persistent chest pain. Severe survivors of COVID-19 compared to nonsevere had a pooled OR of 1.22 [CI 0.69 to 2.16; P = 0.489] and heterogeneity value of I 2 = 69.5%, Q = 32.77 (P = 0.003) (Figure 2e). For persistent symptom of chest pain, 75% (83% overall weight) of the studies produced an insignificant OR, while the weights of the studies with significant risk consisted of 17.3%.
Persistent palpitation
Seven studies including 4752 patients reported persistent palpitation. Severe survivors of COVID-19 compared to nonsevere had a pooled OR of 1.30 [CI 0.93 to 1.81; P = 0.112] and heterogeneity value of I 2 = 18.1%, Q = 6.11 (P < 0.295) (Figure 2f). It was concluded that the OR CI formed by each of the studies included in the meta-analysis for palpitation included one value.
Subgroup analysis
No significant results were found for any persistent symptom when the 12-week time period was accepted as the moderator variable and meta-regression was performed (dyspnea P = 0.914, cough P = 0.752, fatigue P = 0.851, anosmia P = 0.591, chest pain P = 0.106, and palpitation P = 0.684).
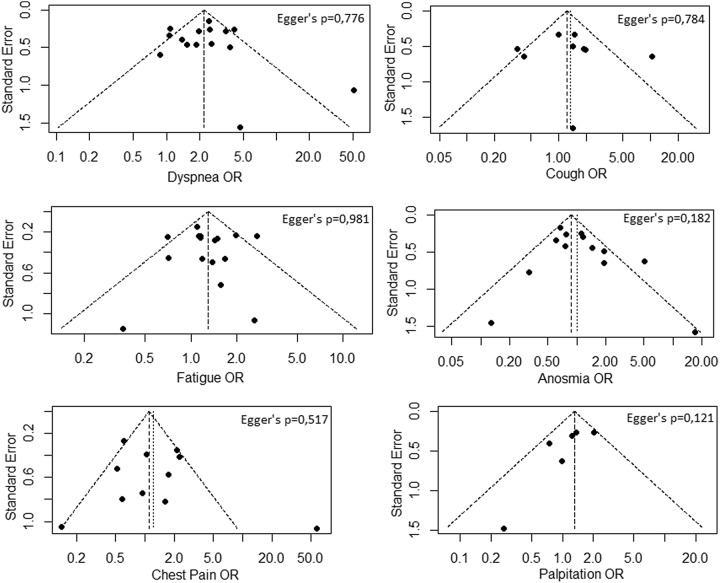
Publication bias and quality analysis
Figure 3 shows the symmetry of funnel plots and the results of the Egger’s regression analysis, which indicated that no significant publication bias found for any persistent symptom. The average NOS score of the included studies was 5.5 (range, 4–7). There were 3 low-quality studies with a NOS score of ≤4 and 17 high-quality studies with a NOS score of ≥5.
Figure 3.
Funnel plots for all persistent symptoms
Discussion
Previous studies evaluating the impact of initial disease severity on the incidence of post-acute and long COVID-19 symptoms have generated inconsistent results (Huang et al., 2021b, 2021a; Kamal et al., 2021). To provide a clearer answer to this question, the current systematic review and meta-analysis aimed to synthesise studies investigating this issue. To the best to our knowledge, the current meta-analysis is the only systematic review to have examined the impact of initial disease severity on the risk of post-acute and long COVID symptoms. The main findings of this meta-analysis were that the risk of cough and chest pain, as well as the risk of anosmia and palpitation, which are common reported respiratory and non-respiratory persistent symptoms, were not affected by the severity of the initial disease. In contrast, compared to patients with less severe acute infection, those who experienced a more severe course were more likely to suffer from persistent dyspnea and fatigue.
The results of this meta-analysis showed that the risk of persistent cough was not affected by the initial disease severity, and this was also valid for chest pain. Although, these findings differ from some previous studies (Song et al., 2021), they are mainly consistent with the current literature (Fernández-de-Las-Peñas et al., 2021). This difference could be due to the subjective nature of self-reporting methods used in these studies. In addition, obtaining a higher risk coefficient for persistent cough and chest pain symptoms in Iqbal’s study compared to other individual studies create heterogeneity. This may be due to the fact that severe cases had a small percentage among all cases in Iqbal’s study, while these patients have experienced relatively higher rates of persistent cough and chest pain symptoms. However, this does not affect the pooled OR much, as these persistent symptoms have a 9.9% versus 5% weight, respectively. Unfortunately, it is rather difficult to interpret these results, since chest pain can be caused by a variety of conditions ranges from myocardial or pulmonary injury to panic attack or musculoskeletal disorders among patients recovering from COVID-19. Furthermore, there are no available data on this issue from previous outbreaks of either SARS-CoV or MERS-CoV infections (Cares-Marambio et al., 2021; Ahmed et al., 2020).
Reports on the risk of persistent palpitation after acute COVID-19 infection regarding the disease severity are highly varied. Qin et al. reported a twofold increased risk of having persistent palpitations associated with more severe disease, while others did not find a significant CI for the OR. (Huang et al., 2021b; Jacobson et al., 2021). Broadly consistent with the available literature, the current meta-analysis showed that there was no link between the initial disease severity and the risk of persistent palpitation. This result is in agreement with Puntman’s findings which showed that cardiac involvement in cardiac MR is not influenced by the initial disease severity in individuals who have recovered from acute COVID-19 infection (Puntmann et al., 2020). This systematic review also found no difference in the risk of persistent anosmia between the severity groups. Although the ORs value for anosmia is very high in Voruz et al.’s study, the number of patients included in this study is quite low compared to other studies. Therefore, even a low number of patients have experienced persistent anosmia that can cause a higher risk ratio coefficients. However, when individual studies were evaluated according to study’s weight, and since the weight of Voruz et al.’s study represents approximately 1%, it was concluded that the pooled OR value for persistent anosmia does not pose a risk. Interestingly, in the study of Maestre et al., moderate–critical disease seemed to be protective against persistent anosmi, and this finding is supported by the results previously reported by Lechien et al. of more persistent anosmia in mild COVID-19 patients than in moderate-to-critical patients. These results were attributed to a better local immunological response in mild patients that limited the virus spread but could result in a stronger impairment of olfactory cells (Lechien et al., 2021).
The current meta-analysis showed that individuals with critical–severe initial disease were more than twice as likely to suffer from persistent dyspnea compared with those had less severe initial disease. Therefore, it seems important that patients with severe/critical initial disease should be followed more closely than patients with moderate/mild initial disease, and these patients should be referred for pulmonary rehabilitation. Another important finding was that although a reduction in the OR was seen over time, severe–critical patients still had a 4.15 and 2.44-fold higher risk of persistent dsypnea compared to less severe patients even beyond 6 and 12 months, respectively (Maestre-Muñiz et al., 2021; Menges et al., 2021). These results are consistent with those of previous studies which have reported prolonged lung diffusion impairment and abnormal findings in the chest CT at 6 and 12 months in critically ill patients (Huang et al., 2021b; Nalbandian et al., 2021). It seems possible that these results are due to prolonged inflammation in critical-severe patients which was not seen in nonsevere patients (Del Valle et al., 2020; Maltezou et al., 2021). This hypothesis is supported by the results of Ortelli et al. showing that patients with post-COVID manifestation had markedly elevated C-reactive protein (CRP) and IL-6 during the acute phase of COVID-19 infection (Ortelli et al., 2021). In summary, the findings reported here suggest that critical–severe patients have a much longer recovery time following acute illness with higher odds of persistent dyspnea than those with less severe acute illness.
It is well known that critical illness with prolonged stay in ICU or hospital is associated with a loss of muscle mass and a decreased lung capacity (Saccheri et al., 2020; Vanhorebeek et al., 2020). Therefore, as expected, a significantly higher likelihood of experiencing persistent fatigue was determined after a more severe course of initial disease compared with a less severe course. However, there are conflicting reports on the role of initial disease severity in the risk of persistent fatigue. While some study results (Huang et al., 2021a; Qin et al., 2021) support the current study findings, others have failed to find a link between the risk of persistent fatigue and initial disease severity (Menges et al., 2021; Sykes et al., 2021). This inconsistency may be due to the variance in patient characteristics such as corticosteroid use during acute infection, which was reported only by Huang et al. as significantly higher in critically ill patients, while there was no differences between two groups regarding age, gender, and the presence of chronic lung disease (Huang et al., 2021a).
Limitations
A major limitation of the present systematic review and meta-analysis was the substantial heterogeneity of the summarized studies in respect of mean ages, gender, the rate of ICU admission, CS use, and follow-up periods. Therefore, the random effects model was used to pool the data. This study was also limited by the absence of randomized studies on this topic, and that all of the reviewed studies were observational in nature. Another limitation of the present study is that, in some included studies, it is not clear whether cases that we have classified as nonsevere include cases with mild initial disease severity, and whether cases that we have classified as severe include cases with critical initial disease severity.
Conclusion
The results of this systematic review and meta-analysis revealed that the risks of persistent cough, chest pain, anosmia, and palpitation were not affected by the initial disease severity. The research has also concluded that the risk of persistent dyspnea and fatigue were significantly higher in COVID-19 survivors with a severe initial disease than in those with a nonsevere initial disease. Moreover, this meta-analysis showed that being in the post-acute or long COVID phase did not affect the risk of symptoms. Clinicians should be alert to potential persistent cough and chest pain as well as anosmia and palpitation in all COVID-19 survivors, which are not limited to patients with critical–severe initial disease.
Acknowledgments
None.
Data availability statement
Data available within the article or its supplementary materials.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethics approval statement
No ethical approval will be required because this study will retrieve and synthesize data from already published studies.
PROSPERO registration number
CRD42021272990.
Patient consent statement
No patient consent statement will be required because this study will retrieve and synthesize data from already published studies.
References
- Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, Eyre L, Breen A, O’connor R, Jones A and Sivan M (2020) Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. Journal of Rehabilitation Medicine 52, jrm00063. [DOI] [PubMed] [Google Scholar]
- Cabrera Martimbianco AL, Pacheco RL, Bagattini  M and Riera R (2021) Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. International Journal of Clinical Practice 75, e14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cares-Marambio K, Montenegro-Jiménez Y, Torres-Castro R, Vera-Uribe R, Torralba Y, Alsina-Restoy X, Vasconcello-Castillo L and Vilaró J (2021) Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Chronic Respiratory Disease 18. doi: 10.1177/14799731211002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf MA, Antoni ML, Ter Kuile MM, Arbous MS, Duinisveld AJF, Feltkamp MCW, Groeneveld GH, Hinnen SCH, Janssen VR, Lijfering WM, Omara S, Postmus PE, Ramai SRS, Rius-Ottenheim N, Schalij MJ, Schiemanck SK, Smid L, Stöger JL, Visser LG, De Vries JJC, Wijngaarden MA, Geelhoed JJM and Roukens AHE (2021) Short-term outpatient follow-up of COVID-19 patients: a multidisciplinary approach. EClinicalMedicine 32, 100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo R, Conte C, Lanzani C, Benedetti F, Roveri L, Mazza MG, Brioni E, Giacalone G, Canti V, Sofia V, D’amico M, Di Napoli D, Ambrosio A, Scarpellini P, Castagna A, Landoni G, Zangrillo A, Bosi E, Tresoldi M, Ciceri F and Rovere-Querini P (2020) Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One 15, e0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M and Gnjatic S (2020) An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature Medicine 26, 1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-De-Las-Peñas C, Guijarro C, Plaza-Canteli S, Hernández-Barrera V and Torres-Macho J (2021) Prevalence of post-COVID-19 cough one year after SARS-CoV-2 infection: a multicenter study. Lung 199, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ga Wells BS, O’connell D, Peterson J, Welch V, Losos M and Tugwell P (2021) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Online]. Retrieved 17 September 2021 from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, Doucet L, Berkani S, Oliosi E, Mallart E, Corre F, Zarrouk V, Moyer JD, Galy A, Honsel V, Fantin B and Nguyen Y (2020) Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. Journal of Infection 81, e4–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin SJ, Mcivor C, Whyatt G, Adams A, Harvey O, Mclean L, Walshaw C, Kemp S, Corrado J, Singh R, Collins T, O’connor RJ and Sivan M (2021) Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. Journal of Medical Virology 93, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E and Buchbinder R (2012) Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. Journal of Clinical Epidemiology 65, 934–939. [DOI] [PubMed] [Google Scholar]
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D and Cao B (2021. a) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, Hu P, Guo L, Liu M, Xu J, Zhang X, Qu Y, Fan Y, Li X, Li C, Yu T, Xia J, Wei M, Chen L, Li Y, Xiao F, Liu D, Wang J, Wang X and Cao B (2021. b) 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 398, 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal A, Iqbal K, Arshad Ali S, Azim D, Farid E, Baig MD, Bin Arif T and Raza M (2021) The COVID-19 sequelae: a cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus 13, e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KB, Rao M, Bonilla H, Subramanian A, Hack I, Madrigal M, Singh U, Jagannathan P and Grant P (2021) Patients with uncomplicated Coronavirus disease 2019 (COVID-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clinical Infectious Diseases 73, e826–e829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal M, Abo Omirah M, Hussein A and Saeed H (2021) Assessment and characterisation of post-COVID-19 manifestations. International Journal of Clinical Practice 75, e13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Bae JY, Bae S, Cha HH, Kwon JS, Suh MH, Lee HJ, Jung J, Kim MJ, Cui C, Park H, Lee J, Park MS and Kim SH (2021) Diagnostic usefulness of subgenomic RNA detection of viable SARS-CoV-2 in patients with COVID-19. Clinical Microbiology and Infection 28, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien JR, Chiesa-Estomba CM, Beckers E, Mustin V, Ducarme M, Journe F, Marchant A, Jouffe L, Barillari MR, Cammaroto G, Circiu MP, Hans S and Saussez S (2021) Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. Journal of Internal Medicine 290, 451–461. [DOI] [PubMed] [Google Scholar]
- Lerum TV, Aaløkken TM, Brønstad E, Aarli B, Ikdahl E, Lund KMA, Durheim MT, Rodriguez JR, Meltzer C, Tonby K, Stavem K, Skjønsberg OH, Ashraf H and Einvik G (2021) Dyspnoea, lung function and CT findings 3-months after hospital admission for COVID-19. European Respiratory Journal 57, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MDM, Foppiani A, Peretti GM, Mangiavini L, Battezzati A, Bertoli S, Martinelli Boneschi F and Zuccotti GV (2021) Long-term Coronavirus disease 2019 complications in inpatients and outpatients: a one-year follow-up cohort study. Open Forum Infectious Diseases 8, ofab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre-Muñiz MM, Arias Á, Mata-Vázquez E, Martín-Toledano M, López-Larramona G, Ruiz-Chicote AM, Nieto-Sandoval B and Lucendo AJ (2021) Long-term outcomes of patients with Coronavirus disease 2019 at one year after hospital discharge. Journal of Clinical Medicine 10, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltezou HC, Pavli A and Tsakris A (2021) Post-COVID syndrome: an insight on its pathogenesis. Vaccines (Basel) 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meije Y, Duarte-Borges A, Sanz X, Clemente M, Ribera A, Ortega L, González-Pérez R, Cid R, Pareja J, Cantero I, Ariño M, Sagués T, LLaberia J, Ayestarán A, Fernández-Hidalgo N and Candás-Estébanez B (2021) Long-term outcomes of patients following hospitalization for coronavirus disease 2019: a prospective observational study. Clinical Microbiology and Infection 27, 1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges D, Ballouz T, Anagnostopoulos A, Aschmann HE, Domenghino A, Fehr JS and Puhan MA (2021) Burden of post-COVID-19 syndrome and implications for healthcare service planning: a population-based cohort study. PLoS One 16, e0254523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J and Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, Gasnier M, Lecoq AL, Meyrignac O, Noel N, Baudry E, Bellin MF, Beurnier A, Choucha W, Corruble E, Dortet L, Hardy-Leger I, Radiguer F, Sportouch S, Verny C, Wyplosz B, Zaidan M, Becquemont L, Montani D and Monnet X (2021) Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 325, 1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, Mcgroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW and Wan EY (2021) Post-acute COVID-19 syndrome. Nature Medicine 27, 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortelli P, Ferrazzoli D, Sebastianelli L, Engl M, Romanello R, Nardone R, Bonini I, Koch G, Saltuari L, Quartarone A, Oliviero A, Kofler M and Versace V (2021) Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. Journal of the Neurological Sciences 420, 117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M and Nagel E (2020) Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from Coronavirus disease 2019 (COVID-19). JAMA Cardiology 5, 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Chen S, Zhang Y, Dong F, Zhang Z, Hu B, Zhu Z, Li F, Wang X, Wang Y, Zhen K, Wang J, Wan Y, Li H, Elalamy I, Li C, Zhai Z and Wang C (2021) Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. European Respiratory Journal 58, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman H, Shilo S, Meir T, Gorfine M, Shalit U and Segal E (2021) COVID-19 dynamics after a national immunization program in Israel. Nature Medicine 27, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Saccheri C, Morawiec E, Delemazure J, Mayaux J, Dubé BP, Similowski T, Demoule A and Dres M (2020) ICU-acquired weakness, diaphragm dysfunction and long-term outcomes of critically ill patients. Annals of Intensive Care 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ramirez DC, Normand K, Zhaoyun Y and Torres-Castro R (2021) Long-term impact of COVID-19: a systematic review of the literature and meta-analysis. Biomedicines 9, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shendy W, Ezzat MM, Elaidy DA and Abdelaziz A (2021) Prevalence of fatigue in patients post Covid. European Journal of Molecular & Clinical Medicine 8, 2021. [Google Scholar]
- Song WJ, Hui CKM, Hull JH, Birring SS, Mcgarvey L, Mazzone SB and Chung KF (2021) Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. The Lancet Respiratory Medicine 9, 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH and Crooks MG (2021) Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung 199, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L, Dowds J, O’brien K, Sheill G, Dyer AH, O’kelly B, Hynes JP, Mooney A, Dunne J, Ni Cheallaigh C, O’farrelly C, Bourke NM, Conlon N, Martin-Loeches I, Bergin C, Nadarajan P and Bannan C (2021) Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Annals of the American Thoracic Society 18, 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhorebeek I, Latronico N and Van Den Berghe G (2020) ICU-acquired weakness. Intensive Care Medicine 46, 637–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voruz P, Allali G, Benzakour L, Nuber-Champier A, Thomasson M, Jacot I, Pierce J, Lalive P, Lovblad K-O and Brallaird O (2021) Long COVID neuropsychological deficits after severe, moderate or mild infection. Clinical and Translational Neuroscience 6, 1–25. [Google Scholar]
- Wang X, Xu H, Jiang H, Wang L, Lu C, Wei X, Liu J and Xu S (2020) Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM 113, 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2020) Clinical management of COVID-19: interim guidance, 27 May 2020: World Health Organization. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 [Google Scholar]
- WHO (2021) Coronavirus (COVID-19) dashboard. Retrieved September 2021 from https://covid19.who.int/ [Google Scholar]
- Xie F, You Y, Huang J, Guan C, Chen Z, Fang M, Yao F and Han J (2021) Association between physical activity and digestive-system cancer: an updated systematic review and meta-analysis. Journal of Sport and Health Science 10, 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayet S, Zahra H, Royer PY, Tipirdamaz C, Mercier J, Gendrin V, Lepiller Q, Marty-Quinternet S, Osman M, Belfeki N, Toko L, Garnier P, Pierron A, Plantin J, Messin L, Villemain M, Bouiller K and Klopfenstein T (2021) Post-COVID-19 Syndrome: nine months after SARS-CoV-2 infection in a cohort of 354 patients: data from the First Wave of COVID-19 in Nord Franche-Comté Hospital, France. Microorganisms 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available within the article or its supplementary materials.