Abstract
Background and Aim:
This study aims to investigate thyroid function-associated parameters and the incidence of thyroid disorders in pregnant women, with the overarching aim to ensure that pregnant women do not develop said disorders due to aberrant iodine levels during the course of pregnancy.
Methods:
A total of 300 pregnant women who underwent routine check-ups at the Yongchuan Hospital Affiliated to Chongqing Medical University from January to December 2021 were enrolled. Venous blood and morning urine were collected. Serum thyroid-stimulating hormone (TSH), free thyroxine (FT4), and free triiodothyronine (FT3) were determined by chemiluminescence immunoassay. Urinary iodine concentration (UIC) was detected by arsenic cerium catalytic spectrophotometry. Thyroid disorders were extrapolated from the measured parameters.
Results:
The overall median UIC was 203 μg/L, which was within normal range. Subgroup analysis revealed that the median UIC in the first trimester was 187.5 μg/L, 211.8 μg/L in the second trimester, and 239.9 μg/L in the third trimester. However, based on the WHO criteria, 32%, 30%, and 18% of pregnant women were iodine deficient during their first, second, and third trimester, respectively. The proportion of women with iodine deficiency in the first and second trimesters was higher compared to the third trimester (P < 0.05). Serum FT3 and FT4 concentrations were higher in subjects in their first and second trimester versus the third trimester, while serum TSH levels were lower in subjects in their first and second trimester versus the third trimester (P < 0.05). The TSH concentration in subjects with inadequate iodine intake (UIC < 150 μg/L) was lower compared to subjects with adequate iodine intake (UIC 150 – 249 μg/L), but higher than in subjects with more than adequate intake (UIC 250 – 499 μg/L) and excess iodine intake (UIC ≥ 500 μg/L) (P < 0.05). TSH concentration and UIC were positively correlated (r = 0.1945, P = 0.0007), while no relationship was observed between UIC and FT3 and FT4 serum levels (r1 = −0.0593, P1 = 0.3053; r2 = −0.0149, P2 = 0.7968). There was no significant difference in FT3 and FT4 concentration between different UIC strata (P > 0.05). The incidence of thyroid disease during pregnancy in iodine-deficient women was greater compared to pregnant women with adequate iodine intake (P < 0.05) and higher in subjects in the more than adequate as well as excessive iodine intake cohorts (P < 0.05).
Conclusion:
The iodine nutritional intake by pregnant women in Yongchuan District, Chongqing, was generally sufficient to meet developmental and metabolic needs. However, about a third of women in their first and second trimester exhibited iodine deficiency. Iodine deficiency was associated with an increased incidence of thyroid diseases.
Relevance for Patients:
In clinical practice, the UIC of pregnant women should be measured during key stages in the pregnancy to prevent the manifestation of thyroid diseases.
Keywords: pregnancy, trimester, urine iodine, thyroid function, thyroid-stimulating hormone, free triiodothyronine, free thyroxine
1. Introduction
Iodine is an essential trace element for the production of thyroid hormones, which are important for maintaining basal metabolism and growth and development of the human body. More than 90% of the iodine required by the human body comes from food. The inorganic iodine in food is soluble in water. It is rapidly absorbed into the bloodstream in the form of iodide ions in the stomach and small intestine, and combines with protein to form iodine, which is enriched in the thyroid gland to facilitate the synthesis of thyroid hormones [1]. About 90% of dietary iodine is filtered by the kidneys and excreted in urine, and about 10% is excreted through feces and sweat [2]. Accordingly, urinary iodine concentration (UIC) is a reliable parameter for the assessment of recent iodine intake. The World Health Organization (WHO) recommends using the median UIC to assess the iodine nutritional status of the population [3].
Pregnancy constitutes an extraordinary physiological state in that iodine metabolism and thyroid function are significantly changed relative to pre-pregnancy. The physiological requirement for iodine of pregnant women increases by 50% compared to non-pregnant women. The risk of pregnant women suffering from iodine deficiency disorders during pregnancy, therefore, is also significantly increased [4]. Both iodine deficiency and iodine excess may have irreversible effects on fetal nervous system development and maternal thyroid morphology and function. The United Nations International Children’s Emergency Fund (UNICEF), the International Council for the Control of Iodine Deficiency Disorders (ICCIDD), and the WHO recommend a daily iodine intake of 250 μg for pregnant and lactating women [3,5]. The latest guidelines issued by the American Thyroid Association (ATA) also suggest that women who plan to become pregnant, are pregnant, or are lactating should take oral supplements containing 150 μg iodine every day [6].
Since the implementation of salt iodization in 1995, China has basically eliminated iodine deficiency-related diseases. In 2011, the China Iodine Monitoring Center reported that the median UIC of school-aged children (SAC) was 238.6 μg/L, the goiter prevalence was 2.4%, and the household iodized salt coverage was 98% [7]. Some studies also pointed out that the excessive intake of iodine in China has persisted for 10 years, which has led to dramatic changes in the prevalence and spectrum of thyroid diseases [8]. SAC is a standard group for evaluating iodine nutrition in the community, but sufficient iodine intake in SAC does not guarantee iodine sufficiency in pregnant women in the same community [9]. Hence, the UIC of SAC is not a good indicator for the iodine nutritional status of pregnant women.
China encompasses a vast territory and the concentration of iodized salt varies from region to region. For example, the concentration of iodized salt in Chongqing is 30 mg/kg, while that in Zhejiang and Jiangsu, it is 25 mg/kg [10]. Yu et al. conducted a cross-sectional study of 625 pregnant women in Zhejiang and found mild-to-moderate iodine deficiency [11]. Zhao et al. monitored the iodine nutrition status in the Yubei district of Chongqing from 2017 to 2021. The median UIC in pregnant women ranged from 150.2 μg/L to 249.9 μg/L [12]. At present, there is no study on iodine nutrition of pregnant women in the Yongchuan area of Chongqing. This study, therefore, aimed to explore the thyroid function and iodine nutrition status of pregnant women in the Yongchuan district.
2. Materials and Methods
2.1. Study population and clinical data curation
In this single-center prospective study, pregnant women who underwent routine obstetric examinations in Yongchuan Hospital of Chongqing Medical University from January to December 2021 were selected and stratified according to gestational weeks: 1st trimester (0 – 13+6 weeks), 2nd trimester (14 – 27+6 weeks), and 3rd trimester (28 – 40+6 weeks). Pregnant women were included in the study who (1) were ≥18 years of age, (2) had lived in the region for ≥3 years, (3) had a normal singleton pregnancy, and (4) were not diagnosed with thyroid disease [13]. Pregnant women who had supplemented iodine during the year before the study and who developed maternal and neonatal adverse outcomes were excluded from the study. All data collections were performed at the Yongchuan Hospital of Chongqing Medical University. The CONSORT flowchart is presented in Figure 1.
Figure 1. CONSORT flowchart of the prospective clinical study.
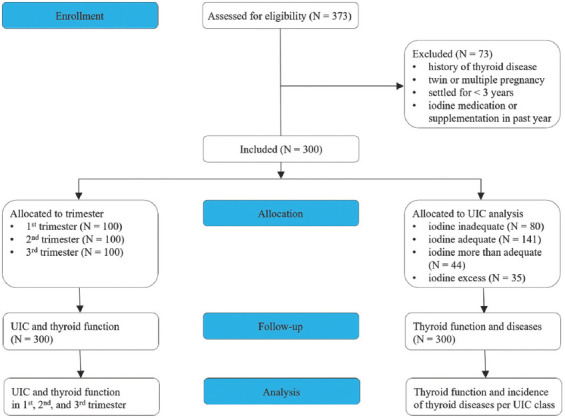
The study was approved by the Institutional Review Board of the Yongchuan Hospital of Chongqing Medical University under protocol number 2021MSXM081. All study participants provided written informed consent and all procedures were performed in accordance with the Declaration of Helsinki on Medical Research involving Human Subjects (2013 edition).
2.2. Sample collection and analysis
Three mL of venous blood was collected in the morning from study subjects following an overnight fast as well as 5 mL of midstream urine. The blood was placed in a centrifuge tube containing separating gel, and serum was collected by centrifugation at 3000 rpm for 10 min. Serum samples were added to labeled test tubes, and an automated chemiluminescence immunoassay was used to determine thyroid-stimulating hormone (TSH), free thyroxine (FT4), and free triiodothyronine (FT3). Urine was transferred to a polyethylene or glass test tube and capped to prevent water from evaporating. UIC was determined at the Department of Clinical Chemistry by arsenic cerium catalytic spectrophotometry (WS/T107.1-2016 standard protocol; MAGLUMI X8 chemiluminescence immunoassay analyzer, Snibe, Guangdong, China). During the testing process, national certified reference material was used for quality assurance. The reference range for each of the tested markers was: FT3, 1.21 – 4.18 pmol/L; FT4, 8.9 – 17.2 pmol/L; and TSH, 0.3 – 4.5 mU/L.
2.3. Diagnostic criteria
In line with the reference standard of our laboratory, diagnosis of abnormal thyroid function in pregnancy entails the following: Clinical hypothyroidism, TSH > 4.5 mU/L, FT4 < 8.9 pmol/L; subclinical hypothyroidism, TSH > 4.5 mU/L, FT4 8.9 – 17.2 pmol/L; clinical hyperthyroidism, TSH < 0.3 mU/L, FT4 > 17.2 pmol/L; subclinical hyperthyroidism, TSH < 0.3 mU/L, FT4 8.9 – 17.2 pmol/L; isolated hypothyroxinemia, TSH 0.3 – 4.5 mU/L, and FT4 < 8.9 pmol/L. The UIC was based on the iodine nutrition standard proposed by the WHO [14], namely, inadequate intake, <150 μg/L; adequate intake, 150 – 249 μg/L; more than adequate iodine intake, 250 – 499 μg/L; and excessive intake, ≥500 μg/L.
2.4. Statistical analysis
Statistical analysis was performed using SPSS v26.0 software (IBM, Armonk, NY, USA). A Kolmogorov–Smirnov Z test was used to determine whether the data were normally distributed. Data that were normally distributed were reported as mean ± standard deviation (SD), whereas data that did not follow a Gaussian profile were as the median. Comparisons groups were performed using the Kruskal–Wallis test with Games-Howell post hoc correction or the two independent samples Wilcoxon rank-sum test. Enumeration data were expressed as rate. Spearman analysis was used to assess the relationship between two variables. P < 0.05 was considered statistically significant.
3. Results
3.1. Cohort characteristics
A total of 300 pregnant women were included in the study. The number of subjects in the first, second, and third term of pregnancy was equal. Table 1 presents the cohort demographics. The overall mean age of the participants was 30.10 ± 3.57 years old. The overall mean BMI was 21.60 ± 2.67 kg/m2. The overall mean number of pregnancies was 1.76 ± 0.89. The overall mean parity was 0.47 ± 0.54.
Table 1. Cohort demographics stratified by trimester.
| Trimester | N | Age (years) | BMI (kg/m2) | Pregnancy (N) | Parity (N) |
|---|---|---|---|---|---|
| 1st | 100 | 30.09±2.81 | 21.23±1.65 | 1.77±0.83 | 0.47±0.52 |
| 2nd | 100 | 29.90±3.00 | 21.74±1.98 | 1.80±0.93 | 0.45±0.55 |
| 3rd | 100 | 30.29±4.60 | 21.84±3.82 | 1.71±0.90 | 0.48±0.54 |
| P-value | - | 0.240 | 0.157 | 0.376 | 0.868 |
Data are reported as mean±SD.
3.2. UIC by trimester
The overall median UIC was 203 μg/L during pregnancy. The median UIC per trimester is presented in Table 2. According to the WHO criteria for pregnant women, the UIC was within the sufficient range in all trimesters. The proportion of pregnant women with inadequate iodine intake in the first, second, and third trimester was 32%, 30%, and 18%. The proportion of subjects with inadequate iodine intake was significantly higher in the 1st and 2nd trimester cohorts compared to women in their 3rd trimester (P < 0.05). There were no significant differences in the proportion of pregnant women in the 1st through 3rd trimester in the cohorts with adequate, more than adequate, and excess iodine intake (P > 0.05).
Table 2. Urinary iodine concentration in pregnant women from Yongchuan, Chongqing, stratified by trimester.
| Trimester | N | Median (µg/L) | < 150 µg/L (%) | 150 – 249 µg/L (%) | 250 – 499 µg/L (%) | ≥ 500 µg/L (%) |
|---|---|---|---|---|---|---|
| 1st | 100 | 187.5a | 32a | 47 | 10 | 11 |
| 2nd | 100 | 211.8a | 30a | 46 | 16 | 8 |
| 3rd | 100 | 239.9 | 18 | 48 | 18 | 16 |
Data are reported as median for continuous variables and as a percentage for categorical variables.
Compared to the 3rd trimester, P < 0.05 (Kruskal–Wallis).
3.3. FT3, FT4, and TSH serum levels by trimester
The median FT3, FT4, and TSH serum levels are presented in Table 3. The FT3 and FT4 concentrations were higher in women in their 1st and 2nd trimester of pregnancy compared to women in their 3rd term (P < 0.05). The TSH concentration was lower during the first 2 trimesters compared to the 3rd trimester (P < 0.05).
Table 3. Thyroid function in pregnant women from Yongchuan, Chongqing, stratified by trimester.
| Trimester | N | FT3 (pmol/L) | FT4 (pmol/L) | TSH (mU/L) |
|---|---|---|---|---|
| 1st | 100 | 3.11 (1.67 – 4.18)a | 12.5 (7.31 – 21.10)a | 1.45 (0.01 – 6.21)a |
| 2nd | 100 | 2.77 (0.96 – 5.36)a | 11.5 (7.10 – 18.40)a | 1.88 (0.01 – 6.34)a |
| 3rd | 100 | 2.57 (0.86 – 6.08) | 9.19 (0.20 – 17.74) | 2.27 (0.00 – 10.8) |
Data are reported as median (minimum - maximum).
Compared to the 3rd trimester, P < 0.05 (Kruskal–Wallis). FT3: Free triiodothyronine, FT4: Free thyroxine, TSH: Thyroid-stimulating hormone
3.4. Relationship between UIC and thyroid function
There was no significant correlation between UIC and FT3 and between UIC and FT4 (r1 = 0.0593, P1 = 0.3053; r2 = 0.0149, P2 = 0.7968, respectively, Figure 2A and B), whereas TSH was positively correlated with UIC (r = 0.1945, P = 0.0007, Figure 2C). Compared to the group with adequate iodine intake (UIC: 150 – 249 μg/L), TSH levels were lower in the iodine-deficient women (UIC < 150 μg/L) and elevated in women with more than adequate intake (UIC 250 – 499 μg/L) and excessive intake (UIC ≥ 500 μg/L) (P < 0.05, Table 4).
Figure 2. The relationship between UIC and FT3 (A), FT4 (B), and TSH (C) in pregnant women from Yongchuan, Chongqing. The Spearman correlation coefficient (r) and the level of significance are provided above the respective graph.
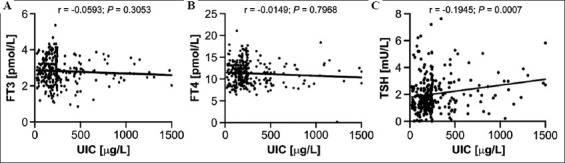
Table 4. Thyroid function-related markers in the plasma of pregnant women from Yongchuan, Chongqing, stratified according to the UIC range.
| Group | FT3 (pmol/L) | FT4 (pmol/L) | TSH (mU/L) |
|---|---|---|---|
| <150 µg/L | 2.76 (1.19 – 3.99) | 11.45 (6.46 – 21.10) | 1.45 (0.01 – 6.34)a |
| 150 – 249 µg/L | 2.87 (1.07 – 6.08) | 11.30 (7.61 – 17.74) | 1.67 (0.01 – 7.21) |
| 250 – 499 µg/L | 2.54 (0.86 – 3.78) | 10.25 (6.41 – 16.40) | 1.87 (0.25 – 10.80)a |
| ≥500 µg/L | 2.74 (1.23 – 0.87) | 10.80 (0.20 – 12.87) | 2.28 (0.21 – 4.35)a |
Data are reported as median (minimum – maximum).
Compared to the adequate iodine intake group (orange), P < 0.05 (Kruskal–Wallis).
3.5. Relationship between thyroid diseases and UIC
The incidence rate of thyroid disease during pregnancy in iodine-deficient women (UIC < 150 μg/L) was greater compared to pregnant women with adequate iodine intake (P < 0.05, Table 5). Similarly, the rate of thyroid diseases was significantly higher in subjects with more than adequate as well as excessive iodine intake cohorts compared to pregnant women with adequate iodine intake (P < 0.05, Table 5).
Table 5. Incidence of thyroid diseases with different urinary iodine concentrations in pregnant women from Yongchuan, Chongqing.
| Thyroid diseases | UIC group | |||
|---|---|---|---|---|
|
| ||||
| <150 µg/L | 150 – 249 µg/L | 250 – 499 µg/L | ≥500 µg/L | |
| Clinical hypothyroidism | 4 (5.00) | 1 (0.71) | 2 (4.50) | 3 (8.5) |
| Subclinical hypothyroidism | 5 (6.25) | 1 (0.71) | 0 (0.00) | 0 (0.00) |
| Clinical hyperthyroidism | 1 (1.25) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Subclinical hyperthyroidism | 3 (3.75) | 1 (0.71) | 0 (0.00) | 1 (2.86) |
| Isolated hypothyroxinemia | 18 (22.5) | 7 (4.96) | 7 (15.91) | 5 (14.29) |
| Total | 31 (38.8) | 10 (7.10) | 9 (20.45) | 9 (25.71) |
| P-value | 0.000 | – | 0.011 | 0.001 |
Data are expressed as n (%). Groups were compared to the adequate iodine intake group (orange) using a Chi-square test with respect to the overall incidence of thyroid diseases.
4. Discussion
Pregnant women are vulnerable to the effects of iodine deficiency. It is imperative to maintain the health of mother and baby by ensuring adequate intake of iodine. Studies have pointed out that insufficient iodine intake during early pregnancy can lead to hypothyroidism, miscarriage, and other pathological manifestations [15]. Pregnant women with insufficient iodine intake in the second and third trimesters are also at increased risk of gestational hypertension, fetal growth restriction, and preterm birth [16,17]. Severe iodine deficiency may also lead to adverse pregnancy outcomes such as stillbirth, perinatal death, and fetal congenital anomalies [18,19]. A long-term follow-up study found that iodine deficiency during pregnancy was associated with offspring neurodevelopmental deficits, including intellectual deficits such as poor school performance, language delay, and hyperactivity disorder miscarriage [20]. In contrast, if iodine is excessively consumed, it may lead to an increased incidence of maternal and infant thyroid dysfunction, such as goiter, thyroiditis, subclinical hypothyroidism, and isolated hypothyroxinemia [21,22]. A meta-analysis conducted in China unveiled that the children in the high iodine-exposed group had severe intellectual impairment, which coincided with an average IQ drop by 1.64 points [23]. Therefore, it is meaningful and necessary to study the iodine nutritional status and thyroid function during pregnancy to deter iodine-related disorders.
Our study evaluated relevant parameters in 300 pregnant women during the three trimesters of pregnancy. We found that the overall median UIC of the participants overall was 203 μg/L, which is within the recommended range. These findings are in line with previous dataderived from a larger survey evaluating iodine status in 2607 pregnant Chongqing women [24]. When compared to studies in Heilongjiang and Hebei province, the UIC in Chongqing was significantly higher [25,26]. It may be that iodine concentration in household table salt started to drop from 35 mg/kg during the 2000 – 2011 era to 20, 25, or 30 mg/kg in 2012 (Chinese standard GB 26878-2011). Each province is mandated to manage its own iodine enrichment policies. Chongqing chose the highest level of 30 mg/kg, while Heilongjiang and Hebei province opted for 25 mg/kg. This may have affected the UIC levels in the general population in those provinces. Although the present results suggest that iodine is generally sufficient, we further explored the UIC stratification per each trimester. We found that the proportion of pregnant women with inadequate iodine intake in the 1st, 2nd, and 3rd trimester was 32%, 30%, and 18%. Compared to the 3rd trimester, the proportion of iodine-deficient pregnant women in their 1st and 2nd trimester was significantly higher, which is consistent with earlier findings [27]. Two potential reasons may explain this phenomenon. First, due to the manifestation of the pregnancy itself in the 1st trimester, women have poor appetite and reduce dietary iodine intake. Some pregnant women also experience pregnancy-related vomiting, resulting in loss of ingested iodine. Second, the fetal thyroid begins to develop at about 10 – 12 weeks of gestation, and the fetus begins to produce thyroid hormones at 18 – 20 weeks of pregnancy [28]. Inasmuch as iodine is key to these processes, the iodine may be withdrawn from the maternal blood and used in fetal metabolism, culminating in a drop in circulatory iodine levels. Therefore, in clinical work, iodine nutrition status detection during pregnancy should be carried out as soon as possible, iodine deficiency should be detected in time, and pregnant women should be reasonably guided to supplement iodine.
Our study further showed that there are term-dependent differences in serum levels of FSH, FT3, and FT4 among pregnant women. A study in Heilongjiang and Shanghai province also showed that FT3 and FF4 decreased with gestational age during pregnancy, while TSH levels increased [25,29]. TSH has a similar molecular structure to serum human chorionic gonadotropin (HCG). HCG appears on the 7th day of pregnancy and peaks at 8 – 10 weeks of gestation [30]. TSH receptors of thyroid follicular cells are able to activate and bind dorsal HCG, stimulating the maternal thyroid to produce T4 and T3 through intracellular messengers such as cAMPK [31]. Ultimately, these lead to the inhibition of TSH production. The degree of thyroid stimulation is affected by the magnitude and duration of HCG. Inhibition of TSH and elevation of serum FT4 concur with prolonged high concentrations of HCG [32]. HCG plays an important role in the marked increase in the need for thyroid hormone production during the 1st trimester. To increase the sensitivity of the uterus to oxytocin in the 3rd trimester in preparation for childbirth, estrogen levels become upregulated in the mother. Thyroxine-binding globulin (TBG) synthesis in the liver increases with elevated estrogen levels, which results in a reduced concentration of FT4 and FT3 [33]. Moreover, deiodinases are a group of enzymes involved in the metabolism of thyroid hormones. The most prominent are type 2 deiodinase and type 3 deiodinase (D3) in the placenta, of which D3 is the most important [34]. Studies have found that D3 was highly expressed in the syncytiotrophoblast and cytotrophoblast of the term placenta, fetal endothelium, and decidua in contact with the maternal circulation [35]. The expression of D3 at this stage and location ensured that the placenta received an adequate supply of iodine for subsequent release into the fetal circulation. Consequently, this process reduces the availability of free thyroid hormones in the maternal circulation. As a result, there are differences in thyroid hormone concentrations during the different stages of pregnancy.
The relationship between iodine nutrition level and thyroid function has been the focus of clinical research in recent years. Based on our UIC data and in accordance with the WHO interpretation thereof, we observed changes in thyroid function as a function of UIC. No significant changes in FT3 and FT4 levels were observed between the different iodine intake groups. This may have been due to the sufficient amount of iodine stored in the thyroid gland to ensure the synthesis of thyroid hormones. A woman’s thyroid gland stores 10 – 20 mg of iodine at the start of pregnancy in an iodine-rich area [35]. Therefore, as long as there is adequate iodine intake during pregnancy, the reserves can accommodate the increased iodine needs during pregnancy. In our study, the median UIC indicated an adequate level of iodine nutrition among pregnant women in Yongchuan, Chongqing. Consequently, there was no significant difference in the levels of FT3 and FT4 among the different iodine intake groups. TSH is one of the biomarkers used for assessing the iodine status of a population [13]. Our study found that serum TSH was positively correlated with urinary iodine levels. Some studies have also pointed out that the relationship between changes in thyroid function and urinary iodine embodies a U-shaped curve. In Shanxi and Inner Mongolia, studies discovered that, when pregnant women are in the state of iodine deficiency and iodine excess, the incidence of thyroid disease during pregnancy was increased, and that the incidence of thyroid disease was low when the UIC was within normal range [36,37]. Our present study reverberated these findings. These results underscore the fact that optimal iodine nutritional status during pregnancy is essential for maintaining normal thyroid function in the mother. At present, pregnant women with abnormal thyroid function are only supplemented with thyroid hormones while urinary iodine testing is not routinely performed, which may lead to an unsubstantiated abuse of drugs and dietary supplements. Reasonable guidance on iodine supplementation for pregnant women is key to preventing iodine deficiency disorders. Therefore, it is necessary to regularly and dynamically monitor the iodine status in clinical practice in pregnant women, particularly during the first 2 trimesters.
The study comes with limitations, which include: (i) The study was conducted in a single center; (ii) postpartum women and neonates were not included in the evaluation; (iii) there was a short follow-up period, and observations related to pregnancy outcomes and long-term follow-up of offspring were not performed; and (iv) the UIC data might not reflect habitual iodine consumption and we did not have data on iodine-containing supplement consumption before pregnancy.
5. Conclusion
Our study adds to a growing body of evidence indicating that iodine deficiency and excess iodine can adversely affect the mother’s thyroid function. Therefore, clinics should dynamically monitor iodine status during pregnancy so as to properly counsel pregnant women in regard to iodine supplementation.
Acknowledgments
We thank Prof. Zhongbang Xiao for his help.
Funding
This project is supported by Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau) (2021MSXM081).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- [1].Shan Z. Interpretation of Iodine Supplementation Guidelines for Chinese Residents. Chin J Pract Int Med. 2019;39:347–350. [Google Scholar]
- [2].Farebrother J, Dalrymple KV, White SL, Gill C, Brockbank A, Lazarus JH, et al. Iodine Status of Pregnant Women with Obesity from Inner City Populations in the United Kingdom. Eur J Clin Nutr. 2021;75:801–8. doi: 10.1038/s41430-020-00796-z. [DOI] [PubMed] [Google Scholar]
- [3].WHO Secretariat. Andersson M, de Benoist B, Delange F, Zupan J. Prevention and Control of Iodine Deficiency in Pregnant and Lactating Women and in Children Less than 2-Years-old:Conclusions and Recommendations of the Technical Consultation. Public Health Nutr. 2007;10:1606–11. doi: 10.1017/S1368980007361004. [DOI] [PubMed] [Google Scholar]
- [4].Glinoer D. The Regulation of Thyroid Function During Normal Pregnancy:Importance of the Iodine Nutrition Status. Best Pract Res Clin Endocrinol Metab. 2004;18:133–52. doi: 10.1016/j.beem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- [5].Azizi F, Smyth P. Breastfeeding and Maternal and Infant Iodine Nutrition. Clin Endocrinol (Oxf) 2009;70:803–9. doi: 10.1111/j.1365-2265.2008.03442.x. [DOI] [PubMed] [Google Scholar]
- [6].Korevaar TI. Evidence-based Tightrope Walking:The 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:309–11. doi: 10.1089/thy.2017.29040.tko. [DOI] [PubMed] [Google Scholar]
- [7].Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, et al. Iodine Status and Prevalence of Thyroid Disorders After Introduction of Mandatory Universal Salt Iodization for 16 Years in China:A Cross-sectional Study in 10 Cities. Thyroid. 2016;26:1125–30. doi: 10.1089/thy.2015.0613. [DOI] [PubMed] [Google Scholar]
- [8].Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of Iodine Intake on Thyroid Diseases in China. N Engl J Med. 2006;354:2783–93. doi: 10.1056/NEJMoa054022. [DOI] [PubMed] [Google Scholar]
- [9].Wang Y, Zhang Z, Chen F, Zhu X, Sun W, Cao Y. Iodine Nutrition Status and Thyroid Function of Women at Different Phases of Gestation in an Iodine Sufficient Rural Area. Asia Pac J Clin Nutr. 2021;30:99–103. doi: 10.6133/apjcn.202103_30(1).0012. [DOI] [PubMed] [Google Scholar]
- [10].Ministry of Health PsRoC. National Food Safety Standards-Iodine Content of Salt (gb26878-2011) Ministry of Health PsRoC. 2011 [Google Scholar]
- [11].Yu Z, Zheng C, Zheng W, Wan Z, Bu Y, Zhang G, et al. Mild-to-moderate Iodine Deficiency in a Sample of Pregnant Women and Salt Iodine Concentration from Zhejiang Province, China. Environ Geochem Health. 2020;42:3811–8. doi: 10.1007/s10653-020-00640-0. [DOI] [PubMed] [Google Scholar]
- [12].Zhao Q, Hu D, Yang J, Huang S, Fan Q, Tang C. Analysis of the Monitoring Results of Iodine Nutrition Status in Yubei District of Chongqing from 201 to 2021. Chin J Ctrl Endem Dis. 2022;37:26–8. [Google Scholar]
- [13].Zhou H, Ma ZF, Lu Y, Pan B, Shao J, Wang L, et al. Assessment of Iodine Status Among Pregnant Women and Neonates Using Neonatal Thyrotropin (tsh) in Mainland China After the Introduction of New Revised Universal Salt Iodisation (USI) in 2012:A Re-emergence of Iodine Deficiency? Int J Endocrinol. 2019;2019:3618169. doi: 10.1155/2019/3618169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring their Elimination:A Guide for Programme Managers. Geneva: World Health Organization; 2007. [Google Scholar]
- [15].Kaile T, Sikateyo B, Phiri MM, Michelo C. Prevalence of Iodine Deficiency Among Pregnant Women in Gwembe and Sinazongwe Districts of Southern Province, Zambia:A Cross-sectional study. BMC Nutr. 2020;6:71. doi: 10.1186/s40795-020-00397-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Businge CB, Longo-Mbenza B, Kengne AP. Iodine Deficiency in Pregnancy along a Concentration Gradient is Associated with Increased Severity of Preeclampsia in Rural Eastern Cape, South Africa. BMC Pregnancy Childbirth. 2022;22:98. doi: 10.1186/s12884-021-04356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gargari SS, Fateh R, Bakhshali-Bakhtiari M, Saleh M, Mirzamoradi M, Bakhtiyari M. Maternal and Neonatal Outcomes and Determinants of Iodine Deficiency in Third Trimester of Pregnancy in an Iodine Sufficient Area. BMC Pregnancy Childbirth. 2020;20:174. doi: 10.1186/s12884-020-02863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eastman CJ, Ma G, Li M. Optimal Assessment and Quantification of Iodine Nutrition in Pregnancy and Lactation:Laboratory and Clinical Methods, Controversies and Future Directions. Nutrients. 2019;11:2378. doi: 10.3390/nu11102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Abel MH, Caspersen IH, Sengpiel V, Jacobsson B, Meltzer HM, Magnus P, et al. Insufficient Maternal Iodine Intake is Associated with Subfecundity, Reduced Foetal Growth, and Adverse Pregnancy Outcomes in the Norwegian Mother, Father and Child Cohort Study. BMC Med. 2020;18:211. doi: 10.1186/s12916-020-01676-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Habibi N, Grieger JA, Bianco-Miotto T. A Review of the Potential Interaction of Selenium and Iodine on Placental and Child Health. Nutrients. 2020;12:2678. doi: 10.3390/nu12092678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Opazo MC, Coronado-Arrázola I, Vallejos OP, Moreno-Reyes R, Fardella C, Mosso L, et al. The Impact of the Micronutrient Iodine in Health and Diseases. Crit Rev Food Sci Nutr. 2022;62:1466–79. doi: 10.1080/10408398.2020.1843398. [DOI] [PubMed] [Google Scholar]
- [22].Shi X, Han C, Li C, Mao J, Wang W, Xie X, et al. Optimal and Safe Upper Limits of Iodine Intake for Early Pregnancy in Iodine-Sufficient Regions:A Cross-sectional Study of 7190 Pregnant Women in China. J Clin Endocrinol Metab. 2015;100:1630–8. doi: 10.1210/jc.2014-3704. [DOI] [PubMed] [Google Scholar]
- [23].Li F, Wan S, Zhang L, Li B, He Y, Shen H, et al. Ameta-analysis of the Effect of Iodine Excess on the Intellectual Development of Children in Areas with High Iodine Levels in their Drinking Water. Biol Trace Elem Res. 2022;200:1580–90. doi: 10.1007/s12011-021-02801-3. [DOI] [PubMed] [Google Scholar]
- [24].Yao N, Zhou C, Xie J, Zhou S. A Cross-sectional Research of Iodine Status of Pregnant Women in Chongqing, South-west China. Public Health Nutr. 2020;23:769–75. doi: 10.1017/S1368980019003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ji C, Bu Y, Tian C, Fan L, Liu S, Liu Y, et al. Determination of Reference Intervals of Ratios of Concentrations of Urinary Iodine to Creatinine and Thyroid Hormone Concentrations in Pregnant Women Consuming Adequate Iodine in Harbin, Heilongjiang Province. Biol Trace Elem Res. 2020;193:36–43. doi: 10.1007/s12011-019-01689-4. [DOI] [PubMed] [Google Scholar]
- [26].Chen Y, Guo W, Pan Z, Zhang D, Gao M, Wu W, et al. Exploration of the Optimal Range of Urinary Iodine Concentration in Chinese Pregnant Women in Mildly Iodine-Deficient and-Sufficient Areas. Eur J Nutr. 2022;61:1221–30. doi: 10.1007/s00394-021-02693-y. [DOI] [PubMed] [Google Scholar]
- [27].Zhang H, Lv S, Mu Z, Li W, Zhang X, Wang Y, Rutherford S. Iodised Salt Contribution to Iodine Nutrition Status of Pregnant and Lactating Women. Br J Nutr. 2015;114:126–33. doi: 10.1017/S0007114515001543. [DOI] [PubMed] [Google Scholar]
- [28].Rodriguez-Diaz E, Pearce EN. Iodine Status and Supplementation Before, During, and After Pregnancy. Best Pract Res Clin Endocrinol Metab. 2020;34:101430. doi: 10.1016/j.beem.2020.101430. [DOI] [PubMed] [Google Scholar]
- [29].Tian W, Yan W, Liu Y, Zhou F, Wang H, Sun W. The Status and Knowledge of Iodine among Pregnant Women in Shanghai. Biol Trace Elem Res. 2021;199:4489–97. doi: 10.1007/s12011-021-02587-4. [DOI] [PubMed] [Google Scholar]
- [30].Guo W, Wang W, Jin Y, Chen W, Chen L, Lin L, et al. Trimester-specific Thyroid Function in Pregnant Women with Different Iodine Statuses. Ann Nutr Metab. 2020;76:165–74. doi: 10.1159/000506276. [DOI] [PubMed] [Google Scholar]
- [31].Stagnaro-Green A. Postpartum Management of Women Begun on Levothyroxine During Pregnancy. Front Endocrinol (Lausanne) 2015;6:183. doi: 10.3389/fendo.2015.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Candido AC, Ribeiro SA, Macedo MS, Fontes EA, Souza EC, Duarte MS, et al. Is Dietary Iodine Intake Excessive According to the Theoretical Model of Healthy Dietary Intake Pattern in Pregnant Women and Schoolchildren:Water, Salt, or Food? Front Nutr. 2021;8:770798. doi: 10.3389/fnut.2021.770798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pan Z, Cui T, Chen W, Gao S, Pearce EN, Wang W, et al. Serum Iodine Concentration in Pregnant Women and its Association with Urinary Iodine Concentration and Thyroid Function. Clin Endocrinol (Oxf) 2019;90:711–8. doi: 10.1111/cen.13945. [DOI] [PubMed] [Google Scholar]
- [34].Adu-Gyamfi EA, Wang YX, Ding YB. The Interplay Between Thyroid Hormones and the Placenta:A Comprehensive Review. Biol Reprod. 2020;102:8–17. doi: 10.1093/biolre/ioz182. [DOI] [PubMed] [Google Scholar]
- [35].Peng S, Li C, Xie X, Zhang X, Wang D, Lu X, et al. Divergence of Iodine and Thyroid Hormones in the Fetal and Maternal Parts of Human-Term Placenta. Biol Trace Elem Res. 2020;195:27–38. doi: 10.1007/s12011-019-01834-z. [DOI] [PubMed] [Google Scholar]
- [36].Ren YT, Jia QZ, Zhang XD, Guo BS, Zhang FF, Cheng XT, et al. Prevalence of Thyroid Function in Pregnant and Lactating Women in Areas with Different Iodine Levels of Shanxi Province. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39:609–13. doi: 10.3760/cma.j.issn.0254-6450.2018.05.013. [DOI] [PubMed] [Google Scholar]
- [37].Li X. Study on the Correlation Between Urinary Iodine Level and Thyroid Function in Women in Different Pregnancy Periods. J Pract Gynecol Endocrinol. 2018;5:13–4. [Google Scholar]


