Abstract
BACKGROUND
Multiple sclerosis (MS) is a common demyelinating disease of the central nervous system, usually presenting with multiple small white matter lesions. In some rare cases, it can present as a singular tumefactive white matter plaque.
OBSERVATIONS
The patient in case 1 was a 33-year-old woman presenting with a restriction of fine motor skills. Magnetic resonance imaging showed a singular round lesion in the left frontal lobe with ring enhancement and moderate perilesional edema. Assuming the diagnosis of a neoplasm, total resection was performed. Histological examination showed an early active inflammatory demyelinating process. A final diagnosis of MS was made. The patient in case 2 was a 65-year-old woman who had been diagnosed with MS 10 years earlier and was experiencing moderate left hemiparesis. She was found to have a progressive right thalamic lesion with contrast enhancement, perilesional edema, and space-occupying effect. Stereotactic biopsy of the lesion was performed. Histological examination revealed a glioblastoma multiforme World Health Organization grade IV, and concomitant chemoradiation was recommended.
LESSONS
On the one hand, tumefactive MS can be a diagnostic challenge because it mimics neoplasms or abscesses. On the other hand, a new lesion in patients with a diagnosis of long-standing demyelinating disease may not necessarily be a new demyelinating lesion and should be closely monitored.
Keywords: tumefactive multiple sclerosis, demyelinating diseases, ring enhancement, differential diagnosis of cerebral neoplasms
ABBREVIATIONS : CSF = cerebrospinal fluid, MRI = magnetic resonance imaging, MS = multiple sclerosis, OCB = oligoclonal band, WHO = World Health Organization
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system, usually affecting young women.1–4 On magnetic resonance imaging (MRI), it usually presents with multiple small plaques in the white matter and is well demarcated and homogeneous.1–4 Some rare cases show tumefactive demyelinating lesions along with atypical clinical presentation.2,3,5–7 These tumefactive demyelinating forms of MS are radiologically and clinically difficult to differentiate from brain tumors.3,5–7 Thorough diagnostic and accurate differentiation is indispensable to avoid aggressive diagnostic and therapeutic procedures.2,5,8,9 Nevertheless, in particular cases, brain biopsy may be inevitable for precise differential diagnosis.2,3,5,6,9
MRI is the standard diagnostic instrument for diagnosis of either typical or atypical demyelinating disorders.7 In tumefactive demyelinating disorders, MRI shows usually singular, infrequently multiple lesions in the white matter, more than 2 cm in size and without involvement of the cortex.2,3,5–8 In contrast to neoplasms, the mass effect of tumefactive demyelinating lesions is less distinct in comparison to their size2,9 In 77% of the cases, tumefactive lesions stand out with a perilesional edema.10 Contrast enhancement can be seen in 95%–100% of cases, but it shows various characteristics.2,7 Focusing on MRI only, however, may lead to misdiagnosis, as presented in the two case reports with tumor-like lesions, which were misinterpreted as a tumor in one case and as an MS lesion in the other case.
Illustrative Cases
Case 1
A 33-year-old woman presented to our neurosurgical outpatient clinic, having experienced rapidly progressive fine motor deficits of the right hand for 2 weeks. Three years earlier, she had a herpes zoster infection accompanied by a right facial nerve paresis that had completely resolved. On inquiry, she also reported an event of a mild right hemiparesis 2 years ago, which also resolved spontaneously. External MRI (Fig. 1) showed a fluid-filled cystic lesion in the left frontal lobe with ring enhancement and a mild perilesional edema but no substantial space-occupying effect. The image was suspicious for either a glioma or an infectious lesion. Therefore, the patient was admitted to our neurosurgical department for resection of the lesion, which was performed without complications. Intraoperatively, the lesion appeared like a low-grade glioma with cystic parts. After resection, the patient developed mild hemiparesis on the right but showed improvement under therapy with intravenous corticosteroids. There were no signs of malignant cells on the histopathological examination, which showed perivascular lymphocyte infiltration and infiltration of macrophages (Fig. 2E–H). It also revealed signs of demyelination accompanied by relative axonal preservation and reactive astrocytes (Fig. 2A–D). For the differential diagnosis, a demyelinating disease was considered, but because this was contradictory to clinical and radiological findings, histological samples were transferred to a reference center for further analysis, and further diagnostic procedures for exclusion of infectious and demyelinating diseases were recommended.
FIG. 1.
Case 1. Fluid-attenuated inversion recovery (FLAIR) and T2-weighted (T2) images show a white matter lesion with perilesional edema but without a space-occupying effect. Outer CSF spaces are not squeezed out. Gadolinium-enhanced (Gad) images stand out with ring enhancement. Diffusion-weighted imaging (DWI) shows no characteristics of an abscess. T1 = T1-weighted.
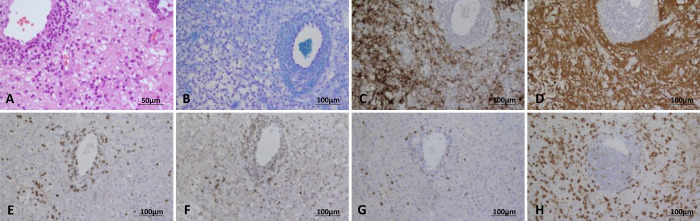
FIG. 2.
Case 1. Hematoxylin and eosin–stained section illustrates reactive astrocytes and macrophages in the brain parenchyma and a perivascular accumulation of lymphocytes and macrophages (A). The myelin stain (Klüver Barrera) documents diminution of myelin (B); neurofilament immunostain identifies intact axons (C); glial fibrillary acid protein highlights long stellated processes of reactive astrocytes (D); many perivascular lymphocytes are T lymphocytes (CD3 [E], CD4 [F], and CD8 [G] immunohistochemistry); and CD68 immunohistochemistry (H) highlights parenchymal macrophages.
Serology and autoimmune diagnostic tests showed no signs of a viral or parasitic infection or autoimmune antibodies. The findings of ophthalmological examination and visually evoked potentials were normal. Additional MRI of the whole spinal axis showed no abnormalities. Cerebrospinal fluid (CSF) examination also revealed no signs of infection or malignancy; however, positive oligoclonal bands (OCBs) were detected. Therefore, demyelinating diseases were again considered. Eventually, the results of the reference pathology center were available, and with regard to signs of active demyelination and myelin-phagocytosing macrophages, the diagnosis of a tumefactive MS was confirmed. After corticosteroid therapy and improvement of the right hemiparesis and fine motor deficits, a final MRI scan revealed regression in the size of the cystic lesion and the perilesional edema. Further treatment with immunomodulatory treatment with omalizumab was initiated.
Case 2
A 65-year-old woman had been diagnosed with MS 10 years earlier, at that time experiencing impaired vision and sensory paraplegia. MRI had shown an inflammatory lesion in the corpus callosum, and OCBs had not been detected in the CSF. After steroid pulse therapy, an immunomodulatory therapy with interferon-β had been initiated.
Eight years after the initial diagnosis, MRI revealed a new small lesion in the right thalamus (Fig. 3, upper). In the absence of new clinical symptoms, no further diagnostic testing or treatment escalation was performed. Besides a slight progression in the size of the right thalamic lesions with new enhancement, subsequent MRI scans showed two new lesions in the right central region and right parietal lobe (Fig. 3, lower). Another 2 years later, a further progression in size of the three new lesions was observed on MRI, with perilesional edema, mass effect, midline shift, and beginning hydrocephalus and uncal herniation (Fig. 4). At this time, the patient was experiencing auditory deficits, slight left hemiparesis, residual visual deficits, and personality changes, so she received steroid pulse therapy. The patient’s case was discussed in the interdisciplinary tumor board, and an indication for biopsy of the lesion was confirmed.
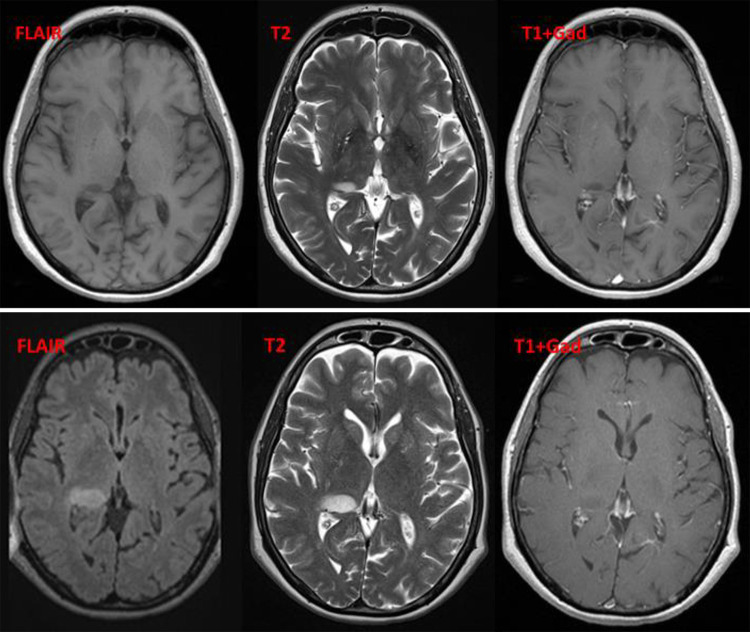
FIG. 3.
Case 2. Upper: Eight years after the initial MS diagnosis, MRI reveals a new small lesion in the right thalamus without enhancement. No perilesional edema can be detected. Lower: Six months later, MRI shows a slight progression in the size of the right thalamic lesions with new enhancement. There is still no perilesional edema. DWI = diffusion-weighted imaging; FLAIR = fluid-attenuated inversion recovery; Gad = gadolinium enhanced; T1 = T1-weighted; T2 = T2-weighted.
FIG. 4.
Case 2. MRI another 2 years later reveals the known white matter lesion arising from the right thalamus, now with perilesional edema, mass effect, midline shift, and beginning hydrocephalus and uncal herniation. DWI = diffusion-weighted imaging; FLAIR = fluid-attenuated inversion recovery; Gad = gadolinium enhanced; GRE = gradient echo sequences; T1 = T1-weighted.
The patient was admitted to our hospital, and biopsy was performed on the next day. One week later, she became intermittently tired and spacy. Due to the suspicion of seizures, anticonvulsant drugs were administered. Computed tomography scan showed no signs of progression of the edema or postoperative complications.
The initial histological examination showed an anaplastic oligodendroglioma, World Health Organization (WHO) grade III (Fig. 5). Radiation of the lesion was planned. Due to the deteriorated general condition of the patient, at first, chemotherapy was not advised.
FIG. 5.

Case 2. Hematoxylin and eosin–stained section illustrates scattered cells with hyperchromatic nuclei (A). Strong diffuse immunoreactivity for glial fibrillary acidic protein in tumor cells (B), a diffuse strong immunoreactivity for p53 (C), and Ki-67 in the range of >5% can be seen (D).
The patient’s clinical status worsened within the next days, so therapy limitation was decided after consultation with her husband, and palliative care was initiated. The patient died 2 weeks after admission. Further histological examination with immunohistochemistry revealed the definite diagnosis of a glioblastoma multiforme, WHO grade IV, isocitrate dehydrogenase mutant.
Discussion
Observations
These two cases serve as impressive examples of the diagnostic challenge concerning tumefactive MS forms. They shed light on the difficulties in differentiation between tumefactive demyelinating lesions on the one hand and neoplasms or abscesses on the other hand.3,5–7 They emphasize the role of thorough diagnostic testing and accurate differentiation to avoid aggressive diagnostic and therapeutic procedures.2,5,8,9
Being a young woman in the fourth decade of life, the first patient fulfills the epidemiological criteria of a typical patient with MS.2,4,7,8 The fact that the patient reported an earlier event with right motor weakness 3 years ago raises the question whether the patient had MS before. Hence, the tumefactive lesion may not have been the initial event.
Tumefactive forms of MS are relatively rare, representing 1–3 of 1,000 MS cases and having a prevalence of 3 in 1 million cases annually.2–4,7 The median age at onset is 37 years, usually arising in the second or third decade of life.2,4 MS mostly affects young women, but female predominance in tumefactive cases is yet not proved.2,4,7,8
The clinical presentation of patients with tumefactive lesions is atypical in comparison with that of patients with typical MS.4,7,8,11 Although patients with typical MS initially present with symptoms such as sensory or motor deficits, hemiparesis or optic neuritis, tumefactive forms can lead to headache, cognitive impairment, speech deficits, cerebellar symptoms, and even seizures.2–4,7–9,11
The second patient was slightly older than the typical age of patients with MS, but given that the first symptoms were reported 10 years ago, she can also be regarded as a typical patient with MS. However, due to the absence of dissemination in time and space (only one relapse and one MS lesion in the corpus callosum), the question arises whether the diagnosis of MS was correct. Nevertheless, the patient’s case highlights the fact that in patients with suspicions of an MS diagnosis, tumefactive lesions deserve closer attention, especially in case of a mass effect.
Tumefactive demyelinating lesions are known to mimic brain tumors on MRI, with low-grade glioma as the most frequent misdiagnosis.1,3,5–7,10,12 In the first case, low-grade glioma was also the first suspicious intraoperative diagnosis, which led to the total resection of the lesion. The patient presented with a single lesion in the left frontal lobe with ring enhancement and mild perilesional edema but no substantial space-occupying effect. As reported for demyelinating tumefactive lesions, in this case, a mild mass effect was detected with a mild perilesional edema in comparison with the lesion size.2,9,10 Ring enhancement was also present, but with a closed ring typical of cerebral neoplasms or abscesses. Concerning the second case, perilesional edema and enhancement were also present, but a massive mass effect, leading to ventricular compression, hydrocephalus, and uncal herniation, was detected. This mass effect, atypical for tumefactive MS, led us to consider a brain-derived tumor, and biopsy was performed.
In both cases with ambiguous clinical presentation and nondistinctive MRI findings, a biopsy was indispensable. The histology of the first patient revealed a perivascular lymphocyte infiltration and myelin-phagocytosing macrophages as signs of active demyelination. Worth mentioning is that there are no reliable characteristics differentiating typical MS lesions from tumefactive demyelinating lesions.2 However, the most frequent misdiagnosis concerning histological examination is low-grade glioma,2,7 because findings such as hypercellularity, atypical reactive astrocytes, mitotic figures, astrocytic pleomorphism, necrosis, cystic changes, and nuclear atypia are also typical histological features of neoplasms.2,4,7 Characteristics certainly applying for tumefactive demyelinating lesions are absence of microvascular proliferation, many macrophages despite missing necrosis, plump reactive astrocytes with multiple micronuclei intermingled with macrophages, and relative preservation of axons.1,2,4,7 Thus, it appears that in the presented case myelin-phagocytosing macrophages suggest a demyelinating lesion, whereas the detected perivascular lymphocyte infiltration represents a neoplastic characteristic. Therefore, because histopathological findings were ambiguous, a final diagnosis could not be made. Eventually, the reference center of pathology confirmed the diagnosis of a demyelinating tumefactive disease. Histological examination concerning the second case showed hypercellularity, atypical reactive astrocytes, astrocytic pleomorphism, necrosis, cystic changes, and nuclear atypia, as mentioned above as typical features of gliomas.2,4,7 Signs of demyelination were not detected. However, malignancy of the tumor and mutation analysis simplified differentiation between a demyelinating and a tumorous lesion in this case.
Interestingly, the first patient’s symptoms had not improved after resection of the lesion but always improved after the application of steroids. The administration of an immune-modifying drug, such as omalizumab in this case, is controversial. Given the fact that the conversion rate to typical MS in cases with initial tumefactive MS occurs in only 30% of the cases, administration of immune-modifying drugs is not coercively necessary.7,9 Due to the fact that the patient had already had an event with neurological symptoms 3 years ago and therefore may have had typical MS before, the administration of an immune-modifying drug seems reasonable. The administration of corticosteroids also in the short term led to an improvement of clinical symptoms in the case of the second patient. This can be attributed to the fact that corticosteroids lead to a reduction of the perilesional edema in brain tumors. At the end, resection, chemotherapy, or radiation would have been necessary for long-term improvement, but the patient’s general condition had already decreased too much for further treatment.
Lessons
Several forms of multiple sclerosis mimic cerebral neoplasm or abscess and therefore lead to misdiagnosis. In the first case, the diagnosis of a cerebral neoplasm could not be definitely excluded, so, in consideration of the rapid progressive neurological deficits, resection was performed. This raises the question whether, if the diagnosis of a demyelinating disease had been conceivable, aggressive procedures might have been avoidable by further noninvasive diagnostic tests. In the second case, a new lesion in a patient with the diagnosis of a long-standing demyelinating disease may be a neoplastic process and should be closely monitored. A biopsy of a lesion in case of ambiguous clinical and radiological findings or insufficient therapeutic effect should be discussed early to confirm the diagnosis.
Acknowledgments
We extend many thanks to the physician team of the Department of Neurosurgery, Justus-Liebig-University Gießen, for collection and preparation of patient data.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Friedrich, Struffert, Uhl. Acquisition of data: Friedrich, Dohmen, Uhl. Analysis and interpretation of data: Friedrich, Dohmen, Uhl. Drafting the article: Friedrich, Struffert, Uhl. Critically revising the article: Struffert, Uhl. Reviewed submitted version of manuscript: Struffert, Uhl. Approved the final version of the manuscript on behalf of all authors: Friedrich. Administrative/technical/material support: Struffert, Uhl.
Supplemental Information
Previous Presentations
This material was presented at the European Association of Neurosurgical Societies eEANS 2021 Virtual Congress, Neurosurgery in Translation, October 3–7, 2021.
References
- 1. Gavra M, Boviatsis E, Stavrinou LC, Sakas D. Pitfalls in the diagnosis of a tumefactive demyelinating lesion: a case report. J Med Case Rep. 2011;5(1):217. doi: 10.1186/1752-1947-5-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lucchinetti CF, Gavrilova RH, Metz I, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131(Pt 7):1759–1775. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiriyama T, Kataoka H, Taoka T, et al. Characteristic neuroimaging in patients with tumefactive demyelinating lesions exceeding 30 mm. J Neuroimaging. 2011;21(2):e69–e77. doi: 10.1111/j.1552-6569.2010.00502.x. [DOI] [PubMed] [Google Scholar]
- 4. Omerhodžić I, Džurlić A, Lisica D, et al. Relapsing tumefactive demyelination: a case report. Acta Med Acad. 2018;47(2):193–198. doi: 10.5644/ama2006-124.231. [DOI] [PubMed] [Google Scholar]
- 5. Cañellas AR, Gols AR, Izquierdo JR, Subirana MT, Gairin XM. Idiopathic inflammatory-demyelinating diseases of the central nervous system. Neuroradiology. 2007;49(5):393–409. doi: 10.1007/s00234-007-0216-2. [DOI] [PubMed] [Google Scholar]
- 6. Kim DS, Na DG, Kim KH, et al. Distinguishing tumefactive demyelinating lesions from glioma or central nervous system lymphoma: added value of unenhanced CT compared with conventional contrast-enhanced MR imaging. Radiology. 2009;251(2):467–475. doi: 10.1148/radiol.2512072071. [DOI] [PubMed] [Google Scholar]
- 7. Algahtani H, Shirah B, Alassiri A. Tumefactive demyelinating lesions: a comprehensive review. Mult Scler Relat Disord. 2017;14:72–79. doi: 10.1016/j.msard.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 8. Turkistani AN, Alshamrani FJ, Shareefi GF, Alsulaiman A. Tumefactive multiple sclerosis masquerade as a central nervous system tumor: a case report. Electron Physician. 2018;10(8):7180–7184. doi: 10.19082/7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Codjia P, Ayrignac X, Carra-Dalliere C, et al. Multiple sclerosis with atypical MRI presentation: results of a nationwide multicenter study in 57 consecutive cases. Mult Scler Relat Disord. 2019;28:109–116. doi: 10.1016/j.msard.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 10. Ikeguchi R, Shimizu Y, Abe K, et al. Proton magnetic resonance spectroscopy differentiates tumefactive demyelinating lesions from gliomas. Mult Scler Relat Disord. 2018;26:77–84. doi: 10.1016/j.msard.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 11. Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun. 2014;48-49:134–142. doi: 10.1016/j.jaut.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 12. Yamada S, Yamada SM, Nakaguchi H, et al. Tumefactive multiple sclerosis requiring emergent biopsy and histological investigation to confirm the diagnosis: a case report. J Med Case Reports. 2012;6(1):104. doi: 10.1186/1752-1947-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]