Abstract
BACKGROUND
Central nervous system (CNS) mature teratoma is a rare disease with symptoms that can vary according to tumor location. Most lesions are benign; rarely, malignancy can develop in any of the somatic components. Elevated levels of tumor markers such as α-fetoprotein and β-human chorionic gonadotropin are not usually found in patients with CNS mature teratoma, and no reports have described an association with carbohydrate antigen 19-9 (CA19-9).
OBSERVATIONS
A 64-year-old woman with headache was found to have a mass lesion in the anterior cranial fossa. Magnetic resonance imaging of the brain suggested a mature teratoma. Serum and cerebrospinal fluid (CSF) tests showed significant CA19-9 elevations (2,770 U/mL and 4,387 U/mL, respectively). Other examinations, including whole-body 18F-fluorodeoxyglucose positron emission tomography, did not detect the origin of elevated CA19-9, suggesting that the high CA19-9 levels were caused by intracranial tumor. The patient underwent tumor removal. The histopathological diagnosis was mature teratoma with positive CA19-9 staining. CA19-9 levels in serum and CSF decreased significantly after tumor removal.
LESSONS
The histopathological findings and postoperative decreased CA19-9 levels established the diagnosis of CA19-9–producing CNS mature teratoma. CNS mature teratoma can cause elevations in CA19-9 in cases with absence of neoplasms in the trunk.
Keywords: central nervous system mature teratoma, tumor marker, carbohydrate antigen 19-9, germ cell tumor
ABBREVIATIONS : AFP = α-fetoprotein, β-hCG = β-human chorionic gonadotropin, CA19-9 = carbohydrate antigen 19-9, CNS = central nervous system, CSF = cerebrospinal fluid, GCT = germ cell tumor, MRI = magnetic resonance imaging, NGGCT = nongerminomatous germ cell tumor, PLAP = placenta-type alkaline phosphatase, T1WI = T1-weighted imaging
Central nervous system (CNS) mature teratomas are very rare neoplasms, classified as germ cell tumors (GCTs) and accounting for only 0.3%–0.6% of all primary intracranial tumors.1 This pathological entity is basically benign with a good prognosis if proper treatment is achieved.2,3 Curative treatment for CNS mature teratoma is limited to complete resection, with chemotherapy and radiotherapy having little to no effect.4,5 So far, three biochemical markers have been identified as tumor markers for GCTs: α-fetoprotein (AFP), β-human chorionic gonadotropin (β-hCG), and placenta-type alkaline phosphatase (PLAP).6,7 However, CNS mature teratomas do not usually produce known tumor markers.2 Carbohydrate antigen 19-9 (CA19-9) is often elevated with pancreatic and biliary tract tumors and is normally produced in the uterine mucosa and salivary glands.8 Two reports have described CA19-9 elevation in both serum and ascites among patients with ovarian mature teratoma.9,10 However, such elevations have never been identified with CNS GCTs, particularly mature teratoma. Here, we report a patient with CA19-9–secreting CNS mature teratoma.
Illustrative Case
Clinical Summary
A 64-year-old woman presented with a 2-week history of headache in the occipital region. Her physical and neurological findings were unremarkable. Computed tomography showed a tumor appearing isodense with fat (Fig. 1A). Magnetic resonance imaging (MRI) showed a large suprasellar tumor, 4.5 × 2.9 × 3.1 cm in size, with scattered droplets in the subarachnoid spaces (Fig. 1B). The tumor extended to the anterior cranial fossa and prepontine cistern, whereas the optic chiasm and pituitary gland were intact. The tumor contents showed prominent hyperintensity on T1-weighted imaging (T1WI) that was decreased on fat-suppressed T1WI (Fig. 1B). T2 sampling perfection with application-optimized contrasts using different flip angle evolution images showed a heterogeneous intense tumor with an isointense nodule (Fig. 1C). Gadolinium-enhanced T1WI showed partial enhancement of the nodule (Fig. 1D). These results from imaging studies were suggestive of CNS teratoma with a ruptured cyst. For further evaluation, we analyzed tumor markers. Notably, CA19-9 was significantly increased in serum (2,770 U/mL) and cerebrospinal fluid (CSF) (4,387 U/mL) compared with normal serum level <37.0 U/mL, although AFP and β-hCG remained within normal limits. Searches were performed for the origin of CA19-9 secretion using whole-body 18F-fluorodeoxyglucose positron emission tomography, gastrointestinal endoscopy, and gynecological consultation. However, the origin of the elevated CA19-9 remained unknown. The significant elevation of CA19-9 seemed to be derived from the intracranial tumor. Because the patient reported worsening headaches, resection was planned.
FIG. 1.

Diagnostic imaging examinations before and after tumor resection. A: Computed tomography shows a fat-density tumor in the suprasellar region without calcification. B: T1WI shows a hyperintense tumor with scattered droplets (white arrowheads) indicating rupture of a tumor cyst. C: T2 sampling perfection with application-optimized contrasts using different flip angle evolution image shows a heterogeneous, intense tumor with isointense nodule (black arrowhead). D: Gadolinium-enhanced T1WI shows partial enhancement of the nodule (white arrow). E: Postoperative T1WI shows total removal of the hyperintense tumor. F: Postoperative gadolinium-enhanced T1WI shows total removal of the nodule tumor.
Bilateral frontal craniotomy and a basal interhemispheric approach to the lesion were performed. After opening the dura mater, disseminated droplets were seen in the subarachnoid space (Fig. 2A). The tumor was mainly located in bilateral anterior cranial fossae with displacement of the olfactory nerve (Fig. 2B). The tumor cyst had yellow creamy contents (Fig. 2C). A small, solid component was found in the suprasellar region (Fig. 2D). Gross total resection was performed, which was also identified by intraoperative MRI (Fig. 1E and D).
FIG. 2.

Intraoperative microscopic images. A: A view of the left frontal cortex shows small droplets in the subarachnoid space (white arrowheads). B: A view of the right subfrontal lobe shows a yellow cyst on the anterior skull base, compressing the left olfactory nerve (cranial nerve [CN] I). C: A view of the incised cyst shows creamy yellow contents spilling over. D: A view after cyst removal shows a tumor nodule (black arrowheads) adhering to the adjacent structures of the left anterior cerebral artery (A2) and optic nerve (CN II) in the suprasellar region. ICA = internal carotid artery.
Histological Findings
Histopathological examination of the tumor nodule showed findings typical of mature teratoma, which comprised fully differentiated tissues resembling epidermis and skin adnexa. None of the components harbored incompletely differentiated tissues or tissues showing an immature appearance. Most of the tumor nodule and cyst wall had a negative finding for immunohistochemical staining of CA19-9, with the exception of the luminal surface of the dermis-like squamous epithelium surrounding the cystic space (Fig. 3).
FIG. 3.
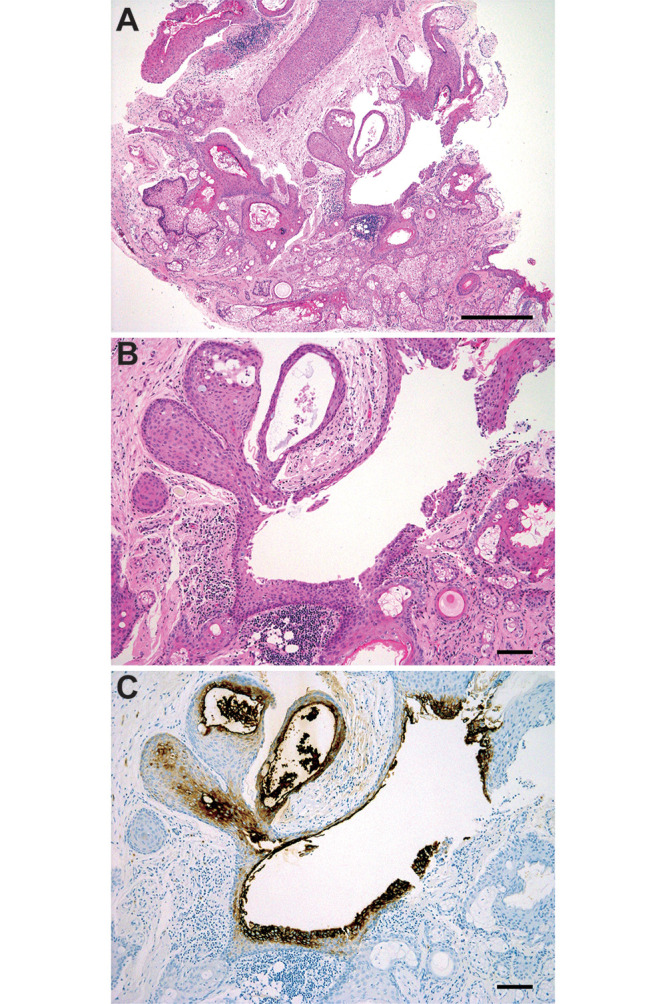
Histopathological examination of the removed tumor. A: Low-power view of the excised tumor nodule shows fully differentiated ectodermal components, including epidermis and skin adnexa (hematoxylin and eosin [H&E], bar = 500 μm). B and C: Middle-power view of the excised tissue reveals that several layers of epithelial cells surrounding the cyst are positive for CA19-9. Meanwhile, the outer layer of the epithelium and skin adnexa-like components appear negative (H&E, B; immunohistochemical staining with CA19-9, C; bars = 100 μm).
Postoperative Course
No complications were observed, and the patient’s headache was improved immediately after surgery. Postoperative MRI revealed total resection. High levels of CA19-9 in serum and CSF decreased significantly to 115 U/mL and 109 U/mL, respectively, by 2 weeks postoperatively. Finally, the patient was discharged home without any complications or neurological deficits. At the 7-month follow-up, the patient was well with no recurrence, and serum CA19-9 was 15 U/mL, within normal limits (Fig. 4).
FIG. 4.

Sequential examinations of CA19-9 in serum and CSF. High levels of CA19-9 in serum and CSF are significantly decreased after tumor removal.
Discussion
Observations
In this article, we have reported the case of a patient with a CA19-9–producing CNS mature teratoma. To the best of our knowledge, prominent elevation of CA19-9 has not previously been reported in association with CNS mature teratoma.
Teratomas are a group of nongerminomatous GCTs (NGGCTs) and are histologically divided into three subtypes: mature, immature, and teratoma with somatic-type malignancy.11 Mature teratomas are well differentiated and consist of three embryonal cell layers. As basically a benign neoplasm, the expected 10-year survival rate of patients is 92.9%.2 The median age of patients diagnosed with mature teratoma is 14.8 years (range 1–46 years).12 Even though clinical manifestations differ according to the location and size of the tumor, ruptured cystic teratoma usually occurs with headache.13–15 In this case, the intraoperative findings of scattered droplets in the subarachnoid space indicated that headache was attributable to cystic rupture. The treatment strategy for mature teratoma is resection. Gross total resection would be ideal, because mature teratomas are resistant to radiotherapy and chemotherapy.16 Postoperative recurrence is very infrequent, affecting one in seven patients.17 However, malignant transformation and recurrence, such as growing teratoma syndrome, can occur.2 Early diagnosis and careful postoperative follow-up are thus very important.
CNS GCTs usually occur in the pineal and suprasellar regions.2 These tumors often contain multiple components. NGGCTs are often mixed tumors that can be composed of any combination of yolk sac tumor, embryonal carcinoma, and choriocarcinoma. Furthermore, NGGCTs can also contain germinoma or teratoma, or both.18 Due to the deep-seated location and the heterogeneity of GCTs, the histopathological diagnosis often depends on a small specimen from a needle biopsy or endoscopic biopsy. Consequently, the classification sometimes remains unconfirmed. GCTs are known to secrete AFP, β-hCG, and PLAP.18 AFP generally reflects the existence of yolk sac tumors, and β-hCG is produced by choriocarcinomas.19 PLAP in CSF has been thought to represent a relatively nonspecific tumor marker for GCT, whereas Aihara et al.20 reported the effectiveness of PLAP for detecting CNS germinoma. Although many investigators have studied diagnostic criteria for tumor markers, no international consensus has been reached regarding clinical diagnostic criteria for biochemical marker levels.21 Furthermore, no reports have clarified the clinical significance of CA19-9 in CNS GCTs.
CA19-9, also called sialyl Lewis A antigen, is a cell surface protein that was found in 1979 using a monoclonal antibody in a human colorectal carcinoma.22 This protein is now widely used as a biochemical tumor marker, particularly for pancreatic ductal adenocarcinoma. CA19-9 is synthesized in the pancreatic parenchyma, biliary tract, and epithelial cells of the gastric, colonic, and uterine mucosa, as well as in the salivary glands. In fact, increased levels of CA19-9 have reportedly been observed in patients with mature cystic teratoma in an ovary.9,10,23–25 In a retrospective study of 80 patients with mature cystic teratoma of the ovary, rates of elevated CA19-9, carbohydrate antigen 125, carcinoembryonic antigen, and AFP were 38.8% (31 of 80), 25% (18 of 72), 9.1% (4 of 44), and 8.7% (4 of 46), respectively, suggesting that CA19-9 may be the only important marker in the diagnosis of mature cystic teratoma of the ovary.25 We found CA 19-9 elevation in this patient because of our test for systemic tumor makers containing CA 19-9. Because it is unusual for neurosurgeons to test CA 19-9 in patients with CNS teratoma, CA 19-9 elevation may be unobserved.
Madaan et al.10 considered that high levels of CA19-9 might be associated with inflammation or weakening of the cyst wall. In the present case, CA19-9–positive cells were observed only in the squamous epithelium surrounding the cystic space, without differentiation into glandular cells. Although not identified on the pathological specimen, glandular tumor cells with CA19-9–producing capacity should have been present, resulting in the accumulation and deposition of CA19-9 into the cystic space and leakage into peripheral blood.
Lessons
This represents a first case report of CA19-9–producing intracranial mature teratoma. In this case, we encountered abnormally high levels of CA19-9, so we first suspected a non-CNS cancer secreting CA19-9. However, examination of the patient’s whole body detected high levels of CA19-9 in CSF. Findings from histopathology and the postoperative decrease in CA19-9 established the diagnosis of CNS mature teratoma producing CA19-9. Although a high level of CA19-9 usually indicates neoplasm of the pancreas, biliary tract, or ovary, CNS mature teratoma is a possible cause of CA19-9 elevation in cases showing no abnormalities in the trunk.
Acknowledgments
We thank the staff of the Department of Neurosurgery, Diagnostic Pathology, Diagnostic Imaging and Nuclear Medicine at Kyoto University for discussions on the manuscript and illustration preparation.
This study was funded by Japan Agency for Medical Research and Development (AMED) (no. JP21ck0106619).
Disclosures
Dr. Arakawa reported grants from Philips, Otsuka, Chugai, Nihon Medi-Physic, Daiichi Sankyo, Stryker, Eisai, Japan Blood Products Organization, Ono Pharmaceutical, Taiho Pharma, Sumitomo Dainippon Pharma, Astellas Pharma, and Sanofi and personal fees from Nippon Kayaku, Novocure, UCB Japan, Ono Pharmaceutica, Brainlab, Merck, Chugai, Eisai, Daiichi Sankyo, Carl Zeiss, and Nihon Medi-Physic outside the submitted work. No other disclosures were reported.
Author Contributions
Conception and design: Arakawa, S Takeuchi, Minamiguchi. Acquisition of data: Arakawa, S Takeuchi, A Takeuchi, Minamiguchi. Drafting the article: S Takeuchi. Critically revising the article: Tanji, Mineharu, Haga, Miyamoto. Reviewed submitted version of manuscript: Arakawa, S Takeuchi, Minamiguchi, Mineharu, Miyamoto. Approved the final version of the manuscript on behalf of all authors: Arakawa. Administrative/technical/material support: Miyamoto. Study supervision: Arakawa. Pathological diagnosis: Minamiguchi.
References
- 1. Romić D, Raguž M, Marčinković P, et al. Intracranial mature teratoma in an adult patient: a case report. J Neurol Surg Rep. 2019;80(1):e14–e17. doi: 10.1055/s-0039-1685213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86(3):446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 3. Aoyama H, Shirato H, Yoshida H, et al. Retrospective multi-institutional study of radiotherapy for intracranial non-germinomatous germ cell tumors. Radiother Oncol. 1998;49(1):55–59. doi: 10.1016/s0167-8140(98)00081-4. [DOI] [PubMed] [Google Scholar]
- 4. Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63(2):155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 5. Regis J, Bouillot P, Rouby-Volot F, Figarella-Branger D, Dufour H, Peragut JC. Pineal region tumors and the role of stereotactic biopsy: review of the mortality, morbidity, and diagnostic rates in 370 cases. Neurosurgery. 1996;39(5):907–914. doi: 10.1097/00006123-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 6. Barlow LJ, Badalato GM, McKiernan JM. Serum tumor markers in the evaluation of male germ cell tumors. Nat Rev Urol. 2010;7(11):610–617. doi: 10.1038/nrurol.2010.166. [DOI] [PubMed] [Google Scholar]
- 7. Calaminus G, Bamberg M, Harms D, et al. AFP/β-HCG secreting CNS germ cell tumors: long-term outcome with respect to initial symptoms and primary tumor resection. Results of the cooperative trial MAKEI 89. Neuropediatrics. 2005;36(2):71–77. doi: 10.1055/s-2005-837582. [DOI] [PubMed] [Google Scholar]
- 8. Duffy MJ. New cancer markers. Ann Clin Biochem. 1989;26(Pt 5):379–387. doi: 10.1177/000456328902600501. [DOI] [PubMed] [Google Scholar]
- 9. Ito K. CA19-9 in mature cystic teratoma. Tohoku J Exp Med. 1994;172(2):133–138. doi: 10.1620/tjem.172.133. [DOI] [PubMed] [Google Scholar]
- 10. Madaan M, Puri M, Sharma R, Kaur H, Trivedi SS. Unusually high levels of CA19-9 associated with mature cystic teratoma of the ovary. Case Rep Obstet Gynecol. 2014;2014:187910. doi: 10.1155/2014/187910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) Editorial Board. Central Nervous System Tumours: WHO Classification of Tumours. 5th ed. Vol. 6. WHO; 2021. [Google Scholar]
- 12. Lee YH, Park EK, Park YS, Shim KW, Choi JU, Kim DS. Treatment and outcomes of primary intracranial teratoma. Childs Nerv Syst. 2009;25(12):1581–1587. doi: 10.1007/s00381-009-0974-8. [DOI] [PubMed] [Google Scholar]
- 13. Xuzhu C, Tao J, Junmei W, Jianping D. Spontaneous rupture of an intracranial teratoma. Neurol India. 2010;58(1):166–167. doi: 10.4103/0028-3886.60391. [DOI] [PubMed] [Google Scholar]
- 14. O’Grady J, Kobayter L, Kaliaperumal C, O’Sullivan M. ‘Teeth in the brain’ — a case of giant intracranial mature cystic teratoma. BMJ Case Rep. 2012;2012:bcr0320126130. doi: 10.1136/bcr.03.2012.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romero LR, Chen BY, Guzman MA, Zhou Y, Lai JP, Chen F. Ruptured intracranial teratoma: a case report and literature review. Clin Med Rev Case Rep. 2015;2:029. [Google Scholar]
- 16. Noudel R, Vinchon M, Dhellemmes P, Litré CF, Rousseaux P. Intracranial teratomas in children: the role and timing of surgical removal. J Neurosurg Pediatr. 2008;2(5):331–338. doi: 10.3171/PED.2008.2.11.331. [DOI] [PubMed] [Google Scholar]
- 17. Sawamura Y, Kato T, Ikeda J, Murata J, Tada M, Shirato H. Teratomas of the central nervous system: treatment considerations based on 34 cases. J Neurosurg. 1998;89(5):728–737. doi: 10.3171/jns.1998.89.5.0728. [DOI] [PubMed] [Google Scholar]
- 18. Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16(9):e470–e477. doi: 10.1016/S1470-2045(15)00244-2. [DOI] [PubMed] [Google Scholar]
- 19. Packer RJ, Cohen BH, Cooney K. Intracranial germ cell tumors. Oncologist. 2000;5(4):312–320. [PubMed] [Google Scholar]
- 20. Aihara Y, Watanabe S, Amano K, et al. Placental alkaline phosphatase levels in cerebrospinal fluid can have a decisive role in the differential diagnosis of intracranial germ cell tumors. J Neurosurg. 2018;131(3):687–694. doi: 10.3171/2018.3.JNS172520. [DOI] [PubMed] [Google Scholar]
- 21. Takami H, Graffeo CS, Perry A, et al. Roles of tumor markers in central nervous system germ cell tumors revisited with histopathology-proven cases in a large international cohort. Cancers (Basel) 2022;14(4):979. doi: 10.3390/cancers14040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 23. Kyung MS, Choi JS, Hong SH, Kim HS. Elevated CA 19-9 levels in mature cystic teratoma of the ovary. Int J Biol Markers. 2009;24(1):52–56. doi: 10.1177/172460080902400108. [DOI] [PubMed] [Google Scholar]
- 24. Wang YQ, Xia WT, Wang F, Zhuang XX, Zheng FY, Lin F. Use of cancer antigen 125, cancer antigen 19-9, and the neutrophil-to-lymphocyte ratio to diagnose mature cystic teratoma with torsion. Int J Gynaecol Obstet. 2017;137(3):332–337. doi: 10.1002/ijgo.12139. [DOI] [PubMed] [Google Scholar]
- 25. Dede M, Gungor S, Yenen MC, Alanbay I, Duru NK, Haşimi A. CA19-9 may have clinical significance in mature cystic teratomas of the ovary. Int J Gynecol Cancer. 2006;16(1):189–193. doi: 10.1111/j.1525-1438.2006.00284.x. [DOI] [PubMed] [Google Scholar]


