Abstract
BACKGROUND
Central neuropathic pain (CNP) of the cervical and/or thoracic spinal cord has many etiologies, both natural and iatrogenic. Frequently, CNP is medically refractory and requires surgical treatment to modulate the perception of pain. Spinal cord stimulation is a modality commonly used in adults to treat this type of refractory pain; however, it is rarely used in the pediatric population.
OBSERVATIONS
The authors reported a case involving a common pediatric condition, Chiari malformation type I with syrinx, that led to a debilitating complex regional pain syndrome. The associated life-altering pain was successfully alleviated following placement of a spinal cord stimulator.
LESSONS
CNP, or the syndromic manifestations of the pain (complex regional pain syndrome), can alter an individual’s life in dramatic ways. Spinal cord stimulator placement in carefully selected pediatric patients should be considered in these difficult pain treatment paradigms.
Keywords: central neuropathic pain, cervical syrinx, Chiari I malformation, complex regional pain syndrome, spinal cord stimulator, syringomyelia
ABBREVIATIONS : CM-I = Chiari malformation type I, CM-I + SM = CM-I and spinal cord syrinx, CNP = central neuropathic pain, CRPS = complex regional pain syndrome, MRI = magnetic resonance imaging, SCS = spinal cord stimulation
Syringomyelia, more commonly known as a “syrinx,” is a dilated cerebrospinal fluid–containing space that develops within the central canal and/or parenchyma of the spinal cord.1 It can be caused by multiple pathological entities, such as Chiari malformation type I (CM-I), trauma, tethered spinal cord, and spinal cord tumors. A syrinx can independently lead to a broad range of clinical signs and symptoms. The clinical presentation mainly depends on the etiology, location, and morphology of the syrinx. Patients are commonly asymptomatic; however, syringomyelia can cause a wide variety of sensory symptoms, lead to progression of scoliotic deformity, and, in severe cases, cause motor weakness of the extremities or lower cranial nerves.2,3
CM-I and spinal cord syrinx (CM-I + SM) are well-known to be associated conditions. The presence of a syrinx as a result of CM-I is estimated to be 40%–75%, and the treatment involves addressing the causative pathology with hopeful resolution or decrease in size of the syrinx.2,4 However, appearance of syrinx improvement on magnetic resonance imaging (MRI) does not always coincide with clinical improvement.5,6 We present a pediatric case of CM-1 + SM in which the syrinx resolved after surgical intervention; however, the central neuropathic pain (CNP) persisted. This condition ultimately led to the diagnosis of complex regional pain syndrome (CRPS), which is an extremely rare diagnosis in the pediatric population, with an estimated incidence of 1 case per 100,000 children.7,8 CRPS is challenging to treat because therapeutic goals normally focus on improvement rather than resolution of a patient’s symptoms.9 Unique to our patient, this is the first reported case of dorsal spinal cord stimulation (SCS) to successfully treat pediatric CNP/CRPS secondary to CM-I + SM.
Illustrative Case
A 14-year-old girl presented with several months of exertional/tussive occipital headaches, posterior neck/shoulder pain, and left radicular arm pain. The left-sided pain radiated to the shoulder and dorsolateral arm and into the thumb. Her quality of life was severely impacted, as evidenced by multiple emergency department visits, difficulty with sleep, and frequent absence from school. Subsequent medical evaluation included MRI of the cervical spine, which demonstrated CM-I + SM (Fig. 1). The syrinx exhibited deviation into the left dorsal horn. She displayed symptoms of allodynia and loss of pinprick/temperature sensation in the left upper extremity. The rest of her physical examination was unremarkable for changes in light touch, proprioception, reflexes, or motor weakness.
FIG. 1.
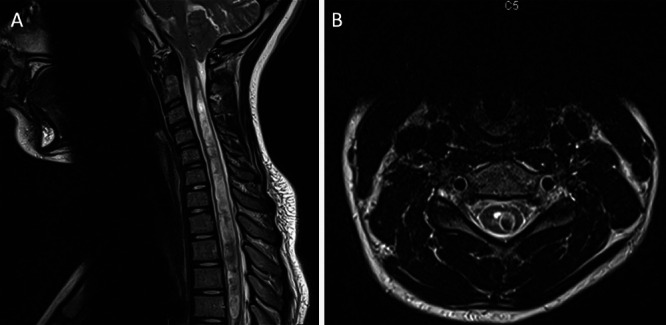
A: Preoperative sagittal MRI of the cervical spine without contrast demonstrating CM-I with an extensive cervicothoracic spinal cord syrinx. B: Preoperative axial MRI of the cervical spine without contrast at C5.
Standard CM-I decompression with pericranial graft duraplasty was performed without complication. Initial improvement of her occipital headaches and left arm dysesthetic pain was noted. At the 2-week follow-up, the central pain in her left arm returned, which was recognized to be worse than it was preoperatively. At that time, gabapentin was prescribed and titrated to 600 mg twice daily, with minimal relief. The patient eventually requested to stop gabapentin because of sedative side effects at school. An MRI at the 15-month follow-up demonstrated adequate CM-I decompression as well as near-complete resolution of the cervicothoracic syrinx (Fig. 2).
FIG. 2.
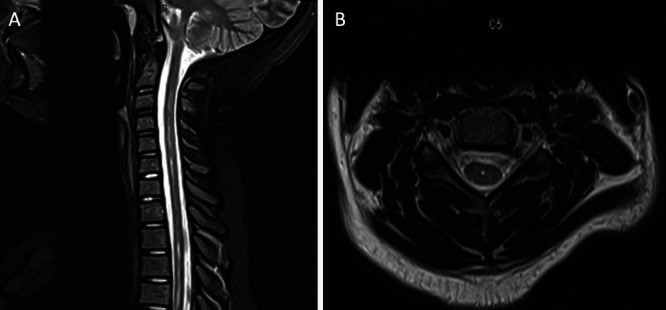
A: Postoperative sagittal MRI of the cervical spine without contrast at the 15-month follow-up showing near-complete resolution of the prior extensive cervicothoracic spinal cord syrinx. B: Postoperative axial MRI of the cervical spine without contrast at C5.
Despite resolution of the syrinx, the left arm pain persisted, causing a significant, unhealthy weight gain due to inactivity, affecting sleep habits, which worsened her academic performance, and even causing a debilitating major depressive episode. Despite such impairing signs and symptoms, the patient was highly motivated to avoid medication for the pain and depression. She was ultimately diagnosed with CRPS Type 1 secondary to cervical syringomyelia. Standard preoperative psychiatric assessment for pain was performed with no identifiable psychiatric causes. The patient was deemed a candidate for dorsal SCS trial and placement.
Cervical SCS percutaneous trial was performed with excellent response, and the patient proceeded to permanent SCS placement. Permanent SCS placement via hemilaminectomy at C6 was performed using the Medtronic Intellis with Adaptivestim Implantable Neurostimulator. Two neurostimulator leads were threaded to C2 under fluoroscopic guidance (Fig. 3). No intraoperative or postoperative complications occurred. At the 6-month follow-up, the patient endorsed significant improvement in upper extremity pain relief and quality of life. She was eventually weaned from gabapentin and did not require medical treatment for depression.
FIG. 3.
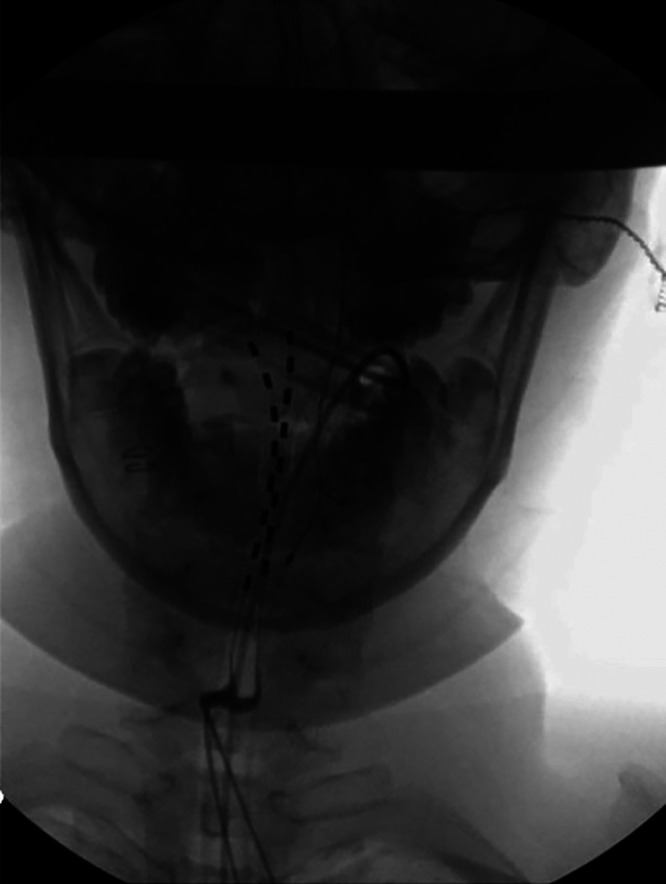
Two permanent neurostimulator leads threaded to C2 under fluoroscopic guidance.
Discussion
Observations
The pathophysiology of CNP in CM-I + SM is not fully understood, but general disruption of lateral spinothalamic fibers is involved.10,11 Preoperative severity can vary, and Chiari decompression that leads to syrinx resolution does not always lead to improvement in pain. In fact, previous studies have noted refractory CNP postoperatively in “deviated” syrinxes in CM-I + SM, similar to the aforementioned patient.12,13 The likely mechanisms involved include necrosis, neuronophagia, and Wallerian degeneration in these deviated or cavitated syrinxes.1
Given the medically refractory CNP and morphology of the syrinx in this case report, SCS to modify the pain response was proposed as a treatment plan. SCS is rarely needed in the pediatric population but has shown benefit in adults with pain due to syrinx from other etiologies.14–16 Evidence for use of SCS in treatment of CRPS in the pediatric population is less vigorous. Bakr et al.17 conducted a literature review of all cases of pediatric CRPS that were treated with SCS and found only 13 cases. There are significant heterogeneity and widely variable characteristics between these reported pediatric cases, which greatly limits their external validity.17 With careful patient selection, SCS should be considered in pediatric cases of medically refractory CNP. Fortunately, CNP in pediatric patients is extremely rare; hence, the level of evidence of a single case report is low. However, CM-I + SM is much more common in the pediatric population, and the incidence may be underreported. To date, there have been no reported cases of SCS treatment for CNP in pediatric patients, and we sought to illustrate an example of an efficacious option for a life-altering problem. We would also like to demonstrate the necessity of a collaborative effort to mend adult- and pediatric-centric practices and surgeons in certain situations.
Lessons
CM-I + SM is more common in the pediatric population, and pain due to syringomyelia can be debilitating. More specifically, central neuropathic pain, or the syndromic manifestations of the pain (CRPS) can alter an individual’s life in dramatic ways. Spinal cord stimulator placement in carefully selected pediatric patients should be considered in these difficult pain treatment paradigms.
Acknowledgments
We thank Dr. David Garcia and Dr. Michael Kinsman for critically reviewing the paper.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Schatmeyer, Kinsman, Garcia. Acquisition of data: Schatmeyer. Analysis and interpretation of data: Dodin, Garcia. Drafting the article: Schatmeyer, Dodin, Garcia. Critically revising the article: Schatmeyer, Dodin, Garcia. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Schatmeyer. Study supervision: Kinsman.
Supplemental Information
Previous Presentations
Portions of this work were presented at the 2021 Midwest Neurosurgical Society Annual Meeting, Omaha, NE, August 14, 2021.
References
- 1. Milhorat TH. Classification of syringomyelia. Neurosurg Focus. 2000;8(3):E1. doi: 10.3171/foc.2000.8.3.1. [DOI] [PubMed] [Google Scholar]
- 2. Tubbs RS, Lyerly MJ, Loukas M, Shoja MM, Oakes WJ. The pediatric Chiari I malformation: a review. Childs Nerv Syst. 2007;23(11):1239–1250. doi: 10.1007/s00381-007-0428-0. [DOI] [PubMed] [Google Scholar]
- 3. Vandertop WP. Syringomyelia. Neuropediatrics. 2014;45(1):3–9. doi: 10.1055/s-0033-1361921. [DOI] [PubMed] [Google Scholar]
- 4. Menezes AH. Chiari I malformations and hydromyelia: complications. Pediatr Neurosurg. 1991-1992;17(3):146–154. doi: 10.1159/000120586. [DOI] [PubMed] [Google Scholar]
- 5. Hale AT, Adelson PD, Albert GW, et al. Factors associated with syrinx size in pediatric patients treated for Chiari malformation type I and syringomyelia: a study from the Park-Reeves Syringomyelia Research Consortium. J Neurosurg Pediatr. 2020;6:1–11. doi: 10.3171/2020.1.PEDS19493. [DOI] [PubMed] [Google Scholar]
- 6. Lu VM, Phan K, Crowley SP, Daniels DJ. The addition of duraplasty to posterior fossa decompression in the surgical treatment of pediatric Chiari malformation Type I: a systematic review and meta-analysis of surgical and performance outcomes. J Neurosurg Pediatr. 2017;20(5):439–449. doi: 10.3171/2017.6.PEDS16367. [DOI] [PubMed] [Google Scholar]
- 7. Baerg K, Tupper SM, Chu LM, et al. Canadian surveillance study of complex regional pain syndrome in children. Pain. 2022;163(6):1060–1069. doi: 10.1097/j.pain.0000000000002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abu-Arafeh H, Abu-Arafeh I. Complex regional pain syndrome in children: incidence and clinical characteristics. Arch Dis Child. 2016;101(8):719–723. doi: 10.1136/archdischild-2015-310233. [DOI] [PubMed] [Google Scholar]
- 9. Watson JC, Sandroni P. Central neuropathic pain syndromes. Mayo Clin Proc. 2016;91(3):372–385. doi: 10.1016/j.mayocp.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 10. Milhorat TH, Kotzen RM, Mu HT, Capocelli AL, Jr, Milhorat RH. Dysesthetic pain in patients with syringomyelia. Neurosurgery. 1996;38(5):940–947. doi: 10.1097/00006123-199605000-00017. [DOI] [PubMed] [Google Scholar]
- 11. Todor DR, Mu HT, Milhorat TH. Pain and syringomyelia: a review. Neurosurg Focus. 2000;8(3):E11. doi: 10.3171/foc.2000.8.3.11. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura M, Chiba K, Nishizawa T, et al. Retrospective study of surgery-related outcomes in patients with syringomyelia associated with Chiari I malformation: clinical significance of changes in the size and localization of syrinx on pain relief. J Neurosurg. 2004;100(3 suppl Spine):241–244. doi: 10.3171/spi.2004.100.3.0241. [DOI] [PubMed] [Google Scholar]
- 13. Seki T, Hamauchi S, Yamazaki M, Hida K, Yano S, Houkin K. Investigation of the neuropathic pain caused by syringomyelia associated with Chiari I malformation. Asian Spine J. 2019;13(4):648–653. doi: 10.31616/asj.2018.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beyaz SG, Bal NS. Spinal cord stimulation for a patient with neuropathic pain related to congenital syringomyelia. Korean J Pain. 2017;30(3):229–230. doi: 10.3344/kjp.2017.30.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campos WK, Almeida de Oliveira YS, Ciampi de Andrade D, Teixeira MJ, Fonoff ET. Spinal cord stimulation for the treatment of neuropathic pain related to syringomyelia. Pain Med. 2013;14(5):767–768. doi: 10.1111/pme.12064. [DOI] [PubMed] [Google Scholar]
- 16. Shu W, Li Y, Tao W, Hu Y. Spinal cord stimulation combined with microsurgical DREZotomy for pain due to syringomyelia. Br J Neurosurg. 2016;30(5):585–587. doi: 10.3109/02688697.2016.1173187. [DOI] [PubMed] [Google Scholar]
- 17. Bakr SM, Knight J, Johnson SK, Williams AE, Tolley JA, Raskin JS. Spinal cord stimulation improves functional outcomes in children with complex regional pain syndrome: case presentation and review of the literature. Pain Pract. 2020;20(6):647–655. doi: 10.1111/papr.12882. [DOI] [PubMed] [Google Scholar]


