Abstract
BACKGROUND
Although the risk of developing malignant lymphoma is higher in patients with rheumatoid arthritis (RA) than in the general population, primary central nervous system lymphoma (PCNSL) in patients with RA is extremely rare. In recent years, there has been concern that biological disease-modifying antirheumatic drugs (bDMARDs), widely administered to patients with RA, might increase the risk of cancer development. The authors report the first case of PCNSL in a patient with RA receiving the bDMARD tocilizumab.
OBSERVATIONS
A 70-year-old man who was diagnosed with RA in 2010 was treated with low-dose methotrexate (MTX) from 2010 to 2015. Tocilizumab was commenced in 2012. In 2018, he developed gait disturbances, and gadolinium-enhanced magnetic resonance imaging showed multiple contrast-enhanced lesions in the basal ganglia and brain stem. Stereotactic brain biopsy led to the diagnosis of diffuse large B-cell lymphoma, and finally PCNSL was diagnosed. He was treated with five courses of MTX 3.5 g/m2, and his disease has been in remission for 34 months.
LESSONS
Low-dose MTX and bDMARDs are associated with the concern of increased cancer risk in patients with RA. Because tocilizumab has been in use for a relatively short time, further accumulation of cases and careful follow-up are necessary.
Keywords: primary central nervous system lymphoma, tocilizumab, rheumatoid arthritis
ABBREVIATIONS : bDMARD = biological disease-modifying antirheumatic drug, DLBCL = diffuse large B-cell lymphoma, IL = interleukin, KPS = Karnofsky performance status, LPD = lymphoproliferative disorder, ML = malignant lymphoma, MRI = magnetic resonance imaging, MTX = methotrexate, PCNSL = primary central nervous system lymphoma, RA = rheumatoid arthritis, Th17 = T-helper type 17, TNF-α= tumor necrosis factor-α, Treg = regulatory T
Tocilizumab, a humanized antihuman interleukin (IL)-6 receptor monoclonal antibody, is a molecular targeted therapy that binds to the IL-6 receptor, inhibiting cellular activity and producing immunosuppressive effects. The most common side effects associated with the use of tocilizumab are upper respiratory tract infections, colds, headaches, high blood pressure, and elevated liver enzymes such as alanine transaminase and cholesterol levels. Tocilizumab is usually used to treat rheumatoid arthritis (RA), systemic juvenile idiopathic arthritis, and Castleman’s disease.
Patients with RA are increasingly being treated with biologics that target T cells or cytokines, such as tumor necrosis factor-α (TNF-α) and IL-6. Patients with RA are suggested to be at a higher risk than the general population of developing malignant lymphoma (ML).1 In addition, drug-related immunodeficiency-associated lymphoproliferative disorder (LPD) is often seen in patients with RA, and methotrexate (MTX)-related lymphoproliferative disorders (MTX-LPDs) are well known. As the use of biological disease-modifying antirheumatic drugs (bDMARDs) has become more common in patients with RA, concerns have been theorized that bDMARDs might increase the risk of lymphoma in patients with RA due to their mechanism of action. Primary central nervous system lymphoma (PCNSL) is a rare variant of extranodal non-Hodgkin’s lymphoma and has one of the poorest prognoses of all lymphomas. We describe a case of PCNSL in a patient with RA who was being treated with tocilizumab. To the best of our knowledge, this is the first reported case in the literature showing the association between an IL-6 inhibitor and PCNSL.
Illustrative Case
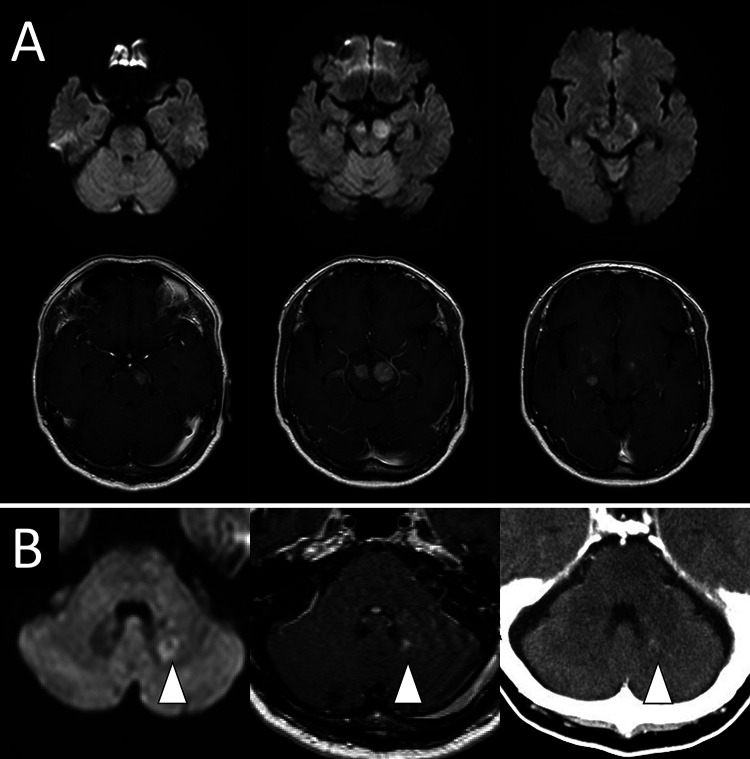
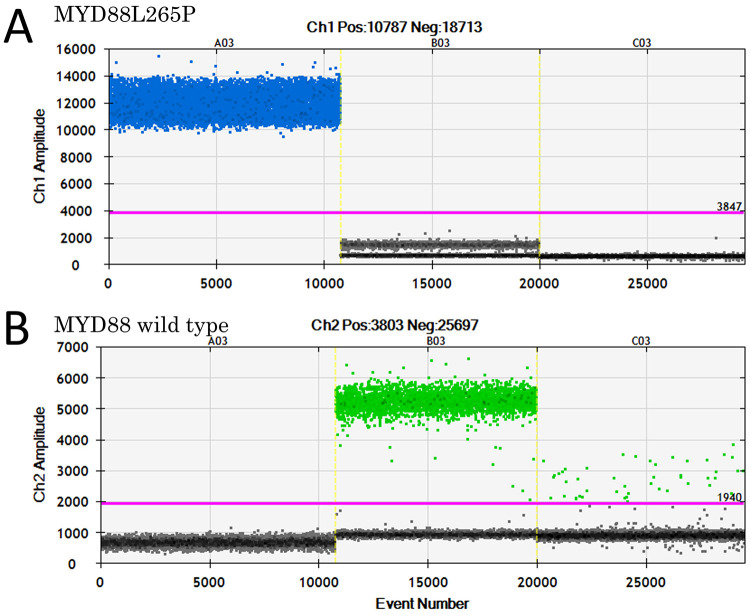
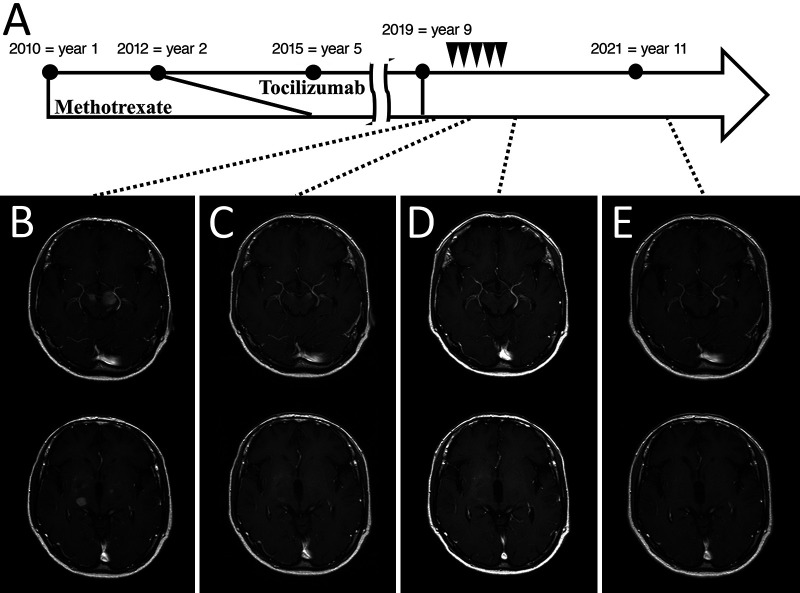
A 70-year-old male was diagnosed with RA and was treated with MTX (8.0 mg orally once every week) for 5 years. Treatment with tocilizumab (162 mg subcutaneously every 2 weeks) was added in year 2. Three years after MTX treatment ended, the patient developed gait disturbances and was diagnosed with lumbar spinal stenosis, for which he underwent surgery for L2–3 posterior fixation and posterior lumbar interbody fusion. However, he subsequently developed paraparesis and dysarthria. A little more than 1 year later, brain magnetic resonance imaging (MRI) showed a neoplastic lesion with homogeneous gadolinium enhancement in the right thalamus, bilateral cerebral peduncles, left pons, and left cerebellar peduncle (Fig. 1A and B), for which he was referred to our hospital and was subsequently admitted to our department. His Karnofsky performance status (KPS) at admission was 50. His physical examination indicated that he was alert, and his cranial nerves II–XI were intact, but his tongue was deviated to the right. Right and left extremity motor strength was 5/5 in the upper limbs and 3/3 in the lower limbs. Serological examination showed mildly elevated IL-2 receptor levels (313 U/ml) and normal levels of lactate dehydrogenase (299 U/L). His human immunodeficiency virus antibody test results were negative. Computed tomography from the neck to the pelvis showed no lesions suspicious for tumor. Biopsy-based diagnosis was planned. However, because there was concern that excision of a large lesion in the brainstem or basal ganglia would result in serious neurological complications due to hemorrhage or nerve damage, a contrast-enhanced lesion 5 mm in diameter in the left cerebellar peduncle was selected for frameless image-guided stereotactic brain biopsy (Stealth Station S7 and Navigus Biopsy Appliance, Medtronic) (Fig. 1B). Pathological examination of the obtained biopsy specimen revealed small areas of higher cell density around the small vessels in the brain parenchyma and large naked nuclei with well-defined nucleoli growing with indistinct boundaries. Because the large, atypical cells were CD20 positive, CD3 negative, cytokeratin glial fibrillary acidic protein negative, and AE1/AE3 negative, a diagnosis of diffuse large B-cell lymphoma (DLBCL) was made (Fig. 2A–E). Additional immunohistochemistry and genetic testing were performed on the remaining tissue sample, but microscopic histopathology of this sample did not contain atypical lymphoid cells (Fig. 2F). Furthermore, genetic mutations in MYD88 L265P and CD79B were not detected (Fig. 3). Because there were no tumor lesions in other organs, a final diagnosis of PCNSL was made. He discontinued tocilizumab treatment and was treated with high-dose MTX (3.5 g/m2) therapy with the addition of rituximab 2 weeks after surgery (Fig. 4A). After five cycles of high-dose MTX therapy, follow-up MRI with contrast enhancement revealed that the multiple lesions had disappeared by September 2019 (Fig. 4B–D). Due to the increased risk of leukoencephalopathy with advanced age, we decided to see him in follow up without whole-brain irradiation. He ultimately achieved a complete response and was discharged with mild paraparesis. Thirty-four months after treatment, his KPS remained at 60, and he had no recurrence of PCNSL (Fig. 4E).
FIG. 1.
A: Axial diffusion-weighted (upper) and postcontrast T1-weighted (lower) MRI showing mass lesions in the right thalamus, bilateral cerebral peduncles, and left pons. B: Axial diffusion-weighted imaging, postcontrast T1-weighted MRI, and contrast-enhanced computed tomography showing the small mass lesion in the left cerebellar peduncle (arrowheads) that was targeted by frameless image-guided stereotactic brain biopsy.
FIG. 2.
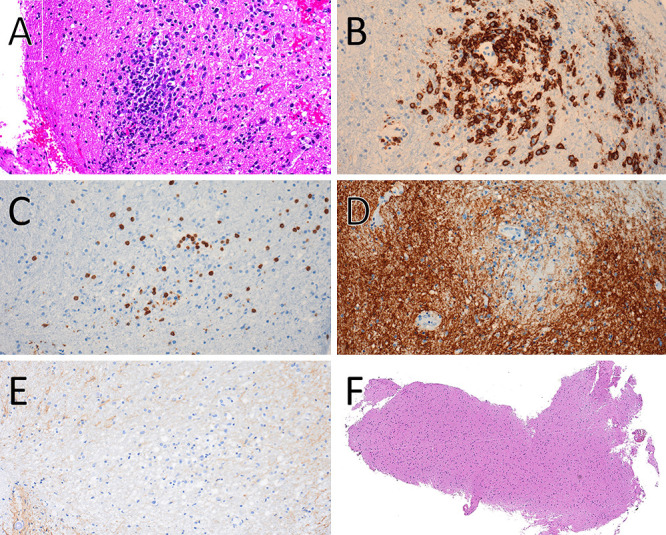
Histopathological findings showing PCNSL. A: Hematoxylin and eosin (H&E) stain, original magnification ×200. B: CD20 stain, original magnification ×200. C: CD3 stain, original magnification ×200. D: Glial fibrillary acidic protein stain, original magnification ×200. E: AE1/AE3 stain, original magnification ×200. F: Histopathological findings in the residual biopsy sample. H&E stain, original magnification ×40.
FIG. 3.
Analysis of MYD88 by droplet digital PCR (ddPCR) using a residual biopsy sample. One-dimensional ddPCR plot for MYD88 L265P mutation (A) and MYD88 wild type (B). A03 and B03 are positive controls for MYD88 L265P and MYD88 wild type, respectively. C03 is the present case. Each point represents a single droplet, which is scored as positive (colored) or negative (gray), depending on the fluorescence amplitude.
FIG. 4.
A: History of drug therapy for RA and the treatment course for PCNSL. R-HD-MTX regimen (arrowheads): rituximab (375 mg/m2), high-dose MTX (3.5 g/m2). B: Brain MRI on admission to the department of neurosurgery. Postcontrast T1-weighted MRI after the second (C) and fifth (D) courses of R-HD-MTX. Postcontrast T1-weighted MRI (E) 2 years after complete remission of PCNSL.
Discussion
Observations
This is the first report in the literature of PCNSL in a patient with RA treated with tocilizumab, a bDMARD. In this case, we opined that PCNSL might have developed as a result of tocilizumab treatment, because it is known that patients with RA are at a high risk of developing ML, and there has been concern for some time that the administration of bDMARDs might increase the risk of developing ML.1,2 Other bDMARDs, such as TNF-α inhibitors, have been used in patients with RA even before the introduction of the IL-6 inhibitor tocilizumab, and many studies have reported on this therapy. In recent years, the use of TNF-α inhibitors has been considered unlikely to increase the risk of development of ML in patients with RA.3–7 However, there are few reports of patients with RA developing ML during treatment with the IL-6 inhibitor tocilizumab.8–13 Because PCNSL is a rare variant of extranodal non-Hodgkin’s lymphoma, reports of DMARD-related PCNSL development in patients with RA are limited. Most reports of PCNSL describe MTX-LPD due to low-dose MTX therapy,14 and there is also a report of a case of PCNSL during treatment with adalimumab, an IL-6 inhibitor, although that was a case of psoriasis vulgaris rather than RA.15
Tocilizumab might affect tumor immunity through inhibition of the IL-6 signaling pathway. IL-6 is an inflammatory cytokine involved in the local and systemic manifestations of RA. IL-6 is also involved in the growth and transformation of multiple myeloma and ovarian, lung, bladder, breast, colon, and prostate cancers. Overexpression of IL-6 is associated with cancer progression by inhibiting apoptosis of cancer cells, promoting angiogenesis, and increasing drug resistance. Therefore, blocking IL-6 signaling is a promising therapeutic strategy for cancers characterized by pathological IL-6 overproduction.16 However, on the one hand, administration of tocilizumab to patients with RA has not been specifically studied with respect to prevention of cancer development and has not been shown to prevent malignancy.17 On the other hand, IL-6 plays a critical role in regulating the balance between IL-17–producing T-helper type 17 (Th17) cells and regulatory T (Treg) cells.18 IL-6 induces Th17 cell differentiation from naïve T cells and inhibits Treg differentiation. Treg cells play an important role in immune tolerance and evasion of an autoimmune response, but they also participate in immune escape of cancer cells and suppression of antitumor immune responses. Therefore, inhibition of the IL-6 signaling pathway by tocilizumab might promote Treg differentiation and contribute to tumor growth and development.
PCNSL, being a specific genetic subtype of DLBCL, might be affected by tocilizumab-induced modification of tumor immunity, leading to the development and growth of PCNSL. DLBCL is composed of seven genetic subtypes, and primary extranodal lymphomas, including PCNSL, are classified as an MCD subtype that is characterized by mutations in MYD88 L265P and CD79B.19 MYD88/CD79B-mutated (MCD) subtypes can appear in various organs other than the lymph nodes, and these organs are typically immune-privileged organs isolated from the systemic immune system.20 MCD subtype tumors genetically abrogate immune responsiveness despite arising in an immune-privileged site. These findings suggest that MCD subtype tumors are vulnerable to immune surveillance, acquire multiple lesions that affect immune recognition in their growth, and must grow in immune-privileged sites. Therefore, promotion of naive CD4-positive T cells to Treg differentiation by tocilizumab might attenuate immune surveillance and facilitate PCNSL development and growth.
Lessons
With the use of many bDMARDs, such as TNF-α inhibitors and tocilizumab, in addition to MTX in patients with RA, it is important to examine the risk of development of ML associated with these drugs. However, there are limited reports on this topic, and it is hoped that further accumulation of case series will clarify whether there is such an association and the clinical characteristics of this association.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Ohno. Acquisition of data: Ohno, Kuramitsu, Iwakoshi, Yamaguchi. Analysis and interpretation of data: Ohno, Iwakoshi, Yamaguchi, Ohka. Drafting the article: Ohno. Critically revising the article: Saito. Reviewed submitted version of manuscript: Kuramitsu, Iwakoshi, Saito. Approved the final version of the manuscript on behalf of all authors: Ohno. Administrative/technical/material support: Kuramitsu, Iwakoshi. Study supervision: Saito.
Supplemental Information
Previous Presentations
This paper was presented as a poster at the 39th Annual Meeting of the Japan Society for Neuro-Oncology, Kobe, Japan, December 5–7, 2021.
References
- 1. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17(1):212. doi: 10.1186/s13075-015-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 3. Hellgren K, Baecklund E, Backlin C, Sundstrom C, Smedby KE, Askling J. Rheumatoid arthritis and risk of malignant lymphoma: is the risk still increased? Arthritis Rheumatol. 2017;69(4):700–708. doi: 10.1002/art.40017. [DOI] [PubMed] [Google Scholar]
- 4. Wadström H, Frisell T, Askling J. Malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: a nationwide cohort study from Sweden. JAMA Intern Med. 2017;177(11):1605–1612. doi: 10.1001/jamainternmed.2017.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein A, Polliack A, Gafter-Gvili A. Rheumatoid arthritis and lymphoma: incidence, pathogenesis, biology, and outcome. Hematol Oncol. 2018;36(5):733–739. doi: 10.1002/hon.2525. [DOI] [PubMed] [Google Scholar]
- 6. Raaschou P, Söderling J, Turesson C, Askling J. Tumor necrosis factor inhibitors and cancer recurrence in Swedish patients with rheumatoid arthritis: a nationwide population-based cohort study. Ann Intern Med. 2018;169(5):291–299. doi: 10.7326/M17-2812. [DOI] [PubMed] [Google Scholar]
- 7. Singh N, Gao Y, Field E, et al. Trends of lymphoma incidence in US veterans with rheumatoid arthritis, 2002-2017. RMD Open. 2020;6(2):3001241. doi: 10.1136/rmdopen-2020-001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamamoto K, Goto H, Hirao K, et al. Longterm safety of tocilizumab: results from 3 years of followup postmarketing surveillance of 5573 patients with rheumatoid arthritis in Japan. J Rheumatol. 2015;42(8):1368–1375. doi: 10.3899/jrheum.141210. [DOI] [PubMed] [Google Scholar]
- 9. Harigai M, Nanki T, Koike R, et al. Risk for malignancy in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs compared to the general population: a nationwide cohort study in Japan. Mod Rheumatol. 2016;26(5):642–650. doi: 10.3109/14397595.2016.1141740. [DOI] [PubMed] [Google Scholar]
- 10. Mercer LK, Regierer AC, Mariette X, et al. Spectrum of lymphomas across different drug treatment groups in rheumatoid arthritis: a European registries collaborative project. Ann Rheum Dis. 2017;76(12):2025–2030. doi: 10.1136/annrheumdis-2017-211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim SC, Pawar A, Desai RJ, et al. Risk of malignancy associated with use of tocilizumab versus other biologics in patients with rheumatoid arthritis: a multi-database cohort study. Semin Arthritis Rheum. 2019;49(2):222–228. doi: 10.1016/j.semarthrit.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura H, Sugai T, Kato M, Hatanaka KC, Atsumi T. Subcutaneous panniculitis-like T-cell lymphoma with haemophagocytic syndrome during tocilizumab therapy for juvenile idiopathic arthritis. Clin Exp Rheumatol. 2017;35(1):174. [PubMed] [Google Scholar]
- 13. Hatanaka N, Sata H, Kusakabe S, Yasumi M, Karasuno T. Development of classical Hodgkin lymphoma in a patient receiving tocilizumab for rheumatoid arthritis. Article in Japanese. Rinsho Ketsueki. 2021;62(10):1505–1509. doi: 10.11406/rinketsu.62.1505. [DOI] [PubMed] [Google Scholar]
- 14. Uneda A, Hirashita K, Kanda T, et al. Primary central nervous system methotrexate-associated lymphoproliferative disorder in a patient with rheumatoid arthritis: case report and review of literature. NMC Case Rep J. 2020;7(3):121–127. doi: 10.2176/nmccrj.cr.2019-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farah RA, Alduaij A, Ugas C, Navarro R. Primary central nervous system lymphoma in a patient on adalimumab therapy for chronic plaque psoriasis. World Neurosurg. 2020;139:260–263. doi: 10.1016/j.wneu.2020.03.155. [DOI] [PubMed] [Google Scholar]
- 16. Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38(7):904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 17. Rubbert-Roth A, Sebba A, Brockwell L, et al. Malignancy rates in patients with rheumatoid arthritis treated with tocilizumab. RMD Open. 2016;2(1):e000213. doi: 10.1136/rmdopen-2015-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 19. Wright GW, Huang DW, Phelan JD, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell. 2020;37(4):551–568.e14. doi: 10.1016/j.ccell.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 2013;13(3):206–218. doi: 10.1038/nri3391. [DOI] [PubMed] [Google Scholar]