This comparative effectiveness research study investigates the outcomes of real-world patients with chronic pain treated with spinal cord stimulators vs conventional medical therapy.
Key Points
Question
What are the outcomes among real-world patients with chronic pain who are treated with spinal cord stimulators compared with conventional medical management?
Findings
In this propensity-matched comparative effectiveness research analysis of 7560 insured individuals, treatment with a spinal cord stimulator was not associated with a reduction in use of opioids, pain injections, radiofrequency ablation, or spine surgery at 2 years. Approximately one-fifth of patients treated with spinal cord stimulators experienced complications and required device revision or removal.
Meaning
Study results suggest that use of spinal cord stimulators is not associated with reductions in opioid use or nonpharmacologic pain interventions.
Abstract
Importance
Spinal cord stimulators (SCSs) are increasingly used for the treatment of chronic pain. There is a need for studies with long-term follow-up.
Objective
To determine the comparative effectiveness and costs of SCSs compared with conventional medical management (CMM) in a large cohort of patients with chronic pain.
Design, Setting, and Participants
This was a 1:5 propensity-matched retrospective comparative effectiveness research analysis of insured individuals from April 1, 2016, to August 31, 2018. This study used administrative claims data, including longitudinal medical and pharmacy claims, from US commercial and Medicare Advantage enrollees 18 years or older in Optum Labs Data Warehouse. Patients with incident diagnosis codes for failed back surgery syndrome, complex regional pain syndrome, chronic pain syndrome, and other chronic postsurgical back and extremity pain were included in this study. Data were analyzed from February 1, 2021, to August 31, 2022.
Exposures
SCSs or CMM.
Main Outcomes and Measures
Surrogate measures for primary chronic pain treatment modalities, including pharmacologic and nonpharmacologic pain interventions (epidural and facet corticosteroid injections, radiofrequency ablation, and spine surgery), as well as total costs.
Results
In the propensity-matched population of 7560 patients, mean (SD) age was 63.5 (12.5) years, 3080 (40.7%) were male, and 4480 (59.3%) were female. Among matched patients, during the first 12 months, patients treated with SCSs had higher odds of chronic opioid use (adjusted odds ratio [aOR], 1.14; 95% CI, 1.01-1.29) compared with patients treated with CMM but lower odds of epidural and facet corticosteroid injections (aOR, 0.44; 95% CI, 0.39-0.51), radiofrequency ablation (aOR, 0.57; 95% CI, 0.44-0.72), and spine surgery (aOR, 0.72; 95% CI, 0.61-0.85). During months 13 to 24, there was no significant difference in chronic opioid use (aOR, 1.06; 95% CI, 0.94-1.20), epidural and facet corticosteroid injections (aOR, 1.00; 95% CI, 0.87-1.14), radiofrequency ablation (aOR, 0.84; 95% CI, 0.66-1.09), or spine surgery (aOR, 0.91; 95% CI, 0.75-1.09) with SCS use compared with CMM. Overall, 226 of 1260 patients (17.9%) treated with SCS experienced SCS-related complications within 2 years, and 279 of 1260 patients (22.1%) had device revisions and/or removals, which were not always for complications. Total costs of care in the first year were $39 000 higher with SCS than CMM and similar between SCS and CMM in the second year.
Conclusions and Relevance
In this large, real-world, comparative effectiveness research study comparing SCS and CMM for chronic pain, SCS placement was not associated with a reduction in opioid use or nonpharmacologic pain interventions at 2 years. SCS was associated with higher costs, and SCS-related complications were common.
Introduction
Spinal cord stimulators (SCSs) are neuromodulation devices implanted in the epidural space with the goal of treating chronic pain that fails to respond to conventional treatment. SCSs have been increasingly used in recent years1,2; approximately 50 000 are implanted annually in the US3 at a cost of approximately $3.5 billion.4 Some have advocated for greater use of SCSs to reduce risks of medications, including opioids and gabapentinoids.5
Despite the increasing utilization of SCSs, there are limitations to the evidence supporting its superiority over usual care, which includes conventional medical management (CMM).6 Most SCS have been authorized by the US Food and Drug Administration (FDA) without clinical data.7 Approximately 85% of large studies of SCSs (ie, >100 patients) are industry funded.8 Independent evaluations have generally been small, single-center, and nonrandomized.9 A recent Cochrane systematic review of randomized trials of SCS found just 1 study (44 patients) examining pain intensity at 1 year or longer follow-up.10 Although some studies have found benefit in pain relief at 6 months from SCSs compared to CMM, benefits often dissipate after 12 to 24 months.11 The comparator group in many SCS trials has not adequately masked a placebo effect; when a placebo control is used, treatment effects are smaller.12
SCSs have potential complications.13 In September 2020, the FDA published a letter to health care professionals stating that more than 107 000 medical device adverse-event reports related to SCSs had been filed between July 2016 and July 2020, including patient injury, device malfunction, and 497 deaths.3 Among 4000 types of medical devices tracked by the FDA, SCSs had the third highest number of adverse events.14
Given the limitations in available data, there is a need for data in a larger, contemporary patient cohort to compare the long-term risks, benefits, and cost-effectiveness of SCSs with CMM. Accordingly, we compared the long-term clinical and health care utilization outcomes among patients treated with permanent SCSs compared with CMM.
Methods
Study Design and Data Source
This was a retrospective comparative effectiveness research study using Optum Labs Data Warehouse (OLDW) data from October 1, 2015, through August 31, 2020. OLDW contains deidentified administrative claims data, including longitudinal medical and pharmacy claims, from US commercial and Medicare Advantage enrollees.15 Because data were deidentified in compliance with the Health Insurance Portability and Accountability Act, institutional review board approval or waiver of authorization was not required. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Cohort Selection
Eligible individuals were 18 years or older with an incident diagnosis of failed back surgery syndrome, complex regional pain syndrome, chronic pain syndrome, and other chronic postsurgical back and extremity pain (for the latter diagnosis, history of spine surgery within 6 months of diagnosis was required) between April 1, 2016, and August 31, 2019 (eTable 1 in the Supplement for codes reviewed by multiple authors).9,16,17,18 The cohort entry date was defined as the first diagnosis claim meeting any of these criteria after a diagnosis-free clean period of 6 months. If individuals had more than 1 qualifying diagnosis, cohort entry diagnosis and date was based on the following hierarchy: (1) failed back surgery syndrome, (2) complex regional pain syndrome, (3) chronic pain syndrome, and (4) other chronic postsurgical back and extremity pain. Individuals without 6 months of contiguous pharmacy and medical coverage before and 12 months after cohort entry were excluded to ensure consistent ascertainment of treatment patterns. Individuals from the all race and ethnicity groups were included and categorized as the following: Asian, Black, Hispanic, White, and unknown or multiple (refers to patients with unknown race or ethnicity or those included in multiple categories).
Treatments
The exposure of interest was permanent (not trial) SCS implantation within 12 months of cohort entry. Patients were assigned 1 of 2 mutually exclusive treatment cohorts (eFigure 1 in the Supplement): (1) a permanent SCS and (2) CMM only, consisting of pain medications, spine surgery, radiofrequency ablation, epidural and facet corticosteroid injections, and conservative nonpharmacologic therapies (physical therapy, chiropractic treatment, and acupuncture) (eTable 1 in the Supplement). Individuals who received both an SCS and CMM in the 12 months after cohort entry were assigned to the SCS group and baseline use of CMM treatments were evaluated as binary covariates. Individuals with no evidence of an SCS or CMM within 12 months after cohort entry were excluded.
For the SCS group, the index date, ie, treatment initiation, was the date of permanent SCS insertion. Individuals in the CMM group were randomly imputed an index date matching the distribution of index dates in the SCS group.
Six months of continuous pharmacy and medical coverage preindex (baseline) and 24 months of continuous coverage postindex date were required for outcome ascertainment in the primary analysis, with all index dates in the final sample between April 1, 2016, and August 31, 2018 (eFigure 2 in the Supplement). Twelve months of continuous enrollment were allowed to increase sample size for propensity score estimation. From both treatment groups, individuals who received an SCS or care for an SCS, diagnosis of malignancy, possible indications for deep brain stimulation (Parkinson disease) or sacral neuromodulation (urinary or fecal incontinence) to avoid including any non-SCS neuromodulation, disabling neurologic deficits including foot drop, and neurogenic bladder during the baseline period were excluded (eTable 2 in the Supplement). Patients without conversion to permanent SCS within 12 months of trial were excluded.
Outcomes
The primary outcomes were chronic opioid use and epidural and facet corticosteroid injection use, surrogates for primary chronic pain treatment modalities, 1 to 12 months and 13 to 24 months after the index date. Chronic opioid use was defined as a binary outcome during each time window if the total length of opioid possession was 90 days or longer and included either (1) greater than or equal to 120 days’ supply or (2) 10 or more fills.19,20 Other outcomes included long-acting opioid use; greater than 50 morphine milligram equivalent (MME) per day; radiofrequency ablations; new spine surgeries; and any fills for nonsteroidal anti-inflammatory drugs (NSAIDs), systemic corticosteroids, antidepressants, gabapentinoids, and benzodiazepines (eTable 3 in the Supplement). Healthcare utilization, including emergency department visits, hospitalizations, and office visits, were examined. Total costs of care (actual) were also assessed; medical costs included both surgical and medical procedures (and represent approximately 75% of total costs), and pharmacy costs were based on outpatient pharmacy claims. Among patients treated with an SCS, postprocedure complications (lead/generator breakdown, displacement, infection or inflammation, and other mechanical complications), SCS revision, and removal were examined (eTable 4 in the Supplement).
Propensity Matching
To balance baseline characteristics between the treatment groups, the probability of receiving a permanent SCS vs CMM was modeled as a function of 65 baseline predictors among patients with 12 months or longer of follow-up. The following variables were assessed for association with SCS treatment: CMM, which included a comprehensive list of surrogates of baseline pain (total number of filled opioid prescriptions, mean opioid MME, days in possession of opioids, epidural and facet corticosteroid injections, radiofrequency ablation, spine surgery, and nonpharmacologic treatments of painful conditions); index calendar year; demographic characteristics, including race and ethnicity, as assessed in the data source used by the investigators21 (because these are important demographic variables and studies have shown differences in treatment of pain by race); clinician specialty for cohort entry; 31 medical and mental health comorbidities using the Elixhauser index22; and additional pain-related and musculoskeletal conditions using Chronic Conditions Data Warehouse algorithm.23 A greedy matching algorithm with a caliper width of 20% of the SD of the logit of the propensity score was used.24 To balance cohort entry diagnosis, matching was performed separately within patients with or without failed back surgery syndrome. Ratio of SCS to CMM matches was 1:5 to achieve optimal power while retaining as many SCS patients as possible. Standardized mean differences were used to evaluate postmatching balance, with values less than 10% considered acceptable.
Statistical Analyses
Patient characteristics for prematch and matched SCS and CMM groups were compared. Using the propensity-matched cohort, outcomes were modeled as a binary variable using generalized linear models with a binomial distribution and a logit link. Total costs of care were modeled using generalized linear models with a gamma distribution and log link. Counts of emergency department visits, hospitalizations, and office visits were modeled using generalized linear models with a Poisson distribution. A generalized estimating equation was used to account for correlation of outcomes within matched clusters during follow-up. Both empirical and robust SEs were examined; as they did not differ, empirical SEs are reported. Outcomes were examined among patients with only either complex regional pain syndrome or chronic pain syndrome at baseline, by patients receiving 7 or fewer days opioids at baseline, and by sex and insurance type. Characteristics of patients excluded due to insufficient post-index follow-up were compared to those included. We also examined the proportion of patients taking opioids at baseline who discontinued these medications at 2 years. All analyses were performed with SAS, version 9.4 (SAS Institute). Significance was considered to be a 2-sided P value <.05. Data were analyzed from February 1, 2021, to August 31, 2022.
Results
Study Cohort
There were 6202 patients in the SCS and 215 686 in the CMM group with a diagnosis of failed back surgery syndrome, complex regional pain syndrome, chronic pain syndrome, and other postsurgical extremity or back pain diagnosis and an adequate diagnosis-free clean period and postincident diagnosis continuous enrollment (eFigure 1 in the Supplement). Overall, 1510 of 4731 patients (32%) who had an SCS within 12 months of the cohort entry date were excluded because they received a trial, but not permanent, SCS within 12 months of cohort entry. After excluding patients with indications for other neuromodulation devices, malignancy-related pain, and without 24 months continuous enrollment, 1419 patients in the SCS and 91 307 in the CMM groups composed the final prepropensity score–matched sample. Using 1:5 matching, the final study cohort included 1260 patients who received an SCS and 6300 CMM. Baseline characteristics of retained patients vs those excluded for disenrollment were similar with clinically insignificant differences (eTable 5 in the Supplement). Similarly, patients with permanent SCS did not differ significantly from those with trial SCS only (eTable 6 in the Supplement). At baseline, 1128 of all patients (79%) treated with an SCS also received opioids, and 219 (15.4%) were receiving rehabilitative therapies. Factors associated with SCS treatment are presented in eTable 7 in the Supplement.
Baseline Patient Characteristics
In the matched population of 7560 total patients, all standardized mean differences between patients receiving SCS and CMM were less than 0.1 (eFigure 3 in the Supplement). The mean (SD) age of patients was 63.5 (12.5) years, 3080 (40.7%) were male, and 4480 (59.3%) were female (Table 1). Patients belonged to the following race and ethnicity groups: 56 Asian (0.7%), 901 Black (11.9%), 484 Hispanic (6.4%), 5888 White (77.9%), and 231 unknown/multiple (3.1%). Diagnosis at cohort entry included 5352 patients (70.8%) with failed back surgery syndrome, 760 patients (10.1%) with complex regional pain syndrome, 1938 patients (25.6%) with chronic pain syndrome, and 63 patients (0.8%) other postsurgical back or extremity pain. Within 6 months before the index date, 5854 of 7560 patients (77.4%) had received opioids. One-third of patients filled prescriptions for each of NSAIDs, muscle relaxants, and benzodiazepines and half for gabapentinoids. Of the 7560 patients, 3003 (39.7%) received epidural and facet corticosteroid injections, and 1235 (16.3%) received any nonpharmacologic, nonintervention therapy. Only 80 of 1260 patients (6.3%) in the postmatch SCS group did not receive any of the CMM treatments during the 6-month baseline period.
Table 1. Patient Characteristics for Prematch and Postmatched Patient Cohorts.
| Characteristic | Final prematch cohort 24 mo | Final postmatch cohort 24 mo | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | SMD | No. (%) | SMD | |||||
| Total (n = 92 726) | SCS (n = 1419) | CMM (n = 91 307) | Total (n = 7560) | SCS (n = 1260) | CMM (n = 6300) | |||
| Age, mean (SD), y | 61.9 (13.3) | 64.3 (11.9) | 61.9 (13.3) | 0.19 | 63.5 (12.5) | 64.0 (12.1) | 63.4 (12.5) | 0.05 |
| Age category | ||||||||
| 18-54 | 25 048 (27.0) | 288 (20.3) | 24 760 (27.1) | −0.16 | 1715 (22.7) | 263 (20.9) | 1452 (23.1) | −0.05 |
| 55-64 | 25 953 (28.0) | 379 (26.7) | 25 574 (28.0) | −0.03 | 2033 (26.9) | 342 (27.1) | 1691 (26.8) | 0.01 |
| 65-74 | 25 321 (27.3) | 463 (32.6) | 24 858 (27.2) | 0.12 | 2270 (30.0) | 400 (31.8) | 1870 (29.7) | 0.04 |
| 75+ | 16 404 (17.7) | 289 (20.4) | 16 115 (17.7) | 0.07 | 1542 (20.4) | 255 (20.2) | 1287 (20.4) | −0.00 |
| Sex | ||||||||
| Male | 36 379 (39.2) | 561 (39.5) | 35 818 (39.2) | 0.01 | 3080 (40.7) | 493 (39.1) | 2587 (41.1) | −0.04 |
| Female | 56 347 (60.8) | 858 (60.5) | 55 489 (60.8) | −0.01 | 4480 (59.3) | 767 (60.9) | 3713 (58.9) | 0.04 |
| Insurance type | ||||||||
| Commercially insured | 29 417 (31.7) | 353 (24.9) | 29 064 (31.8) | −0.15 | 2101 (27.8) | 315 (25.0) | 1786 (28.4) | −0.08 |
| Medicare Advantage | 63 309 (68.3) | 1066 (75.1) | 62 243 (68.2) | 0.15 | 5459 (72.2) | 945 (75) | 4514 (71.7) | 0.08 |
| Geographic location | ||||||||
| Northeast | 7565 (8.2) | 76 (5.4) | 7489 (8.2) | −0.11 | 489 (6.5) | 74 (5.9) | 415 (6.6) | −0.03 |
| Midwest | 18 153 (19.6) | 411 (29.0) | 17 742 (19.4) | 0.22 | 2073 (27.4) | 342 (27.1) | 1731 (27.5) | −0.01 |
| South | 55 794 (60.2) | 729 (51.4) | 55 065 (60.3) | −0.18 | 3971 (52.5) | 671 (53.3) | 3300 (52.4) | 0.02 |
| West | 11 214 (12.1) | 203 (14.3) | 11 011 (12.1) | 0.07 | 1027 (13.6) | 173 (13.7) | 854 (13.6) | 0.01 |
| Race and ethnicity | ||||||||
| Asian | 1329 (1.4) | <11 (NA)a | >1318 (NA) | −0.08 | 56 (0.7) | <11 (NA) | >45 (NA) | −0.02 |
| Black | 15 148 (16.3) | 157 (11.1) | 14 991 (16.4) | −0.16 | 901 (11.9) | 150 (11.9) | 751 (11.9) | −0.00 |
| Hispanic | 8016 (8.6) | >90 (NA) | >7926 (NA) | −0.08 | 484 (6.4) | >85 (NA) | >399 (NA) | 0.03 |
| White | 65 513 (70.7) | 1114 (78.5) | 64 399 (70.5) | 0.18 | 5888 (77.9) | 973 (77.2) | 4915 (78.0) | −0.02 |
| Unknown/multipleb | 2720 (2.9) | 47 (3.3) | 2673 (2.9) | 0.02 | 231 (3.1) | 41 (3.3) | 190 (3.0) | 0.01 |
| Index year | ||||||||
| 2016 | 23 689 (25.6) | 282 (19.9) | 23 407 (25.6) | −0.14 | 1637 (21.7) | 261 (20.7) | 1376 (21.8) | −0.03 |
| 2017 | 42 662 (46.0) | 664 (46.8) | 41 998 (46) | 0.02 | 3553 (47) | 595 (47.2) | 2958 (47.0) | 0.01 |
| 2018 | 26 375 (28.4) | 473 (33.3) | 25 902 (28.4) | 0.11 | 2370 (31.4) | 404 (32.1) | 1966 (31.2) | 0.02 |
| Cohort entry diagnosis | ||||||||
| Failed back surgery | 22 739 (24.5) | 1028 (72.5) | 21 711 (23.8) | 1.12 | 5352 (70.8) | 892 (70.8) | 4460 (70.8) | 0.00 |
| Complex regional pain | 5239 (5.7) | 123 (8.7) | 5116 (5.6) | 0.12 | 760 (10.1) | 94 (7.5) | 666 (10.6) | −0.11 |
| Chronic pain | 63 790 (68.8) | 398 (28.1) | 63 392 (69.4) | −0.91 | 1938 (25.6) | 365 (29.0) | 1573 (25.0) | 0.09 |
| Other chronic back/extremity pain | 2775 (3.0) | 13 (0.9) | 2762 (3.0) | −0.15 | 63 (0.8) | 12 (1.0) | 51 (0.8) | 0.02 |
| Clinician type on day of cohort entry | ||||||||
| Primary care | 41 097 (44.3) | 222 (15.6) | 40 875 (44.8) | −0.67 | 1299 (17.2) | 207 (16.4) | 1092 (17.3) | −0.02 |
| Anesthesiologist | 32 020 (34.5) | 991 (69.8) | 31 029 (34.0) | 0.77 | 4890 (64.7) | 847 (67.2) | 4043 (64.2) | 0.06 |
| Neurosurgeon | 4808 (5.2) | 141 (9.9) | 4667 (5.1) | 0.18 | 745 (9.9) | 115 (9.1) | 630 (10) | −0.03 |
| Orthopedic surgeon | 4600 (5.0) | 70 (4.9) | 4530 (5.0) | −0.00 | 394 (5.2) | 67 (5.3) | 327 (5.2) | 0.01 |
| Physiatrist | 8317 (9.0) | 157 (11.1) | 8160 (8.9) | 0.07 | 908 (12.0) | 144 (11.4) | 764 (12.1) | −0.02 |
| Other medical physician | 10 720 (11.6) | 65 (4.6) | 10 655 (11.7) | −0.26 | 344 (4.6) | 58 (4.6) | 286 (4.5) | 0.00 |
| Non–medical physician | 3973 (4.3) | 51 (3.6) | 3922 (4.3) | −0.04 | 305 (4.0) | 48 (3.8) | 257 (4.1) | −0.01 |
| Surrogates of baseline pain | ||||||||
| Total baseline filled prescriptions for opioids | ||||||||
| Mean (SD) | 4.2 (4.2) | 4.7 (4.2) | 4.2 (4.2) | 0.12 | 4.5 (4.3) | 4.6 (4.1) | 4.5 (4.3) | 0.02 |
| Median (IQR) | 3 (1-6) | 4 (1-7) | 3 (1-6) | 4 (1-7) | 4 (1-7) | 4 (1-7) | ||
| Average opioid MME baseline | ||||||||
| Mean (SD) | 29.9 (62.6) | 35.5 (68.2) | 29.8 (62.5) | 0.09 | 34.9 (69.1) | 35.5 (69.7) | 34.8 (69.0) | 0.01 |
| Median (IQR) | 8.0 (0.4-30.7) | 12.7 (1.1-38.9) | 7.9 (0.4-30.6) | 10.1 (0.7-38.0) | 12.2 (1.1-38.6) | 9.9 (0.6-37.8) | ||
| Baseline opioid days | ||||||||
| Mean (SD) | 76.5 (68.3) | 85.8 (67.8) | 76.3 (68.3) | 0.14 | 81.1 (68.8) | 84.3 (67.7) | 80.4 (69.0) | 0.06 |
| Median (IQR) | 61 (3-150) | 90 (9-155) | 61 (3-150) | 76 (5-154) | 90 (8-155) | 74 (4-154) | ||
| Baseline quartile of days supply of opioids | ||||||||
| Quartile 1 | 21 980 (23.7) | 291 (20.5) | 21 689 (23.8) | −0.08 | 1706 (22.6) | 264 (21.0) | 1442 (22.9) | −0.05 |
| Quartile 2 | 22 421 (24.2) | 296 (20.9) | 22 125 (24.2) | −0.08 | 1699 (22.5) | 270 (21.4) | 1429 (22.7) | −0.03 |
| Quartile 3 | 24 502 (26.4) | 406 (28.6) | 24 096 (26.4) | 0.05 | 2006 (26.5) | 355 (28.2) | 1651 (26.2) | 0.04 |
| Quartile 4 | 23 823 (25.7) | 426 (30.0) | 23 397 (25.6) | 0.10 | 2149 (28.4) | 371 (29.4) | 1778 (28.2) | 0.03 |
| Epidural and facet corticosteroid injections | 18 178 (19.6) | 610 (43.0) | 17 568 (19.2) | 0.53 | 3003 (39.7) | 507 (40.2) | 2496 (39.6) | 0.01 |
| Radiofrequency ablation | 3325 (3.6) | 126 (8.9) | 3199 (3.5) | 0.22 | 495 (6.6) | 92 (7.3) | 403 (6.4) | 0.04 |
| Spine surgery | 8645 (9.3) | 193 (13.6) | 8452 (9.3) | 0.14 | 853 (11.3) | 159 (12.6) | 694 (11.0) | 0.05 |
| Other nonpharmacologic, nonintervention treatments during baseline | ||||||||
| Physical therapy | 10 170 (11.0) | 152 (10.7) | 10 018 (11.0) | −0.01 | 874 (11.6) | 139 (11.0) | 735 (11.7) | −0.02 |
| Acupuncture | 595 (0.6) | <11 (NA) | >584 (NA) | −0.04 | 27 (0.36) | <11 (NA) | >16 (NA) | 0.01 |
| Chiropractor | 5672 (6.1) | 76 (5.4) | 5596 (6.1) | −0.03 | 422 (5.6) | 69 (5.5) | 353 (5.6) | −0.01 |
| Any nonpharmacologic, nonintervention treatment | 15 047 (16.2) | 219 (15.4) | 14 828 (16.2) | −0.02 | 1235 (16.3) | 199 (15.8) | 1036 (16.4) | −0.02 |
| Pharmacologic treatment during baseline | ||||||||
| Opioids | 70 746 (76.3) | 1128 (79.5) | 69 618 (76.2) | 0.08 | 5854 (77.4) | 996 (79.1) | 4858 (77.1) | 0.05 |
| NSAIDs | 29 921 (32.3) | 458 (32.3) | 29 463 (32.3) | 0.00 | 2507 (33.2) | 416 (33.0) | 2091 (33.2) | −0.00 |
| Muscle relaxants | 27 975 (30.2) | 463 (32.6) | 27 512 (30.1) | 0.05 | 2526 (33.4) | 413 (32.8) | 2113 (33.5) | −0.02 |
| TCA/SNRI antidepressants | 19 536 (21.1) | 446 (31.4) | 19 090 (20.9) | 0.24 | 2141 (28.3) | 370 (29.4) | 1771 (28.1) | 0.03 |
| Gabapentinoids | 34 732 (37.5) | 786 (55.4) | 33 946 (37.2) | 0.37 | 3891 (51.5) | 674 (53.5) | 3217 (51.1) | 0.05 |
| Benzodiazepines | 29 721 (32.1) | 512 (36.1) | 29 209 (32.0) | 0.09 | 2615 (34.6) | 457 (36.3) | 2158 (34.3) | 0.04 |
| Oral steroids | 22 849 (24.6) | 344 (24.2) | 22 505 (24.7) | −0.01 | 1824 (24.1) | 316 (25.1) | 1508 (23.9) | 0.03 |
| Musculoskeletal comorbidities | ||||||||
| Fibromyalgia | 7199 (7.8) | 114 (8.0) | 7085 (7.8) | 0.01 | 542 (7.2) | 101 (8.0) | 441 (7) | 0.04 |
| Spine disk disease | 66 987 (72.2) | 1339 (94.4) | 65 648 (71.9) | 0.63 | 7074 (93.6) | 1181 (93.7) | 5893 (93.5) | 0.01 |
| Traumatic spine injury | 6057 (6.5) | 129 (9.1) | 5928 (6.5) | 0.10 | 595 (7.9) | 111 (8.8) | 484 (7.7) | 0.04 |
| Osteoporosis | 5544 (6.0) | 89 (6.3) | 5455 (6.0) | 0.01 | 486 (6.4) | 76 (6.0) | 410 (6.5) | −0.02 |
| Osteoarthritis | 14 182 (15.3) | 320 (22.6) | 13 862 (15.2) | 0.19 | 1579 (20.9) | 275 (21.8) | 1304 (20.7) | 0.03 |
| Mental health comorbidities | ||||||||
| Anxiety | 23 530 (25.4) | 406 (28.6) | 23 124 (25.3) | 0.07 | 2124 (28.1) | 359 (28.5) | 1765 (28.0) | 0.01 |
| History of benzodiazepine use disorder | 538 (0.6) | 11 (0.8) | 527 (0.6) | 0.02 | 43 (0.6) | 11 (0.9) | 32 (0.5) | 0.04 |
| Alcohol use disorder | 2090 (2.3) | 21 (1.5) | 2069 (2.3) | −0.06 | 132 (1.75) | 20 (1.59) | 112 (1.78) | −0.01 |
| Depression | 22 706 (24.5) | 660 (46.5) | 22 046 (24.1) | 0.48 | 2999 (39.7) | 515 (40.9) | 2484 (39.4) | 0.03 |
| Psychosis | 1466 (1.6) | 13 (0.9) | 1453 (1.6) | −0.06 | 73 (1.0) | 13 (1.0) | 60 (1.0) | 0.01 |
| Substance abuse disorder | 9168 (9.9) | 156 (11.0) | 9012 (9.9) | 0.04 | 797 (10.5) | 136 (10.8) | 661 (10.5) | 0.01 |
| Other comorbidities | ||||||||
| Pregnancy | 264 (0.3) | 0 (0.0) | 264 (0.3) | −0.08 | NA (NA) | NA (NA) | NA (NA) | −0.02 |
| Blood loss anemia | 1097 (1.2) | 12 (0.9) | 1085 (1.2) | −0.03 | 58 (0.8) | 11 (0.9) | 47 (0.8) | 0.01 |
| Cardiac arrhythmias | 13 024 (14.1) | 222 (15.6) | 12 802 (14.0) | 0.05 | 1069 (14.1) | 198 (15.7) | 871 (13.8) | 0.05 |
| Congestive heart failure | 8112 (8.8) | 114 (8.0) | 7998 (8.8) | −0.03 | 507 (6.7) | 107 (8.5) | 400 (6.4) | 0.08 |
| Coagulopathy | 2401 (2.6) | 44 (3.1) | 2357 (2.6) | 0.03 | 189 (2.5) | 37 (2.9) | 152 (2.4) | 0.03 |
| Chronic pulmonary disease | 22 193 (23.9) | 341 (24.0) | 21 852 (23.9) | 0.00 | 1768 (23.4) | 311 (24.7) | 1457 (23.1) | 0.04 |
| Deficiency anemia | 5795 (6.3) | 81 (5.7) | 5714 (6.3) | −0.02 | 396 (5.2) | 77 (6.1) | 319 (5.1) | 0.05 |
| Diabetes, uncomplicated | 23 380 (25.2) | 371 (26.2) | 23 009 (25.2) | 0.02 | 1923 (25.4) | 336 (26.7) | 1587 (25.2) | 0.03 |
| Diabetes, complicated | 17 133 (18.5) | 267 (18.8) | 16 866 (18.5) | 0.01 | 1407 (18.6) | 243 (19.3) | 1164 (18.5) | 0.02 |
| Diabetes | 27 257 (29.4) | 432 (30.4) | 26 825 (29.4) | 0.02 | 2227 (29.5) | 390 (31.0) | 1837 (29.2) | 0.04 |
| Fluid and electrolyte disorders | 9125 (9.8) | 106 (7.5) | 9019 (9.9) | −0.09 | 555 (7.3) | 98 (7.8) | 457 (7.3) | 0.02 |
| HIV | 379 (0.4) | <11 (NA) | >368 (NA) | −0.02 | 21 (0.3) | <11 (NA) | >10 (NA) | −0.01 |
| Hypertension, uncomplicated | 55 671 (60.0) | 913 (64.3) | 54 758 (60.0) | 0.09 | 4684 (62.0) | 811 (64.4) | 3873 (61.5) | 0.06 |
| Hypertension, complicated | 9503 (10.3) | 126 (8.9) | 9377 (10.3) | −0.05 | 626 (8.3) | 116 (9.2) | 510 (8.1) | 0.04 |
| Hypertension | 56 886 (61.4) | 934 (65.8) | 55 952 (61.3) | 0.09 | 4766 (63.0) | 830 (65.9) | 3936 (62.5) | 0.07 |
| Hypothyroidism | 15 392 (16.6) | 259 (18.3) | 15 133 (16.6) | 0.04 | 1294 (17.1) | 230 (18.3) | 1064 (16.9) | 0.04 |
| Liver disease | 4602 (5.0) | 73 (5.1) | 4529 (5.0) | 0.01 | 382 (5.1) | 63 (5) | 319 (5.1) | −0.00 |
| Obesity | 15 034 (16.2) | 251 (17.7) | 14 783 (16.2) | 0.04 | 1276 (16.9) | 221 (17.5) | 1055 (16.8) | 0.02 |
| Other neurological deficits | 5950 (6.4) | 88 (6.2) | 5862 (6.4) | −0.01 | 428 (5.7) | 77 (6.1) | 351 (5.6) | 0.02 |
| Pulmonary circulation disorders | 2383 (2.6) | 31 (2.2) | 2352 (2.6) | −0.03 | 146 (1.9) | 29 (2.3) | 117 (1.9) | 0.03 |
| Peptic ulcer disease | 1091 (1.2) | 18 (1.3) | 1073 (1.2) | 0.01 | 93 (1.2) | 16 (1.3) | 77 (1.2) | 0.00 |
| Peripheral vascular disease | 9948 (10.7) | 152 (10.7) | 9796 (10.7) | −0.00 | 733 (9.7) | 135 (10.7) | 598 (9.5) | 0.04 |
| Paralysis | 1012 (1.1) | <11 (NA) | >1001 (NA) | −0.06 | 41 (0.54) | <11 (NA) | >30 (NA) | 0.01 |
| Kidney failure | 9377 (10.1) | 142 (10.0) | 9235 (10.1) | −0.00 | 698 (9.2) | 129 (10.2) | 569 (9.0) | 0.04 |
| Valvular disease | 5981 (6.5) | 85 (6.0) | 5896 (6.5) | −0.02 | 457 (6.0) | 74 (5.9) | 383 (6.1) | −0.01 |
| Weight loss | 2771 (3.0) | 31 (2.2) | 2740 (3) | −0.05 | 166 (2.2) | 28 (2.2) | 138 (2.2) | 0.00 |
| Health care utilization and costs | ||||||||
| All-cause cost of care, $ | ||||||||
| Baseline total costs, PMPM | ||||||||
| Mean (SD) | 2261 (4639) | 2003 (3513) | 2265 (4654) | −0.06 | 2138 (4241) | 1993 (3487) | 2167 (4376) | −0.04 |
| Median (IQR) | 958 (436-2312) | 1162 (646-2181) | 954 (433-2316) | 1060 (557-2253) | 1139 (619-2142) | 1045 (544-2283) | ||
| Baseline medical costs, PMPM | ||||||||
| Mean (SD) | 1718 (4292) | 1406 (3288) | 1723 (4306) | −0.08 | 1550 (3570) | 1389 (3242) | 1582 (3632) | −0.06 |
| Median (IQR) | 542 (222-1496) | 691 (371-1324) | 539 (221-1501) | 625 (310-1429) | 679 (363-1300) | 613 (300-1464) | ||
| Baseline outpatient pharmacy costs, PMPM | ||||||||
| Mean (SD) | 543 (1595) | 597 (1031) | 542 (1603) | 0.04 | 588 (2215) | 604 (1064) | 585 (2379) | 0.01 |
| Median (IQR) | 193 (71-532) | 275 (107-662) | 192 (70-530) | 229 (86-602) | 281 (102-665) | 219 (83-590) | ||
| All-cause health care resource utilization | ||||||||
| Baseline emergency department stays | ||||||||
| Mean (SD) | 0.6 (1.4) | 0.5 (1.2) | 0.6 (1.4) | −0.09 | 0.5 (1.3) | 0.5 (1.2) | 0.5 (1.3) | −0.04 |
| Median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) | ||
| Baseline emergency department days | ||||||||
| Mean (SD) | 0.7 (3.4) | 0.6 (1.5) | 0.7 (3.4) | −0.07 | 0.6 (1.7) | 0.5 (1.5) | 0.6 (1.8) | −0.06 |
| Median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) | ||
| Baseline inpatient stays | ||||||||
| Mean (SD) | 0.2 (0.6) | 0.1 (0.4) | 0.2 (0.6) | −0.20 | 0.1 (0.4) | 0.1 (0.4) | 0.2 (0.4) | −0.09 |
| Median (IQR) | 0 | 0 | 0 | 0 | 0 | 0 | ||
Abbreviations: MME, morphine milligram equivalent; NA, not available; NSAID, nonsteroidal anti-inflammatory drug; PMPM, per member per month; SMD, standardized mean difference; SNRI, serotonin and norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
Small numbers (n <11) cannot be reported according to the Optum Labs cell size suppression policy.
Unknown/multiple refers to patients with unknown race or ethnicity or included in multiple categories.
Outcomes of SCS vs CMM
Pharmacologic Treatments for Pain
After achieving baseline balance, during the first 12 months, patients treated with SCSs filled a higher number of opioid prescriptions, were more likely to have chronic opioid use (54.9% vs 51.8%; adjusted odds ratio [aOR], 1.14; 95% CI, 1.01-1.29) (Table 2 and Table 3) and long-acting opioid use (22.5% vs 18.5%; aOR, 1.28; 95% CI, 1.11-1.49) compared with those treated with CMM. During months 13 to 24, there were no significant reductions across pharmacologic treatments for pain among patients treated with SCS; patients treated with SCS had similar adjusted odds of chronic opioid use (49.0% vs 47.6%; aOR, 1.06; 95% CI, 0.94-1.20) and long-acting opioid use (18.3% vs 16.3%; aOR, 1.16; 95% CI, 0.99-1.36). Among patients taking opioids during the 6-month baseline period, SCS was not associated with a higher rate of opioid discontinuation during months 13 to 24 (eTable 8 in the Supplement).
Table 2. Pain and Health Care Utilization 24 Months After Permanent Spinal Cord Stimulator (SCS) Implantation vs Conventional Medical Management (CMM).
| Variable | Follow-up, mo | Total (n = 7560) | SCS (n = 1260) | CMM (n = 6300) |
|---|---|---|---|---|
| Surrogates of pain | ||||
| Average MME | 1-12 | |||
| Mean (SD) | 33.5 (65.5) | 33.0 (60.7) | 33.6 (66.4) | |
| Median (IQR) | 9.6 (0.7-39.1) | 11.8 (1.9-38.2) | 9.0 (0.5-39.2) | |
| Average MME | 13-24 | |||
| Mean (SD) | 28.3 (55.6) | 27.1 (49.2) | 28.5 (56.8) | |
| Median (IQR) | 5.3 (0.0-35.1) | 6.0 (0.0-34.4) | 5.2 (0.0-35.1) | |
| No. of opioid scripts | 1-12 | |||
| Mean (SD) | 8.3 (8.2) | 8.9 (7.8) | 8.2 (8.2) | |
| Median (IQR) | 7 (1-13) | 7 (2-13) | 6 (1-13) | |
| No. of opioid scripts | 13-24 | |||
| Mean (SD) | 7.4 (8.0) | 7.4 (7.6) | 7.4 (8.0) | |
| Median (IQR) | 5 (0-12) | 5 (0-12) | 5 (0-12) | |
| Chronic opioid use | 1-12 | 3952 (52.3) | 692 (54.9) | 3260 (51.8) |
| 13-24 | 3615 (47.8) | 617 (49.0) | 2998 (47.6) | |
| Long-acting opioid use | 1-12 | 1449 (19.2) | 284 (22.5) | 1165 (18.5) |
| 13-24 | 1259 (16.7) | 231 (18.3) | 1028 (16.3) | |
| High MME | 1-12 | 3984 (52.7) | 815 (64.7) | 3169 (50.3) |
| 13-24 | 3318 (43.9) | 563 (44.7) | 2755 (43.7) | |
| Epidural and facet corticosteroid injections | 1-12 | 2693 (35.6) | 273 (21.7) | 2420 (38.4) |
| 13-24 | 1895 (25.1) | 314 (24.9) | 1581 (25.1) | |
| Radiofrequency ablation | 1-12 | 644 (8.5) | 67 (5.3) | 577 (9.2) |
| 13-24 | 494 (6.5) | 72 (5.7) | 422 (6.7) | |
| Advanced imaging | 1-12 | 2440 (32.3) | 367 (29.1) | 2073 (32.9) |
| 13-24 | 2194 (29.0) | 357 (28.3) | 1837 (29.2) | |
| Spine surgery | 1-12 | 1364 (18.0) | 179 (14.2) | 1185 (18.8) |
| 13-24 | 957 (12.7) | 148 (11.8) | 809 (12.8) | |
| Pharmacologic treatment during follow-up | ||||
| NSAIDs | 1-12 | 2944 (38.9) | 476 (37.8) | 2468 (39.2) |
| 13-24 | 2674 (35.4) | 442 (35.1) | 2232 (35.4) | |
| Muscle relaxants | 1-12 | 3158 (41.8) | 558 (44.3) | 2600 (41.3) |
| 13-24 | 2909 (38.5) | 495 (39.3) | 2414 (38.3) | |
| Systemic steroids | 1-12 | 2614 (34.6) | 422 (33.5) | 2192 (34.8) |
| 13-24 | 2532 (33.5) | 444 (35.2) | 2088 (33.1) | |
| TCA/SNRI antidepressants | 1-12 | 2397 (31.7) | 412 (32.7) | 1985 (31.5) |
| 13-24 | 2305 (30.5) | 419 (33.3) | 1886 (29.9) | |
| Gabapentinoids | 1-12 | 3996 (52.9) | 681 (54.1) | 3315 (52.6) |
| 13-24 | 3714 (49.1) | 671 (53.3) | 3043 (48.3) | |
| Benzodiazepines | 1-12 | 2702 (35.7) | 451 (35.8) | 2251 (35.7) |
| 13-24 | 2407 (31.8) | 371 (29.4) | 2036 (32.3) | |
| Health care utilization and costs, $ | ||||
| All-cause cost of care | ||||
| Total costs, PMPM | Baseline | |||
| Mean (SD) | 2138 (4241) | 1993 (3487) | 2167 (4376) | |
| Median (IQR) | 1060 (557-2253) | 1139 (619-2142) | 1045 (544-2283) | |
| Follow-up total costs, PMPM | 1-12 | |||
| Mean (SD) | 2789 (4220) | 5531 (4188) | 2240 (4008) | |
| Median (IQR) | 1500 (649-3641) | 4488 (3319-6436) | 1182 (559-2552) | |
| Follow-up total costs, PMPM | 13-24 | |||
| Mean (SD) | 2120 (3682) | 2171 (2845) | 2109 (3827) | |
| Median (IQR) | 1070 (479-2434) | 1263 (548-2638) | 1035 (464-2398) | |
| Medical costs, PMPM | Baseline | |||
| Mean (SD) | 1550 (3571) | 1389 (3242) | 1582 (3632) | |
| Median (IQR) | 625 (310-1429) | 679 (363-1300) | 613 (300-1464) | |
| Follow-up medical costs, PMPM | 1-12 | |||
| Mean (SD) | 2184 (3492) | 4916 (3917) | 1638 (3127) | |
| Median (IQR) | 921 (362-2932) | 3910 (2987-5616) | 690 (307-1738) | |
| Follow-up medical costs, PMPM | 13-24 | |||
| Mean (SD) | 1498 (2785) | 1557 (2487) | 1486 (2840) | |
| Median (IQR) | 595 (253-1618) | 695 (278-1786) | 579 (247-1583) | |
| Outpatient pharmacy costs, PMPM | Baseline | |||
| Mean (SD) | 588 (2215) | 604 (1064) | 585 (2379) | |
| Median (IQR) | 229 (86-602) | 281 (102-665) | 219 (83-590) | |
| Follow-up outpatient pharmacy costs, PMPM | 1-12 | |||
| Mean (SD) | 604 (2249) | 615.1 (1120) | 602 (2412) | |
| Median (IQR) | 240 (93-610) | 290 (111-648) | 231 (90-601) | |
| Follow-up outpatient pharmacy costs, PMPM | 13-24 | |||
| Mean (SD) | 622 (2282) | 614 (1097) | 623.6 (2451) | |
| Median (IQR) | 232 (87-604) | 283 (108-662) | 223 (85-590) | |
| All-cause health care resource utilization | ||||
| Follow-up inpatient stays | 1-12 | |||
| Mean (SD) | 0.3 (0.8) | 0.3 (0.7) | 0.3 (0.8) | |
| Median (IQR) | 0 (0-0) | 0 (0-0) | 0 (0-0) | |
| Follow-up inpatient stays | 13-24 | |||
| Mean (SD) | 0.3 (0.8) | 0.3 (0.7) | 0.3 (0.8) | |
| Median (IQR) | 0 (0-0) | 0 (0-0) | 0 (0-0) | |
| Follow-up ED stays | 1-12 | |||
| Mean (SD) | 1.0 (2.2) | 0.9 (2.0) | 1.0 (2.2) | |
| Median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-1) | |
| Follow-up ED stays | 13-24 | |||
| Mean (SD) | 0.9 (2.0) | 0.9 (1.8) | 0.9 (2.1) | |
| Median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-1) | |
| Follow-up ED, d | 1-12 | |||
| Mean (SD) | 1.2 (3.0) | 1.2 (2.7) | 1.2 (3.0) | |
| Median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-1) | |
| Follow-up ED, d | 13-24 | |||
| Mean (SD) | 1.2 (3.0) | 1.1 (2.4) | 1.2 (3.1) | |
| Median (IQR) | 0 (0-1) | 0 (0-1) | 0 (0-1) | |
| Office visits | Baseline | |||
| Mean (SD) | 12.1 (9.0) | 13.3 (8.7) | 11.9 (9.0) | |
| Median (IQR) | 10 (6-16) | 11 (7-17) | 10 (6-15) | |
| Follow-up office visits | 1-12 | |||
| Mean (SD) | 22.5 (16.6) | 23.0 (16.2) | 22.5 (16.7) | |
| Median (IQR) | 19 (11-29) | 20 (12-30) | 19 (11-29) | |
| Follow-up office visits | 13-24 | |||
| Mean (SD) | 21.1 (16.8) | 22.4 (18.0) | 20.8 (16.5) | |
| Median (IQR) | 17 (10-27) | 18 (11-29) | 17 (10-27) | |
Abbreviations: ED, emergency department; MME, morphine milligram equivalent; NSAID, nonsteroidal anti-inflammatory drug; PMPM, per member per month; SNRI, serotonin and norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
Table 3. Propensity Score–Matched Generalized Estimating Equation Model for Clinical Outcomes Within 24 Months After Permanent Spinal Cord Stimulator Placement vs Conventional Medical Management.
| Outcome | Follow-up, mo | Odds ratio (95% CI) |
|---|---|---|
| Chronic opioid use | 1-12 | 1.14 (1.01-1.29) |
| 13-24 | 1.06 (0.94-1.20) | |
| Long-acting opioid use | 1-12 | 1.28 (1.11-1.49) |
| 13-24 | 1.16 (0.99-1.36) | |
| High MME | 1-12 | 1.81 (1.60-2.04) |
| 13-24 | 1.04 (0.92-1.18) | |
| Epidural and facet corticosteroid injections | 1-12 | 0.44 (0.39-0.51) |
| 13-24 | 1.00 (0.87-1.14) | |
| Radiofrequency ablation | 1-12 | 0.57 (0.44-0.72) |
| 13-24 | 0.84 (0.66-1.09) | |
| Advanced imaging | 1-12 | 0.84 (0.74-0.96) |
| 13-24 | 0.97 (0.85-1.11) | |
| Spine surgery | 1-12 | 0.72 (0.61-0.85) |
| 13-24 | 0.91 (0.75-1.09) | |
| NSAIDs | 1-12 | 0.95 (0.83-1.07) |
| 13-24 | 0.99 (0.87-1.13) | |
| Muscle relaxants | 1-12 | 1.13 (0.99-1.28) |
| 13-24 | 1.03 (0.91-1.17) | |
| Systemic steroids | 1-12 | 0.94 (0.83-1.07) |
| 13-24 | 1.09 (0.97-1.24) | |
| TCA/SNRI antidepressants | 1-12 | 1.05 (0.92-1.20) |
| 13-24 | 1.16 (1.02-1.32) | |
| Gabapentinoids | 1-12 | 1.06 (0.94-1.20) |
| 13-24 | 1.22 (1.08-1.37) | |
| Benzodiazepines | 1-12 | 1.01 (0.88-1.14) |
| 13-24 | 0.87 (0.76-1.00) |
Abbreviations: MME, morphine milligram equivalents; NSAID, nonsteroidal anti-inflammatory drug; SNRI, serotonin and norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
During the first 12 months, there were no significant differences in the use of NSAIDs, muscle relaxants, steroids, TCA/SNRI antidepressants, gabapentinoids, or benzodiazepines. During months 13 to 24, patients treated with SCSs had no difference in the likelihood of receiving NSAIDs or muscle relaxants. However, these patients were more likely to fill a prescription for TCA/SNRI antidepressants (33.3% vs 29.9%; aOR, 1.16; 95% CI, 1.02-1.32) and gabapentinoids (53.3% vs 48.3%; aOR, 1.22; 95% CI, 1.08-1.37), although less likely to fill a benzodiazepine prescription (29.4% vs 32.3%; aOR, 0.87; 95% CI, 0.76-1.00). Results were generally consistent among propensity-matched comparisons by sex and type of insurance coverage (commercial and Medicare Advantage). Results were also consistent when limited to patients matched based on chronic regional pain syndrome or chronic pain syndrome diagnoses (eTable 9 in the Supplement) and when limited to patients who had received opioids for 7 or fewer days during the 6-month baseline period (eTable 10 in the Supplement).
Nonpharmacologic Pain Interventions
Fewer patients with SCSs received epidural and facet corticosteroid injections within the first 12 months compared with CMM (21.7% vs 38.4%; aOR, 0.44; 95% CI, 0.39-0.51) (Table 2 and Table 3), but this difference was not present by months 13 to 24 (24.9% vs 25.1%; aOR, 1.00; 95% CI, 0.87-1.14). Similarly, fewer patients with SCS underwent a radiofrequency ablation within the first 12 months compared with CMM (5.3% vs 9.2%; aOR, 0.57; 95% CI, 0.44-0.72), with no significant difference during months 13 to 24 (5.7% vs 6.7%; aOR, 0.84; 95% CI, 0.66-1.09). Results were consistent by sex and insurance type.
Health Care Utilization and Cost Outcomes
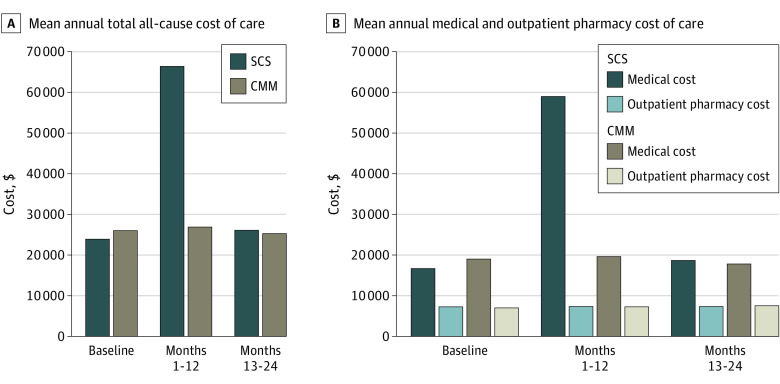
There were no significant differences between patients treated with SCSs or CMM in emergency department visits or hospitalizations in either the first or second year of follow-up (Table 2). The mean (SD) total cost of care per member per month during the first year was $5531 ($4188) for patients treated with SCS vs CMM $2240 ($4008) (P < .001); this difference was driven entirely by significantly higher medical costs for patients treated with SCS. Over 12 months, this represents over $39 000 in higher health care costs within the first year post-SCS placement (Figure). Stratified by type of insurance coverage, total costs were approximately $60 000 and $33 000 higher for commercially insured and Medicare Advantage enrollees, respectively. During months 13 to 24, the total costs were similar between the 2 groups ($2171 SCS vs $2109 CMM; P = .51) and adjusted cost ratios were also similar. Among all patients receiving SCS, out-of-pocket medical (ie, nonpharmacy) costs were approximately $2215 at baseline, increasing to $3695 in the first 12 months after SCS placement, and $1781 in the second year after device placement.
Figure. Costs of Care Among Propensity-Matched Patients Treated With Spinal Cord Stimulators (SCSs) vs Conventional Medical Management (CMM).
A, Mean annual total all-cause cost of care. B, Mean annual medical and outpatient pharmacy cost of care.
SCS-Related Complications and Removal
Among the 1260 patients treated with SCS, 226 (17.9%) experienced complications within the first 2 years after placement (Table 4). These complications included breakdown, displacement, other mechanical complications, and infection of the lead and/or generator. During the first 2 years, 279 patients (22.1%) had an SCS removal and/or revision; 126 (10%) of these were in the absence of a complication, suggesting lack of effectiveness.
Table 4. Spinal Cord Stimulator (SCS)–Related Complications and Revisions or Removals Among 1260 Patients Within 24 Months After Permanent Device Implantation.
| Complications/revisions or removals | No. of months after SCS placement | No. (%) |
|---|---|---|
| Complications | ||
| Breakdown of lead/generator | 1-12 | 56 (4.4) |
| 13-24 | 16 (1.3) | |
| Displacement of lead/generator | 1-12 | 22 (1.8) |
| 13-24 | NA (NA)a | |
| Infection/inflammation of lead/generator | 1-12 | 26 (2.1) |
| 13-24 | NA (NA) | |
| Other mechanical complications of lead/generator | 1-12 | 117 (9.3) |
| 13-24 | 51 (4.1) | |
| Any complication of lead/generator | 1-12 | 176 (14.0) |
| 1-24 | 226 (17.9) | |
| Revision or removals | ||
| Revision of lead/generator | 1-12 | 184 (14.6) |
| 13-24 | 75 (6.0) | |
| Lead removal | 1-12 | 95 (7.5) |
| 13-24 | 50 (4.0) | |
| Generator removal | 1-12 | 23 (1.8) |
| 13-24 | NA (NA) | |
| Any removal/revision of lead/generator | 1-12 | 217 (17.2) |
| 1-24 | 279 (22.1) |
Abbreviation: NA, not applicable.
Small numbers (n <11) cannot be reported according to the Optum Labs cell size suppression policy.
Discussion
In this large, real-world, comparative effectiveness research study comparing well-matched SCS and CMM patients, permanent SCS placement was not associated with a meaningful reduction in use of pharmacologic (including opioids) or nonpharmacologic interventions used for chronic pain at 2 years. Although patients treated with SCS received fewer epidural and facet corticosteroid injections and radiofrequency ablations within the first year after permanent device placement, perhaps due to time spent on efforts to establish SCS effectiveness for pain treatment, these differences were not present in the second year. SCS was also associated with risk, including device removal or revision in more than one-fifth of patients.
The lack of reduction in pharmacotherapy, epidural and facet corticosteroid injections, and radiofrequency ablations at 2 years among patients receiving SCS compared with those receiving CMM suggests that SCS was providing insufficient pain relief to forego other therapies or improve rates of depression or anxiety, as prescriptions for these drug classes did not decline. There is often a significant placebo effect to pain management procedures,25 including SCS.12 A systematic review of RCTs of SCS vs placebo found low to very low certainty of benefits on pain intensity.10 Because most patients still had their permanent SCS in place at 2 years, some may receive prolonged benefit from this modality, although we were not able to identify this through reductions in opioid use or nonpharmacologic pain interventions. Future research should seek to identify these possible subgroups and examine other endpoints that may be important to patients.
These findings also suggest that, despite recommendations that SCS be placed to reduce the need for opioids,5 this may not occur successfully in most patients who are receiving a contemporary SCS. In May 2018, the FDA announced an initiative to encourage device innovation to target pain26; however, all but a single SCS within the past 20 years have been approved based on literature reviews and not original clinical trials7; this means limited data support SCS that are used in clinical practice. A prior meta-analysis of 5 clinical trials, 4 of which were industry funded, found a minor reduction in opioid use after SCSs compared with CMM.27 In contrast, a recent independent study with 1-year follow-up of patients postlaminectomy found small, clinically questionable opioid discontinuation associated with SCSs.28 We extend these findings to 2 years and several additional endpoints among a broader population receiving SCS for multiple indications.
SCSs may also be associated with harm in some patients. Nearly one-fifth of patients treated with SCSs experienced device-related complications within 2 years. Even more had their devices removed or revised. More than two-fifths of SCS explants are for lack of pain relief.29 In this context, the greater than 100 000 adverse event reports filed with FDA over the past 4 years3 and 49 SCS-related recalls in the past 20 years7 indicate significant risks to patients.
SCS also have high costs: $39 000 more in the first year among patients treated with SCS than CMM. This additional spending was not recouped in the second year after SCS placement because patients continued to receive similar amounts of both pharmacologic and nonpharmacologic treatment. Although we did not conduct a formal cost-effectiveness analysis, some prior research (primarily industry-funded) has found these devices to be cost-effective,30,31,32 whereas those conducted by independent investigators have found SCSs to not be cost-effective.33
Back pain, with or without extremity pain, has high prevalence: more than one-fourth of patients report back pain within the past 3 months.34 With more than $100 billion in annual total costs,35 health plans must support use of safe and beneficial evidence-based therapies.6,36,37 The higher total costs of care that we observed associated with SCSs were primarily borne by health plans, particularly commercial insurance, and could result in higher premiums for all beneficiaries. Clinical practice guidelines provide strong recommendations that patients with chronic low back pain should initially use nonpharmacologic therapies such as exercise, rehabilitation, and cognitive behavioral therapy and then carefully selected pharmacologic treatment.38 Treatment of concurrent conditions, such as anxiety and depression, is also essential to effective pain treatment. A recent investigation by the US Department of Health and Human Services Office of Inspector General found that Medicare had overpaid by more than $600 million for neurostimulator implantation procedures, primarily because other treatments had not been trialed and a multidisciplinary approach to pain management had not been used.39
Limitations
Our findings should be considered in the context of study limitations. First, as with any observational study, results could be subject to residual confounding; patients receiving SCSs were a small group overall and may differ in unmeasured ways from patients who did not receive SCS. However, we used 65 variables for propensity matching. Although we were unable to account for pain scores within the matching process, we did include both pharmacologic and nonpharmacologic treatments that are strong surrogates for pain, with small standardized mean differences indicating a robust match, including by underlying pain diagnosis. Observational studies will be the sole source of long-term comparative data because SCS are widely available, and the FDA has not required new clinical trials for SCS approvals. Second, there is a movement toward ascertaining more holistic outcomes as a composite of multiple factors to evaluate success of SCS.40 Although these outcomes could not be evaluated using our data source, prospective studies should evaluate the benefits of SCS on holistic outcomes.40 Third, it is possible that patients with chronic pain could have received benefit from SCS but required medications and procedures for other areas of pain. Fourth, our data set did not include functional measures such as quality of life or ability to return to work, nor the impact of measured complications on patients. However, ascertainment of these outcomes is only possible for prospective studies that have dedicated mechanisms to ascertain these data. Fifth, our study population did not include individuals with Medicare fee-for-service or Medicaid insurance. Sixth, chronic pain is a diagnosis that often lasts longer than the 6-month clean period that we used and some patients were excluded because of insufficient longitudinal data, which may limit study generalizability; however, characteristics between included and excluded patients were not clinically different.
Conclusions
In conclusion, results of this large comparative effectiveness research study examining SCSs compared with CMM for chronic pain suggest a lack of clinical benefit for most patients and possible harm to some. There may be opportunities to redeploy the high—and increasing—use and spending associated with SCS toward more evidence-based interventions for chronic pain relief.
eTable 1. Cohort Identification and Evidence of Spinal Cord Stimulator Treatment and Conventional Medical Management
eTable 2. Identification of Individuals with Conditions for Study Exclusion
eTable 3. Code Lists for Pain Management Outcomes
eTable 4. Code List for Spinal Cord Stimulator-Related Complications, Revisions, and Removals
eTable 5. Comparison of Disenrolled and Retained Enrollees
eTable 6. Comparison of Patients With Trial Spinal Cord Stimulator Only and Patients Who Received Permanent SCS Placement
eTable 7. Complete List of Predictors and Odds Ratios Associated With Spinal Cord Stimulator Treatment
eTable 8. Rates of Opioid Discontinuation Among Patients With At Least 1 Opioid Fill During 6-Month Baseline Period
eTable 9. Pain and Health Care Utilization 24 Months After Permanent Spinal Cord Stimulator Implantation vs Conventional Medical Management Among Patients With Complex Regional Pain Syndrome and Chronic Pain Syndrome Diagnoses Only
eTable 10. Pain and Health Care Utilization 24 Months After Permanent Spinal Cord Stimulator Implantation vs Conventional Medical Management Among Patients With ≤7 Days Opioid Prescription During 6-Month Baseline Period
eFigure 1. Flow Diagram of Cohort Construction
eFigure 2. Overall Study Design Key Time Periods and Events
eFigure 3. Prematch and Postmatch Standardized Mean Difference
References
- 1.Gharibo C, Laux G, Forzani BR, Sellars C, Kim E, Zou S. State of the field survey: spinal cord stimulator use by academic pain medicine practices. Pain Med. 2014;15(2):188-195. doi: 10.1111/pme.12264 [DOI] [PubMed] [Google Scholar]
- 2.Leung N, Tsourmas NF, Yuspeh L, et al. Increased spinal cord stimulator use and continued opioid treatment among injured workers: a regional pilot study. J Occup Environ Med. 2020;62(8):e436-e441. doi: 10.1097/JOM.0000000000001933 [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration . Conduct a trial stimulation period before implanting a spinal cord stimulator (SCS)—letter to health care providers. Accessed May 20, 2022. https://www.fda.gov/medical-devices/letters-health-care-providers/conduct-trial-stimulation-period-implanting-spinal-cord-stimulator-scs-letter-health-care-providers
- 4.Global Market Insights Inc . Neurostimulation devices market value to hit $19 billion by 2025: Global Market Insights, Inc. Accessed May 20, 2022. https://www.prnewswire.com/news-releases/neurostimulation-devices-market-value-to-hit-19-billion-by-2025-global-market-insights-inc-300937560.html
- 5.Poree L, Krames E, Pope J, Deer TR, Levy R, Schultz L. Spinal cord stimulation as treatment for complex regional pain syndrome should be considered earlier than last resort therapy. Neuromodulation. 2013;16(2):125-141. doi: 10.1111/ner.12035 [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166(7):493-505. doi: 10.7326/M16-2459 [DOI] [PubMed] [Google Scholar]
- 7.Carome MA. Implanted spinal cord stimulators for pain relief. Accessed May 20, 2022. https://www.citizen.org/wp-content/uploads/2526_200610_Spinal-Cord-Stimulator-Report_FINAL.pdf
- 8.Harmsen IE, Hasanova D, Elias GJB, et al. Trends in clinical trials for spinal cord stimulation. Stereotact Funct Neurosurg. 2021;99(2):123-134. doi: 10.1159/000510775 [DOI] [PubMed] [Google Scholar]
- 9.Odonkor CA, Orman S, Orhurhu V, Stone ME, Ahmed S. Spinal cord stimulation vs conventional therapies for the treatment of chronic low back and leg pain: a systematic review of health care resource utilization and outcomes in the last decade. Pain Med. 2019;20(12):2479-2494. doi: 10.1093/pm/pnz185 [DOI] [PubMed] [Google Scholar]
- 10.O’Connell NE, Ferraro MC, Gibson W, et al. Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database Syst Rev. 2021;12:CD013756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner JA, Hollingworth W, Comstock BA, Deyo RA. Spinal cord stimulation for failed back surgery syndrome: outcomes in a workers’ compensation setting. Pain. 2010;148(1):14-25. doi: 10.1016/j.pain.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 12.Duarte RV, Nevitt S, McNicol E, et al. Systematic review and meta-analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain. 2020;161(1):24-35. doi: 10.1097/j.pain.0000000000001689 [DOI] [PubMed] [Google Scholar]
- 13.Simopoulos T, Aner M, Sharma S, Ghosh P, Gill JS. Explantation of percutaneous spinal cord stimulator devices: a retrospective descriptive analysis of a single-center 15-year experience. Pain Med. 2019;20(7):1355-1361. doi: 10.1093/pm/pny245 [DOI] [PubMed] [Google Scholar]
- 14.Weiss M, Mohr H. Spinal-cord stimulators help some patients, injure others. Accessed May 20, 2022. https://apnews.com/article/wv-state-wire-us-news-ap-top-news-sc-state-wire-health-86ba45b0a4ad443fad1214622d13e6cb
- 15.Optum Labs . Optum Labs and Optum Labs Data Warehouse (OLDW) Descriptions and Citation. Optum Labs; 2022. [Google Scholar]
- 16.Desai MJ, Hargens LM, Breitenfeldt MD, et al. The rate of magnetic resonance imaging in patients with spinal cord stimulation. Spine (Phila Pa 1976). 2015;40(9):E531-E537. doi: 10.1097/BRS.0000000000000805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farber SH, Han JL, Petraglia Iii FW, et al. Increasing rates of imaging in failed back surgery syndrome patients: implications for spinal cord stimulation. Pain Physician. 2017;20(6):E969-E977. [PMC free article] [PubMed] [Google Scholar]
- 18.Petraglia FW III, Farber SH, Gramer R, et al. The incidence of spinal cord injury in implantation of percutaneous and paddle electrodes for spinal cord stimulation. Neuromodulation. 2016;19(1):85-90. doi: 10.1111/ner.12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeffery MM, Hooten WM, Henk HJ, et al. Trends in opioid use in commercially insured and Medicare Advantage populations in 2007-16: retrospective cohort study. BMJ. 2018;362:k2833. doi: 10.1136/bmj.k2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521-527. doi: 10.1097/AJP.0b013e318169d03b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillespie CW, Morin PE, Tucker JM, Purvis L. Medication adherence, health care utilization, and spending among privately insured adults with chronic conditions in the US, 2010-2016. Am J Med. 2020;133(6):690-704.e19. doi: 10.1016/j.amjmed.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 22.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 23.US Centers for Medicare & Medicaid Services. Chronic conditions data warehouse . Accessed June 29, 2021. https://www2.ccwdata.org/web/guest/condition-categories
- 24.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redberg RF. Sham controls in medical device trials. N Engl J Med. 2014;371(10):892-893. doi: 10.1056/NEJMp1406388 [DOI] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration . As part of efforts to combat opioid crisis, FDA launches innovation challenge to spur development of medical devices—including digital health and diagnostics—that target pain, addiction, and diversion. Accessed May 20, 2022. https://www.fda.gov/news-events/press-announcements/part-efforts-combat-opioid-crisis-fda-launches-innovation-challenge-spur-development-medical-devices
- 27.Pollard EM, Lamer TJ, Moeschler SM, et al. The effect of spinal cord stimulation on pain medication reduction in intractable spine and limb pain: a systematic review of randomized controlled trials and meta-analysis. J Pain Res. 2019;12:1311-1324. doi: 10.2147/JPR.S186662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vu TN, Khunsriraksakul C, Vorobeychik Y, et al. Association of spinal cord stimulator implantation with persistent opioid use in patients with postlaminectomy syndrome. JAMA Netw Open. 2022;5(1):e2145876. doi: 10.1001/jamanetworkopen.2021.45876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of 8 years of experience from an academic center database. Neuromodulation. 2015;18(7):603-608. doi: 10.1111/ner.12312 [DOI] [PubMed] [Google Scholar]
- 30.Farber SH, Han JL, Elsamadicy AA, et al. Long-term cost utility of spinal cord stimulation in patients with failed back surgery syndrome. Pain Physician. 2017;20(6):E797-E805. [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor RS, Taylor RJ, Van Buyten JP, Buchser E, North R, Bayliss S. The cost effectiveness of spinal cord stimulation in the treatment of pain: a systematic review of the literature. J Pain Symptom Manage. 2004;27(4):370-378. doi: 10.1016/j.jpainsymman.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 32.Mekhail NA, Aeschbach A, Stanton-Hicks M. Cost benefit analysis of neurostimulation for chronic pain. Clin J Pain. 2004;20(6):462-468. doi: 10.1097/00002508-200411000-00012 [DOI] [PubMed] [Google Scholar]
- 33.Hollingworth W, Turner JA, Welton NJ, Comstock BA, Deyo RA. Costs and cost-effectiveness of spinal cord stimulation (SCS) for failed back surgery syndrome: an observational study in a workers’ compensation population. Spine (Phila Pa 1976). 2011;36(24):2076-2083. doi: 10.1097/BRS.0b013e31822a867c [DOI] [PubMed] [Google Scholar]
- 34.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila Pa 1976). 2006;31(23):2724-2727. doi: 10.1097/01.brs.0000244618.06877.cd [DOI] [PubMed] [Google Scholar]
- 35.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24. doi: 10.2106/00004623-200604002-00005 [DOI] [PubMed] [Google Scholar]
- 36.Chou R, Deyo R, Friedly J, et al. Systemic pharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2017;166(7):480-492. doi: 10.7326/M16-2458 [DOI] [PubMed] [Google Scholar]
- 37.Cohen KR. Management of chronic low back pain. JAMA Intern Med. 2022;182(2):222-223. doi: 10.1001/jamainternmed.2021.7359 [DOI] [PubMed] [Google Scholar]
- 38.Qaseem A, Wilt TJ, McLean RM, et al. ; Clinical Guidelines Committee of the American College of Physicians . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514-530. doi: 10.7326/M16-2367 [DOI] [PubMed] [Google Scholar]
- 39.Department of Health and Human Services Office of Inspector General . Medicare overpaid more than $636 million for neurostimulator implantation surgeries. Accessed May 20, 2022. https://oig.hhs.gov/oas/reports/region1/11800500.pdf
- 40.Goudman L, Billot M, Duarte RV, Eldabe S, Rigoard P, Moens M. Gradation of clinical holistic response as new composite outcome to evaluate success in spinal cord stimulation studies for pain. Neuromodulation. 2021;S1094-7159(21)06395-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Cohort Identification and Evidence of Spinal Cord Stimulator Treatment and Conventional Medical Management
eTable 2. Identification of Individuals with Conditions for Study Exclusion
eTable 3. Code Lists for Pain Management Outcomes
eTable 4. Code List for Spinal Cord Stimulator-Related Complications, Revisions, and Removals
eTable 5. Comparison of Disenrolled and Retained Enrollees
eTable 6. Comparison of Patients With Trial Spinal Cord Stimulator Only and Patients Who Received Permanent SCS Placement
eTable 7. Complete List of Predictors and Odds Ratios Associated With Spinal Cord Stimulator Treatment
eTable 8. Rates of Opioid Discontinuation Among Patients With At Least 1 Opioid Fill During 6-Month Baseline Period
eTable 9. Pain and Health Care Utilization 24 Months After Permanent Spinal Cord Stimulator Implantation vs Conventional Medical Management Among Patients With Complex Regional Pain Syndrome and Chronic Pain Syndrome Diagnoses Only
eTable 10. Pain and Health Care Utilization 24 Months After Permanent Spinal Cord Stimulator Implantation vs Conventional Medical Management Among Patients With ≤7 Days Opioid Prescription During 6-Month Baseline Period
eFigure 1. Flow Diagram of Cohort Construction
eFigure 2. Overall Study Design Key Time Periods and Events
eFigure 3. Prematch and Postmatch Standardized Mean Difference