Abstract
Previous estimates determined prevalence of hypothyroidism (HT) to be 4.6% of the US population. This study aimed to update estimates of HT prevalence in the United States by retrospective analysis of 2 datasets. Data on HT type (overt or subclinical HT) and treatment were collected from the 2009-2010 and 2011-2012 National Health and Nutrition Examination Survey (NHANES) cycles. From the Optum administrative claims database, medical and pharmacy claims were collected between January 1, 2012, and December 31, 2019. Patients were defined as having HT if, per given year, they had >1 prescription for HT treatment, >1 claim indicating an HT diagnosis, or thyroid-stimulating hormone levels >4.0 mIU/L (NHANES arm). For both studies, treatment was defined as any evidence of synthetic or natural thyroid hormone replacement, identified by pharmacy claims or patient surveys. Data are reported as percentage of patients with HT and treatments received. Between 2009 and 2012, HT prevalence remained around 9.6% of the US population. The administrative claims dataset showed that HT prevalence grew from 9.5% in 2012 to 11.7% in 2019 and that >78% of patients received thyroxine (T4) monotherapy. Similarly, the NHANES dataset showed that T4 replacement therapy was the most common treatment for HT. From 2012–2019, patients with untreated HT grew from 11.8% to 14.4%. The prevalence of HT in the United States has steadily increased since 2009. Likewise, the percentage of hypothyroid-diagnosed patients not receiving treatment also increased, suggesting that the increased prevalence may be due to increased cases of subclinical HT.
Keywords: hypothyroidism, epidemiology, retrospective study, administrative claims database
Hypothyroidism (HT) is one of the most common endocrine disorders, with clinical symptoms ranging from mild, such as fatigue, weight gain, cold intolerance, and depression, to more severe manifestations that may include myxedema and death [1, 2].
There are 2 major forms of endogenous HT—primary and secondary. Primary HT occurs when the thyroid cannot produce enough thyroid hormones. In geographic regions with insufficient iodine, primary HT is most often due to iodine deficiency. In iodine-sufficient regions, autoimmune thyroid disease is the most common cause of primary HT. Secondary, or central, HT is less common and generally stems from a dysfunctional pituitary gland or hypothalamus [1, 3]. Exogenous HT is commonly a side effect of medications (eg, amiodarone, thalidomide, iodine- or lithium-containing drugs, anti-cancer immunotherapies), surgery, or radiotherapy to the head/neck region [1, 3].
HT can be classified as either overt or subclinical. Overt HT is defined as thyroid-stimulating hormone (TSH) levels above the reference range with free thyroxine (FT4) levels below the reference. Subclinical HT is defined as elevated TSH with normal FT4 levels [1]. Patients with subclinical HT may or may not present with symptoms, thus subclinical HT is typically diagnosed by laboratory tests. There is an ongoing discussion regarding how, if at all, to treat subclinical HT. A wait-and-see strategy is most common among patients with subclinical HT, as up to 4% of patients may go on to develop overt HT [2, 4, 5].
The most commonly prescribed treatment for HT is thyroid hormone replacement therapy with levothyroxine, a synthetic form of thyroxine (T4) [1-3, 6]. Some patients may also be prescribed liothyronine, a synthetic triiodothyronine (T3) replacement therapy, despite clinical trials demonstrating little to no effect on symptoms of HT [6-8]. Indeed, the National Institute for Health and Care Excellence guidelines do not recommend T3 replacement therapy, either alone or in combination with levothyroxine [9]. There is also a subgroup of patients being treated with desiccated thyroid extracts, which are animal-derived versions that contain both T3 and T4 [10] and have demonstrated similar efficacy to that of synthetic replacement therapies [11].
The most-cited estimation of HT prevalence in the United States stems from analyses of survey data obtained by the National Health and Nutrition Examination Survey (NHANES) from 1988 to 1994 published in 2002. At that time, HT prevalence was estimated to be 4.6% (0.3% overt HT and 4.3% subclinical) of the US population [12]. The NHANES dataset is a robust and diverse sample that combines data from interviews and physical examinations of participants to estimate vital and health statistics of the general US population. As of 2012, the NHANES is no longer collecting thyroid profiles from participants. Estimates of HT have not been systematically updated since those published in 2002, and thus currently cited values may underestimate the true prevalence of HT in the United States and prevent healthcare professionals from adequate screening to facilitate timely diagnosis and treatment [12].
The aim of this study was to estimate the prevalence of HT in the United States and to determine the proportion of patients receiving HT treatment, as well as the type of therapies most often prescribed. Two methods were used to assess this—data from the 2009-2010 and 2011-2012 NHANES cycles and retrospective data analysis using medical/pharmacy claims data from 2012 to 2019 from the Optum administrative claims database.
Materials and Methods
Study Design and Participants
In order to provide the most up-to-date estimation of HT prevalence in the United States, this study assessed HT prevalence via 2 methods. After 2012, NHANES stopped collecting thyroid function tests data, thus no further HT prevalence assessments could be done. An alternative method of continuing to assess HT prevalence was through database claims. In this study, prevalence results for 2012 were similar between methods, suggesting that the database analysis was sufficient for estimating prevalence from 2013 onward. First, survey data from the 2009-2010 and 2011-2012 NHANES cycles were obtained. Patients ages ≥12 years old were included in the NHANES thyroid profile subsample; women who were pregnant were excluded. Disease status (eg, overt or subclinical HT, disease-free) was determined from blood samples. Overt HT, based on laboratory measures, was defined as presence of elevated TSH (>4.0 mIU/L) and a subnormal or low level of FT4 ( < 0.8 ng/dL); patients with a documented prescription for HT treatment were also categorized with overt HT. Subclinical HT was defined as elevated TSH (>4.0 mIU/L) and normal FT4 (≥0.8 ng/dL), without a documented prescription for HT treatment. Patients with subclinical HT (based on laboratory results) with a documented prescription for HT treatment were classified as overt HT. Disease-free patients had TSH levels between 0.4 and 4.0 mIU/L and no prescription for HT treatment. Eligible HT therapies were levothyroxine, liothyronine, or a combination of the 2.
Second, using the Optum administrative claims database, which collects longitudinal, patient-level information on medical and pharmacy claims from a geographically diverse large set of commercial electronic claims processors across the United States, data on patients ≥18 years of age, with continuous enrollment for the entirety of the calendar year, were collected between 2012 and 2019. Eligible patients had >1 medical claim indicating HT using an International Classification of Diseases 9 Clinical Modification/International Classification of Diseases 10 Clinical Modification code or >1 prescription fill for HT treatment. Information obtained from the database included patients with private, commercial insurance and government-provided insurance (ie, Medicaid). These HT treatments were manufactured versions of T4 (ie, levothyroxine sodium, Synthroid®, Euthyrox®, Unithroid®, Levoxyl®, Levothroid®, Levo-T®, Tirosint®), T3 (ie, liothyronine, Cytomel®), or T3/T4 combination drugs (ie, Thyrolar®, Armour Thyroid®, Nature-Throid®, NP Thyroid®, Westhroid®, WP Thyroid®).
Outcomes
The primary outcome of this study was to estimate the prevalence of HT in the United States. Using NHANES data from the 2009-2010 and 2011-2012 cycles, the prevalence and percentage of HT stratified by overt or subclinical disease, as well as percentage of patients receiving treatment and the types of therapies prescribed, were assessed. Using medical and pharmacy claims data from a commercially insured population, the annual prevalence of HT, the percentage of patients with HT receiving treatment, and the type of treatments they were prescribed were evaluated. Reported treatments are categorized by type: T3 replacement therapy, T4 replacement therapy, T3/T4 combination therapy (ie, 1 therapy that combines both T3 and T4 replacement or treatment with separate T3 and T4 replacement therapies within the same year), or no evidence of treatment.
NHANES Variables
Presence and type of HT were defined by elevated TSH and subnormal/low FT4 levels identified using the NHANES variables LBDTSH1S and LBXT4F, respectively. Covariables, such as patient demographic characteristics, insurance status, and self-reported comorbidities, were indexed using the NHANES variable codes listed in Table 1.
Table 1.
Definition on measurement of variables and covariables: Search criteria from the NHANES 2009-2010 and 2011-2012 survey results
| Patient characteristic | NHANES variable code | Notes |
|---|---|---|
| TSH (mIU/L) | LBDTSH1S | Overt HT: TSH >4.0 mIU/L + FT4 < 0.8 ng/dL or RX utilization to treat HT Subclinical HT: TSH >4.0 mIU/L + FT4 ≥ 0.8 ng/dL Without RX to treat HT Disease free: TSH 0.4-4.0 mIU/L |
| FT4 (ug/dL) | LBXT4F | |
| Age (years) | RIDAGYR | |
| Sex | RIAGENDR | Male = 1; female = 2 |
| Race | RIDETH1 | Non-Hispanic White = 3; Non-Hispanic Black = 4; Mexican American or other Hispanic = 1 or 2; other = 5 |
| Insurance statusa | ||
| Private | HIQ031A | =14 |
| Medicare | HIQ031B | =15 |
| Medi-Gap | HIQ031C | =16 |
| Medicaid | HIQ031D | =17 |
| SCHIP | HIQ031E | =18 |
| Military | HIQ031F | =19 |
| Indian Health Service | HIQ031G | =20 |
| State-sponsored | HIQ031H | =21 |
| Other government | HIQ031I | =22 |
| Other | HIQ031J | =23 |
| No coverage | HIQ031AA | =40 |
| Comorbidities, n (%) | ||
| Angina | MCQ160D | =1 (yes) |
| Arthritis | MCQ160A | =1 (yes) |
| Asthma | MCQ010 | =1 (yes) |
| Cancer | MCQ220 | =1 (yes) |
| Cerebrovascular accidents | MCQ160F | =1 (yes) |
| COPDb | MCQ160G, MCQ160K | =1 (yes) |
| Chronic renal failure | KIQ022 | =1 (yes) |
| Congestive heart failure | MCQ160B | =1 (yes) |
| Coronary heart disease | MCQ160C | =1 (yes) |
| Diabetes | DIQ010 | =1 (yes) |
| Hepatic conditions | MCQ160I | =1 (yes) |
| Hypertension | BPQ020 | =1 (yes) |
| Hyperlipidemia | BPQ080 | =1 (yes) |
| Ischemic heart diseasec | MCQ160D, MCQ160E | =1 (yes) |
| BMI | BMXBMI | |
| <18.5 | ||
| 18.5-24.9 | ||
| 25-29.9 | ||
| ≥30 | ||
| Doctor ever said you were overweight | MCQ080 | =1 (yes) |
| Osteoporosis | OSQ060 | =1 (yes) |
| Ever had thyroid problems | MCQ160M | =1 (yes) |
| Still have thyroid problems | MCQ170M | =1 (yes) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; FT4, free thyroxine; HT, hypothyroidism; NHANES, National Health and Nutrition Examination Survey; RX, prescription; SCHIP, State Children’s Health Insurance Program; TSH, thyroid stimulating hormone.
Survey responses may not be mutually exclusive. Where multiple forms of insurance were reported, the hierarchy of payment used by the National Center for Health Statistics in NHANES was employed (Medicare, Medicaid/SCHIP, private insurance, worker's compensation, self-pay, no charge/charity, other, unknown).
Includes emphysema and chronic bronchitis.
Includes coronary heart disease, angina, angina pectoris, and myocardial infarction.
Statistical Analysis of Data
The total number of patients satisfying the inclusion criteria each year were included in the denominator of prevalence estimates for that year. The total number of patients identified as having HT each year were included in the numerator for prevalence estimates for that year. Descriptive analysis was used to assess HT prevalence measures from the NHANES dataset. HT was determined either as (1) evidence of current medication to treat HT or (2) evidence of laboratory results consistent with HT. Weights were applied according to the variable with the smallest sample across all variables used in a calculation (ie, the thyroid panel). Combined years weighting followed rules as detailed in NHANES weighting tutorials [13]. HT prevalence and overall medications used to treat HT were estimated, by cycle and combined cycles, as the overall count and percentage.
Using data obtained from the Optum administrative claims database, prevalence was calculated for each year as the number of patients ≥18 years old who met the HT criteria (ie, >1 medical claim indication HT or >1 prescription fill for HT treatment) divided by the total number of patients in the database for that year. These data were further stratified and presented as the proportion of patients with HT by sex, age group, and geographical region. Similarly, the proportion of patients with HT receiving treatment each year, and the types of treatment, are reported; treatment types were identified using national drug codes from pharmacy claims. All data are defined descriptively.
Results
Baseline Patient Demographic and Clinical Characteristics
Of patients enrolled in the NHANES 2009-2010 and 2011-2012 cycles, approximately one-third of patients were ages 12 to 29 years (n = 75 375 591; 29.6%), 30 to 49 years (n = 82 141 239; 32.2%), and 50 to 79 years (n = 87 480 954; 34.3%), with only 3.9% of patients (n = 10 039 565) ≥ 80 years old (Table 2). The majority of patients were non-Hispanic White (n = 167 196 440; 65.6%), and almost half had private insurance coverage (n = 122 585 418; 48.1%). The most common comorbidities were arthritis (n = 52 156 831; 20.5%), hypertension (n = 69 596 983; 27.3%), hyperlipidemia (n = 69 140 550; 27.1%), and obesity (n = 101 962 554; 40.0%). Nearly 10% of survey participants reported ever having thyroid problems (n = 24 762 196), and 77% (n = 14 052 118) of those still experiencing thyroid problems showed evidence of HT. Among survey participants, 2.1% (n = 5 381 196) had evidence of untreated overt HT, of which 56.2% were female (n = 3 023 221) (data not shown). Among males with untreated overt HT, 41.9% (n = 988 098) were >60 years old and 32.9% (n = 775 292) were 12 to 44 years old. In contrast, 47.8% (n = 1 446 282) of females with untreated overt HT were 12 to 44 years old and 34.5% (n = 1 044 048) were 44 to 59 years old.
Table 2.
Baseline patient demographic and clinical characteristics: Data from NHANES 2009-2010 and 2011-2012 survey results
| Overall population N = 255 037 348 | Overt HT (labs or RX) N = 21 052 081 | Subclinical HT (labs) N = 3 500 678 | Overall HT N = 24 552 760 | Disease-free N = 230 484 588 | |
|---|---|---|---|---|---|
| Age (years), n (%) | |||||
| 12-29 | 75 375 591 (29.6) | 1 790 951 (2.4) | 903 253 (1.2) | 2 694 203 (3.6) | 72 681 387 (96.4) |
| 30-49 | 82 141 239 (32.2) | 3 856 096 (4.7) | 1 490 855 (1.8) | 5 346 951 (6.5) | 76 794 288 (93.5) |
| 50-79 | 87 480 954 (34.3) | 12 630 694 (14.4) | 792 231 (0.9) | 13 422 925 (15.3) | 74 058 028 (84.7) |
| ≥80 | 10 039 565 (3.9) | 2 774 340 (27.6) | 314 340 (3.1) | 3 088 680 (30.8) | 6 950 885 (69.2) |
| Gender, n (%) | |||||
| Female | 131 404 101 (51.5) | 15 175 187 (11.6) | 1 827 201 (1.4) | 17 002 388 (12.9) | 114 401 713 (87.1) |
| Male | 123 633 247 (48.5) | 5 876 895 (4.8) | 1 673 477 (1.4) | 7 550 371 (6.1) | 116 082 875 (93.9) |
| Race, n (%) | |||||
| Mexican American or other Hispanic | 37 751 178 (14.8) | 1 535 638 (4.1) | 561 011 (1.5) | 2 096 649 (5.6) | 35 654 529 (94.5) |
| Non-Hispanic White | 167 196 440 (65.6) | 17 991 330 (10.8) | 2 670 018 (1.6) | 20 661 348 (12.4) | 146 535 092 (87.6) |
| Non-Hispanic Black | 30 303 402 (11.9) | 921 944 (3.04) | 104 005 (0.3) | 1 025 949 (3.4) | 29 277 452 (96.6) |
| Other | 19 786 328 (7.8) | 603 169 (3.05) | 165 644 (0.8) | 768 813 (3.9) | 19 017 515 (96.1) |
| Insurance status, n (%) | |||||
| Private | 122 585 418 (48.1) | 8 352 351 (6.8) | 1 452 559 (1.2) | 9 804 910 (8.0) | 112 780 508 (92.0) |
| Public | 74 618 047 (29.3) | 10 305 855 (13.8) | 1 354 905 (1.8) | 11 660 760 (15.6) | 62 957 287 (84.4) |
| Other | 57 050 612 (22.4) | 2 316 644 (4.1) | 693 214 (1.2) | 3 009 858 (5.3) | 54 040 754 (94.7) |
| Comorbidities, n (%) | |||||
| Angina | 4 113 995 (1.6) | 600 884 (14.6) | 159 306 (3.9) | 760 190 (18.5) | 3 353 806 (81.5) |
| Arthritis | 52 156 831 (20.5) | 7 581 181 (14.5) | 491 011 (0.9) | 8 072 192 (15.5) | 44 084 639 (84.5) |
| Asthma | 37 369 901 (14.7) | 3 987 128 (10.7) | 407 626 (1.1) | 4 394 755 (11.8) | 32 975 147 (88.2) |
| Cancer | 22 257 497 (8.7) | 3 636 881 (16.3) | 631 018 (2.8) | 4 267 899 (19.2) | 17 989 598 (80.8) |
| Cerebrovascular accidents | 5 346 329 (2.1) | 758 194 (14.2) | 148 154 (2.8) | 906 348 (17.0) | 4 439 981 (83.1) |
| COPD | 14 165 550 (5.6) | 2 397 549 (16.9) | 175 409 (1.2) | 2 572 958 (18.2) | 11 592 591 (81.8) |
| Diabetes | 21 489 118 (8.4) | 3 642 055 (17.0) | 201 342 (0.9) | 3 843 397 (17.9) | 17 645 721 (82.1) |
| Hepatic conditions | 6 912 385 (2.7) | 1 210 722 (17.5) | 34 239 (0.5) | 1 244 961 (18.0) | 5 667 423 (82.0) |
| Hypertension | 69 596 983 (27.3) | 8 982 770 (12.9) | 888 701 (1.3) | 9 871 471 (14.2) | 59 725 512 (85.8) |
| Hyperlipidemia | 69 140 550 (27.1) | 9 150 153 (13.2) | 586 564 (0.9) | 9 736 718 (14.1) | 59 403 832 (85.9) |
| Ischemic heart disease | 12 763 796 (5.0) | 2 272 353 (17.8) | 236 283 (1.9) | 2 508 635 (19.7) | 10 255 161 (80.4) |
| Obesity | 101 962 554 (40.0) | 10 889 669 (10.7) | 1 495 016 (1.5) | 12 384 685 (12.2) | 89 577 869 (87.9) |
| Thyroid problems ever | 24 762 196 (9.7) | 15 404 101 (62.2) | 202 993 (0.8) | 15 607 094 (63.0) | 9 155 102 (37.0) |
| Thyroid problems currently | 18 256 198 (7.2) | 13 987 904 (76.6) | 64 214 (0.4) | 14 052 118 (77.0) | 4 204 079 (23.0) |
Abbreviations: COPD, chronic obstructive pulmonary disorder (including emphysema and bronchitis); HT, hypothyroidism; NHANES, National Health and Nutrition Examination Survey; RX, prescription.
Among patients with HT in the 2019 Optum dataset, 76.1% (n = 1 054 083) were female, and the most common comorbidities were cardiovascular disease (CVD; 71.7%, n = 992 682), hypertension (63.7%, n = 882 522), and lipid disorders (65.3%, n = 903 360; Table 3). More patients (78.1%, n = 1 081 840) received T4 monotherapy compared with T3/T4 combination therapy (3.3%, n = 46 190). Patients receiving T4 monotherapy were older (66.3 ± 14.4 vs 51.5 ± 12.4 years) and had greater comorbidity than patients receiving T3/T4 combination therapy.
Table 3.
Baseline patient demographic and clinical characteristics: Data from Optum administrative claims database (2019)
| Overall population N = 11 796 796 | Total HT population N = 1 384 369 | HT population: T4 monotherapy N = 1 081 840 | HT population: T3/T4 combination N = 46 190 | |
|---|---|---|---|---|
| Age (years), mean ± SD | 55.5 ± 19.3 | 66.4 ± 14.7 | 66.3 ± 14.4 | 51.5 ± 12.4 |
| Gender, n (%) | ||||
| Female | 6 200 921 (52.6) | 1 054 083 (76.1) | 824 635 (76.2) | 40 379 (87.4) |
| Male | 5 595 309 (47.4) | 330 255 (23.9) | 257 193 (23.8) | 5809 (12.6) |
| Geographical region, n (%) | ||||
| South | 4 844 444 (42.0) | 611 897 (44.2) | 476 029 (44.0) | 24 950 (54.1) |
| West | 2 732 509 (23.7) | 325 094 (26.5) | 266 366 (24.6) | 11 665 (25.3) |
| Midwest | 2 657 887 (23.1) | 283 774 (20.5) | 220 515 (20.4) | 7417 (16.1) |
| Northeast | 1 295 721 (11.2) | 162 588 (11.7) | 118 265 (10.9) | 2114 (4.6) |
| Comorbidities, n (%) | ||||
| Cardiovascular disease | 5 333 948 (45.2) | 992 682 (71.7) | 775 481 (71.7) | 16 773 (36.3) |
| Dementia | 293 617 (2.5) | 79 083 (5.7) | 58 419 (5.4) | 254 (0.5) |
| Diabetes | 1 959 960 (16.6) | 401 626 (29) | 314 751 (29.1) | 4203 (9.1) |
| Hypertension | 4 667 994 (39.6) | 882 522 (63.7) | 691 169 (63.9) | 12 435 (26.9) |
| Lipid disorders | 4 569 827 (38.7) | 903 360 (65.3) | 701 059 (64.8) | 16 400 (35.5) |
| Metabolic syndrome | 58 978 (0.5) | 15 377 (1.1) | 10 050 (0.9) | 1558 (3.4) |
| Obesity | 1 562 138 (13.2) | 287 374 (20.8) | 226 062 (20.9) | 7989 (17.3) |
| Stroke | 246 414 (2.1) | 57 373 (4.1) | 42 087 (3.9) | 564 (1.2) |
Abbreviations: HT, hypothyroidism; SD, standard deviation; T3, triiodothyronine; T4, thyroxine.
HT Prevalence Estimates in the United States Based on NHANES Data
Combining NHANES data from the 2009-2010 and 2011-2012 survey cycles showed that over half of patients with HT were female (51.5%), the majority of patients identified as non-Hispanic White (65.6%), and nearly half of patients had private insurance (48.1%; Table 2).
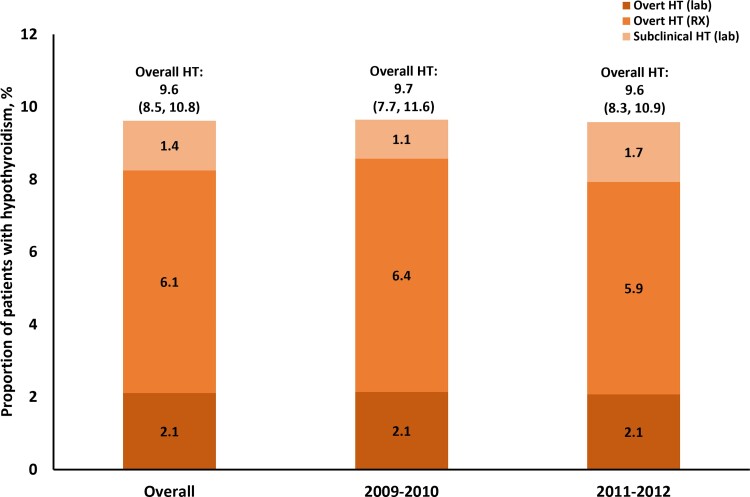
Analysis showed that 2.1% (n = 5 381 197) and 6.1% (n = 15 670 884) of survey participants had overt HT based on laboratory results (TSH >4.0 mIU/L and FT4 < 0.8 ng/dL) or current prescriptions, respectively; 1.4% (n = 3 500 678) of survey participants demonstrated evidence of subclinical HT and were not receiving treatment (Fig. 1) (based on TSH >4.0 mIU/L and FT4 ≥ 0.8 ng/dL). Overall, this analysis estimated HT prevalence (overt or subclinical) to be 9.6%.
Figure 1.
Proportion of patients with hypothyroidism from 2009 to 2012: Estimates from the National Health and Nutrition Examination Survey 2009-2010 and 2011-2012 survey results. Abbreviations: HT, hypothyroidism; RX, prescription.
When stratified to only include patients with TSH >4.5 mIU/L, as was done in the previous NHANES study [12], the prevalence of overt HT based on prescriptions remained the same; however, prevalence based on laboratory results was 2.0% (n = 4 988 115) and subclinical HT prevalence was 0.9 (n = 2 288 406).
When broken up by NHANES cycle, prevalence of HT generally remained stable between the 2009-2010 and 2011-2012 surveys. Prevalence of overt HT, defined by laboratory results, remained stable at 2.1% (n = 5 419 103 in 2009-2010; n = 5 343 292 in 2011-2012) between the 2009-2010 and 2011-2012 cycles; prevalence based on hypothyroid drug prescriptions decreased slightly from 6.4% (n = 16 228 763) to 5.9% (n = 15 113 005). Prevalence of untreated subclinical HT increased from 1.1% (n = 2 727 223) to 1.7% (n = 4 274 134) between the 2 survey cycles. Overall, HT prevalence remained stable at 9.7% (n = 24 375 089) in 2009-2010 and 9.6% (n = 24 730 431) in 2011-2012.
HT Prevalence Estimates in the United States Based on Claims Data
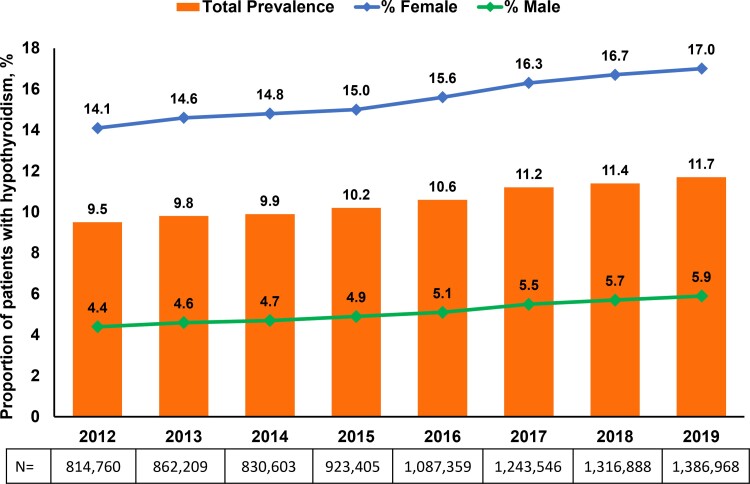
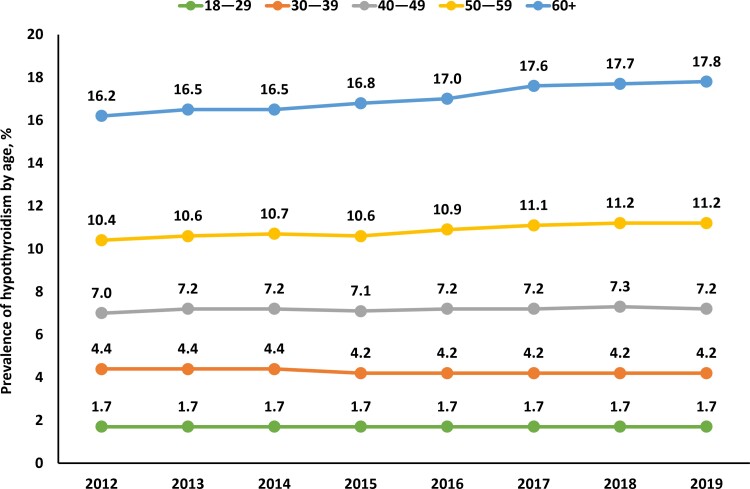
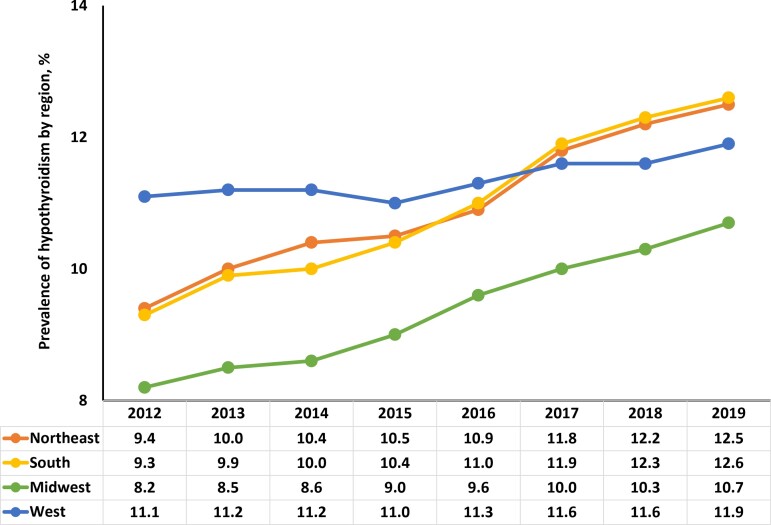
Analysis of data from the Optum administrative claims database estimated overall HT prevalence as 9.5% (n = 813 251) in 2012 (Fig. 2). Prevalence of HT steadily increased from 9.8% (n = 857 571) in 2013 to 11.7% (n = 1 384 369) in 2019. Among females, HT prevalence increased steadily from 14.1% in 2012 to 17.0% in 2019, while, among males, HT prevalence increased from 4.4% in 2012 to 5.9% in 2019 (Fig. 2). When stratified by age, HT prevalence remained consistent in patients <50 years old between 2012 and 2019 (Fig. 3). Over the course of the study, HT prevalence modestly increased in patients ages 50 to 59 (10.4-11.2%) and ≥60 years (16.2-17.8%). HT prevalence also steadily increased in the Northeast (9.4-12.5%), South (9.3-12.6%), and Midwest (8.2-10.7%) regions of the United States from 2012 to 2019 (Fig. 4); prevalence remained relatively stable in the Western region (11.1% in 2012 vs 11.9% in 2019).
Figure 2.
Proportion of patients with hypothyroidism from 2012 to 2019: Estimates from the Optum administrative claims database.
Figure 3.
Proportion of patients with hypothyroidism by age from 2012 to 2019: Estimates from the Optum administrative claims database.
Figure 4.
Proportion of patients with hypothyroidism in the United States by geographic region from 2012 to 2019: Estimates from the Optum administrative claims database.
Thyroid Hormone Replacement Formulations Use Among Patients with HT
Among NHANES participants, 6.1% (n = 15 507 465) were prescribed levothyroxine (T4 replacement) and 0.1% (n = 235 472) were prescribed liothyronine (T3 replacement) (data not shown).
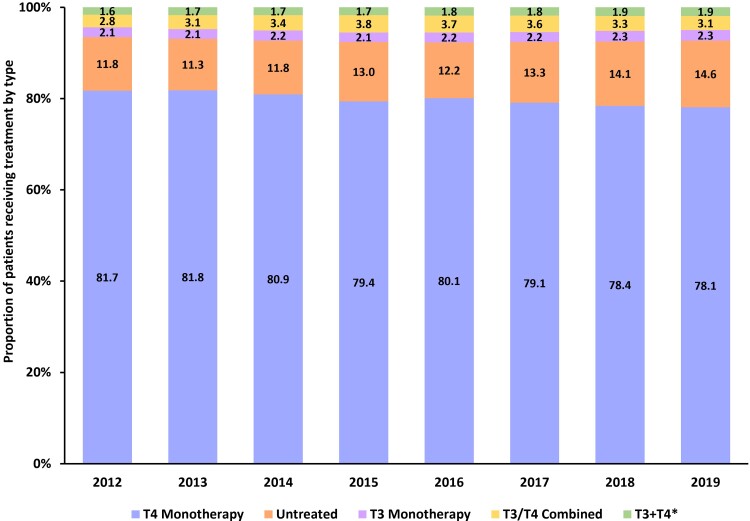
From 2012 to 2019, the majority of patients with HT (>78%) in the Optum administrative claims database had pharmacy claims for T4 replacement monotherapy, while the proportion of patients receiving a T3 replacement therapy (approximately 2.1%) or combination T3 and T4 replacement therapies (approximately 3.4%) remained consistent throughout the study period (Fig. 5). The proportion of patients with HT who were not receiving treatment appeared to increase from 11.8% in 2012 to 14.4% in 2019 (Fig. 5).
Figure 5.
Proportion of hypothyroid drugs used by class from 2012 to 2019: Estimates from the Optum administrative claims database. *Patients had a fill for both a T3 therapy and a separate T4 therapy within the same year. Abbreviations: T3, triiodothyronine; T4, thyroxine.
Discussion
This study determined, through 2 methods, that prevalence of HT in the United States in 2012 was approximately 9.5% (Figs. 1 and 2). According to medical/pharmacy claims data, this value rose from 9.8% in 2013 to 11.7% in 2019. Based on these data, prevalence estimates of HT in the United States have more than doubled since the last systematic evaluation [12]. This study also demonstrated that T4 replacement therapy dominates prescribed treatment for HT in the United States with nearly 80% of patients receiving T4 monotherapy.
Even though a number of publications have shown different HT prevalence rates, the most cited has been the 2002 publication of NHANES data from 1988 to 1994, which estimated that 4.6% of the US population suffered from HT, with 0.3% diagnosed with overt HT [12]. Based on laboratory data from the NHANES 2009-2010 and 2011-2012 cycles, and stratified using TSH >4.5 mIU/L, this study estimated overt HT prevalence to be 2.0%—vastly greater than previous estimates. It is important to note that the 2002 NHANES study estimated HT prevalence based on laboratory values, regardless of treatment status [12]. As such, patients who were receiving treatment and had normalized laboratory values were not included, and thus the prevalence findings may have been an underestimation. Using the same parameters, this study also estimated that 0.9% of the US population had untreated subclinical HT. In this study, patients with subclinical HT laboratory values who were receiving thyroid hormone replacement therapy were classified as overt HT and thus may explain why our estimate is considerably lower than previous NHANES estimates of 4.3% [13]. However, this study consistently demonstrated, using NHANES data, that overall HT prevalence had more than doubled by 2012 (4.6% vs 9.6%). Moreover, using administrative claims data, this study also demonstrated that overall prevalence has continued to rise (11.7% in 2019). There were no changes to clinical practice guidelines regarding HT that may account for these increases, suggesting more research is needed to identify the underlying drivers of the prevalence increment. Interestingly, prevalence among younger patients (<50 years) remained low and stable (2–7%) from 2012 to 2019 (Fig. 3). Overall prevalence increases to 10% to 11% in patients ≥50 years and further to 16% to 18% in patients ≥60 years old. Increasing prevalence of HT with increasing age is in line with previous reports and highlights the importance of routine screening in older patients [1, 2, 12]. This study also showed interesting trends in regional differences in HT prevalence. The cause of these trends cannot be elucidated from our dataset; however, it is possible that differences in population demographics, such as age, or iodine status may play a role. Indeed, other studies have provided conflicting reports on the association between iodine status and thyroid abnormalities [14, 15], thus further study on this phenomenon is warranted.
While there is ongoing discussion about how, if at all, to treat patients with subclinical HT, this study demonstrated that 14.4% of patients diagnosed with HT (overt or subclinical) in 2019 remained untreated. HT has been linked to several other chronic diseases, and it is possible that untreated HT may contribute to increased morbidity. According to the US Department of Health and Human Services’ National Diabetes Statistics Report, approximately 13% of all US adults have diabetes mellitus (DM), with 21.4% unaware of their disease [16]. Moreover, prevalence of DM has steadily increased since 1999 when prevalence was estimated at 9.5% of adults in the United States [16]. Prevalence of subclinical HT in patients with type 2 DM approaches 20% [17]. Both type 1 and type 2 DM have been associated, and commonly coexist, with HT [17, 18]. Type 1 diabetes, an autoimmune disease, is associated with autoimmune thyroid disease given the overlapping pathology of each disease. Type 2 DM and HT have similar symptoms that may mask the presence of the other. The hepatic glucose transporter type 2 gene, an enzyme necessary for insulin-mediated glucose transport, has been shown to be downregulated in HT, resulting in reduced insulin sensitivity [18, 19].
Lipid metabolism has also been shown to be affected by alterations in thyroid hormone levels. Thyroid hormones induce enzymes necessary for cholesterol biosynthesis and regulate receptors necessary for metabolism of lipoproteins. In patients with HT, biosynthesis of new cholesterol is downregulated, yet activity of low-density lipoprotein receptors is also reduced, resulting in increased levels of low-density lipoprotein and total cholesterol; some studies have also shown increased triglyceride and high-density lipoprotein (HDL) levels [20, 21]. In 2016, it was estimated that 12.4% and 18.4% of adults (≥20 years) had high total cholesterol and low high-density lipoprotein levels, respectively—both of which are risk factors for CVD [22]. These values are marked declines from those first reported in 2000 (18.3% and 22.2%, respectively), most likely due to advancements in therapies (ie, statins) [22, 23]. It is important to note that over half of patients diagnosed with hyperlipidemia are receiving treatment [23, 24].
The link between HT and poor cardiovascular outcomes has long been established. Thyroid hormones have a substantial effect on cardiovascular function, and there is evidence that long-term alteration in thyroid hormones produces lasting effects on cardiovascular health [25]. Namely, HT results in decreased cardiac output, impaired relaxation of vascular smooth muscle, and increased arterial stiffness and systemic vascular resistance. Altogether, these effects impact cardiac function and promote heart failure. These changes also lead to increased sodium-sensitive diastolic blood pressure, which puts more stress on cardiac tissue [19, 26]. Moreover, thyroid hormones play a role in cardiac electrophysiology, and HT has been associated with cardiac arrhythmias, especially Torsades de pointes and atrial fibrillation [19, 25]. Like HT, prevalence of CVD (including coronary heart disease, heart failure, and stroke but excluding hypertension) was 9.3% (approximately 26.1 million people) in 2018 [27]; this is a notable increase from statistics published in 2015, which estimated 15.5 million adults in the United States had CVD. Likewise, in 2018, hypertension was estimated to affect 45.4% of adults in the United States, an increase from 41.7% in 2014 [28]. Thus, the increasing prevalence of HT coincides with increases in the prevalence of risks for CVD.
The prevalence of concurrent DM, hypertension, and hyperlipidemia increased from 3.0% in 2000 to 6.3% in 2012 [29]. While the data presented here are not causal evidence, the argument can be made that, given the underlying disease mechanisms and biochemical roles of thyroid hormones in homeostatic function, increasing prevalence of HT may play a role in the increasing prevalence of several other diseases. However, studies specifically on metabolic syndrome, which encompasses all 3 diseases, are lacking, and more research is needed to establish a link between HT and metabolic syndrome [30].
A strength of this study is the use of 2 large, geographically diverse datasets that provide a good representation of the US population. The NHANES dataset is robust data from interviews and physical examinations of participants to consistently estimate vital and health statistics of the general US population. The methodology to characterize HT within the NHANES dataset in this study allowed for inclusion of the successfully treated HT population (with laboratory values in normal range) in the overall HT estimate, whereas the NHANES III estimates relied solely on laboratory values to determine HT status, both clinical and subclinical [12]. The retrospective claims database had a large sample, allowing for a robust prevalence estimate. Year-after-year estimates allow for the examination of trends in prevalence. Claims-based analysis allows for the examination of diagnosed prevalence, which reduces the risk of overestimating the rate of HT. Additionally, we examined the actual prescription or medication fills to determine the rate and type of treatment patients received.
There are general limitations inherent to retrospective study designs and in the secondary use of data. This study used cross-sectional, retrospective data and cannot determine causality for HT. While associations can be observed regarding comorbidities with treated or untreated HT, temporality cannot be determined as related to development of HT. Likewise, using cross-sectional data does not allow for retesting for overt HT or subclinical HT, which is important for confirming a diagnosis of HT. NHANES data that contain the thyroid panel, while ideal in estimating US prevalence of treated and untreated HT, are somewhat dated. Current cycles of NHANES do not collect thyroid panels, and it is unknown if these panels will be collected in subsequent cycles. Likewise, data obtained from patient surveys rely on patient perspectives and memory and are, as a result, subject to recall bias, which could affect survey outcomes [31]. Because patients can be included in the study by either evidence of medication use or lab results and diagnosis rates were estimated by evidence of treatment with thyroid medication, the diagnosis rate may be slightly inflated and overestimate the actual prevalence of HT due to the utilization of desiccated therapies for off-label uses, such as weight loss, as well as the inability to separate patients with overt HT receiving treatment from those with subclinical HT receiving treatment. Moreover, data regarding thyroid function tests prior to the start of replacement therapy was not available, thus it is possible that patients with normal TSH/FT4 levels but prescribed therapy are included in and may inflate the estimation of the prevalence of overt HT. Prevalence of subclinical HT may be underestimated in this sample since any patient receiving HT treatment was classified as overt HT. For the Optum administrative claims data, which includes both private (ie, commercial) and public (ie, government-provided) insurance, it is possible that there is a delay in filing medical claims by the providers. Exposures and outcomes of interest are only captured if a patient has an interaction with the healthcare system. Due to the open nature of the claims data used in this study, continuous enrollment is approximated by patterns of encounters for individual patients during the study period. Filled prescriptions are only proxies for actual consumption; it is assumed the patients took their medication as directed. The commercial claims database may not be representative of the general HT population and may underestimate overall HT prevalence.
Conclusions
Since the last systematic evaluation, overall prevalence (including overt and subclinical HT) has more than doubled in the United States based on NHANES and medical claims data. The prevalence of overt HT in the United States has significantly increased over the past 2 decades and, as of 2019, has continued to steadily rise, affecting around 30 million people age 18+ in the United States based on prevalence estimates from this analysis projected onto the 2019 US population [32]. The cause/causes of this prevalence rate increment have not yet been assessed but may point to a more conservative wait-and-see approach to treating asymptomatic or subclinical HT patients. These data demonstrated that T4 monotherapy has remained the standard of care for HT and maintain that screening for HT continues to be an important aspect of routine care, particularly in the elderly where prevalence is highest. Given the impact subclinical HT may have on several other diseases, further research regarding treatment of patients with subclinical HT is important to further assess the need to mitigate morbidity risk among the US population.
Acknowledgments
The authors would like to thank Varinder Singh for their contribution to statistical coding of the NHANES analyses. Medical writing services were provided by Samantha D. Francis Stuart, PhD, of Fishawack Facilitate Ltd., part of Fishawack Health, and funded by AbbVie.
Contributor Information
Kathleen L Wyne, Division of Endocrinology, Diabetes, and Metabolism, The Ohio State University Wexner Medical Center, Columbus, OH, USA.
Lekshmi Nair, Division of Endocrinology, Department of Internal Medicine, Diabetes, and Metabolism, Wexner Medical Center, The Ohio State University, Columbus, OH, USA.
Chris P Schneiderman, AbbVie, Inc., Chicago, IL, USA.
Brett Pinsky, AbbVie, Inc., Chicago, IL, USA.
Oscar Antunez Flores, AbbVie, Inc., Chicago, IL, USA.
Dianlin Guo, AbbVie, Inc., Chicago, IL, USA.
Bruce Barger, AbbVie, Inc., Chicago, IL, USA.
Alexander H Tessnow, Email: alex.tessnow@utsouthwestern.edu, Division of Endocrinology, Diabetes, and Metabolism, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Author Contributions
All authors participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. All authors had access to the data results, and participated in the development, review, and approval of this manuscript. No honoraria or payments were made for authorship.
Funding
This work/study was funded by AbbVie Inc. AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. All authors had access to the data results and participated in the development, review, and approval of this abstract. No honoraria or payments were made for authorship.
Disclosure
C.P.S., B.P., O.A.F., D.G., and B.B. are full-time employees of AbbVie and own AbbVie stock and/or stock options. K.L.W. has no disclosures to report. L.N. has no disclosures to report. A.H.T. has received consultant fees from Spectrix Therapeutics and Arbor Pharmaceuticals and has served on the advisory board of Horizon Pharmaceuticals.
Data Availability
Data from the NHANES is publicly available through the National Center for Health Statistics (https://wwwn.cdc.gov/nchs/nhanes/). Data from the Optum database portion of this study contains proprietary elements owned by Optum and, therefore, cannot be broadly disclosed or made publicly available. This data can, however, be made available to a third party via the corresponding author upon reasonable request assuming certain data security and privacy protocols are in place and that the third party has executed Optum's standard license agreement, which includes restrictive covenants governing the use of the data.
References
- 1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550–1562. Doi: 10.1016/S0140-6736(17)30703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calissendorff J, Falhammar H. To treat or not to treat subclinical hypothyroidism, what is the evidence? Medicina (Kaunas). 2020;56(1):40. Doi: 10.3390/medicina56010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patil N, Rehman A, Jialal I. Hypothyroidism. Accessed August 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK519536/
- 4. Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf). 1995;43(1):55–68. Doi: 10.1111/j.1365-2265.1995.tb01894.x [DOI] [PubMed] [Google Scholar]
- 5. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215–228. Doi: 10.1159/000356507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim MI. Hypothyroidism in older adults. Accessed August 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK279005/
- 7. Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J Clin Endocrinol Metab. 2005;90(2):805–812. Doi: 10.1210/jc.2004-1672 [DOI] [PubMed] [Google Scholar]
- 8. Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006;91(7):2592–2599. Doi: 10.1210/jc.2006-0448 [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Healthcare and Excellence . Thyroid disease: assessment and management. NICE Guideline.2019. [PubMed] [Google Scholar]
- 10. Garber JR, Cobin RH, Gharib H, . Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18(6):988–1028. Doi: 10.4158/EP12280.GL [DOI] [PubMed] [Google Scholar]
- 11. McAninch EA, Bianco AC. The history and future of treatment of hypothyroidism. Ann Intern Med. 2016;164(1):50–56. Doi: 10.7326/M15-1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):11. Doi: 10.1210/jcem.87.2.8182 [DOI] [PubMed] [Google Scholar]
- 13. National Health and Nutrition Examination Survey . Module 3: Weighting. Accessed August 4, 2021. https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx
- 14. Meisinger C, Ittermann T, Wallaschofski H, et al. Geographic variations in the frequency of thyroid disorders and thyroid peroxidase antibodies in persons without former thyroid disease within Germany. Eur J Endocrinol. 2012;167(3):363–371. Doi: 10.1530/EJE-12-0111 [DOI] [PubMed] [Google Scholar]
- 15. Wang X, Mo Z, Mao G, et al. Geographical influences on thyroid abnormalities in adult population from iodine-replete regions: a cross-sectional study. Sci Rep. 2021;11(1):994. Doi: 10.1038/s41598-020-80248-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 17. Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res. 2013;2013:390534. Doi: 10.1155/2013/390534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalra S, Aggarwal S, Khandelwal D. Thyroid dysfunction and type 2 diabetes mellitus: screening strategies and implications for management. Diabetes Ther. 2019;10(6):2035–2044. Doi: 10.1007/s13300-019-00700-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Udovcic M, Herrera Pena R, Patham B, Tabatabai L, Kansara A. Hypothyroidism in the heart. Methodist Debakey Cardiovasc J. 2017;13(2):55–59. Doi: 10.14797/mdcj-13-2-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duntas LH, Brenta G. A renewed focus on the association between thyroid hormones and lipid metabolism. Front Endocrinol (Lausanne). 2018;9:511. Doi: 10.3389/fendo.2018.00511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rizos CP, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J. 2011;5:76–84. Doi: 10.2174/1874192401105010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carroll MD, Fryar CD, Nguyen DT. High Total and Low High-Density Lipoprotein Cholesterol in Adults: United States, 2015-2016. NCHS Data Brief No 290. Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 23. Mercado C, DeSimone AK, Odom E, Gillespie C, Ayala C, Loustalot F. Prevalence of cholesterol treatment eligibility and medication use among adults—United States, 2005–2012. MMWR. 2015;64(47):1305–1311. Doi: 10.15585/mmwr.mm6447a1 [DOI] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. High cholesterol facts . Accessed August 5, 2021. https://www.cdc.gov/cholesterol/facts.htm
- 25. Cappola AR, Desai AS, Medici M, et al. Thyroid and cardiovascular disease research agenda for enhancing knowledge, prevention, and treatment. Circulation. 2019;139:2892–2909. Doi: 10.1161/CIRCULATIONAHA.118.036859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berta E, Lengyel I, Halmi S, et al. Hypertension in thyroid disorders. Front Endocrinol (Lausanne). 2019;10:482. Doi: 10.3389/fendo.2019.00482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Virani SS, Alonso A, Aparicio HJ, . Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. Doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 28. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018. NCHS Data Brief No 364. Hyattsville, MD: National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
- 29. Song Y, Liu X, Zhu X, et al. Increasing trend of diabetes combined with hypertension or hypercholesterolemia: NHANES data analysis 1999-2012. Sci Rep. 2016;6:36093. Doi: 10.1038/srep36093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwen KA, Schroder E, Brabant G. Thyroid hormones and the metabolic syndrome. Eur Thyroid J. 2013;2(2):83–92. Doi: 10.1159/000351249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stull DE, Leidy NK, Parasuraman B, Chassany O. Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr Med Res Opin. 2009;25(4):929–942. Doi: 10.1185/03007990902774765 [DOI] [PubMed] [Google Scholar]
- 32. US Census Bureau . ACS demographic and housing estimates. Accessed January 4, 2022. https://data.census.gov/cedsci/table?q=DP05#
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the NHANES is publicly available through the National Center for Health Statistics (https://wwwn.cdc.gov/nchs/nhanes/). Data from the Optum database portion of this study contains proprietary elements owned by Optum and, therefore, cannot be broadly disclosed or made publicly available. This data can, however, be made available to a third party via the corresponding author upon reasonable request assuming certain data security and privacy protocols are in place and that the third party has executed Optum's standard license agreement, which includes restrictive covenants governing the use of the data.
References
- 1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550–1562. Doi: 10.1016/S0140-6736(17)30703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calissendorff J, Falhammar H. To treat or not to treat subclinical hypothyroidism, what is the evidence? Medicina (Kaunas). 2020;56(1):40. Doi: 10.3390/medicina56010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patil N, Rehman A, Jialal I. Hypothyroidism. Accessed August 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK519536/
- 4. Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf). 1995;43(1):55–68. Doi: 10.1111/j.1365-2265.1995.tb01894.x [DOI] [PubMed] [Google Scholar]
- 5. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215–228. Doi: 10.1159/000356507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim MI. Hypothyroidism in older adults. Accessed August 4, 2021. https://www.ncbi.nlm.nih.gov/books/NBK279005/
- 7. Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J Clin Endocrinol Metab. 2005;90(2):805–812. Doi: 10.1210/jc.2004-1672 [DOI] [PubMed] [Google Scholar]
- 8. Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006;91(7):2592–2599. Doi: 10.1210/jc.2006-0448 [DOI] [PubMed] [Google Scholar]
- 9. National Institute for Healthcare and Excellence . Thyroid disease: assessment and management. NICE Guideline.2019. [PubMed] [Google Scholar]
- 10. Garber JR, Cobin RH, Gharib H, . Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18(6):988–1028. Doi: 10.4158/EP12280.GL [DOI] [PubMed] [Google Scholar]
- 11. McAninch EA, Bianco AC. The history and future of treatment of hypothyroidism. Ann Intern Med. 2016;164(1):50–56. Doi: 10.7326/M15-1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):11. Doi: 10.1210/jcem.87.2.8182 [DOI] [PubMed] [Google Scholar]
- 13. National Health and Nutrition Examination Survey . Module 3: Weighting. Accessed August 4, 2021. https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx
- 14. Meisinger C, Ittermann T, Wallaschofski H, et al. Geographic variations in the frequency of thyroid disorders and thyroid peroxidase antibodies in persons without former thyroid disease within Germany. Eur J Endocrinol. 2012;167(3):363–371. Doi: 10.1530/EJE-12-0111 [DOI] [PubMed] [Google Scholar]
- 15. Wang X, Mo Z, Mao G, et al. Geographical influences on thyroid abnormalities in adult population from iodine-replete regions: a cross-sectional study. Sci Rep. 2021;11(1):994. Doi: 10.1038/s41598-020-80248-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 17. Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res. 2013;2013:390534. Doi: 10.1155/2013/390534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalra S, Aggarwal S, Khandelwal D. Thyroid dysfunction and type 2 diabetes mellitus: screening strategies and implications for management. Diabetes Ther. 2019;10(6):2035–2044. Doi: 10.1007/s13300-019-00700-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Udovcic M, Herrera Pena R, Patham B, Tabatabai L, Kansara A. Hypothyroidism in the heart. Methodist Debakey Cardiovasc J. 2017;13(2):55–59. Doi: 10.14797/mdcj-13-2-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duntas LH, Brenta G. A renewed focus on the association between thyroid hormones and lipid metabolism. Front Endocrinol (Lausanne). 2018;9:511. Doi: 10.3389/fendo.2018.00511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rizos CP, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J. 2011;5:76–84. Doi: 10.2174/1874192401105010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carroll MD, Fryar CD, Nguyen DT. High Total and Low High-Density Lipoprotein Cholesterol in Adults: United States, 2015-2016. NCHS Data Brief No 290. Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 23. Mercado C, DeSimone AK, Odom E, Gillespie C, Ayala C, Loustalot F. Prevalence of cholesterol treatment eligibility and medication use among adults—United States, 2005–2012. MMWR. 2015;64(47):1305–1311. Doi: 10.15585/mmwr.mm6447a1 [DOI] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention. High cholesterol facts . Accessed August 5, 2021. https://www.cdc.gov/cholesterol/facts.htm
- 25. Cappola AR, Desai AS, Medici M, et al. Thyroid and cardiovascular disease research agenda for enhancing knowledge, prevention, and treatment. Circulation. 2019;139:2892–2909. Doi: 10.1161/CIRCULATIONAHA.118.036859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berta E, Lengyel I, Halmi S, et al. Hypertension in thyroid disorders. Front Endocrinol (Lausanne). 2019;10:482. Doi: 10.3389/fendo.2019.00482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Virani SS, Alonso A, Aparicio HJ, . Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. Doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 28. Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018. NCHS Data Brief No 364. Hyattsville, MD: National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
- 29. Song Y, Liu X, Zhu X, et al. Increasing trend of diabetes combined with hypertension or hypercholesterolemia: NHANES data analysis 1999-2012. Sci Rep. 2016;6:36093. Doi: 10.1038/srep36093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwen KA, Schroder E, Brabant G. Thyroid hormones and the metabolic syndrome. Eur Thyroid J. 2013;2(2):83–92. Doi: 10.1159/000351249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stull DE, Leidy NK, Parasuraman B, Chassany O. Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr Med Res Opin. 2009;25(4):929–942. Doi: 10.1185/03007990902774765 [DOI] [PubMed] [Google Scholar]
- 32. US Census Bureau . ACS demographic and housing estimates. Accessed January 4, 2022. https://data.census.gov/cedsci/table?q=DP05#