Manganese (Mn) is an essential plant micronutrient, and Mn deficiency can be a major limiting factor for crop yield, particularly in dry, well-aerated, and alkaline soils. For example, Mn deficiency is prevalent in Texas, Northern Europe, China, and large areas in southern Australia. In addition, Mn deficiency frequently occurs in combination with other micro(nutrient) deficiencies, such as iron (Fe), making it often challenging to identify among the array of other deficiency symptoms (Broadley et al., 2012; Alejandro et al., 2020). Yet, ensuring optimal Mn nutrition is pivotal for plant growth, development, and reproduction.
The best-known role of Mn in plants is in the oxygen-evolving complex of photosystem II, where it is part of the Mn4Ca cluster that oxidizes and subsequently splits water molecules. One of the first Mn-deficiency symptoms in many plants is therefore leaf chlorosis (Broadley et al., 2012; Alejandro et al., 2020). Apart from its role in photosynthesis, Mn ions (Mn2+) are essential cofactors for different enzymes, including the Mn2+-dependent glycosyltransferases that synthesize cell wall precursors. These enzymes are Golgi-resident proteins that produce, among others, pectin precursors, which are then delivered to the apoplast. The Golgi localization of the Mn2+-dependent glycosyltransferases requires Mn2+ import into the lumen of this endomembrane compartment. A Mn2+-permeable ATPase called ER localised Calcium pump (ECA3) has been previously identified as a candidate for Mn2+ import into the Golgi lumen, and Arabidopsis (Arabidopsis thaliana) eca3 knockout plants show severe chlorosis under Mn2+ deficiency (Mills et al., 2008). This suggests that chloroplasts might be primarily affected in eca3 plants, hinting at the complexity of endomembrane Mn2+ transport and allocation.
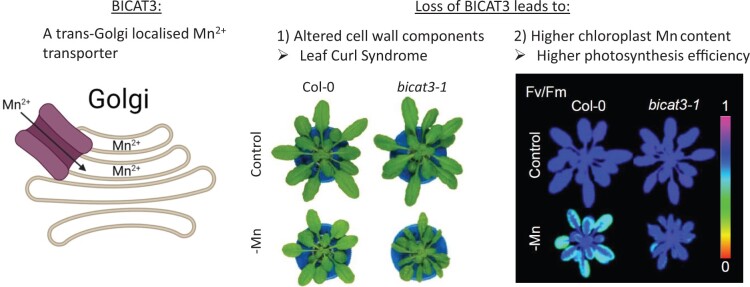
In this issue of Plant Physiology, (He et al. (2022), characterized the Arabidopsis Bivalent Cation Transporter 3 (BICAT3) as a principal manganese transporter localized specifically in trans-Golgi cisternae (Figure 1). They showed that BICAT3 is ubiquitously expressed in both areal and nonareal tissues, and that its function is crucial for pollen tube growth and for plant performance under Mn2+ deficiency, confirming and expanding on previous work (Hoecker et al., 2020; Zhang et al., 2021). Under control conditions, bicat3 knockout plants developed similarly to wild-type plants during vegetative growth, but bicat3 siliques were shorter. Semi in vivo pollen tube growth assays using excised pistils demonstrated that the bicat3 male gametophyte is defective, and pollen tubes showed a strong growth defect.
Figure 1.
The BICAT3 can transport Mn2+ and is localized to trans-Golgi cisternae. Loss of BICAT3 impacts cell wall matrix polysaccharide biosynthesis in the Golgi and leads to defective cell wall component linkage and composition, resulting in Leaf Curl Syndrome. Additionally, bicat3 plants take up more Mn2+, which is allocated to chloroplasts, improving photosynthesis. Partly created using biorender, adapted from He et al. (2022).
While vegetative growth was similar to wild-type under control conditions, plants without BICAT3 developed severe phenotypic alterations under Mn2+ deficiency. Monosaccharaide analysis and sugar linkage determination revealed that bicat3 plants had substantial modifications of cell wall matrix components compared to the wild-type. These cell wall alterations had developmental consequences, and bicat3 plants displayed strong anatomical deformities under low Mn2+ conditions, such as Leaf Curl Syndrome.
Intuitively, one might assume that the phenotype of bicat3 plants is connected to lower Mn2+ content in plants. Yet, changes in endomembrane ion transport often lead to surprising and unexpected downstream effects, and connecting transporter function to phenotype is often challenging (David et al., 2019). The phenotype of bicat3 plants is no exception.
He et al. (2022) found that the loss of BICAT3 led to higher Mn2+ concentration in shoots under Mn deficiency, and photosynthesis was improved in bicat3. Mn2+ analysis of isolated chloroplasts revealed higher chloroplast Mn2+ content, strongly suggesting that this is the reason for the higher overall shoot Mn2+ concentration. The higher overall Mn2+ concentration in bicat3 indicates that bicat3 plants are more efficient in Mn2+ uptake from media with very low Mn2+ concentrations and that the increase in cellular Mn2+ is channeled toward the chloroplasts. This observation might suggest that BICAT3 is involved in or upstream of signaling events connected to plasma membrane Mn2+ uptake.
The improved photosynthesis and higher Mn2+ concentration might sound positive at first glance, yet, bicat3 plants showed a reduction in cell size due to cell wall defects. These cell wall defects in bicat3 are likely the result of reduced glycosyltransferase activity in the Golgi and suggest that other Mn2+-transport proteins, like ECA3, cannot (fully) replace BICAT3 function. Surprisingly, comparing bicat3 and eca3 plants, He et al. 2022 found that photosynthesis was improved in eca3 in their hydroponic experimental set-up, albeit less pronounced than in bicat3. This contrasts with previous findings using in vitro grown seedlings that show severely reduced chlorophyll content in eca3 (Mills et al., 2008), suggesting decreased photosynthesis. The different observations demonstrate the complexity of ion transport and the importance of investigating plants using a diverse set of growth conditions.
Several very interesting questions arise from the characterization of bicat3 plants. One could speculate that loss of Golgi Mn2+ transport might lead to a signaling mechanism in the Golgi, which detects insufficient Mn2+ or activity alterations dependent on Mn2+ processes. Through yet unknown mechanisms, this signal seems to result in increased (capacity of?) cellular Mn2+ uptake at the plasma membrane under Mn2+-deficient conditions, and bicat3 plants contain more Mn2+ compared to the wild-type. Communication between ion transport at endomembranes and the plasma membrane has been observed for other ions, and research in this area is a field that is rapidly developing (Horaruang et al., 2020).
One could further speculate that in bicat3 plants, which are unable to sufficiently import the now increased cytosolic Mn2+ into the Golgi, Mn2+ is then alternatively channeled into the chloroplasts, improving photosynthesis. This is speculative, and there are other plausible interpretations of the observed phenotype of bicat3. Yet, the higher overall Mn2+ concentration in bicat3 plants demonstrates that cellular processes to increase Mn2+ are present in plants and more Mn2+ can be allocated to chloroplasts under Mn deficiency. It is yet unknown why wild-type Arabidopsis plants do not increase Mn2+ uptake under Mn deficiency. If we could identify the signaling mechanism for cross-talk between Golgi Mn2+ uptake and plasma membrane Mn2+ uptake without reducing Golgi Mn2+ content and disrupting cell wall biosynthesis, we might be able to generate plants that grow more efficiently in soils with low Mn2+. This would be a substantial advantage in agriculture that could lead to reduced yield losses and fertilizer usage. Expanding our knowledge on the complexity and cross-talk of ion uptake and cellular ion compartmentation is therefore an important step in this direction.
Conflict of interest statement. There is no conflict of interest.
References
- Alejandro S, Höller S, Meier B, Peiter E (2020) Manganese in plants: from acquisition to subcellular allocation. Front Plant Sci 11: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F (2012) Function of nutrients: micronutrients. InMarschner P, ed, Marschner’s Mineral Nutrition of Higher Plants. Academic Press, London, pp 191–248 [Google Scholar]
- David R, Byrt CS, Tyerman SD, Gilliham M, Wege S (2019) Roles of membrane transporters: connecting the dots from sequence to phenotype. Ann Bot 124: 201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Yang B, Hause G, Rössner N, Peiter-Volk T, Schattat MH, Voiniciuc C, Peiter E (2022) The trans-Golgi-localized protein BICAT3 regulates manganese allocation and matrix polysaccharide biosynthesis. Plant Physiol 190: 2579–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoecker N, Honke A, Frey K, Leister D, Schneider A (2020) Homologous proteins of the manganese transporter PAM71 are localized in the golgi apparatus and endoplasmic reticulum. Plants 9: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horaruang W, Hills A, Blatt MR (2020) Communication between the plasma membrane and tonoplast is an emergent property of ion transport1 [OPEN ]. Plant Physiol 182: 1833–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RF, Doherty ML, López-Marqués RL, Weimar T, Dupree P, Palmgren MG, Pittman JK, Williams LE (2008) ECA3, a Golgi-Localized P2A-Type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol 146: 116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zhang C, Liu C, Fu A, Luan S (2021) A Golgi-localized manganese transporter functions in pollen tube tip growth to control male fertility in Arabidopsis. Plant Commun 2: 100178. [DOI] [PMC free article] [PubMed] [Google Scholar]