Abstract
Homologous recombination-mediated gene targeting (GT) enables precise sequence knockin or sequence replacement, and thus is a powerful tool for heritable precision genome engineering. We recently established a clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeats-associated protein 9 (CRISPR/Cas9)-mediated approach for heritable GT in Arabidopsis (Arabidopsis thaliana), but its broad utility was not tested, and the underlying molecular mechanism was unclear. Here, we achieved precise GT at 14 out of 27 tested endogenous target loci using the sequential transformation approach and obtained vector-free GT plants by backcrossing. Thus, the sequential transformation GT method provides a broadly applicable technology for precise genome manipulation. We show that our approach generates heritable GT in the egg cell or early embryo of T1 Arabidopsis plants. Analysis of imprecise GT events suggested that single-stranded transfer DNA (T-DNA)/VirD2 complexes produced during the Agrobacterium (Agrobacterium tumefaciens) transformation process may serve as the donor templates for homologous recombination-mediated repair in the GT process. This study provides new insights into the molecular mechanisms of CRISPR/Cas9-mediated GT in Arabidopsis.
The sequential transformation gene targeting method provides a broadly applicable technology for precise genome manipulation in Arabidopsis.
Introduction
Genome engineering is a powerful tool for plant research and promises to have multiple biotechnological applications in agriculture in the near future. Homologous recombination (HR)-mediated gene targeting (GT) is widely used in many organisms, including mammals (Thomas and Capecchi, 1987) (Chandrasegaran and Carroll, 2016), although low HR frequencies have limited its use in seed plants (Fauser et al., 2012). We recently described an efficient (5.3%–9.1%) and precise homology-directed repair (HDR)-mediated method for GT in A. thaliana (Arabidopsis) (Miki et al., 2018). Our method employed sequential transformation to express a single-guide RNA (sgRNA) and donor template into transgenic parental plants stably expressing the clustered regularly interspaced short palindromic repeats-associated protein 9 (Cas9) nuclease in egg cells and the early embryo (Miki et al., 2018). These Cas9 parental lines have been donated to the Arabidopsis Biological Resource Center (ABRC, stock numbers CS69955 and CS69956). Success in HDR-mediated GT using sequence-specific nucleases was recently reported in some plant species (Miki et al., 2021), such as Arabidopsis (Permyakova et al., 2021; van Tol et al., 2022), Nicotiana tabacum (Nishizawa-Yokoi et al., 2020), Nicotiana benthamiana (Hsu et al., 2021; Eini et al., 2022), Solanum lycopersicum (Vu et al., 2021), Oryza sativa (Nishizawa-Yokoi et al., 2020), Zea mays (Gao et al., 2020; Peterson et al., 2021), Triticum aestivum (Čermák et al., 2017), Hordeum vulgare (Lawrenson et al., 2021), Brassica oleracea (Hsu et al., 2021), and Saccharum spp. (Oz et al., 2021). However, most of these methods relied on the selection for antibiotic markers or herbicide resistance genes at the target loci. Unlike these other approaches, our method does not require a selection marker at the GT locus and is potentially applicable to any genomic locus. Nevertheless, the molecular mechanisms underpinning our method are still unclear, particularly the timing at which GT is established and the nature of the donor repair template for HDR.
Here, we show that our sequential transformation method can establish GT in the T1 generation in Arabidopsis. We attempted to generate knockin and base substitutions in 27 endogenous loci and obtained precise GT at 14 of them. In addition to precise GT events, we observed imprecise GT events in several target sites. Analysis of the imprecise GT events suggests that single-stranded transfer DNA (T-DNA) is a potential donor template for HDR. Importantly, we show that the sequential transformation strategy can yield vector-free GT Arabidopsis plants after backcrossing.
Results
Precise and imprecise heritable GT is achieved in the T1 generation in Arabidopsis
The parental plants of our sequential transformation GT method stably express a human codon-optimized Streptococcus pyogenes Cas9 (hSpCas9; hereafter Cas9) in egg cells and early embryos (Figure 1A) (Miki et al., 2018). In earlier work, we performed bulk screening of T2 seedlings based on published data showing that the DD45pro::Cas9 system mutation efficiency in Arabidopsis was drastically enhanced in the T2 population compared to the T1 (Mao et al., 2016). Nevertheless, given that the DD45 promoter drives Cas9 expression in the egg/early embryo of the parental plants, we hypothesized that GT could be established in as early as the T1 generation. To test our hypothesis, we created HDR donor/sgRNA constructs to generate ROS1-3xFlag and ROS1-4xMyc GT lines (Figure 1B; Supplemental Figure S1), using a previously described sgRNA targeting repressor of silencing 1 (ROS1) (Miki et al., 2018). The constructs were integrated into the genome of the parental line DD45-#58 (Miki et al., 2018) (ABRC stock CS69955) (here after parental line, and PL in Figures) using the Agrobacterium flower dipping method (Figure 1A).
Figure 1.

ROS1-3xFlag and ROS1-4xMyc GT. A, Outline of the sequential transformation strategy for GT and screening procedure. A parental transgenic line containing a DD45 promoter::Cas9 expression cassette was re-transformed with a binary construct containing donor DNA and sgRNA expression cassettes, using Basta as selectable marker. T1 transgenic lines were selected with Basta, and individual T1 plants were analyzed. The positive lines were used for further experiments. B, Schematic representation of the ROS1-3xFlag and ROS1-4xMyc donor constructs and target endogenous ROS1 locus. The bold horizontal line indicates the probe used for Southern blotting. C, Primer design for GT screening. GT-specific primers are specific to the knockin GT alleles, while external and full-length primers can amplify both endogenous and knockin GT alleles. D and E, Genotyping PCR and Southern blot hybridization for ROS1-3xFlag (D) and ROS1-4xMyc (E) in T1 plants. The amplification product from the full length primer set was digested with ClaI and PacI for ROS1-3xFlag and ROS1-4xMyc, respectively (Supplemental Figure S1). Line numbers for successful GT events are shown at the top. F and G, Genotyping PCR and Southern blot hybridization for ROS1-3xFlag (F) and ROS1-4xMyc (G) T2 plants. H, qChop-PCR at the At1g26400 and At1g03890 loci for Col-0, PL, ROS1-3xFlag lines #71 and #16 and ROS1-4xMyc lines #12 and #23. The error bars indicate standard deviation of Student’s t-test (n = 4). I, Western blot analysis of ROS1-3xFlag T2 seedlings of the two precise knockin lines (#71 and #155). J, ChIP assay of ROS1-3xFlag GT T2 homozygous plant at the At1g26400 and At1g03890 loci. Act7 was used as negative control. The error bars indicate standard deviation of Student’s t-test (n = 4).
Polymerase chain reaction (PCR) and Southern blot analysis of 183 T1 transformants identified a total of 16 GT-positive plants for ROS1-3xFlag (8.7% efficiency) while screening of 70 T1 transformants identified seven GT-positive plants for ROS1-4xMyc (10% efficiency) (Figure 1, D and E and Table 1). Two independent biallelic ROS1-3xFlag GT plants were obtained in the T1 population (Figure 1D, lines #57 and #83 and Table 1). Three primer sets were designed for the PCR screening. GT-specific primers contained a primer within the knockin sequence and another one upstream of the 5′-homology arm, resulting in amplification only in the presence of a knockin allele (Figure 1C; Supplemental Figure S1A). The 5′-external primer set was designed to contain a primer upstream of the 5′-homology arm and another one within the 3′-homology arm sequence, theoretically capable of amplifying both the endogenous and knockin alleles (Figure 1C; Supplemental Figure S1A). Finally, full-length primers were designed to anneal to the upstream and downstream of the homology arms, capable of amplifying endogenous and knockin alleles (Figure 1C; Supplemental Figure S1A). The GT-specific primers produced some false-positive signals that were not detected by the 5′-external primer set (Figure 1, D and E). All GT-positive plants detected by the 5′-external primer were confirmed by Southern blotting of genomic DNA digested with HindIII (Figure 1, D and E; Supplemental Figure S1A). Sanger sequencing of PCR products amplified with the 5′-external primers showed that the 5′-homology arm and the repeat sequence 3xFlag or 4xMyc epitope tags were precisely incorporated into the ROS1 target locus (Supplemental Figure S1, B and C).
Table 1.
Knockin GT efficiencies for the ROS1 locus
| Construct | Transformant | 5′-external primer set |
Full-length primer set |
||
|---|---|---|---|---|---|
| GT Positive (Biallelic) | GT Efficiency (%) | Precise GT | Precise GT Efficiency (%) | ||
| ROS1-3xFlag | 183 | 16 (2) | 8.7 | 2 | 1.09 |
| ROS1-4xMyc | 70 | 7 | 10 | 0 | 0 |
GT efficiency was calculated based on the number of individual T1 transformants examined.
Several reports have described the incidence of imprecise GT events, with one homology arm being replaced in a precise manner, while the second arm showing incorrect incorporation (Wolter et al., 2018; Wolter and Puchta, 2019; Gao et al., 2020; Huang et al., 2021; Peterson et al., 2021. Analysis of the 3′-homology arm region of the knockin events by PCR genotyping with 3′-external primers and full-length primers (Figure 1C; Supplemental Figure S1A) indicated that the majority of knockin events were imprecise. Fourteen of the 16 positive ROS1-3xFlag GT plants, including the two biallelic knockin lines, as well as all 7 positive ROS1-4xMyc tag lines harbored incomplete integration at the 3′-homology arm (Figure 1, D and E). Precise integration was detected in ROS1-3xFlag lines #71 and #155 (Figure 1D; Supplemental Figure S1). All GT events, precise and imprecise, were stably inherited and showed Mendelian segregation in the T2 generation (Figure 1, F and G).
Bulk screening of T2 seedlings failed to detect any new GT events, suggesting that the sequential transformation strategy achieves GT mostly in the T1 generation, probably in egg cells immediately after transformation and/or in early embryos after fertilization of the new transformant.
To determine whether the ROS1 GT plants retained active DNA demethylation activity, we used quantitative Chop-PCR to analyze the DNA methylation status of two genomic loci known to become hypermethylated in loss-of-function ros1 mutants (Qian et al., 2012; Miki et al., 2018). Analysis of two, one precise and one imprecise, T2 homozygous ROS1-3xFlag and two imprecise T2 homozygous ROS1-4xMyc GT plants did not show hypermethylation at these loci (Figure 1H), suggesting that the in-frame knockin of the 3xFlag and 4xMyc tags did not interfere with ROS1 function. Even though three of those lines harbored imprecise GT at the 3′-region, the 3′-homology arm is downstream of the stop codon and the genomic defects did not appear to interfere with ROS1 expression or function in the knockin lines. ROS1-3xFlag protein was detected by western blot analysis in T2 seedlings of the two precise knockin lines (#71 and #155) (Figure 1I), indicating that the knockin ROS1 allele was expressed. To determine whether ROS1-3xFlag localizes properly in the chromatin, we performed chromatin immunoprecipitation (ChIP) with anti-flag antibodies in ROS1-3xFlag precise knockin GT plants. We observed an enrichment of ROS1-3xFlag at two loci that become hypermethylated in ros1 mutants, but not at the negative control locus Actin7 (Figure 1J), suggesting that ROS1-3xFlag has the expected chromatin distribution in the genome.
Wide range of application of GT in Arabidopsis provides valuable tools for functional research in vivo
To test the broad utility and overall efficiency of the sequential transformation method we individually targeted 26 additional genetic loci in Arabidopsis (Supplemental Table S1). We engineered different constructs aiming to incorporate green fluorescence protein (GFP), 3xFlag, mCherry, or Luciferase (Luc) knockins as well as amino acid substitutions using the parental DD45pro::Cas9 (CS69955) transgenic line (Figure 2A). Since the specific primer sets detected some false positives in the ROS1-3xFlag and ROS1-4xMyc screening (Figure 1, C–E), we opted to use 5′-external primer sets in the initial screening for positive GT events in T1 transgenic plants. Precise GT events were detected in 13 of the 26 target loci using the full-length primer set followed by sequencing of the amplicon (Tables 2 and 3). Precise 3xFlag knockin was detected at the nuclear RNA polymerase D 1 (NRPD1), calmodulin-binding transcription activator 3 (CAMTA3), indole-3-acetic acid 7 (IAA7), pyrabactin resistance (PYR1)-like 2 (PYL2), and PYL4 loci, and precise GFP knockin was obtained at the embryo defective 2410 (EMB2410), sold overly sensitive 1 (SOS1), and silent information regulator 2 (SRT2) loci, while precise amino acid substitutions were generated at the domains rearranged methylase 2 (DRM2) (C584A), NRPD1 (D447A), ROS1 (C1054S), and PYL2 (E147L) loci (Tables 2 and 3). The genotyping analyses for the 3xFlag knockins in the NRPD1, CAMTA3, and IAA7 loci in T2 homozygous plants are shown in Figure 2B. These precise knockins were detected by 5′-external and full-length primer sets and confirmed by the Sanger sequencing of the amplicons.
Figure 2.
Characterization of precise knockin and amino acid substitution GT events in the NRPD1, CAMTA3, IAA7, ROS1, and DRM2 endogenous loci. A, Schematic representation of the knockin (left) and base substitution (right) constructs and target genomic locus. The donor constructs contain 800 bp homology arms. B, PCR genotyping of precise 3xFlag knockins into NRPD1, CAMTA3, and IAA7 loci. C, F, and I, PCR genotyping of precise GT amino acid substitution of ROS1-C1054S (C), DRM2-C587A (F), and NRPD1-D447A (I), respectively. D, G, and J, Sequence confirmation of the GT events in ROS1-C1054S (D) DRM2-C587A (G) and NRPD1-D447A (J), respectively. Red letters in the Col-0 sequence indicate the PAM. Intended amino acid substitutions, silent mutations, and not introduced substitutions are indicated by red, blue and green rectangles, respectively. E, H, and K, Chop-PCR analysis in the generated gene targeted plants for ROS1-C1054S (E), DRM2-C587A (H), and NRPD1-D447A (K), respectively. M, marker.
Table 2.
GT efficiencies for 22 Arabidopsis endogenous loci
| Construct | Number of Transformants Analyzed | 5′-Arm GT | Precise GT (Biallelic) | Precise GT Efficiency (%) |
|---|---|---|---|---|
| NRPD1-3xFlag | 97 | 2 | 2 | 2.0 |
| CAMTA3-3xFlag | 155 | 2 | 1 | 0.6 |
| EMB2410-GFP | 378 | 14 | 8 (1) | 2.1 |
| IAA7-3xFlag | 188 | 1 | 1 | 0.53 |
| DRM2-C587A | 185 | 1 | 1 | 0.54 |
| NRPD1-D447A | 208 | 3 | 2 | 0.9 |
| ROS1-C1054S | 244 | 2 | 2 | 0.81 |
| PYL2-E147L | 706 | 2 | 2 | 0.28 |
| ICE1-3xFlag | 712 | 1 | 0 | 0 |
| DRM2-3xFlag | 909 | 2 | 0 | 0 |
| SnRK2.6-3xFlag | 816 | 2 | 0 | 0 |
| ABI1-3xFlag | 581 | 0 | 0 | 0 |
| CBF2-3xFlag | 662 | 0 | 0 | 0 |
| HKT1-mCherry | 163 | 0 | 0 | 0 |
| LRX3-3xFlag | 118 | 0 | 0 | 0 |
| NRPE1-3xFlag | 534 | 0 | 0 | 0 |
| RALFL22-3xFlag | 617 | 0 | 0 | 0 |
| SOS2-3xFlag | 134 | 0 | 0 | 0 |
| At2g35050-3xFlag | 160 | 0 | 0 | 0 |
| Luc-Ago4 | 557 | 0 | 0 | 0 |
| 3xFlag-SUMO1 | 668 | 0 | 0 | 0 |
| NRPE1-D449A | 297 | 0 | 0 | 0 |
GT efficiency was calculated based on the number of individual T1 transformant populations examined.
Table 3.
Precise and imprecise knockin GT efficiencies
|
Construct |
Transformant | 5′-Arm GT | 3′-Arm GT | Precise GT | GT Efficiency |
|---|---|---|---|---|---|
| SOS1-GFP | 384 | 37 | 13 | 6 | 1.5% |
| SRT2-GFP | 977 | 9 | 11 | 2 | 0.2% |
| PYL2-3xFlag | 167 | N.D. | 2 | 1 | 0.6% |
| PYL4-3xFlag | 177 | 3 | N.D. | 1 | 0.5% |
GT efficiency was calculated based on the number of individual T1 transformants examined.
Precise GT is invaluable for in vivo functional studies as shown by three of the GT targets in which we introduced amino acid substitutions in the native proteins. The Fe–S-binding motif, highly conserved in the ROS1 protein family, is required for in vitro activity and contains four proximally located cysteine residues, C1038, C1045, C1048, and C1054 (Mok et al., 2010; Duan et al., 2015). Two independent GT lines containing a substitution of the highly conserved C1045 cysteine with serine (C1054S) were produced, and were confirmed by restriction fragment length polymorphism (RFLP) with a full-length primer set in the T2 generation (Figure 2C) followed by sequencing of the amplicon. In addition to the amino acid substitution, we introduced a silent mutation within the protospacer-adjacent motif (PAM) sequence to prevent sgRNA binding and double-strand break (DSB)-mediated mutations following precise GT (Paquet et al., 2016; Miki et al., 2018) (Figure 2D). Chop-PCR analysis of two loci showing hypermethylation in ros1 mutants revealed similar levels of hypermethylation in T2 ROS1-C1054S GT plants and the ros1-4 SALK mutant (Figure 2E). The results indicate that the highly conserved C1054 is essential for the homolog of yeast MET 18 (MET18)-dependent transfer of the iron–sulfur cluster to the cysteine(s) in the Fe–S motif and is thus critical for ROS1 DNA glycosylase activity in vivo (Duan et al., 2015) (Wang et al., 2016).
DRM2 is required for both the de novo establishment and maintenance of DNA methylation in all sequence contexts in the RNA-directed DNA methylation (RdDM) pathway. To establish whether the highly conserved catalytic C587 cysteine in the C-terminal motif IV is required for the methyltransferase activity of DRM2, we replaced C587 with alanine (Henderson et al., 2010). One precise amino acid substitution line, DRM2-C587A, was identified from 185 T1 plants (Table 2) and T2 homozygous plants were obtained (Figure 2F). Sanger sequencing confirmed that the intended C587A substitution and two additional silent mutations were precisely incorporated (Figure 2G). Hypomethylation was observed at two tested RdDM target loci, AtSN1 and AtMu1, in drm2-2 mutants as well as in the DRM2-C587A GT plants (Figure 2H), indicating that the highly conserved cysteine C587 is required for the methyltransferase activity of DRM2 in vivo (Henderson et al., 2010).
NRPD1 is the largest subunit of the plant-specific RNA polymerase IV (Pol IV), required for the silencing of transposons and other repetitive sequences by the 24-nt small interfering RNA (siRNA)-mediated RdDM pathway. Pol IV acts at the beginning of the RdDM pathway to generate 24-nt siRNAs at the target loci. Complementation studies have identified three aspartic acid residues (D447, 449, and 451) in the Metal A motif of NRPD1 required for siRNA accumulation and DNA methylation (Haag et al., 2009). We aimed to substitute all three residues with alanine (D447A, D449A, and D451A) simultaneously in NRPD1 via GT, and therefore designed a donor template harboring the substitutions as well as a silent mutation introducing a SacII enzyme restriction site to facilitate screening. Three independent T1 GT lines were identified from 208 T1 transformants (Table 2) and homozygous T2 GT plants were produced (Figure 2I). Interestingly, Sanger sequencing revealed that the D447A substitution and SacII site were precisely incorporated but the D449A and D451A substitutions were not generated in any of the GT lines (Figure 2J). Although the two out of three intended amino acid substitutions were not made, both homology arms were correctly incorporated. Therefore, the obtained three NRPD1-D447A lines were classified as precise GT (Table 2). Functional studies were performed using the previously described NRPD1-3xFlag knockin GT lines (Figure 2B), the newly generated NRPD1-D447A substitution lines and the nrpd1-3 mutant. Chop-PCR analysis of two RdDM target loci showed hypomethylation of both loci in the nrpd1-3 mutant and NRPD1-D447A lines but not in the NRPD1-3xFlag lines (Figure 2K). These results show that aspartic acid-447 of the core DFDGD sequence in the Metal A site is essential for the catalytic activity of NRPD1, and that the in-frame addition of a 3xFlag motif does not interfere with the NRPD1 catalytic activity (Figure 2, B and K).
Detailed characterization of imprecise knockin events
In order to add a GFP tag to the SOS1 protein, we designed a donor construct (Figure 3A) and produced 384 independent T1 transgenic lines. External (5′ and 3′) and full-length primer sets were used to screen and genotype the transgenic lines for SOS1-GFP knockin events. The screening detected 37 precise 5′-homology arm GT events, 13 precise 3′-homology arm GT events and 6 precise SOS1-GFP knockin lines (Figure 3B and Table 3). Mendelian inheritance was observed in the T2 progenies and detailed genotyping identified three types of events, precise GT at both ends, precise 5′-arm/imprecise 3′-arm and imprecise 5′-arm/precise 3′-arm (hereafter referred as precise, 5′-precise, and 3′-precise, respectively) (Figure 3, C and D; Table 4). To study the nature of imprecise GT events, Sanger sequencing was conducted for the PCR product of full length and 3′-external primer sets of 5′-precise T2 homozygous line #29 (Figure 3D). Sequence analysis confirmed the presence of a precise knockin at the 5′-homology arm region but revealed unwanted integration of the sequences downstream of GFP in the T-DNA cassette (Figure 3E; Supplemental Figure S2A). Unfortunately, we were unable to obtain PCR products using 5′-external, 3′-external, or full-length primers for other imprecise GT events (Figure 3, B and D) and could not determine the nature of these integrations.
Figure 3.
Characterization of imprecise knockin events in the SOS1, SRT2, PYL2 and PYL4 loci. A, Schematic representation of the SOS1-GFP donor construct and target region of the SOS1 genomic locus. The donor construct contains an in-frame GFP sequence with the endogenous SOS1 gene and 800-bp homology arms. The bold horizontal line indicates the probe used for Southern blotting. B–D, PCR genotyping and Southern blot hybridization of SOS1-GFP T1 plants (B), precise knockin T2 plants (C), and imprecise knockin T2 plants (D). E, Schematic representation of the imprecise knockin SOS1-GFP events. F, Schematic representation of the GFP and 3xFlag knockin donor constructs used for SRT2 and PYL2. G, PCR genotyping of the precise and imprecise SRT2-GFP, PYL2-3xFlag, and PYL4-3xFlag knockin events in the T2 generation. H and I, Schematic representations of the imprecise knockin SRT2-GFP (H) and PYL2-3xFlag (I) events. M, marker.
Table 4.
Segregation ratio of GT events in the T2 progenies
| GT line | Genotype |
||
|---|---|---|---|
| GT Homozygous | GT Heterozygous | Wild Type | |
| (+/+) | (+/−) | (−/−) | |
| SOS1-GFP | |||
| #13 | 9 | 18 | 9 |
| #45 | 12 | 15 | 9 |
| #139 | 9 | 24 | 3 |
| #27 (Imprecise) | 21 | 46 | 27 |
| #137 (Imprecise) | 15 | 51 | 29 |
| #29 (Imprecise) | 18 | 52 | 15 |
| #60 (Imprecise) | 22 | 42 | 25 |
| SRT2-GFP | |||
| #17 | 7 | 8 | 9 |
To study the nature of 3′-precise (i.e. 5′-imprecise) GT events we analyzed two additional GT targets, where we aimed to knockin GFP into the SRT2 locus and 3xFlag into the PYL2 locus (Figure 3F and Table 3). Amplification products were obtained with the 5′-specific or full-length primer sets in T2 SRT2-GFP and PYL2-3xFlag lines (Figure 3G), and Sanger sequencing was performed to determine the structure of the GT in both loci. Similar to the observation in the SOS1-GFP GT line, a partial T-DNA insertion from the donor GFP to the RB sequence was observed in the 5′-homology arm imprecise SRT2-GFP line (Figure 3H; Supplemental Figure S2B). In the case of the PYL2-3xFlag line, the T-DNA insertion continues a 137-bp fragment of the pCambia3301 binary vector (Figure 3I; Supplemental Figure S2C). Combined, the analysis of imprecise knockins for SOS1-GFP, SRT2-GFP, and PYL2-3xFlag lines show the precise integration of one homology arm by the HDR pathway, while the site on the other arm was T-DNA insertion. These results indicate that the single-stranded T-DNA released from the binary vector by Agrobacterium likely provides a repair donor template for GT in our experimental system.
CRISPR-mediated DSB efficiency influences GT frequency
The factors influencing the GT efficiency of our sequential transformation method are unknown. We hypothesized that CRISPR/Cas9-mediated DSB efficiency influences GT efficiency. DSB efficiency in the CRISPR/Cas9 system is mainly determined by the target sequence and we had used the online CRISPOR web resource (http://crispor.tefor.net/crispor.py) to select sgRNAs with high specificity and high predicted efficiency for the GT donor constructs (Concordet and Haeussler, 2018). CRISPOR predicts sgRNA efficiency providing scores from 10 different algorithms and we have summarized GT efficiencies, sgRNA sequences, and predicted CRISPOR-based scores for our targets in Supplemental Table S1. An analysis of this data did not show any correlation between GT efficiency and predicted sgRNA scores. It is nevertheless important to note that according to the CRISPOR instructions, sgRNA efficiency “predictions are not very accurate.”
An alternative method to estimate DSB efficiency is to determine CRISPR/Cas9-mediated mutation frequency; therefore, we studied mutation frequencies in lines with successful GT events and lines with no GT events. For this purpose we pooled purified genomic DNA from 10 randomly selected, independent GT-negative T1 lines, performed PCR to amplify the genomic fragments surrounding the targets and sequenced the amplicons to estimate the non-homologous end joining (NHEJ)-mediated mutation frequencies at the different target loci. The samples from ROS1-3xFlag and SOS1-GFP transgenic populations exhibited high mutation frequency, as indicated by the multiple sequencing peaks observed close to the PAM, even though these lines did not contain GT events (Figure 4). On the other hand, analysis of At2g35050-3xFlag, high-affinity potassium transporter 1 (HKT1)-mCherry, leucine-rich repeat/extensin 3 (LRX3)-3xFlag, and SOS2-3xFlag T1 transformants where we were unable to detect any successful GT event showed almost no CRISPR-generated mutations (Figure 4). We have increased the numbers of analyzed HKT1-mCherry and At2g35050-3xFlag T1 samples to 100 without detecting any mutations. The lower sgRNA efficiencies observed for At2g35050, HKT1, LRX3, and sold overly sensitive 2 (SOS2) compared to ROS1 and SOS1 support our hypothesis that CRISPR/Cas9-mediated DSB activity affects GT establishment when using our sequential transformation method (Gao et al., 2020; Merker et al., 2020).
Figure 4.

DSB frequency in GT-negative T1 plants. To determine DSB frequency of Cas9, mutation efficiency of the target loci were amplified by PCR and analyzed by Sanger sequencing. Tissue from ten GT negative T1 lines from ROS1-3xFlag, SOS1-GFP, At2g35050-3xFlag, HKT1-mCherry, LRX3-3xFlag, and SOS2-3xFlag was pooled together and DNA extracted followed by PCR amplification and sequencing. sgRNA sequences are shown above the chromatograms and PAMs are indicated in red letters.
To investigate whether features of the donor T-DNA integration, such as transgene expression levels or copy number may influence GT efficiency, we analyzed T1 lines from the ROS1-3xFlag and ROS1-4xMyc experiments, including the 2 precise and 14 imprecise GT-positive ROS1-3xFlag lines and 30 randomly picked GT-negative lines from the same experiment, as well as the 7 imprecise GT-positive ROS1-4xMyc and 39 GT-negative lines. Our results did not reveal a link between GT efficiency, including precise or imprecise GT, and donor T-DNA copy numbers or expression levels of the resistance marker (Supplemental Figure S3).
GT frequency in the sequential transformation method is not associated with proximity of the donor and target genes
It has been proposed that GT frequencies are enhanced when both donor and target genes are located closely on the same chromosome in Arabidopsis (Fauser et al., 2012) and barley (Lawrenson et al., 2021). We reasoned that if a short physical distance between the target and donor loci improves GT efficiency, we should expect a strong genetic linkage between the endogenous target locus and the integrated donor transgene in our GT method. To test this hypothesis, we backcrossed two previously published GT lines, DME-GFP and ROS1-Luc (Miki et al., 2018), and two GT lines produced in this research, precise ROS1-3xFlag and imprecise ROS1-4xMyc, with non-transgenic Col-0 wild-type plants. After two backcrosses, we obtained GT heterozygous plants lacking both the donor and the DD45pro::Cas9 transgenes, as well as the gl2 mutation present in the parental line (Figure 5, A–E) (Mao et al., 2016; Miki et al., 2018). In addition, self-pollination of heterozygous demeter (DME)-GFP plants produced homozygous GT segregating progeny without any of the transgenes (Figure 5F). The ease of obtaining donor transgene-free GT plants in the backcrossed population suggests that the GT locus and donor transgene were not tightly linked in the chromosome. This observation is consistent with our hypothesis that the sequential transformation method establishes GT mainly in the T1 generation, perhaps occurring right after Agrobacteria deliver the T-DNA into fertilized eggs and even before the donor transgene is integrated into the genome. Furthermore, it has been reported that transgene-free GT plants were obtained from self-pollinated rice and tobacco (Nishizawa-Yokoi et al., 2020).
Figure 5.
Production of transgene free GT plants by backcrossing. A–E, Genotyping PCR for 2× backcrossed individual plants of DME-GFP (A), ROS1-Luc (B), ROS1-3xFlag (C), ROS1-4xMyc (D), and DME-3xFlag (E), respectively. The parental CRISPR/Cas9 and the GT donor T-DNAs were successfully removed by two times backcrossing to Col-0. F, Genotyping PCR for the self-crossing progeny of two times backcrossed DME-GFP in (A). M, marker.
DNA methylation in GT plants
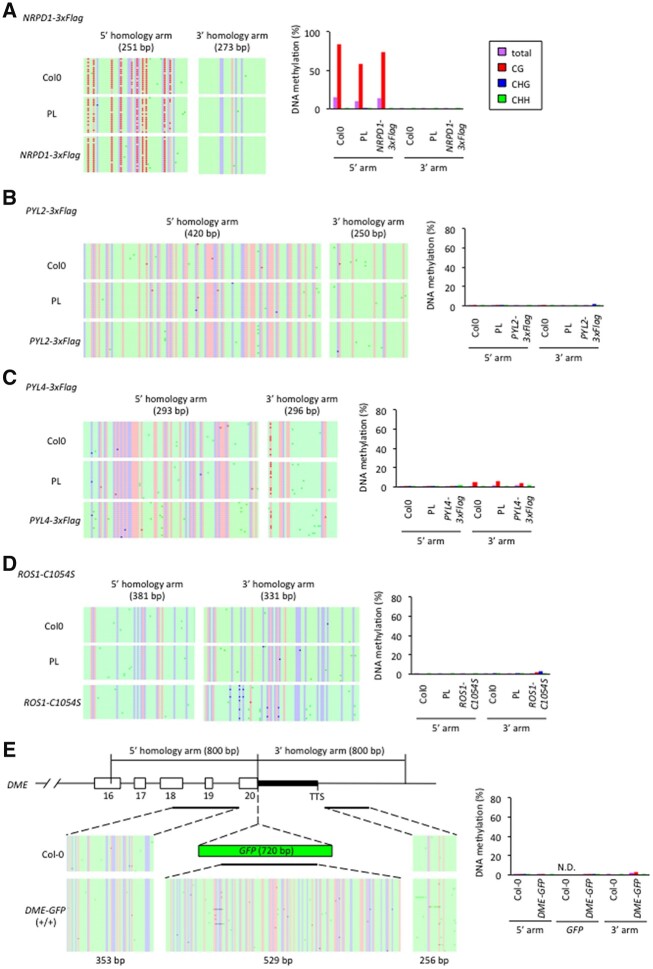
We have previously shown that GT does not affect cytosine DNA methylation at the homology arms of homozygous ROS1-GFP T4 plants (Miki et al., 2018). To determine whether DNA methylation is disrupted in T1 plants, we performed bisulfite sequencing of ROS1-3xFlag T1 plants, including two biallelic lines, two heterozygous lines with precise GT events, and two heterozygous lines with 5′-precise GT events. No substantial changes in cytosine methylation in either the 5′- or 3′-homology arm region was observed in any of the analyzed plants (Supplemental Figure S4), confirming that GT establishment does not affect the DNA methylation status at the target locus (Miki et al., 2018). Analysis of four additional precise GT events in different loci, NRPD1-3xFlag, PYL2-3xFlag, PYL4-3xFlag, and ROS1-C1054S, showed that cytosine methylation patterns in T2 homozygous precise GT plants were similar to non-transgenic Col-0 and the parental DD45pro::Cas9 transgenic line (Figure 6, A–D).
Figure 6.
Epigenetic effects at the GT target loci. A–D, Individual locus bisulfite sequencing analysis. Bisulfite sequencing at 5′- and 3′-homology arms are shown for NRPD1-3xFlag (A), PYL2-3xFlag (B), PYL4-3xFlag (C), and ROS1-C1054S (D), respectively. E, Individual locus bisulfite sequencing analysis for transgene free homozygous DME-GFP plants in Figure 5F. Purple, total C methylation; red, CG methylation; blue, CHG methylation; green, CHH methylation. At least independent 20 clones were sequenced for each sample for the bisulfite sequencing analysis. Sequencing results were analyzed using Kismeth (Gruntman et al., 2008).
Southern blot analysis of T2 ROS1-GFP GT lines produced using an all-in-one strategy has previously suggested the absence of de novo methylation in the knockin sequence (Peng et al., 2020). In this work we performed individual bisulfite sequencing of vector-free DME-GFP homozygous plants (Figure 5F) and Col-0. We did not observe substantial changes in cytosine methylation in either the 5′- or 3′-homology arm region or de novo methylation at the introduced GFP sequence (Figure 6E). Altogether, our results suggest that GT in Arabidopsis does not affect DNA methylation of the homology arms, and that the knockin sequence somehow escaped from RdDM.
Discussion
We have performed an in-depth characterization of the sequential transformation approach previously developed by our group to generate GT in plants via HDR (Miki et al., 2018). Our results suggest that when Arabidopsis plants bearing the DD45pro::Cas9 cassette are transformed by flower dipping with constructs containing the donor DNA and a targeting sgRNA, heritable GT is established mainly in T1 egg cells and/or early embryos. However, we cannot exclude that GT events may also be generated at lower frequencies in subsequent generations.
The GT frequency of sequential transformation strategy in the present research is much lower than previously reported (Miki et al., 2018), even though we have used the same parental line (DD45-#58, ABRC stock CS69955) and the same sgRNA for the ROS1-3xFlag and ROS1-4xMyc GT constructs. We hypothesize that the decrease in GT efficiency could be due to a reduction in the expression of the DD45pro::Cas9 transgene in the parental lines as a result of gene silencing. While the original report used a T2 generation DD45-#58 parental line, grown under selection on Murashige and Skoog (MS) plates containing 25 mg L−1 hygromycin (Miki et al., 2018), the T4 and later generation DD45-#58 parental line used in the present work could only survive on plates with <10 mg L−1 hygromycin. This fact indicates that the transgene expression level of the parental line has been markedly reduced in the T4 generation, which is bound to have adverse consequences in the GT efficiency. Future work may need to use freshly generated parental lines with strong transgene expression.
Specific primers mistakenly detected some false-positive GT events that were confirmed by sequencing of the PCR products. These false-positive PCR products were not heritable and we speculate that GT events can occur in some somatic cells, thus these minor GT events could be detected due to the sensitivity of PCR amplification. For this reason, external primers are more appropriate for the primary screening of plants obtained by the sequential transformation method followed by the use of full-length primers to identify precise GT events (Miki et al., 2021).
Similar to other reports (Wolter et al., 2018) (Wolter and Puchta, 2019; Gao et al., 2020; Huang et al., 2021; Peterson et al., 2021), we have identified imprecise knockin events for the SOS1-GFP, SRT2-GFP, and PYL2-3xFlag GT targets. In our study, all three imprecise knockin events were non-homologous end-joining (NHEJ)-mediated T-DNA integrations into the target sites. Our inability to obtain PCR amplicons for other imprecise GT events may be due to possible multicopy T-DNA integrations, or possible T-DNA rearrangements. Agrobacterium-mediated integration of the T-DNA into the host genome involves the production of single-stranded T-DNA (ssT-DNA) which associates with VirD2 and it has been proposed that the complex could recognize DSB sites for integration (Gelvin, 2017; Singer, 2018). In fact, high frequencies of T-DNA integrations into CRISPR/Cas9 cleavage sites has been observed (Zhang et al., 2018), indicating the presence of ssT-DNA/VirD2 complexes near the DSB site, which could be used as a donor template for HDR (Figure 7, left). However, according to our observations, occasionally one homology arm is precisely integrated via HDR, while another arm is integrated by NHEJ-mediated T-DNA fragment insertion by the same mechanism as Agrobacterium-mediated transgene integration (Figure 7, middle and right). If the source of the donor template for GT is the fully integrated genomic T-DNA cassette, we should expect to find genomic sequences flanking the T-DNA in the imprecise GT events, but such genomic sequences were never observed. Thus, we propose that the Agrobacterium-delivered extrachromosomal ssT-DNA/VirD2 complex (Singer, 2018), and not the genome-integrated T-DNA, serves as donor template for HDR-mediated repair (Figure 7) (Miki et al., 2021). This hypothesis is consistent with recent reports of Agrobacterium-mediated transgene-free GT in Arabidopsis and tomato, suggesting that integration of the donor T-DNA into genomic sequence might not be necessary for the establishment of GT in plants (van Tol et al., 2022) (Danilo et al., 2019). The finding that GT efficiency is substantially increased by the use of a Cas9–VirD2 fusion protein compared to the WT Cas9 in rice provides further support to our hypothesis (Ali et al., 2020).
Figure 7.
A hypothetical working model for Agrobacterium-mediated GT in plants. The released ssT-DNA and VirD2 complex from Agrobacterium can act as a repair donor template for HDR-mediated GT in plants (left). The 5′- or 3′-arm imprecise GT events can also happen via NHEJ-mediated T-DNA integration machinery (middle and right). Green lines represent 5′- and 3′-homology arm sequences; blue represents knockin sequence; purple indicates other T-DNA regions; red arrows indicate newly synthesized DNA strand by repair machinery.
Understanding the causes affecting GT efficiency at different loci and developing rules for the design of GT targets is critical to achieve the full potential of GT. Here, we successfully achieved precise GT for 14 out of 27 loci, and found a positive association between CRISPR/Cas9-mediated DSB efficiency and GT frequency, in agreement with previous reports (Gao et al., 2020; Merker et al., 2020). The sgRNA design is therefore critical for GT efficiency and needs to take into account not only its nucleotide sequence but also the chromosomal environment at the target site. For example, nucleosome occupancy can influence the accessibility of the target in yeast (Yarrington et al., 2018), while chromatin structure can influence the efficiency of CRISPR/Cas9-mediated DSB (Kuscu et al., 2014; Wu et al., 2014; Liu et al., 2016). However, a high DSB efficiency is not always associated with a high frequency of GT (Gao et al., 2020; Vu et al., 2021). Unfortunately, the available data currently do not allow the development of efficiency prediction models for GT and more research is sorely needed.
One of the GT targets in this work aimed to introduce three amino acid substitutions in the NRPD1 locus. In addition to the substitutions, we introduced a silent mutation generating a SacII restriction enzyme site that was used for screening purposes. Interestingly, we obtained three independent D447A substitution GT lines, but none of them contained the remaining two substitutions, D449A and D451A. The D447A substitution site is 5-bp downstream of the SacII restriction site used for screening, whereas the D449A and D451A substitution sites are 6 and 12 bp further downstream, respectively. Partial substitution events are not uncommon and have been previously reported, with incorporated substitutions tending to be proximal to the DSB, or at least closer to the DSB than the screening marker sites (Sun et al., 2016; Wolter et al., 2018; Li et al., 2019; Huang et al., 2021; Eini et al., 2022; van Tol et al., 2022). In tobacco, substitution efficiency has been linked to the distance to the DSB (Huang et al., 2021). The accumulated data suggest that a good strategy to screen for base substitutions is to place a marker restriction enzyme site far from the DSB and locate the substitution sites between the DSB and marker restriction enzyme sites, as close as possible to the DSB. Alternatively, using two sgRNAs at each end of the flanking sides for the replacement could be attempted. Precise amino acid substitutions were achieved for ROS1-C1054S, DRM2-C587A, and NRPD1-D447A in the present study and characterization of the GT lines provided robust data about the importance of the respective amino acids for the enzymatic activity of the target proteins in vivo. These results emphasize the usefulness of GT approaches for gene function research providing multiple advantages over in vitro methods and in vivo strategies based on complementation with mutated proteins.
In summary, we have used the sequential transformation GT strategy targeting 27 loci and successfully obtained precise GT in 14 of the targets. This wide range application of GT technology in plants has been rarely reported. Genome-wide GT has recently been tested in 74 target sites using site-specific insertion landing pads, and knockin events were achieved in 93% of the tested sites (69 out of 74) in maize (Gao et al., 2020). Nevertheless, our sequential transformation strategy does not rely on any selection marker at the target loci (Miki et al., 2018) and yields vector-free GT plants by Mendelian segregation in a backcrossed population. Our work suggests that this GT method can be widely applied for precise genome engineering in plants.
Materials and methods
Plant materials and growth condition
The Arabidopsis (A. thaliana) accession Col-0 and ABRC donated the stock number CS69955 parental line, previously named DD45-#58, were used for all experiments. All plants were grown at 22°C on half MS medium with 1% (w/v) sucrose or in soil with a 16-h light/8-h dark photoperiod. The new transformants of sequential transformation T1 lines were directly sowed in soil, and selected by three times Basta spray.
Plasmid construction
For GT constructs for the sequential transformation strategy were followed published protocol (Miki et al., 2018) (Miki et al., 2021) (Supplemental Table S2). Briefly, AtU6-26 promoter driven sgRNA cassette and donor sequence were constructed in pCambia3301. All transformants were generated by the flower dipping method.
DNA analysis
Total DNA was extracted by the cethyltrimethyl ammonium bromide (CTAB) method. Extracted DNA was used for analysis of GT events by PCR and Southern blotting (Supplemental Table S2). Southern blotting was performed according to published protocols. Briefly, extracted DNA was digested overnight with chosen restriction enzymes, then separated on a 1.5%–2% agarose gel, visualized by Image Lab Software and Gel Doc XR (BIO-RAD), and then transferred to nylon membrane (GE Healthcare). The probes were labeled with 32P-α-dCTP by using the Random primer DNA labeling kit (Takara). The hybridization signals were detected with a phosphor imager (Fuji).
DNA methylation analysis
DNA methylation was analyzed by bisulfite sequencing. For individual bisulfite sequencing, total DNA was extracted by the CTAB method, and un-methylated cytosines were converted into uracil by using EZ DNA Methylation-Gold Kit (ZYMO RESEARCH). Genomic regions of interest were amplified by specific primers (Supplemental Table S2), then the amplicons were cloned into pMD-18 (Takara), and independent colonies were sequenced. The Sanger sequencing results were analyzed by Kismeth (Gruntman et al., 2008).
Chop-PCR is a method for detecting DNA methylation using restriction enzymes and PCR. Extracted DNA was digested with a DNA methylation-sensitive restriction enzyme such as BstUI, HaeIII, and DdeI followed by PCR or qPCR (Supplemental Table S2). The AtAct7 locus was used as an internal control.
ChIP assay
ChIP assay was performed according to a published protocol (Saleh et al., 2008). Briefly, chromatin was extracted from 1 gm of cross-linked plant materials. Immunoprecipitation was performed using anti-DDDK-tag conjugated magnetic beads (MBL International). ChIP products were analyzed by quantitative PCR (Supplemental Table S2).
Data availability
The authors declare that all the data supporting the findings of this study are available within the paper and its supplementary information files. The data sets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers (Supplemental Table S3).
Supplemental data
The following materials are available in the online version of this article:
Supplemental Figure S1. Detailed ROS1 GT constructs and primers.
Supplemental Figure S2. Detailed Sanger sequence results of the imprecise knockin events.
Supplemental Figure S3. Donor T-DNA relative expression levels and copy numbers in T1 GT plants.
Supplemental Figure S4. DNA methylation analysis of the ROS1-3xFlag target locus in T1 plants.
Supplemental Table S1. Predicted sgRNA activity.
Supplemental Table S2. Sequences of primers used in this study.
Supplemental Table S3. Gene accession numbers.
Supplementary Material
Acknowledgments
We would like to thank all lab members and the Shanghai Center for Plant Stress Biology, Chinese Academy of Sciences for assistance.
Funding
This work was supported by the Shanghai Science and Technology Innovation Plan (20ZR1467000) to D.M. and by the Chinese Academy of Sciences to J-K.Z.
Conflict of interest statement. The authors declare no conflict of interests.
Contributor Information
Zhengjing Zhang, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China.
Wenjie Zeng, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Wenxin Zhang, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Jing Li, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Dali Kong, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Lei Zhang, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Rui Wang, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Fangnan Peng, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Zhe Kong, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Yongping Ke, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Heng Zhang, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China.
Chanhong Kim, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China.
Huiming Zhang, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China.
Jose Ramón Botella, School of Agriculture and Food Sciences, University of Queensland, Brisbane 4072, Australia.
Jian-Kang Zhu, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China; Institute of Advanced Biotechnology and School of Life Sciences, Southern University of Science and Technology, Shenzhen 518055, China; Center for Advanced Bioindustry Technologies, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Daisuke Miki, Shanghai Center for Plant Stress Biology, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Shanghai 200032, China.
Z.Z., Wj.Z., Wx.Z., and D.M. designed the research; Z.Z., Wj.Z., Wx.Z., and D.M. performed the experiments with assistance from J.L., D.K., L.Z., R.W., F.P., Z.K., Y.K., H.Z., and C.K.; D.M. supervised the project; D.M., Z.Z., Wj.Z., Wx.Z., He.Z, Hu.Z., C.K., J.R.B., and J-K.Z. wrote the article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is Daisuke Miki (daisukemiki@psc.ac.cn).
References
- Ali Z, Shami A, Sedeek K, Kamel R, Alhabsi A, Tehseen M, Hassan N, Butt H, Kababji A, Hamdan SM, et al. (2020) Fusion of the Cas9 endonuclease and the VirD2 relaxase facilitates homology-directed repair for precise genome engineering in rice. Commun Biol 3: 44–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čermák T, Curtin SJ, Gil-Humanes J, Čegan R, Kono TJY, Konečná E, Belanto JJ, Starker CG, Mathre JW, Greenstein RL, et al. (2017) A multipurpose toolkit to enable advanced genome engineering in plants. The Plant Cell 29: 1196–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasegaran S, Carroll D (2016) Origins of programmable nucleases for genome engineering. J Mol Biol 428: 963–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concordet J-P, Haeussler M (2018) CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res 46: W242–W245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilo B, Perrot L, Mara K, Botton E, Nogué F, Mazier M (2019) Efficient and transgene-free gene targeting using Agrobacterium-mediated delivery of the CRISPR/Cas9 system in tomato. Plant Cell Rep [DOI] [PubMed] [Google Scholar]
- Duan CG, Wang X, Tang K, Zhang H, Mangrauthia SK, Lei M, Hsu CC, Hou YJ, Wang C, Li Y, et al. (2015) MET18 connects the cytosolic iron-sulfur cluster assembly pathway to active DNA demethylation in Arabidopsis. PLoS Genet 11: e1005559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eini O, Schumann N, Niessen M, Varrelmann M (2022) Targeted mutagenesis in plants using Beet curly top virus for efficient delivery of CRISPR/Cas12a components. New Biotechnol 67: 1–11 [DOI] [PubMed] [Google Scholar]
- Fauser F, Roth N, Pacher M, Ilg G, Sanchez-Fernandez R, Biesgen C, Puchta H (2012) In planta gene targeting. Proc Natl Acad Sci USA 109: 7535–7540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Mutti J, Young JK, Yang M, Schroder M, Lenderts B, Wang L, Peterson D, St. Clair G, Jones S, et al. (2020) Complex trait loci in maize enabled by CRISPR-Cas9 mediated gene insertion. Front Plant Sci 11: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelvin SB (2017) Integration of Agrobacterium T-DNA into the plant genome. Ann Rev Genet 51: 195–217 [DOI] [PubMed] [Google Scholar]
- Gruntman E, Qi Y, Slotkin RK, Roeder T, Martienssen RA, Sachidanandam R (2008) Kismeth: Analyzer of plant methylation states through bisulfite sequencing. BMC Bioinform 9: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag JR, Pontes O, Pikaard CS (2009) Metal A and metal B sites of nuclear RNA polymerases Pol IV and Pol V are required for siRNA-dependent DNA methylation and gene silencing. PloS one 4: e4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson IR, Deleris A, Wong W, Zhong X, Chin HG, Horwitz GA, Kelly KA, Pradhan S, Jacobsen SE (2010) The de novo cytosine methyltransferase DRM2 requires intact UBA domains and a catalytically mutated paralog DRM3 during RNA–directed DNA methylation in Arabidopsis thaliana. PLOS Genet 6: e1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-T, Yuan Y-H, Lin Y-C, Lin S, Cheng Q-W, Wu F-H, Sheen J, Shih M-C, Lin C-S (2021) Efficient and economical targeted insertion in plant genomes via protoplast regeneration. The CRISPR J 4: 752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T-K, Armstrong B, Schindele P, Puchta H (2021) Efficient gene targeting in Nicotiana tabacum using CRISPR/SaCas9 and temperature tolerant LbCas12a. Plant Biotechnol J 19: 1314–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuscu C, Arslan S, Singh R, Thorpe J, Adli M (2014) Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nature Biotechnol 32: 677–683 [DOI] [PubMed] [Google Scholar]
- Lawrenson T, Hinchliffe A, Clarke M, Morgan Y, Harwood W (2021) In-planta gene targeting in barley using Cas9 with and without geminiviral replicons. Front Genome Edit 3: 663380–663380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Li J, He Y, Xu M, Zhang J, Du W, Zhao Y, Xia L (2019) Precise gene replacement in rice by RNA transcript-templated homologous recombination. Nature Biotechnol 37: 445–450 [DOI] [PubMed] [Google Scholar]
- Liu X, Homma A, Sayadi J, Yang S, Ohashi J, Takumi T (2016) Sequence features associated with the cleavage efficiency of CRISPR/Cas9 system. Sci Rep 6: 19675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Zhang Z, Feng Z, Wei P, Zhang H, Botella JR, Zhu JK (2016) Development of germ-line-specific CRISPR-Cas9 systems to improve the production of heritable gene modifications in Arabidopsis. Plant Biotechnol J 14: 519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker L, Schindele P, Puchta H (2020) Using CRISPR/ttLbCas12a for in planta Gene Targeting in A. thaliana. Curr Protoc Plant Biol 5: e20117. [DOI] [PubMed] [Google Scholar]
- Miki D, Wang R, Li J, Kong D, Zhang L, Zhu J-K (2021) Gene targeting facilitated by engineered sequence-specific nucleases: potential applications for crop improvement. Plant Cell Physiol 62: 752–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki D, Zhang W, Zeng W, Feng Z, Zhu J-K (2018) CRISPR/Cas9-mediated gene targeting in Arabidopsis using sequential transformation. Nat Commun 9: 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki D, Zinta G, Zhang W, Peng F, Feng Z, Zhu J-K (2021) CRISPR/Cas9-based genome editing toolbox for Arabidopsis thaliana. InSanchez-Serrano JJ, Salinas J, eds, Arabidopsis Protocols. Springer US, New York, NY, pp 121–146 [DOI] [PubMed] [Google Scholar]
- Mok YG, Uzawa R, Fau-Lee J, Lee J Fau-Weiner GM, Weiner Gm Fau-Eichman BF, Eichman Bf Fau-Fischer RL, Fischer Rl Fau-Huh JH, Huh JH (2010) Domain structure of the DEMETER 5-methylcytosine DNA glycosylase. Proc Natl Acad Sci USA 107: 19225–19230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa-Yokoi A, Mikami M, Toki S (2020) A universal system of CRISPR/Cas9-mediated gene targeting using all-in-one vector in plants. Front Genome Edit 2: 604289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz MT, Altpeter A, Karan R, Merotto A, Altpeter F (2021) CRISPR/Cas9-mediated multi-allelic gene targeting in sugarcane confers herbicide tolerance. Front Genome Edit 3: 673566–673566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, Olsen KM, Gregg A, Noggle S, Tessier-Lavigne M (2016) Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533: 125–129 [DOI] [PubMed] [Google Scholar]
- Peng F, Zhang W, Zeng W, Zhu J-K, Miki D (2020) Gene targeting in Arabidopsis via an all-in-one strategy that uses a translational enhancer to aid Cas9 expression. Plant Biotechnol J 18: 892–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permyakova NV, Marenkova TV, Belavin PA, Zagorskaya AA, Sidorchuk YV, Uvarova EA, Kuznetsov VV, Rozov SM, Deineko EV (2021) Assessment of the level of accumulation of the dIFN protein integrated by the knock-in method into the region of the histone H3.3 Gene of Arabidopsis thaliana. Cells 10: 2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D, Barone P, Lenderts B, Schwartz C, Feigenbutz L, St. Clair G, Jones S, Svitashev S (2021) Advances in Agrobacterium transformation and vector design result in high-frequency targeted gene insertion in maize. Plant Biotechnol J 19: 2000–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Miki D, Zhang H, Liu Y, Zhang X, Tang K, Kan Y, La H, Li X, Li S, et al. (2012) A histone acetyltransferase regulates active DNA demethylation in Arabidopsis. Science 336: 1445–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Avramova Z (2008) An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat Protoc 3: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Singer K (2018) The mechanism of T-DNA integration: Some major unresolved questions. Curr Top Microbiol Immunol 418: 287–317 [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang X, Wu C, He Y, Ma Y, Hou H, Guo X, Du W, Zhao Y, Xia L (2016) Engineering herbicide-resistant rice plants through CRISPR/Cas9-mediated homologous recombination of acetolactate synthase. Mol Plant 9: 628–631 [DOI] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR (1987) Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51: 503–512 [DOI] [PubMed] [Google Scholar]
- van Tol N, van Schendel R, Bos A, van Kregten M, de Pater S, Hooykaas PJJ, Tijsterman M (2022) Gene targeting in polymerase theta-deficient Arabidopsis thaliana. Plant J 109: 112–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TV, Doan DTH, Tran MT, Sung YW, Song YJ, Kim J-Y (2021) Improvement of the LbCas12a-crRNA System for Efficient Gene Targeting in Tomato. Front Plant Sci 12: 722552–722552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li Q, Yuan W, Cao Z, Qi B, Kumar S, Li Y, Qian W (2016) The cytosolic Fe-S cluster assembly component MET18 is required for the full enzymatic activity of ROS1 in active DNA demethylation. Sci Rep 6: 26443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter F, Klemm J, Puchta H (2018) Efficient in planta gene targeting in Arabidopsis using egg cell-specific expression of the Cas9 nuclease of Staphylococcus aureus. Plant J 94: 735–746 [DOI] [PubMed] [Google Scholar]
- Wolter F, Puchta H (2019) In planta gene targeting can be enhanced by the use of CRISPR/Cas12a. Plant J 100: 1083–1094 [DOI] [PubMed] [Google Scholar]
- Wu X, Scott DA, Kriz AJ, Chiu AC, Hsu PD, Dadon DB, Cheng AW, Trevino AE, Konermann S, Chen S, et al. (2014) Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nature Biotechnol 32: 670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarrington RM, Verma S, Schwartz S, Trautman JK, Carroll D (2018) Nucleosomes inhibit target cleavage by CRISPR-Cas9 in vivo. Proc Nat Acad Sci 115: 9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Xing H-L, Wang Z-P, Zhang H-Y, Yang F, Wang X-C, Chen Q-J (2018) Potential high-frequency off-target mutagenesis induced by CRISPR/Cas9 in Arabidopsis and its prevention. Plant Mol Biol 96: 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within the paper and its supplementary information files. The data sets generated or analyzed during the current study are available from the corresponding author on reasonable request.