Summary
Background.
Stigma is a formidable social-structural barrier to HIV testing, and yet the effect of stigma on HIV testing is rarely examined at the community-level. The aim of this study was to examine the geospatial relationships of perceived HIV stigma and HIV testing among men and women living in rural Uganda.
Methods.
Women (n=5381) and men (n = 4359) residing in rural areas of Uganda who self-identified as HIV negative completed measures that included HIV testing history and HIV stigma. Geospatial cluster analyses identified areas of higher stigma (hot-spots) and lower stigma (cold-spots) and their geographical dispersion. Gender stratified Poisson regression models tested individual and community-level stigma in relation to frequency of HIV testing in the previous 2-years.
Findings.
Among the 9,740 participants, 940 (9%) had never been tested for HIV, and among those who had been tested, 1131(12%) had not been tested in the previous 2-years; men were less likely to have been tested than women. Multi-level modeling showed that women demonstrated significant individual and community-level associations between lower stigma predicting higher rates of HIV testing. In contrast, individual-level stigma was not associated with testing frequency for men, whereas higher community-level stigma was significantly associated with higher rates of testing.
Interpretation.
Results suggest that HIV stigma exerts differential influence on testing for women and men. HIV testing campaigns targeted to men and women in rural Uganda will require gender tailoring fit to local contexts.
Funding.
National Institute of Mental Health (NIMH) grant number R01MH10639 awarded to Susan M. Kiene and Rhoda K. Waynenze, PIs.
Keywords: HIV stigma, Uptake of HIV testing, Geospatial analyses, HIV in Africa, HIV prevention, Health services research
Introduction
Current efforts to end HIV depend on at least 90% of people living with HIV being aware of their status and engaging in HIV care. Testing is therefore square one in controlling HIV. The global scale-up of HIV testing demands removing physical-structural constraints by offering testing at convenient locations and reducing stigma-related barriers by offering opportunities for rapid home or self-testing (1, 2). Sub-Saharan Africa has the highest rates of HIV infection in the world, and stigma-related concerns about testing HIV positive may be increasing over time (3). As a barrier to HIV testing, as well as an impediment to achieving mental health and well-being, stigma is a leading cause of late HIV diagnosis in Africa. Studies conducted throughout sub-Saharan Africa indicate that greater HIV stigma is associated with lower rates of testing (4–9). In Ethiopia, 69% of people with HIV are diagnosed late, of which 32% report fear of stigma as the basis for delayed testing (10). Similarly, a case control study in Zimbabwe found that stigma-related experiences posed an independent risk for late presentation to HIV care (11). In Uganda, HIV testing is associated with the degree to which individuals anticipate experiencing stigma if they to test HIV positive (12).
There are also well-established gender differences in HIV testing, with men testing less frequently than women. Multiple factors likely contribute to gender differences in HIV testing, including utilizing health services and culturally defined masculinity (13), as well as the financial consequences HIV poses to women and their children. Perceived HIV stigma, specifically the negative and stigmatizing views that individuals hold toward people living with HIV, may also contribute to gender differences in HIV testing. In some studies men report greater HIV stigma than women (7), while others report that women perceive greater HIV stigma than men (14). Research in South Africa indicates stigma in the surrounding community is significantly related to HIV testing among women, but not men (7). Furthermore, HIV testing and stigma are not evenly distributed across geographical areas. HIV stigma, for example, is more common in rural than urban areas (15–17). While stigma may vary between rural and urban settings, the influence of stigma may be more localized, with persons in closest proximity exerting the most influence on both perceived stigma and HIV testing (18).
The current study was conducted to examine the geospatial relationships of perceived HIV stigma and HIV testing among men and women living in rural Uganda, an area with one of the largest HIV epidemics in sub-Saharan Africa (19).We hypothesized that individuals who perceive greater HIV stigma relative to others in proximity to them would test less frequently for HIV. In addition, we hypothesized that communities with greater perceived HIV stigma (i.e., stigma hot-spots) would demonstrate lower HIV testing relative to communities with lower perceived HIV stigma (i.e., stigma cold-spots). We also hypothesized that individual-level stigma would interact with community-level stigma. Specifically, we hypothesized that individuals who perceive higher stigma and reside in stigma hot-spots would demonstrate the least frequent HIV testing, whereas individuals who perceive lower HIV stigma and reside in stigma cold-spots would demonstrate the most frequent HIV testing.
Methods
Study Design and Participants
We screened participants during the first recruitment waves of the PATH/Ekkubo study, an enhanced linkage to HIV care cluster randomized trial conducted in Uganda [Clinical Trial Registry NCT02545673 (20)]. For this study, data were collected in 13 villages in the central Uganda districts of Butambala and Mpigi. Study eligibility criteria were: age 18 or older or emancipated minor, household residence, and speaking the local language of Luganda or English. There were 10,474 potentially eligible individuals, of which, 438 (4%) were not found at home after up-to-three visits and 296 (3%) declined to participate. Participants were therefore 5381 women and 4359 men aged 18 to 59, including 42 female and 1 male emancipated minors (< 18 and ≥14 years), who reported that they were HIV negative or unknown HIV status and consented to complete an interviewer administered questionnaire and accepted taking a rapid HIV test. Health services, including HIV testing are provided free at government and not-for-profit health facilities and non-governmental organizations in the districts included in this study.
Procedures
We administered interviews before conducting rapid HIV testing as part of the trial (see Appendix, page 10). We conducted 95% of interviews in private areas inside households, and the remaining 5% were conducted in private areas outside of households or another location. All interviews regardless of location were conducted between the participant and interviewer without any other persons present, including household members and neighbors. The median number of persons per household interviewed was between 2 and 3. Geolocation data, specifically latitude and longitude coordinates, were captured via interviewer’s tablet computers. The geolocation data were linked to individual participants and we statistically controlled the number of interviews conducted within households. All participants provided written informed consent and all study procedures were approved by San Diego State University, and Makerere University School of Public Health ethical review committees and the Uganda National Council for Science and Technology.
Measures
Demographic characteristics.
We collected participant gender, age, marital status, education, and sources of income. Participants also reported whether their home has electricity as an economic indicator and whether a spouse, family member or other person living with HIV resided in their household.
HIV testing history.
Participants were first asked whether they had ever been tested for HIV prior to participating in the study. For participants who indicated that they had been tested, a series of follow-up questions included how many times they had ever been tested and the months and years for any tests received using an open response format. These data were used to quantify the frequency of HIV tests in the previous 2-years. Specifically, participants who had never been tested as well as those who had been tested but not in the previous 2-years were defined as not recently tested. For participants who had been tested in the previous 2-years, frequency counts of reported number of tests were used as a continuous variable in Poisson regression models. For descriptive analyses, participants were grouped on the basis of natural breaks in the frequency distribution of HIV testing in the past 2-years; (a) 1 to 2 times, (b) 3 to 5 times, and (c) having been tested 6 or more times.
Perceived HIV stigma.
We administered an 8-item perceived HIV Stigma Scale developed for use in sub-Saharan Africa (21). Interviewers instructed participants with the following script: “I’m going to read you some statements about what some people may believe about people living with HIV and I want you to tell me if you agree or disagree with the statements.” Example items include, “A person with HIV must have done something wrong and deserves to be punished” and “People who have HIV should be ashamed”. The perceived HIV stigma items were responded to on 5-point rating scales from “0 = strongly disagree” to “4 = strongly agree”, with higher scores representing greater perceived stigma, alpha = 0.87.
Statistical analysis
Because women are more likely than men to receive health services that offer routine HIV testing, such as family planning and antenatal services, we stratified all analyses by participant gender. Initial descriptive comparisons between women and men on demographic and health characteristics were performed using contingency table X2 tests for categorical variables and independent t-tests for continuous variables. For geospatial analyses, we conducted Local Moran’s I tool using ArcGIS to define individual and community-level perceived HIV stigma. Our research question concerns the social influence of HIV stigma on HIV testing frequency within higher and lower stigma areas. Moran’s I identifies clustering based on concentrations of continuous values in relation to the distance between values (22). Specifically, Moran’s I calculates the local cluster mean based on proximal scores removing the influence of the individual score. Clustering was therefore determined on the basis of perceived HIV stigma scores and participant geolocation. Because we assumed that individuals with greater perceived stigma would exert greater influence on others closer, relative to farther away, we used an inverse distance weighted interpolation. This procedure gives greater weight to stigma scores most proximal to the predicted value, with weights diminishing as a function of distance. We set the distance of maximum weighting to one kilometer (KM) ‘as a crow flies’ given the observed size of villages sampled and the distances between villages. We constrained the area of influence to 1 KM based on the assumption that social influences of stigma would be greater with proximity. By definition, interviews conducted among household members are the most proximal and therefore potentially most influential. We included women and men in a single geospatial analysis to account for the social influence of perceived HIV stigma across genders. Local Moran’s I defines spatial areas as statistically significant clusters using z-scores (p <.05) based on nearby values, referred to here as communities, with: (a) clusters of nearby values with similarly higher stigma scores (hot-spots); (b) clusters of nearby values with similarly lower stigma scores (cold-spots). Furthermore, the analysis identifies individuals as outliers when they have statistically significantly lower or higher stigma scores relative to dissimilar ‘neighboring’ scores in hot-spots and cold-spots, respectively (23). Individuals are therefore identified as having higher or lower stigma scores in higher or lower stigma communities on the basis of relative scores within and between clusters. In addition, the analysis identifies dispersed stigma scores as occurring outside of hot-spots and cold-spots, that is without statistically significant spatial clustering. Descriptive analyses among cluster groups for categorical variables used contingency table X2 tests and for continuous variables we used analysis of variance.
Next, we conducted Poisson regression models to test study hypotheses regarding the effects of individual-level and community-level stigma on HIV testing frequency counts. We modeled the main effects of (a) individual-level (higher vs. lower perceived HIV stigma scores) and (b) community-level (perceived stigma hot-spots vs cold-spots) as defined by statistically significant values of Moran’s I. We also modeled the individual-level X community-level stigma interactions. To test the main effects and interactions we performed non-redundant multi-level Poisson regressions with robust estimators, specifying lower stigma categories as the reference group. These models included all participants designated in hot-spots or cold-spots. The models controlled for participant age, years of education, whether they had electricity in their home, whether the participant reported living with a person who has HIV, the participant’s own post-survey HIV test results, and the number of household members interviewed. Likelihood ratio X2 tests for model effects, associated p values and parameter estimates are reported. Modeling was conducted using SPSS v.24 and statistical significance was defined as p < .05.
Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Data for this study were collected between November 25, 2015 and May 26, 2017. A total of 940/9740 (9%) had never been tested for HIV, and among those participants who had been previously tested, 1,131/9740 (12%) had not been tested in the previous 2-years. Taken together, 2071/9740 (21%) participants formed the group that had not been tested in the past 2-years. As shown in Table 1, women were significantly more likely to have been tested in the previous 2-years 4535/5381 (84%) compared to men 3134/4359 (72%). Furthermore, women were more likely to have been tested multiple times compared to men. Women were also more likely than men to be married, unemployed, less educated, not to have electricity, living with an HIV positive person, test positive after completing the survey, and had higher perceived HIV stigma scores.
Table 1.
Demographics and HIV testing characteristics of men and women in rural Uganda communities.
| Men N = 4359 |
Women N = 5381 |
|||
|---|---|---|---|---|
|
|
||||
| Characteristic | N (%) | N (%) | X2 | p |
|
| ||||
| Marital status | ||||
| Never Married | 1710 (39) | 1024 (19) | 486.64 | <.0001 |
| Divorced | 443 (10) | 893 (17) | 84.19 | <.0001 |
| Widowed | 30 (1) | 201 (4) | 96.57 | <.0001 |
| Married - separate | 417 (10) | 857 (16) | 85.67 | <.0001 |
| Married - together | 1759 (40) | 2406 (45) | 18.70 | <.0001 |
| Never work for money | 367 (8) | 1605 (30) | 684.49 | <.0001 |
| Household without electricity | 185242) | 2480 (46) | 12.64 | <.0001 |
| HIV positive household member | 129 (3) | 217 (4) | 8.09 | <.0001 |
| Tested HIV+ post-survey | 123 (3) | 286 (5) | 37.2 | < .0001 |
| Times HIV tested in past 2-years | ||||
| Not Tested in past 2-years | 1225 (28) | 846 (15) | 220.48 | <.0001 |
| Tested 1–2 times | 1459 (34) | 1479 (28) | 40.95 | <.0001 |
| Tested 3–5 times | 1116 (26) | 1549 (29) | 12.28 | <.0001 |
| Tested 6+ times | 559 (12) | 1507 (28) | 3323.1 | <.0001 |
| M (SD) | M (SD) | t | ||
|
|
||||
| Age | 29.2 (10.01) | 29.10 ( 10.13) | 0.46 | .639 |
| Years of education | 8.0 (3.36) | 7.80 (3.37) | 2.94 | .003 |
| Perceived stigma score | 0.89 (0.60) | 0.92 (0.59) | 2.31 | .021 |
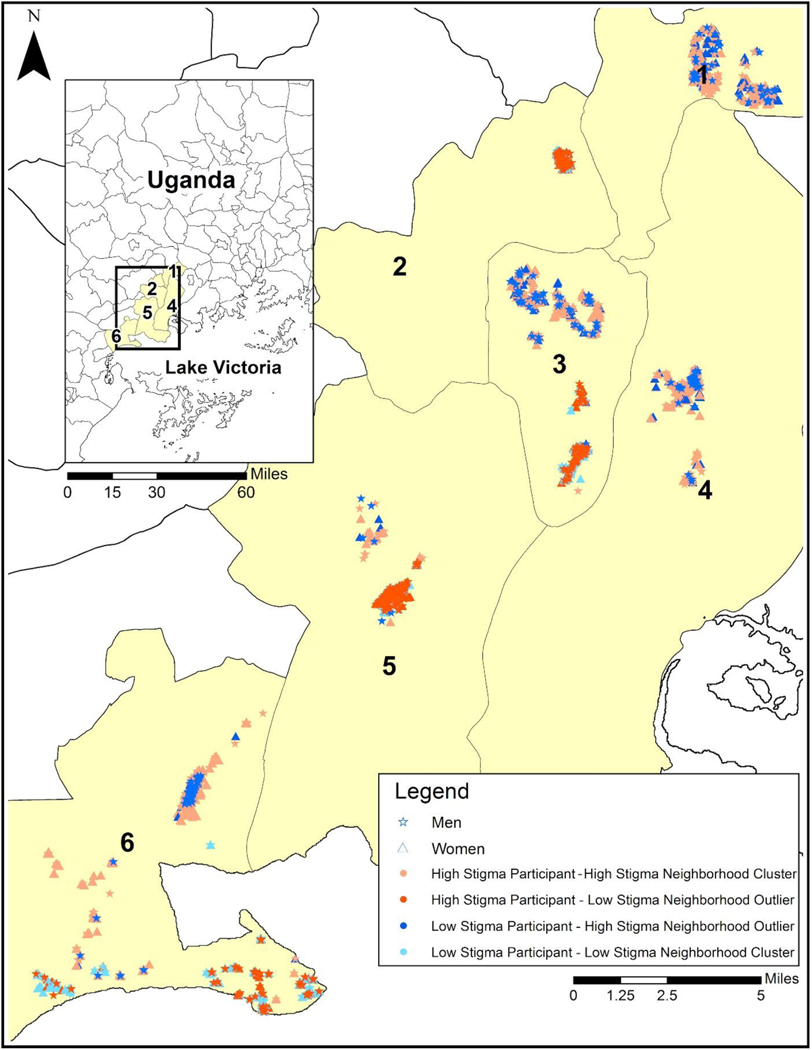
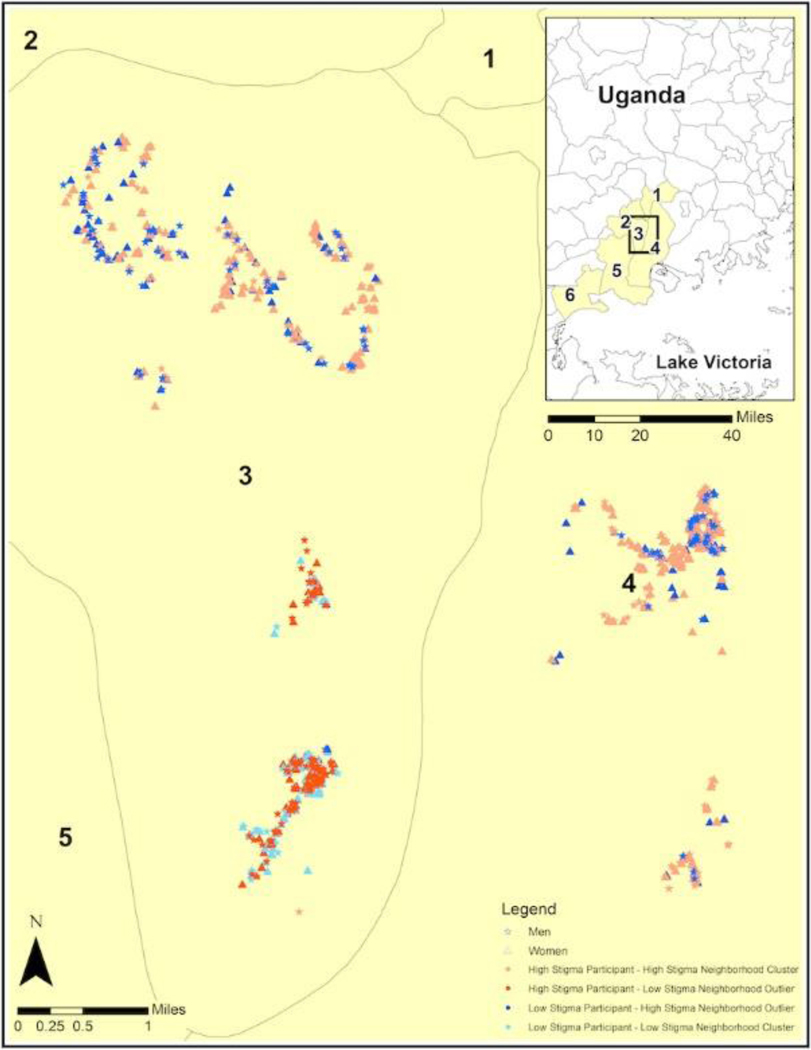
Results of the GIS clustering analysis on perceived HIV stigma scores indicated that 8207/9740) (84%) participants were located in areas of significantly higher stigma (hot-spots) or lower stigma (cold-spots). Specifically, 3850/9740 (39%) of participants clustered in stigma hot-spots and 4357/9740 (45%) clustered in stigma cold-spots. The remaining 1533/9740 (16%) of participants were in areas of dispersed HIV stigma (i.e., non-significant). Figure 1 shows the location of stigma hot-spots and cold-spots represented within the six study sub-counties. Stigma hot-spots and cold-spots were distributed across the sampling area with a mean of 7.4 KM (range 3.5 −11.5) between clusters. Figure 2 shows one sub-county with a closer view of stigma hot-spots and cold-spots with markers differentiating men and women, visualizing individuals with significantly dissimilar (p < .05) perceived stigma scores relative to persons in their proximity (e.g., outliers).
Figure 1.
Stigma hot-spots and cold-spots represented in the 6 sub-counties in the Butambala and Mpigi districts of Uganda that participated in the study.
Figure 2.
Stigma hot-spots and cold-spots with markers differentiating men and women in stigma hot-spots and cold-spots, as well as differentiating individuals with lower stigma in stigma hot-spots and individuals with higher stigma in stigma cold-spots.
Table 2 shows the descriptive analyses for participant characteristics and HIV testing in association with geospatially defined stigma hot-spots and cold-spots, as well as non-significant areas, for women and men. For women (upper-panel), stigma areas were associated with HIV testing groups. Women in stigma hot-spots were significantly least likely to have been tested in the past 2-years, whereas women in stigma cold-spots were more likely and more frequently tested. Women with the highest rates of testing, that is having been tested 3 or more times in the past 2-years, were in stigma cold-spots, whereas women with the lowest rates of multiple tests were in stigma hot-spots.
Table 2.
Percentages of men and women in each HIV testing frequency category within geospatial stigma clusters.
| Geolocation Stigma Clusters | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Higher Stigma in Stigma Hot-spots N=1670 | Lower Stigma in Stigma Hot-spots N=563 | Lower Stigma in Stigma Cold-spots N=1138 | Higher Stigma in Stigma Cold-spots N=1102 | Non-Significant N=908 | |||
|
|
|||||||
| Women | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | F | p |
|
| |||||||
| Age | 29.5 (10.1) | 30.5 (10.4) | 27.9 (9.2) | 27.1 (9.3) | 31.1 (11.1) | 26.9 | <.0001 |
| Education | 8.0 (3.5) | 8.4 (3.2) | 7.6 (3.0) | 8.1 (3.1) | 7.0 (3.5) | 21.6 | <.0001 |
| Interviews per household | 2.7 (1.3) | 2.5 (1.3) | 2.7 (1.1) | 3.2 (1.4) | 3.21.5) | 51.5 | <.0001 |
| Times tested in past 2-years | 3.9(4.6) | 4.6 (5.3) | 5.6 (5.2) | 4.5 (4.1) | 3.5 (4.7) | 29.0 | <.0001 |
| N (%) | N (%) | N (%) | N (%) | N (%) | X2 | ||
|
|
|||||||
| Without electricity | 887 (53) | 296 (52) | 359 (31) | 346 (31) | 592 (65) | 368.7 | <.0001 |
| HIV+ household member | 28 (2) | 17 (3) | 35 (3) | 74 (6) | 63 (7) | 68.4 | <.0001 |
| Tested HIV+ post-survey | 53 (3) | 14 (3) | 51 (4) | 34 (3) | 134 (15) | 197.3 | <.0001 |
| Not tested past 2-years | 293 (17) | 100 (18) | 133 (12) | 152 (14) | 168 (18) | 28.3 | <.0001 |
| Tested 1–2 times | 542 (33) | 143 (25) | 234 (20) | 232 (16) | 328 (36) | 106.1 | <.0001 |
| Tested 3–5 times | 446 (26) | 161 (29) | 319 (28) | 399 (26) | 224 (24) | 40.9 | <.0001 |
| Tested 6+ times | 389 (23) | 159 (28) | 452 (40) | 319 (28) | 188 (21) | 120.3 | <.0001 |
|
|
|||||||
| Men | N=1191 | N=426 | N=1053 | N=1064 | N=625 | ||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | F | ||
|
|
|||||||
| Age | 29.9 (10.4) | 30.3 (10.3) | 28.6 (9.4) | 27.5 (9.2) | 30.8 (10.5) | 15.9 | <.0001 |
| Education | 8.1(3.6) | 8.2 (3.6) | 7.7 (2.8) | 8.3 (3.2) | 7.5 (3.5) | 7.6 | <.0001 |
| Interviews per household | 3.1(1.3) | 3.0 (1.4) | 3.1 (1.1) | 3.5 (1.4) | 1.5 (3.1) | 20.5 | <.0001 |
| Times tested in past 2-years | 2.9 (4.3) | 3.1 (4.1) | 2.7 (3.7) | 2.73.4) | 2.4 (3.1) | 2.4 | .049 |
| N (%) | N (%) | N (%) | N (%) | N (%) | X2 | ||
|
|
|||||||
| Without electricity | 649 (54) | 238 (56) | 258 (24) | 313 (29) | 394 (63) | 423.2 | <.0001 |
| HIV+ household member | 24 (2) | 8 (2) | 16 (2) | 41 (4) | 40 (8) | 41.7 | <.0001 |
| Tested HIV+ post-survey | 21 (2) | 9 (2) | 28 (3) | 12 (1) | 53 (9) | 89.8 | <.0001 |
| Not tested past 2-years | 342 (28) | 125 (29) | 276 (26) | 315 (29) | 167 (26) | 4.1 | .381 |
| Tested 1–2 times | 396 (33) | 113 (26) | 365 (35) | 327 (31) | 258 (41) | 30.6 | <.0001 |
| Tested 3–5 times | 285 (24) | 116 (27) | 286 (27) | 293 (28) | 136 (22) | 10.6 | <.0001 |
| Tested 6+ times | 168 (14) | 72 (17) | 126 (12) | 129 (12) | 64 (10) | 12.9 | <.0001 |
For men, stigma areas were also associated with HIV testing history. As shown in the lower panel of Table 2, there was a mixed pattern of association between geospatial stigma areas and HIV testing for men. Men in stigma hot-spots were more likely to have been tested six or more times, but men with higher individual stigma scores in stigma hot-spots were least likely tested 3–5 times. For men, there was no association between having not been tested in the past 2-years and perceived HIV stigma.
Results of the Poisson regression models testing the effects of individual and community-level stigma predicting HIV testing are shown in Table 3. Among women, the model effects for both individual and community-level stigma were significant. The individual x community-level stigma interaction was not significant. At the individual-level, women with higher perceived stigma had lower rates of HIV testing than women with lower perceived stigma. Similarly, women in stigma hot-spots had lower rates of testing than women in stigma cold-spots.
Table 3.
Multivariable models predicting HIV testing frequency from individual-level and community-level stigma geolocation clusters stratified by gender.
| Women | Men | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictor | B | Effect X2 | p | B | Effect X2 | p |
| Age | .016 | 489.6 | < .0001 | .024 | 663.5 | < .0001 |
| Education | .012 | 28.6 | < .0001 | .038 | 155.1 | < .0001 |
| Without electricity | .153 | 99.9 | < .0001 | .065 | 8.8 | .003 |
| HIV+ household member | −.009 | 0.1 | .811 | .016 | .1 | .793 |
| Tested HIV+ post-survey | −.407 | 81.7 | < .0001 | −.906 | 87.6 | < .0001 |
| Interviews per household | −.045 | 67.9 | < .0001 | .036 | 24.1 | < .0001 |
| Individual-level stigma | −.173 | 94.4 | < .0001 | −.030 | 5.5 | .018 |
| Community-level stigma | −.223 | 178.9 | < .0001 | .077 | 7.0 | .008 |
| Individual-level X Community-level stigma | 2.9 | .087 | 0.8 | .349 | ||
For men, the main effect of individual-level stigma on HIV testing frequency was also statistically significant; men with higher stigma scores were tested less frequently. The main effect of community-level stigma was also significant, but the direction of the association between perceived stigma and HIV testing was the opposite of expected; men in stigma hot-spots had significantly higher rates of HIV testing than men in stigma cold-spots. The individual x community-level stigma interaction was not significant.
Discussion
Regular and repeated HIV testing in places of high-HIV prevalence is essential to achieving HIV control (24). The current study was conducted in communities with 8% HIV prevalence, among the highest in the world. We found that one-in-five participants in our population-based survey had not been tested for HIV in the previous 2-years, with HIV testing significantly less frequent among men than women. Gender differences in overall HIV testing are universally reported in sub-Saharan Africa and are likely explained by women being tested in family planning, antenatal care, and other health services. Our findings therefore support the a priori decision to stratify the models by gender. We also observed that individuals with higher perceived stigma in cold-spots (outliers), for both men and women were more common than outliers with lower stigma in hot-spots. The greater number of individuals with higher stigma scores may be explained by the overall prevalence of perceived stigma, including people with higher perceived stigma in relatively lower stigma social contexts. We also found that HIV testing was associated with HIV stigma. As predicted, women who individually perceived lower stigma, as well as women located in stigma cold-spots demonstrated the highest rates of HIV testing. Women with lower individual perceived stigma residing in stigma cold-spots were the most frequently tested. Our results for women, specifically that higher stigma is associated with lower HIV testing, are consistent with other studies of women in sub-Saharan Africa, including Uganda (25–27).
Similar to the current study, previous research has reported mixed patterns of associations between HIV stigma and HIV testing among men and women in southern Africa. For example, in South Africa, while higher stigma is associated with lower testing among women, this association is not consistently reported for men (28). Also, compared to men, sub-Saharan African women desire greater social distance from people living with HIV and express greater anticipated HIV stigma (29). While men with higher individual-level stigma were less often tested than men with lower individual-level stigma, men demonstrated the opposite direction of association at the community-level; men in stigma hot-spots had significantly higher HIV testing than men in stigma cold-spots. One potential explanation for higher community-level being associated with greater testing for men may be that repeatedly testing HIV negative may coincide with a greater sense of distancing from HIV, a form of ‘othering’ (30). Alternatively, higher community-level stigma may reflect observing more acts of stigma against people living with HIV, a factor that has been associated with greater HIV testing in past research (31, 32). Further research is needed to replicate these findings and explain gender differences in the association between perceived stigma and HIV testing.
Research conducted in South Africa has also found that different measures of stigma are differentially related to HIV testing. For example, women with greater perceived stigma have lower rates of testing, as was the case in this study, whereas women with greater stigma-attitudes are more likely to have been tested for HIV (29). Our findings also suggest that different conceptualizations of stigma may lead to different associations with HIV testing, depending on gender and local contexts. Theories of stigma emphasize the importance of intersecting factors, where the combination of the multiple dimensions, in this case gender and perceived HIV stigma, may be differentially related to HIV testing among women (33) and men (34). A limitation of our study is that our measure of perceived HIV stigma was not intersectional, in that the items were gender neutral, referring to people living with HIV. The intersection of gender and HIV stigma, and how intersectional stigma is related to HIV testing should be the subject of future research.
The current study’s findings should be interpreted in light of its methodological limitations. Our data were collected cross-sectionally and cannot inform causal directions. We also relied on self-reported history of HIV testing as well as responses to questions about HIV stigma, both of which may be influenced by social expectancies and recall biases. HIV testing has become normative in many places and there are widespread messages regarding the importance of testing. Thus, the social expectancies for HIV testing may have biased responses making the rates of HIV testing observed in our study upper-bound estimates. In contrast, discriminating against people living with HIV is biased toward under-reporting (3), and therefore our measures may under-estimate perceived stigma. We are also unable to account for the potential effects of programs and campaigns to increase HIV testing in Uganda that may have different effects on men and women. The accuracy of the number of HIV tests reported may have been influenced by the 2-year retrospective period. It should be noted that our geospatial analysis of stigma scores, particularly use of Local Moran’s I, requires replication. This study was also conducted in one country with its own unique culture and HIV epidemic. The degree to which these findings are generalizable to other settings is unknown. We did not collect data on sexual behavior or other indicators of HIV risk, limiting our ability to explain HIV testing rates. Also, our study focused on perceived stigma, just one dimension of stigma. The degree to which our findings generalize to other stigma dimensions is unknown. Furthermore, the restricted range of responses to our perceived stigma measure reduced our power to detect associations and should be considered a limitation.
We conclude that this study points toward extending available interventions that are designed to alleviate the effects of stigma among people living with HIV to increase HIV-testing (35). Broad public health education campaigns to reduce stigma and increase HIV testing have shown promise in sub-Saharan Africa and may provide a foundation for continued efforts. Reducing community-level stigma may also extend beyond testing to ultimately improve linkage and retention to HIV care. Our findings are consistent with past studies, indicating HIV testing interventions require gender tailoring as well as local contextualization. Treating stigma as a monolithic barrier to HIV testing has yielded interventions with uneven success. For men additional factors, such as healthcare access and masculinity may likely prove critical to increasing HIV testing. HIV testing campaigns may better appeal to men if they are designed by men and aim to avoid reinforcing perceived stigma while not promoting masculine stereotypes.
Supplementary Material
Research in Context.
Evidence before this study
We searched PubMed on January 10, 2020 for empirical studies with the terms “HIV stigma” and “HIV testing”, “sub-Saharan Africa”, “Africa” and no date or language restrictions. Previous research in sub-Saharan Africa shows that HIV testing is suppressed by perceived HIV stigma at the individual-level, such that greater stigma beliefs are predictive of lower rates of HIV testing. One study has also shown that women are more likely to be sensitive to the impact of stigma in their community than men. Few studies have examined the interaction between individual and community stigma as a barrier to HIV testing.
Added value of this study
HIV stigma impacts HIV testing differently for men and women. For women, individuals’ own perceived stigma is related to lower testing rates and community-level perceived stigma has a similar effect. For men, greater perceived stigma at the individual-level also predicts less frequent testing. In contrast, among men higher community-level perceived stigma is related to more frequent HIV testing, possibly because repeatedly testing HIV negative may foster perceived stigma as a form of social distancing, or ‘othering’.
Implications of all available evidence
Efforts to increase HIV testing require strategies for reducing HIV stigma among women and men. Interventions to increase HIV testing may benefit from gender tailoring, with social influence operating differently for women and men.
Acknowledgements
Funding for this study was provided by National Institute of Mental Health (NIMH) grant R01MH10639 to Susan Kiene and Rhoda K. Waynenze, PIs.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Prof Seth C. Kalichman, Institute for Collaboration on Health, Intervention and Policy, University of Connecticut, Storrs, CT USA.
Bruno Shkembi, Institute for Collaboration on Health, Intervention and Policy, University of Connecticut, Storrs, CT USA.
Prof Rhoda K. Wanyenze, Department of Disease Control and Environmental Health, Makerere University School of Public Health, Kampala, Uganda.
Rose Naigino, Department of Disease Control and Environmental Health, Makerere University School of Public Health, Kampala, Uganda.
Moses H. Bateganya, FHI 360, Durham, NC, USA.
Prof Nicolas A. Menzies, Department of Global Health and Population, Harvard T. H. Chan School of Public Health, Boston, MA USA.
Prof Chii-Dean Lin, Department of Mathematics and Statistics, San Diego State University, San Diego, CA USA.
Haruna Lule, Formerly: Gombe Hospital, Gombe, Uganda.
Prof Susan M. Kiene, Division of Epidemiology and Biostatistics, School of Public Health, San Diego State University, San Diego, CA USA.
References
- 1.Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med. 2012;9(12):e1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hensen B, Taoka S, Lewis JJ, Weiss HA, Hargreaves J. Systematic review of strategies to increase men’s HIV-testing in sub-Saharan Africa. AIDS. 2014;28(14):2133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan BT, Tsai AC. HIV stigma trends in the general population during antiretroviral treatment expansion: analysis of 31 countries in sub-Saharan Africa, 2003–2013. J Acquir Immune Defic Syndr. 2016;72(5):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly JD, Weiser SD, Tsai AC. Proximate Context of HIV Stigma and Its Association with HIV Testing in Sierra Leone: A Population-Based Study. AIDS Behav. 2016;20(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalichman SC, Simbayi L. HIV Testing Attitudes, AIDS Stigma and Voluntary HIV Counseling and Testing in a Black Township in Cape Town, South Africa. Sexually Transmitted Infections. 2003;79(6):442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roura M, Urassa M, Busza J, Mbata D, Wringe A, Zaba B. Scaling up stigma? The effects of antiretroviral roll-out on stigma and HIV testing. Early evidence from rural Tanzania. Sex Transm Infect. 2009;85(4):308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treves-Kagan S, El Ayadi AM, Pettifor A, MacPhail C, Twine R, Maman S, et al. Gender, HIV Testing and Stigma: The Association of HIV Testing Behaviors and Community-Level and Individual-Level Stigma in Rural South Africa Differ for Men and Women. AIDS Behav. 2017;21(9):2579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genberg BL, Hlavka Z, Konda KA, Maman S, Chariyalertsak S, Chingono A, et al. A comparison of HIV/AIDS-related stigma in four countries: negative attitudes and perceived acts of discrimination towards people living with HIV/AIDS. Soc Sci Med. 2009;68(12):2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukolo A, Blevins M, Victor B, Paulin HN, Vaz LM, Sidat M, et al. Community stigma endorsement and voluntary counseling and testing behavior and attitudes among female heads of household in Zambezia Province, Mozambique. BMC Public Health. 2013;13:1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assen A, Molla F, Wondimu A, Abrha S, Melkam W, Tadesse E, et al. Late presentation for diagnosis of HIV infection among HIV positive patients in South Tigray Zone, Ethiopia. BMC Public Health. 2016;16:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamasiya B, Nyikahadzoi K, Mukamuri BB. Factors influencing smallholder farmers’ behavioural intention towards adaptation to climate change in transitional climatic zones: A case study of Hwedza District in Zimbabwe. J Environ Manage. 2017;198(Pt 1):233–9. [DOI] [PubMed] [Google Scholar]
- 12.Chan BT, Weiser SD, Boum Y, Siedner MJ, Mocello AR, Haberer JE, et al. Persistent HIV-related stigma in rural Uganda during a period of increasing HIV incidence despite treatment expansion. AIDS. 2015;29(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mambanga P, Sirwali RN, Tshitangano T. Factors contributing to men’s reluctance to seek HIV counselling and testing at Primary Health Care facilities in Vhembe District of South Africa. Afr J Prim Health Care Fam Med. 2016;8(2):e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mugoya GC, Ernst K. Gender differences in HIV-related stigma in Kenya. AIDS Care. 2014;26(2):206–13. [DOI] [PubMed] [Google Scholar]
- 15.Kalichman S, Katner H, Banas E, Kalichman M. Population Density and AIDS-Related Stigma in Large-Urban, Small-Urban, and Rural Communities of the Southeastern USA. Prev Sci. 2017;18(5):517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girma E, Gebretsadik LA, Kaufman MR, Rimal RN, Morankar SN, Limaye RJ. Stigma against people with HIV/AIDS in rural Ethiopia, 2005 to 2011: signs and predictors of improvement. AIDS Behav. 2014;18(6):1046–53. [DOI] [PubMed] [Google Scholar]
- 17.Lyons A, Hosking W, Rozbroj T. Rural-urban differences in mental health, resilience, stigma, and social support among young Australian gay men. J Rural Health. 2015;31(1):89–97. [DOI] [PubMed] [Google Scholar]
- 18.Ledgerwood A, Wang YA. Achieving local and global shared realities: distance guides alignment to specific or general social influences. Curr Opin Psychol. 2018;23:62–5. [DOI] [PubMed] [Google Scholar]
- 19.UNAIDS. UNAIDS Data: UNAIDS; 2017. [Available from: https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf.
- 20.Kiene SM, Kalichman SC, Sileo KM, Menzies NA, Naigino R, Lin CD, et al. Efficacy of an enhanced linkage to HIV care intervention at improving linkage to HIV care and achieving viral suppression following home-based HIV testing in rural Uganda: study protocol for the Ekkubo/PATH cluster randomized controlled trial. BMC Infect Dis. 2017;17(1):460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalichman SC, Simbayi LC, Jooste S, Toefy Y, Cain D, Cherry C, et al. Development of a brief scale to measure AIDS-related stigma in South Africa. AIDS Behav. 2005;9(2):135–43. [DOI] [PubMed] [Google Scholar]
- 22.Anselin L. Local indciators of spatial association -LISA. Geographical Analysis. 1995;27:92–115. [Google Scholar]
- 23.esri. Cluster and Outlier Analysis (Anselin Local Moran’s I): esri; 2018. [Available from: https://pro.arcgis.com/en/pro-app/tool-reference/spatial-statistics/cluster-and-outlier-analysis-anselin-local-moran-s.htm. [Google Scholar]
- 24.UNAIDS. 90–90-90: An ambitious treatment target to help end the AIDS epidemic. UNAIDS; 2014. [Google Scholar]
- 25.Gunn JK, Asaolu IO, Center KE, Gibson SJ, Wightman P, Ezeanolue EE, et al. Antenatal care and uptake of HIV testing among pregnant women in sub-Saharan Africa: a cross-sectional study. J Int AIDS Soc. 2016;19(1):20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebregziabher M, Dai L, Vrana-Diaz C, Teklehaimanot A, Sweat M. Gender Disparities in Receipt of HIV Testing Results in Six Sub-Saharan African Countries. Health Equity. 2018;2(1):384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkins JM, Nyakato VN, Kakuhikire B, Mbabazi PK, Perkins HW, Tsai AC, et al. Actual Versus Perceived HIV Testing Norms, and Personal HIV Testing Uptake: A Cross-Sectional, Population-Based Study in Rural Uganda. AIDS Behav. 2018;22(2):616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chimoyi L, Tshuma N, Muloongo K, Setswe G, Sarfo B, Nyasulu PS. HIV-related knowledge, perceptions, attitudes, and utilisation of HIV counselling and testing: a venue-based intercept commuter population survey in the inner city of Johannesburg, South Africa. Glob Health Action. 2015;8:26950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan BT, Tsai AC. Personal contact with HIV-positive persons is associated with reduced HIV-related stigma: cross-sectional analysis of general population surveys from 26 countries in sub-Saharan Africa. J Int AIDS Soc. 2017;20(1):21395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winskell K, Hill E, Obyerodhyambo O. Comparing HIV-related symbolic stigma in six African countries: social representations in young people’s narratives. Soc Sci Med. 2011;73(8):1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambisa W, Curtis S, Mishra V. AIDS stigma as an obstacle to uptake of HIV testing: evidence from a Zimbabwean national population-based survey. AIDS Care. 2010;22(2):170–86. [DOI] [PubMed] [Google Scholar]
- 32.Bond L, Lauby J, Batson H. HIV testing and the role of individual- and structural-level barriers and facilitators. AIDS Care. 2005;17(2):125–40. [DOI] [PubMed] [Google Scholar]
- 33.Turan B, Hatcher AM, Weiser SD, Johnson MO, Rice WS, Turan JM. Framing Mechanisms Linking HIV-Related Stigma, Adherence to Treatment, and Health Outcomes. Am J Public Health. 2017;107(6):863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sileo KM, Reed E, Kizito W, Wagman JA, Stockman JK, Wanyenze RK, et al. Masculinity and engagement in HIV care among male fisherfolk on HIV treatment in Uganda. Cult Health Sex. 2019;21(7):774–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson GZ, Reinius M, Eriksson LE, Svedhem V, Esfahani FM, Deuba K, et al. Stigma reduction interventions in people living with HIV to improve health-related quality of life. Lancet HIV. 2020;7(2):e129–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.