Abstract
The roles of mitogen-activated protein kinases (MAPKs) in plant–fungal pathogenic interactions are poorly understood in crops. Here, microscopic, phenotypic, proteomic, and biochemical analyses revealed that roots of independent transcription activator-like effector nuclease (TALEN)-based knockout lines of barley (Hordeum vulgare L.) MAPK 3 (HvMPK3 KO) were resistant against Fusarium graminearum infection. When co-cultured with roots of the HvMPK3 KO lines, F. graminearum hyphae were excluded to the extracellular space, the growth pattern of extracellular hyphae was considerably deregulated, mycelia development was less efficient, and number of appressoria-like structures and their penetration potential were substantially reduced. Intracellular penetration of hyphae was preceded by the massive production of reactive oxygen species (ROS) in attacked cells of the wild-type (WT), but ROS production was mitigated in the HvMPK3 KO lines. Suppression of ROS production in these lines coincided with elevated abundance of catalase (CAT) and ascorbate peroxidase (APX). Moreover, differential proteomic analysis revealed downregulation of several defense-related proteins in WT, and the upregulation of pathogenesis-related protein 1 (PR-1) and cysteine proteases in HvMPK3 KO lines. Proteins involved in suberin formation, such as peroxidases, lipid transfer proteins (LTPs), and the GDSL esterase/lipase (containing “GDSL” aminosequence motif) were differentially regulated in HvMPK3 KO lines after F. graminearum inoculation. Consistent with proteomic analysis, microscopic observations showed enhanced suberin accumulation in roots of HvMPK3 KO lines, most likely contributing to the arrested infection by F. graminearum. These results suggest that TALEN-based knockout of HvMPK3 leads to barley root resistance against Fusarium root rot.
TALEN-based knockout lines of HvMPK3, showing elevated antioxidant capacity mitigating ROS production and root cell wall stiffening by suberin, confer barley root resistance to Fusarium graminearum.
Introduction
Cereals are a major food staple for the world population; however, there is a substantial reduction in the annual yield because of pathogens, weeds, temperature extremes, high salt concentrations, drought, and arid conditions (Sewelam et al., 2016). Diseases caused by fungal pathogens have been responsible for the destruction of cereal crop yield worldwide in the last years (Rózewicz et al., 2021).
To combat invading pathogens, plants recognize microbe-associated molecular patterns (MAMPs), and damage-associated molecular patterns (DAMPs), which are essential for triggering plant immunity (Tsuda and Katagiri, 2010). MAMPs and DAMPs elicit the so-called pattern-triggered immunity (PTI), which is mediated by plant pattern recognition receptors (Zipfel, 2014). PTI is characterized by a series of events, including signaling through protein kinase cascades, generation of reactive oxygen species (ROS), calcium ion influx, transcriptional reprogramming, cell wall appositions, and hormonal changes (Zipfel, 2009; Bigeard et al., 2015). ROS play a dual role during plant immune responses. In addition to signaling functions (Qi et al., 2017), their over-accumulation leads to the hypersensitive response, which hinders the progression of the biotrophic pathogens to the plant tissues (Camejo et al., 2016; Camagna and Takemoto, 2018). In contrast, necrotrophic pathogens may benefit from nutrients provided by the damaged tissues, and thus ROS accumulation facilitates the invasion of the pathogen into the tissues (Barna et al., 2012; Kámán-Tóth et al., 2019). During the biotrophic growth phase, hemibiotrophic fungi may trigger ROS production in the host cells leading to the elicitation of defense responses. Nevertheless, robust ROS accumulation associated with cell death occurs during the necrotrophic stage, allowing the pathogen invasion to the host tissues (Kumar et al., 2002; Shetty et al., 2007).
Mitogen-activated protein kinases (MAPKs) are integrated in signaling cascades responsible for conveying signals generated by extracellular and intracellular stimuli (Komis et al., 2011, 2018). In Arabidopsis (Arabidopsis thaliana L.) and rice (Oryza sativa L.), there are several MAPKs described, of which mostly A. thaliana MAPK 3 (AtMPK3), AtMPK4, and AtMPK6 and their rice orthologs are responsible for plant resistance against pathogens (Kishi-Kaboshi et al., 2010; Meng and Zhang, 2013; Bigeard et al., 2015). The signaling pathway involving AtMPK3/AtMPK6 pair is responsible for the regulation of camalexin biosynthesis (a major phytoalexin found in Cruciferae plants) during infection of Arabidopsis by necrotrophic fungal pathogens (Mao et al., 2011). However, in the case of bacterial infection, AtMPK4 is responsible for its regulation (Qiu et al., 2008). MPK3/MPK6 in different plant species also regulate hypersensitive cell death responses and the generation of ROS (Ren et al., 2002; Liu et al., 2007). The importance of MAPK signaling in plant–pathogen interactions is also supported by studies of bacterial effectors, several of which target and inhibit plant MAPK cascades (Cui et al., 2010; Eschen-Lippold et al., 2016). Last genome-wide study using an updated reference genome reported 20 MAPKs, 6 MAPKKs, and 156 MAPKKKs in barley (Hordeum vulgare L.), adopting a nomenclature based on orthology relationships to Arabidopsis MAPKs (Cui et al., 2019). Recently, we showed that TALEN-induced knockout mutations in HvMPK3, an ortholog of AtMPK3, attenuated the responsivity of barley to bacterial PAMP flagellin 22 (flg22), manifested by decreased abundance of chitinases and pathogenesis-related (PR) proteins (Takáč et al., 2021).
Fusarium graminearum causes a major loss of barley yield due to head blight and root rot diseases. This fungus is considered a hemibiotrophic pathogen, living in the host plants for a short period (hours to several days), before switching to necrotrophic form, which retrieves nutrients from dead cells (Trail, 2009;Walter et al., 2010; Ma et al., 2013). Although Fusarium head blight was earlier believed as a primary disease caused by F. graminearum, the root colonization by this pathogen is currently recognized as very important for immense economic losses. Fusarium root rot causes rapid necrosis, leading to a substantial reduction in root growth and biomass, which is accompanied by the progression of the pathogen to the stem base (Wang et al., 2015). The growth-inhibiting impact of the pathogen was assigned to the production of the mycotoxin deoxynivalenol (DON) (Masuda et al., 2007). The hyphae colonize intracellular and intercellular spaces in the root cortex in sensitive wheat (Triticum aestivum L.) cultivars, while the invasion in resistant cultivar is stopped at the epidermal cells (Wang et al., 2015). Barley defense mechanisms against Fusarium root rot are poorly understood. So far, these resistance strategies involved de novo biosynthesis of barley root exudates (Lanoue et al., 2010), activation of jasmonic acid (JA)-dependent defense genes and genes related to DON detoxification (Wang et al., 2018). The role of MAPKs in barley responses to Fusarium has not been elucidated, and their participation in Fusarium-induced signal transduction is unknown.
In this study, we discovered that independent barley lines with TALEN-based knockout mutations of HvMPK3 exhibited higher root resistance to F. graminearum and produced less ROS than wild-type (WT) plants. Fusarium hyphae potential to penetrate the root cells of HvMPK3 KO lines substantially decreased. This was accompanied by the attenuation of defense responses in WT plants and upregulated levels of cysteine proteases and secretory peroxidases (PRXs) in HvMPK3 KO lines, as documented by proteomic analysis. Our results indicate that ROS generation, might facilitate the invasion of WT plants by F. graminearum, but elevated abundances of cytosolic ascorbate peroxidase (cAPX) and catalase might reduce ROS levels, thus contributing to the higher resistance of HvMPK3 KO lines. Finally, the stiffening of the cell walls by suberin deposition likely represents a barrier, which prevents pathogen invasion to the root cells of HvMPK3 KO lines.
Results
Phenotype of WT and HvMPK3 KO plants infected by F. graminearum
Fungal mycelium developed extensively after infecting the roots of 5-day-old barley seedlings by F. graminearum conidia. Ten days after infection, densely developed mycelium surrounded seeds and basal parts of the root system in WT plants (Supplemental Figure S1A). Roots of WT plants were largely arrested in their growth (Supplemental Figure S1A, black arrows). In contrast, the root system of HvMPK3 KO-A (Supplemental Figure S1B), HvMPK3 KO-B (Supplemental Figure S1C), and HvMPK3 KO-D (Supplemental Figure S1D) lines inoculated by F. graminearum developed well without any visible reduction of root growth. In addition, the development of F. graminearum mycelia itself was inhibited, particularly around the seeds and roots (Supplemental Figure S1, B–D). Dark brown coloration of F. graminearum mycelia appeared close to the seeds and the basal parts of the root system of HvMPK3 KO lines (Supplemental Figure S1, B–D). On WT genotypes, however, F. graminearum mycelia showed pale white color and massive coverage of infected plants (Supplemental Figure S1A).
Inoculation of control seedling roots (Figure 1, A–D) with F. graminearum spores led to their germination and subsequent colonization of roots by growing mycelia. In a period of 24 h after inoculation (Figure 1, E–H), the root apex of WT plants was massively invaded by green fluorescent protein (GFP)-expressing F. graminearum hyphae (Figure 1E). However, only sparse hyphae were present on the root surface of HvMPK3 KO lines (Figure 1, F–H). Propidium iodide (PI) staining of cell walls in living cells of uninfected plants (Figure 1, A–D) served as a marker of root tissue organization, while accumulation of PI in nuclei in dead cells of infected plants (Figure 1, E–H) indicated their mortality after penetration by F. graminearum hyphae. The cell death in the epidermis of WT roots colonized by F. graminearum was evident (Figure 1E), while the amount of dead epidermal cells in inoculated roots of HvMPK3 KO lines was considerably low (Figure 1, F–H). The quantitative analysis of cell death rate revealed a statistically significant increase in infected WT plants. In contrast, there was no statistical difference in the number of dead root epidermal cells between uninfected plants and plants analyzed 24 h after inoculation in HvMPK3 KO lines (Figure 1I).
Figure 1.
Colonization of root in barley wild-type (WT) and HvMPK3 KO lines by F. graminearum mycelia 24 and 48 h after inoculation with spores. A–H, Overview of propidium iodide-labeled uninfected root apex of WT (A), HvMPK3 KO-A (B), HvMPK3 KO-B (C), and HvMPK3 KO-D (D) lines under control conditions, and 24 h after inoculation of roots with spores of GFP-expressing F. graminearum in WT (E), HvMPK3 KO-A (F), HvMPK3 KO-B (G), and HvMPK3 KO-D (H) lines. Note propidium iodide staining of cell walls in living cells and nuclei in dead cells. I, Quantitative evaluation of the proportion of dead cells in control and infected root apices among tested barley lines. Data are presented as means ± standard deviation (sd); n = 20 roots/line, 70–90 epidermal cells evaluated in each root. Different lowercase letters above the error bars (I) represent statistical significance according to one-way analysis of variance (ANOVA) and subsequent the least significant difference (LSD) test at P-value < 0.05. J–M, Roots of WT (J), HvMPK3 KO-A (K), HvMPK3 KO-B (L), and HvMPK3 KO-D (M) lines stained with propidium iodide 48 h after inoculation with spores of GFP-expressing F. graminearum. Hyphae growing on root surface in parallel orientation with elongated root epidermal cells are indicated by white arrows, hyphae growing away and not touching roots are indicated by yellow arrows, zones occupied by malformed mycelia with wavy growth pattern, growing perpendicularly to elongated root epidermal cells are indicated by asterisks. Composite images in (J–M) were created from three consequential frames in y-axis. Scale bars: 50 µm (A–H) and 100 µm (J–M).
Examination of plants 48 h after inoculation with F. graminearum showed that roots of WT plants were massively colonized by the fungal mycelium (Figure 1J), but mycelium was much less developed around the infected area on the root surface in HvMPK3 KO lines (Figure 1, K–M). Surprisingly, this fact was related to different growth pattern of hyphae, including considerable changes in their density, but also in the shape of developing mycelium. On the surface of WT roots, the extracellular hyphae were growing in parallel with longitudinal root axis and closely associated with the surface of root epidermal cells (Figure 1J). In contrast to Fusarium-treated WT, hyphae were growing away from the roots and often without touching the root surface of HvMPK3 KO lines (Figure 1, K–M). In addition, mycelium growing around HvMPK3 KO roots was malformed with unusual wavy growth pattern of hyphae, and showed changed growth direction oriented perpendicularly to the axis of elongated root epidermal cells (Figure 1, K–M). Using analysis of the angular distribution of growing mycelia, we quantitatively determined a degree of anisotropy of hyphal distribution at the root surface. In the WT roots, the graph shows almost uniform longitudinal orientation of hyphae relative to longitudinal axis of the root (Supplemental Figure S2A). Conversely, mycelia around root surfaces of HvMPK3 KO lines showed more random organization (Supplemental Figure S2, B–D). Quantitative evaluation revealed higher average values for angular distribution of hyphae on WT roots (Supplemental Figure S2E), suggesting prevalence of hyphae arrangement in particular orientation. On the other hand, statistically significant lowering of values determining an isotropy characterized by random distribution of hyphae with no prevalent orientation, was found in HvMPK3 KO lines (Supplemental Figure S2E). We also analyzed fluorescence skewness, defining a pattern of fluorescence intensity distribution on the root surface and comparing how a ratio between high and low fluorescence intensities distribution is changing. This analysis revealed a high degree of fluorescence uniformity (referred by lower values) on the surface of WT roots (Supplemental Figure S2F). This indicates that WT root surface was rather uniformly covered by a high density of fluorescent mycelia. On the other hand, a high degree of nonuniformity of the fluorescence intensity distribution was documented by higher values of fluorescence skewness in HvMPK3 KO roots (Supplemental Figure S2F), indicating that the root surface of the HvMPK3 KO lines was only sparsely covered with a low density of fluorescent mycelium.
In comparison to uninfected WT plants (Figure 2A), analysis of root system phenotypes of F. graminearum-treated WT plants (10 days after inoculation) revealed a reddish-brown coloration and malformation of some roots (Figure 2E). However, a comparison of uninfected roots of HvMPK3 KO lines (Figure 2, B–D) with infected ones (Figure 2, F–H) showed no change. Roots in the basal part of the root systems of HvMPK3 KO lines did not exhibit a reddish-brown coloration (Figure 2, F–H). The morphology (Supplemental Figure S3) and the length (Figure 2I) of roots measured 10 days after inoculation revealed that F. graminearum infection significantly reduced the root length in the WT plants, but there was no significant difference between control versus infected HvMPK3 KO lines (Figure 2I). These data indicate insensitivity of HvMPK3 KO plants to F. graminearum infection in early seedling stages, and sustained root growth without deleterious effects of the pathogen.
Figure 2.
Comparison of root phenotypes in barley wild-type (WT) and HvMPK3 KO lines between nontreated and F. graminearum-treated plants 10 days after inoculation with spores. A–H, Morphology of roots in the basal part of the root system of the uninfected plant of WT (A), HvMPK3 KO-A (B), HvMPK3 KO-B (C), and HvMPK3 KO-D (D) lines under control conditions, and 10 days after inoculation in WT (E), HvMPK3 KO-A (F), HvMPK3 KO-B (G), and HvMPK3 KO-D (H) lines. Progression of infection and the rate of root damage is indicated by a reddish-brown coloration originating from the F. graminearum mycelia. Note apparent morphological changes in roots of WT (E, arrowheads) that are not present in roots of HvMPK3 KO-A (F), HvMPK3 KO-B (G), and HvMPK3 KO-D (H) lines. Uncropped original images of the tested barley plants are presented in Supplemental Figure S3. Scale bars: 1 cm. I, Quantitative evaluation of the root length in WT and HvMPK3 KO lines without infection (Control), and 10 days after infection with F. graminearum spores (with Fusarium). Data are presented as means ± standard deviation (sd); n = 30 plants/line. Different lowercase letters above the error bars (I) represent statistical significance according to one-way ANOVA and subsequent LSD test at P < 0.05.
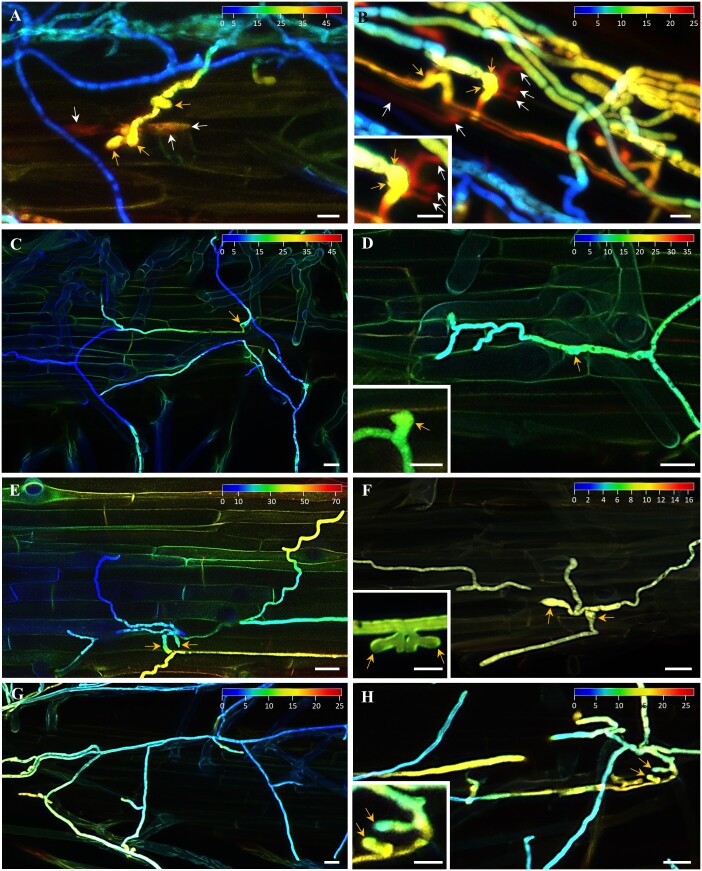
Growth of the mycelium on the root surface precedes the hyphae invasion to the root cells by cell wall penetration. Volumetric fluorescence visualization of GFP-expressing F. graminearum hyphae on the root surface allowed not only qualitatively determine the distribution and density of mycelium, but also its penetration ability. Therefore, we documented the mycelium distribution on the root surface and the depth of hyphal invasion to root epidermal cells by a color-coded fluorescence intensity distribution along the Z-axis of imaging. In WT roots 48 h after inoculation with spores, such analysis revealed formation of appressoria-like structures and infection hyphae invading the root epidermal cells. Close contacts of appressoria-like structures with root cell surface and intracellular location of invading infection hyphae were clearly distinguished from external mycelium according to distinct fluorescence color coding (Figure 3, A and B). In sharp contrast, the same analysis did not reveal penetration and intracellular infection hyphae formation in the root epidermis of HvMPK3 KO lines (Figure 3, C–H). Overall, microscopic analysis revealed formation of dense mycelium on the surface of WT roots, with frequent infection hyphae penetration to the root epidermal cells (Supplemental Figure S4, A and B). In contrary, low-density surface mycelium with different growth pattern, and no infection hyphae penetration in the root epidermal cells were revealed in HvMPK3 KO lines (Supplemental Figure S4, C–H). Moreover, occasional F. graminearum appressoria-like structures developed on root surfaces of HvMPK3 KO lines were often abnormal in shape and size. While the appressoria-like structures on WT roots were more complex (yellow arrows in Figure 3, A and B, inset in 3B), running hyphae produced rather foot structures only, with no visible penetration to root epidermal cells in HvMPK3 KO lines (yellow arrows in Figure 3, C–H, insets in 3, D, F, and H). Differences in appressoria-like structures morphology between F. graminearum mycelium growing on the surface of WT roots (Figures 3, A, B and 4, A) and roots of HvMPK3 KO lines (Figures 3, C and H and 4, B–D) prompted us to analyze appressoria-like structure formation capacity, which may be directly related also with mycelium penetration ability to the root epidermal cells. We have found significantly lower numbers of appressoria-like structures on the surface of HvMPK3 KO roots compared to the WT (Figure 4E). Therefore, data from phenotypical analysis revealed different responses of WT and HvMPK3 KO roots to F. graminearum infection.
Figure 3.
Visualization of volumetric fluorescence signal distribution of F. graminearum hyphae growing on root surface of barley wild-type (WT) and HvMPK3 KO lines 48 h after inoculation with spores. Depth of the hyphae localization is indicated by a color-coding depicted from the root surface to deep inside the root tissue. The color range in each image is indicated in µm. A and B, Formation of appressoria-like structures (yellow arrows) and infection hyphae (white arrows) penetrating cell wall of epidermal cells in roots of WT plants. Inset in (B) shows detailed 3D visualization of appressorium-like structure (yellow arrows) and infection hyphae (white arrows). C–H, Developed mycelia on root surface of HvMPK3 KO-A (C and D), HvMPK3 KO-B (E and F), and HvMPK3 KO-D (G and H) lines. Appressoria-like structures and foot structures are indicated by yellow arrows, cell wall penetration, and formation of infection hyphae were not detected by color-coded analysis. Insets in (D, F, and H) show detailed 3D visualization of foot structures (yellow arrows). The inset in (H) shows the magnified structures indicated by arrows in the larger image, whereas insets in (D) and (F) are different from the structures shown in the larger images. Scale bars: 10 µm (A and B), 10 µm (insets in B, D, F, and H) and 20 µm (C–H).
Figure 4.
Morphology of F. graminearum appressoria-like structures and their number on root surface of barley wild-type (WT) and HvMPK3 KO lines 48 h after inoculation with spores. A–D, Mycelium density and the appressoria-like structure morphology 48 h after inoculation with F. graminearum spores on the root surface of WT (A), HvMPK3 KO-A (B), HvMPK3 KO-B (C), and HvMPK3 KO-D (D) lines. Appressoria-like structures are indicated by arrows. Scale bars: 20 µm. E, Quantitative evaluation of the average number of appressoria-like structures developed on the surface of infected roots. Data are represented as mean ± standard deviation (sd); n = 10 images/line. Different lowercase letters above the error bars represent statistical significance according to one-way ANOVA and subsequent LSD test at P < 0.05.
Fusarium-induced ROS production in barley roots
ROS production in infected plant cells is associated with the early recognition of pathogen at the apoplast, and is accompanied by sudden oxidative burst (Qi et al., 2017). Therefore, ROS production in roots of WT and HvMPK3 KO lines was analyzed by CM-H2DCFDA fluorescence detection 24 h after inoculation with F. graminearum spores. This analysis revealed a substantially higher ROS accumulation in WT (Figure 5A), compared to the HvMPK3 KO lines (Figure 5, B–D). Consequently, quantitative evaluation of the fluorescent staining levels showed that ROS accumulation was substantially higher in the infected WT roots than in infected roots of HvMPK3 KO lines (Figure 5E). Independent spectrophotometric examination of ROS levels in the infected WT and HvMPK3 KO lines showed results consistent with these microscopic observations (Supplemental Figure S5). In WT plants, ROS production in cells attacked by fungus preceded fungal hyphae penetration (Figure 5F). Such cells in the preinvasion stage were still alive and accumulated a high amount of ROS, while collapsed protoplasts and disintegrated cytoplasm in other surrounding cells with absent ROS fluorescence signal indicated that they were already dead (Figure 5F). In the root apex of WT plants, ROS production and accumulation occurred in cells not yet penetrated with F. graminearum infection hyphae, while in most of the root epidermal cells, ROS production was not detected (Figure 5G). Based on the staining of nuclei by PI, these cells were already dead and penetrated by F. graminearum infection hyphae (Figure 5G). Conversely, only a limited number of root epidermal cells appeared dead after PI staining of HvMPK3 KO lines 24 h after inoculation with F. graminearum spores (Figure 5, H–J). Fluorescence signal for ROS detection was not observed, which was related to the low number of F. graminearum hyphae growing around the roots and their minimal contact with the surface of root epidermal cells in HvMPK3 KO lines (Figure 5, H–J).
Figure 5.
Fluorescence detection of ROS production in roots of barley wild-type (WT) and HvMPK3 KO lines 24 h after inoculation with F. graminearum spores. A–D, Overview of the root apex labeled by CM-H2DCFDA probe 24 h after inoculation in WT (A), HvMPK3 KO-A (B), HvMPK3 KO-B (C), and HvMPK3 KO-D (D) lines. E, Quantitative evaluation of the fluorescence intensity determined in arbitrary units (AU) after CM-H2DCFDA labeling of control and inoculated roots. Quantification was performed 24 h after inoculation with F. graminearum. Data are presented as means ± standard deviation (sd); n = 15 plants/line. Different lowercase letters above the error bars represent statistical significance according to one-way ANOVA and subsequent LSD test at P < 0.05. F, Increased ROS production in root epidermal cells of WT plant treated with mCherry-expressing F. graminearum (arrows) for 24 h. Note the close contact of ROS-producing cell and the mycelia (arrowhead) before infection. G–J, Local view of the infected root zone 24 h after inoculation with GFP-expressing F. graminearum and subsequently labeled by CM-H2DCFDA for ROS and by propidium iodide for cell walls in living cells and for nuclei in dead cells in WT (G), HvMPK3 KO-A (H), HvMPK3 KO-B (I), and HvMPK3 KO-D (J) lines. ROS-producing cells are indicated by arrows. Scale bars: 1 mm (A–D), 50 µm (G–J), 10 µm (F).
Differential proteomic analysis
To elucidate resistance mechanisms of HvMPK3 KO lines against F. graminearum at molecular level, root proteomes of WT and HvMPK3 KO lines infected with F. graminearum were compared to mock controls 24 h after infection.
In total, we have identified 133 differentially abundant proteins in F. graminearum-treated WT plants, of which 43 were upregulated and 90 were downregulated in infected versus control plants. In HvMPK3 KO lines, 94 differentially abundant proteins were found, from which 47 were upregulated and the same number (47) was downregulated in infected versus control plants (Supplemental Tables S1 and S2). We evaluated the differential proteomes of WT and HvMPK3 KO lines using gene ontology (GO) annotation analysis (Supplemental Figures S6 and S7).
Here we compared the previously published differential proteome of HvMPK3 KO lines exposed to flg22 (Takáč et al., 2021) with the differential proteome of the same lines treated with Fusarium (Figures 6 and 7). In contrast to flg22, Fusarium caused the differential abundance of lipid transfer proteins (LTPs), histone isoforms, and proteins involved in ROS regulation (Figure 6). In more detail, abundances of histone isoforms were upregulated in WT, but downregulated in HvMPK3 KO lines treated with Fusarium (Figure 6). LTPs were distinctly affected in HvMPK3 KO lines compared to WT (Figure 6). Putative lipid transfer-like protein DEFECTIVE IN INDUCED RESISTANCE (DIR1) and nonspecific LTP1 were upregulated while YLS3 was downregulated in infected WT lines. In HvMPK3 KO lines, Fusarium caused upregulations of two VAS lipid transfer-like proteins, and downregulation of CW21 nonspecific LTP.
Figure 6.
Heat map depicting the fold changes of defense-related proteins, histones, and proteins involved in redox regulation differentially regulated in wild-type (WT) and HvMPK3 KO plants in response to F. graminearum (this study) and flg22 treatment (Takáč et al., 2021). Fold changes are presented in color as indicated by the color key on the right. White color indicates that the protein was not detected, or the difference in protein abundance was not statistically significant. Proteins unique for either control or treated samples were found solely in the proteomes of control or treated samples, considering all analyzed replicates (JA, jasmonic acid; PR, pathogenesis-related; PRX, peroxidases; LTPs, lipid transfer proteins).
Figure 7.
Heat map depicting the fold changes of protein involved in protein degradation, abiotic stress response, cell wall modification, membrane transport and cytoskeleton, differentially regulated in wild-type (WT) and HvMPK3 KO plants in response to F. graminearum (this study) and flg22 treatment (Takáč et al., 2021). Fold changes are presented in color as indicated by the color key on the right. White color indicates that the protein was not detected, or the difference in protein abundance was not statistically significant. Proteins unique for either control or treated samples were found solely in the proteomes of control or treated samples, considering all analyzed replicates.
Concerning proteins involved in redox regulation, peptide methionine sulfoxide reductase B5 and thioredoxin domain-containing protein 9 were downregulated, while glutathione S-transferase U6 (GSTU6) was upregulated in Fusarium-treated WT (Figure 6). Peptide methionine sulfoxide reductase B3 and GSTU1 were negatively affected by Fusarium treatment in HvMPK3 KO lines (Figure 6). Monodehydroascorbate reductase 5 (involved in ascorbate regeneration) was downregulated, while thioredoxin M2 (a chloroplastic redox buffering protein) was upregulated in HvMPK3 KO plants (Figure 6).
Fusarium also deregulated secretory PRXs in barley. The differential proteome of HvMPK3 KO lines contained five secretory PRXs and WT only three. Notably, abundances of secretory PRXs were decreased in WT, while three out of five secretory PRXs were upregulated in the Fusarium-treated HvMPK3 KO lines (Figure 6). Using specific activity staining on native gels, we have found that HvMPK3 KO lines exhibited also increased activities of three peroxidase isoforms (Rf 0.63, 0.90, and 0.98), as compared to the WT plants after Fusarium treatment (Figure 8, A and B; Supplemental Figure S8). To assess the abundance of major H2O2-decomposing enzymes, cAPX and catalase were analyzed using immunoblot assays. We detected cAPX in the barley root extracts by employing anti-APX antibody (Figure 8, C and D; Supplemental Figure S9). Remarkably, the abundance of cAPX increased in HvMPK3 KO lines, while it decreased in WT plants. In addition, we observed an increase of catalase abundance in both analyzed HvMPK3 KO lines, while it slightly decreased in WT (Figure 8, E and F; Supplemental Figure S10). These results suggest that HvMPK3 KO lines likely exert an increased capacity to decompose H2O2 during F. graminearum infection.
Figure 8.
Specific activity of peroxidases and abundances of ascorbate peroxidase and catalase in roots of barley wild-type (WT) and HvMPK3 KO lines (KO-A and KO-B) 24 h after inoculation with F. graminearum. A, Images of native polyacrylamide gels showing activity of peroxidases. B, Graphs showing quantifications of band optical intensities in (A). C, E, and G, Immunoblotting analyses of cytosolic ascorbate peroxidase (cAPX; C), catalase (E), and pathogenesis-related protein 1 (HvPR-1; G). M—marker, Ponceau S staining shows equal protein amount on the membrane. D, F, and H, Graphs showing quantifications of band optical intensities in (C, E, and G). Data are presented as means ± standard deviation (sd). Asterisks above the error bars represent statistically significant differences between control and treated samples according to Student’s t test at P < 0.05. Uncropped, full original images of the gels are documented in Supplemental Figures S8–S11.
Moreover, cysteine proteases were more affected by Fusarium than by flg22. They were downregulated in WT but mostly upregulated in HvMPK3 KO lines (Figure 6). Next, we identified more proteins belonging to the family of heat shock proteins in the Fusarium-induced differential proteome. The majority of them was downregulated in HvMPK3 KO lines (Figure 7).
Proteins involved in cell wall modifications and abiotic stress response were more affected by Fusarium as compared to flg22, and these protein groups mostly showed reduced abundances in WT. Finally, enzymes with ubiquitination and de-ubiquitination activities were also affected by Fusarium, but without differences between WT and HvMPK3 KO lines (Figure 7).
As HvMPK3 KO lines exhibited reduced colonization of roots by F. graminearum hyphae, we focused on proteins predicted to be localized to extracellular space (Supplemental Table S3). These included three cysteine proteinases, which were upregulated in HvMPK3 KO lines, and downregulated in WT. Furthermore, PR proteins (PR-1, thionin 2, and glucan endo-1,3-beta-glucosidase 4) were found downregulated in WT. Notably, PR-1 protein was upregulated in HvMPK3 KO lines. Immunoblotting analysis validated this differential abundance of PR-1 in WT and HvMPK3 KO lines (Figure 8, G and H; Supplemental Figure S11). Some of the above-mentioned LTPs were predicted as extracellular, including DIR1 putative lipid transfer-like protein, nonspecific LTP1, and two VAS lipid transfer-like proteins (Supplemental Table S3). LTPs together with another extracellular protein, GDSL esterase/lipase At5g33370, are involved in cutin and suberin formation in plants (Hong et al., 2017; Edqvist et al., 2018). Therefore, we examined suberin accumulation in roots of F. graminearum-treated WT and HvMPK3 KO lines using specific fluorescent dye Fluorol Yellow 088. This histochemical analysis showed stronger suberin deposition to the root surface cell layers of noninfected HvMPK3 KO lines (Figure 9, B, C, and G), unlike WT plants (Figure 9, A and G). The suberin histochemical staining at root surface cell layers was considerably enhanced in HvMPK3 KO lines after F. graminearum infection (Figure 9, E–G). However, in WT only a slight increase in suberin staining was observed (Figure 9, D and G). In addition, the suberin staining also increased in the root endodermis and stele of HvMPK3 KO lines (Figure 9, E and F). In contrast, histochemical staining of lignin with basic fuchsin showed no differences between WT and HvMPK3 KO lines under control conditions (Supplemental Figure S12, A–C and G). Fluorescence intensity of lignin staining of WT after treatment with F. graminearum reached only 45% when compared with control condition (Supplemental Figure S12, A, D, and G). On the other hand, both HvMPK3 KO lines showed only a moderate decrease in lignin staining, achieving 72% (HvMPK3 KO-A) and 82% (HvMPK3 KO-B), respectively, when compared to control conditions (Supplemental Figure S12, B, C, and E–G). These results suggest that a suberized cell wall barrier against F. graminearum invasion was built up at the surface layers of HvMPK3 KO roots.
Figure 9.
Suberin staining of barley roots 24 h after inoculation with F. graminearum spores. Freehand cross-sections of roots were stained with fluorescent suberin dye Fluorol Yellow 088 under control conditions (upper) and 24 h after inoculation with F. graminearum spores (lower). A and B, WT. C and D, HvMPK3 KO-A line. E and F, HvMPK3 KO-B line. Note strongly increased suberin staining in surface cell layers, endodermis and xylem of both HvMPK3 KO lines. G, Quantification of suberin staining in freehand barley root cross-sections. Fluorescence mean intensity is determined in arbitrary units (AU). Data are presented as means ± standard deviation (sd) from at least two roots of each barley line in three biological replicates (n = 6). Asterisks indicate statistically significant differences between control and treated samples of respective line, *P < 0.05, **P < 0.01, Student’s t test. Scale bars: 50 µm.
In conclusion, proteomic analysis showed that the resistance of HvMPK3 KO lines to F. graminearum was accompanied by increased abundances of defense-related proteins such as PR-1 and cysteine proteases, as well as differential regulation of proteins involved in suberin formation including GDSL esterase/lipase, secretory PRXs, and LTPs. Furthermore, it was corroborated by overabundances of cAPX and catalase. To summarize, our results indicate that the cell wall stiffening by suberin in surface root cells, most likely supported by the above-mentioned proteome changes, might substantially contribute to the resistance of HvMPK3 KO lines against F. graminearum (Figure 10).
Figure 10.
A proposed working model for barley HvMPK3 KO transgenic lines resistance to F. graminearum. After inoculation of barley wild-type (WT) seedling roots with F. graminearum spores, root cells are massively invaded by intracellular penetration hyphae, which is preceded by the massive production of ROS. Colonized root cells of WT were characterized by downregulation of pathogenesis-related (PR) proteins (type-1 PR protein, chitinase CLP, thionin THI2), cysteine proteases (CPs), peroxidases (PRXs), and lipid transfer proteins (LTPs, YLS3). In inoculated roots of HvMPK3 KO lines, growth of F. graminearum hyphae is excluded to the extracellular space with no infection hyphae penetration. It is preceded by the suppression of ROS production. Upregulation of PR protein 1, CPs, PRXs (PER12, cationic peroxidase PNC1, peroxidase HRPN), LTPs (lipid transfer-like protein VAS), and extracellular protein GDSL esterase/lipase contribute to altered defense response and especially to enhanced suberin production and cell wall deposition leading to the resistance against fungus.
Generally, we did not observe any changes in the above-ground phenotypes of WT versus HvMPK3 KO plants from early stages of development (Supplemental Figure S13A) to maturity (Supplemental Figure S13B). Nevertheless, when detached leaves of young plants (12 days old) were treated with F. graminearum spores, we observed that WT plants were susceptible to the pathogen (Supplemental Figure S14, A, B, and I), while at least partial resistance to F. graminearum was recorded in HvMPK3 KO lines (Supplemental Figure S14, C–I).
Discussion
The diversified family of MAPKs lies in the core of plant defense mechanisms due to their important role in extracellular stimuli perception. In addition, MAPK cascades are responsible for plant resistance to abiotic stresses and exert multiple developmental functions (Bigeard et al., 2015; Komis et al., 2018). Despite a substantial number of MAPK-related studies in dicots like Arabidopsis, the current understanding of MAPK roles in defense of cereal crops against pathogens is still limited. In cereals, MAPKs play either positive or negative roles in immune reactions against fungal pathogens (Křenek et al., 2015; Chen et al., 2021). In rice, the OsMKK4–OsMPK3/OsMPK6 module participates in transduction of a fungal chitin elicitor signal and regulates defense responses leading to the biosynthesis of diterpenoid phytoalexins and lignin, and progression of plant cell death (Kishi-Kaboshi et al., 2010). OsMPK3 negatively regulates the expression of defense genes and the defense reactions against hemibiotrophic blast fungus Magnaporthe grisea (Xiong and Yang, 2003). It was reported that this negative regulation is mediated through interaction with, and by phosphorylation of OsMPK3 via calcium-dependent protein kinase OsCPK18 (Xie et al., 2014). Wheat TaMPK3, unlike TaMPK6, is specifically activated, and its transcript levels as well as abundance are increased during compatible interactions with necrotrophic fungus Mycosphaerella graminicola (Rudd et al., 2008). This increase was directly associated with the onset of programmed cell death (PCD) and the generation of related PCD symptoms (Rudd et al., 2008). Barley MPK3 is upregulated in response to the rust fungus Puccinia hordei inoculation, especially during effector-triggered immunity (Křenek et al., 2015). Remarkably, HvMPK3 KO lines showed attenuated response to flg22 treatment in terms of defense-related proteins such as chitinases, indicating some positive role of HvMPK3 in PTI (Takáč et al., 2021).
Although Fusarium root rot is capable to cause severe yield losses, the plant defense mechanism against this disease has not been sufficiently investigated and understood yet. Role of MAPKs in cereal responses to Fusarium were not studied before. The resistance against Fusarium root rot was previously shown to be mediated by hindering the penetration of the fungi at the wheat epidermal cells (Ma et al., 2013; Wang et al., 2015). Our results suggest that the exclusion of F. graminearum to the extracellular space in the roots of HvMPK3 KO lines is likely caused by suberin deposition to the root surface of these lines, which is also supported by upregulation of proteins involved in suberin formation. We also observed decrease in lignin deposition, similarly as previously published for F. oxysporum treatment of flax and F. solani treatment of soybean (Lozovaya et al., 2006; Wojtasik et al., 2016). These developmental changes in roots may directly promote defense reactions against F. graminearum in HvMPK3 KO lines. It was reflected by nontargeted growth of F. graminearum hyphae toward the root, repelling the root surface mycelium from the touching, and by reducing the number of events of root epidermal cell penetration by specialized infection hyphae.
When compared to flg22 treatment (Takáč et al., 2021), F. graminearum infection had more obvious impact on the barley proteome, especially concerning changed abundance of extracellular proteins. This might be assigned to the hemibiotrophic pathosystem of the fungus, eliciting more complex defense mechanisms compared to bacterial elicitor flg22. Hence, this proteomic analysis allowed to gain a more detailed insight into defense mechanisms occurring in HvMPK3 KO lines and suggested that WT roots were compromised in defense response, most likely due to fungus-secreted toxin effectors. It was illustrated by the downregulation of PR17c precursor and several isoforms of cysteine proteases, representing well-known pathogen effector targets (Zhang et al., 2012; Mueller et al., 2013). Cysteine proteases are major regulators of plant defense responses (Misas-Villamil et al., 2016) and in contrast to WT, their abundance considerably increased in HvMPK3 KO lines, indicating that they might mediate resistance of these lines against F. graminearum.
Specialized infection structures called appressoria and appressoria-like structures are necessary for the F. graminearum infection. It was reported in plants like wheat, rice, maize (Zea mays L.), or barley attacked by different fungal pathogens, including rusts, powdery mildew, and blast diseases (Boenisch and Schäfer 2011; Qiu et al., 2019). To facilitate host penetration, appressoria of Magnaporthe oryzae generate enormous pressure up to 8.0 MPa that drive physically rupture of the host cell wall (Talbot, 2019). F. graminearum can produce unicellular swollen foot structures or multicellular appressoria-like structures called infection cushions (Boenisch and Schäfer 2011; Wang et al., 2015; Qiu et al., 2019), whereas the biology of these structures is not completely known. Here, the number of formed and invading appressoria-like structures was substantially higher in the case of WT as compared to the HvMPK3 KO lines. Twenty-four hours after infection, fungal hyphae successfully invaded the epidermal root cells of WT plants, which was preceded by the dramatic ROS increase and accumulation, leading to hyphae penetration to cells. This is entirely consistent with the previously described model of hemibiotrophic and necrotrophic fungal pathosystems (Desmond et al., 2008). In contrast, the production of ROS was substantially lower in infected HvMPK3 KO plants, partly due to prevention of the physical interaction between the pathogen and the root surface of transgenic plants, and partly also due to their elevated antioxidant capacity mediated by cAPX and catalase.
The ROS generated and released during infection can affect both counterparts, the host and the microbe (Qi et al., 2017). ROS are indispensable for the activation of plant defense responses as a signaling component (Lee et al., 2020) or they can cause peroxidation of lipids, oxidation of proteins, damage to nucleic acids, enzyme inhibition, and activation of PCD pathway during hypersensitive response (Camagna and Takemoto, 2018), thus leading to the inhibition of biotrophic pathogens. However, the Fusarium infection represents another example, since ROS accumulation is induced by its toxins in the host plants (Desmond et al., 2008). ROS produced by the host cells cause enhanced production of fungal toxins enabling hyphae penetration into the host cells (Walter et al., 2010).
Secretory PRXs were one of the most affected protein groups in both HvMPK3 KO and WT lines after F. graminearum infection. All peroxidase isoforms were downregulated in WT, but three out of five PRXs were upregulated in HvMPK3 KO lines in response to F. graminearum. Secretory PRXs belong to class III of plant PRXs, having a broad spectrum of activities in plants (Sasaki et al., 2004; Passardi et al., 2005; Almagro et al., 2009). Such PRXs belong to a PR protein 9 subfamily and are supposed to reduce the progression of pathogens by a generation of structural barriers. They are bifunctional because they exert either ROS-scavenging or ROS-producing activity (Passardi et al., 2004). Peroxidase activity contributes to the ROS generated during oxidative burst (O’Brien et al., 2012). ROS produced by PRXs may either have signaling roles or contribute to the toxic environment for the pathogen (Camejo et al., 2016).
One of the crucial features of secretory PRXs is to mediate cross-linking of cell wall components leading to cell wall reinforcement. They may cross-link extensins leading to the formation of large oligomers (Mishler-Elmore et al., 2021). The cross-linking of ferulic acid by covalent bonds requires the presence of cell wall PRXs. Such cross-linked ferulic acid may create intra polysaccharide or interpolysaccharide bonds leading to cell wall stiffening (Fry, 2004). Lignin formation depends on oxidative cross-linking of monolignols (Ralph et al., 2004), and expression of PRXs was shown to be associated with lignification (Sorokan et al., 2014; Cosio et al., 2017). Similarly, suberin poly-phenolic domain assembly requires peroxidase-mediated oxidative coupling reactions (Bernards et al., 2004). Moreover, transient overexpression of HvPrx40, a gene encoding class III peroxidase, in barley epidermis improves resistance against Blumeria graminis f.sp. hordei pathogen (Johrde and Schweizer, 2008). It is likely that PRXs similar to peroxidase 12 (A. thaliana), cationic peroxidase PNC1 (Arachis hypogaea), and peroxidase HRPN (Armoracia rusticana) contribute to the formation of suberized structural barriers which exclude F. graminearum to the extracellular space in HvMPK3 KO plants. Moreover, a genomic study reported a possible link of GDSL esterase/lipases to suberin biosynthesis (Soler et al., 2007). Protein similar to GDSL esterase/lipase At5g33370 (A. thaliana) was substantially upregulated in HvMPK3 KO plants. According to our results, HvMPK3 KO lines activated a different set of LTPs compared to WT. In Arabidopsis, LTP named AZI1 directly interacts with AtMPK3, and confers salinity tolerance (Pitzschke et al., 2014). In our study, a protein similar to lipid transfer-like protein VAS, implicated in suberin formation (Edstam et al., 2013), was upregulated in HvMPK3 KO lines.
This might suggest a connection between HvMPK3 and LTPs as a part of barley defense mechanism against F. graminearum infection. In contrast to the shootless phenotype of the clustered regularly interspaced short palindromic repeats/CRISPR-associated endonuclease (CRISPR/Cas9)-induced homozygous knockout mutant in the HvMPK6 gene (Křenek et al., 2021), we did not observe obvious phenotypical differences in the above-ground plant parts among HvMPK3 KO lines and control WT lines. This suggests that HvMPK3 knockout has no substantial effect on barley growth and development. Considering that TALEN-based knockout of HvMPK3 causes root resistance to F. graminearum, it provides a reliable genetic and molecular tool for targeted biotechnological application toward improving barley as an important cereal crop.
Materials and methods
Characterization and cultivation of plant material
Three independent homozygous knockout lines of barley (H. vulgare L.) designated as HvMPK3 KO-A, HvMPK3 KO-B, and HvMPK3 KO-D, and control WT lines (Takáč et al., 2021) were used for the experiments. The knockout lines were generated by the Z1 TALEN gene pair and contain a 5-bp deletion (HvMPK3 KO-A and HvMPK3 KO-D) or a 20-bp deletion (HvMPK3 KO-B) in the target region of the HvMPK3 gene annotated according to the cv. Morex genome assembly version IBSC_v2 under the Ensembl Plants code HORVU4Hr1G057200 (Mascher et al., 2017; Takáč et al., 2021). These mutations cause frameshifts after decoding of the 27th codon (5-bp deletion) or 26th codon (20-bp deletion) in the HORVU4Hr1G057200.4 splicing variant and introduce early premature translation termination (stop) codons into it. HORVU4Hr1G057200.4 codes for the largest HvMPK3 isoform, a 369-amino acid (aa) long protein, which corresponds to the A. thaliana L. MPK3 (370 aa). Both deletions are also present in the coding region of HORVU4Hr1G057200.3, which produces the second largest HvMPK3 isoform (336 aa). The knockout effect of the introduced mutations was demonstrated at the level of phosphorylated HvMPK3 protein (pHvMPK3), which was not present in the protein extracts of the flg22-treated roots of HvMPK3 KO lines (Takáč et al., 2021). The Z1 TALEN gene pair T-DNA cassette is segregated away from the lines HvMPK3 KO-A and HvMPK3 KO-B but not from the line HvMPK3 KO-D.
Seeds were surface sterilized using 5% (v/v) sodium hypochlorite and 70% (v/v) ethanol, followed by an additional incubation with 2.5-mM (v/v) H2O2 overnight. After sterilization, imbibed seeds were washed in sterile milliQ H2O to clean the residual H2O2 before transferring to plates with agar-solidified nitrogen-free medium (Fåhraeus, 1957) for stratification at 4°C for synchronous germination. The stratified sterile seeds on plates were transferred to phytotron (Weiss-Gallenkamp, Loughborough, UK) and cultivated at 21°C for 16 h in the light (day), and 8 h in darkness (night), 70% relative humidity with light levels of 150 μmol.m−2.s−1 provided by cool white fluorescent tubes (Philips Master tl-d 36W/840).
For documentation of above-ground phenotypes, plants of WT and HvMPK3 KO lines were cultivated in a greenhouse from November 2021 till May 2022. Plants were grown in the Topf + LF30 + TonXL professional substrate (Gramoflor, Vechta, Germany) supplemented with additional perlite (Perlit Ltd., Šenov u Nového Jičína, Czech Republic) in pots (Pöppelmann TEKU, Lohne, Germany) and fertilized several times with YaraMila Complex NPK (Mg;S) 12 11 18 (2.7;8) containing microelements B, Fe, Mn, and Zn (Yara International ASA, Oslo, Norway) during vegetation. Natural lighting conditions were supplemented with red-yellow sodium-vapor lamps (Osram Vialox NAV-T Super 4Y 600W) to maintain the 16-h day/8-h night regime during the whole cultivation period.
Preparation of fluorescent F. graminearum
Mycelia of F. graminearum were cultivated in Mung bean soup (140 rpm, 20°C, 3 days), subsequently filtered through glass wool and centrifuged (300 rpm, room temperature (RT), 5 min). Conidia obtained as such, were resuspended in sterile distilled water and inoculated on yeast extract–peptone–dextrose medium. After overnight cultivation (180 rpm, 30°C), the mycelium was filtered through Miracloth (Merck, Darmstadt, Germany) and washed with sterile distilled water, and 1.2-M KCl. For protoplast isolation, mycelia were cultivated in a solution containing 250-mg driselase, 1-mg chitinase, and 100-mg lysing enzyme from Trichoderma harzianum (all from Sigma-Aldrich, Heidelberg, Germany). After 3 h (90 rpm, 30°C), the enzyme-protoplast solution was run over the frit and the flow-through was centrifuged (3,000 rpm, 4°C, 10 min). Obtained pellet was washed with 1.2-M KCl, then resuspended in 1.2-M sorbitol supplemented with 50-mM CaCl2.
Plasmid (5 μg of pNDN-OGG and pNDH-OCT containing GFP; Schumacher, 2012) was mixed with 2× STC buffer, F. graminearum protoplasts, and 50% (w/v) polyethylene glycol (PEG) 4,000. After 25 min on ice, 50% (w/v) PEG 4,000 was added. Next, the mixture was incubated 10 min at RT, followed by addition of 1× STC buffer. Prepared samples were mixed with regeneration agar containing nourseothricin (300 μg.mL−1, Jena BioScience, Jena, Germany for pNDN-OGG) or hygromycin (100 μg.mL−1, InvivoGen, San Diego, CA, USA for pNDH-OCT). After 5 days at 20°C, nourseothricin- and hygromycin-resistant transformants were transferred into complete medium (CM) agar plates containing antibiotics in the final concentration of 100 μg.mL−1.
Genomic DNA was extracted from lyophilized F. graminearum mycelia according to Cenis (1992). Diagnostic polymerase chain reaction (PCR) was performed using GoTaq G2 Flexi DNA polymerase according to a manufacturer's protocol (Promega, Madison, WI, USA). Primers were designed using Primer3 0.4.0 software and synthesized by Sigma-Aldrich. The presence of pNDN-OGG or pNDH-OCT in F. graminearum transformants was checked with primers PoliC_fw (5′-CCCGGAAACTCAGTCTCCTT-3″) and TgluC_rev (5′-GTCTTCCGCTAAAACACCCC-3″) (1,295 bp) or PoliC_fw and Ttub_rev (5′-GAGGTGTGAGCATGGAAGTGATG-3″) (1,593 bp), respectively.
Homokaryots of OE:GFP transformants were obtained by single spore isolation. Briefly, fungi were cultivated in Mung bean soup (20°C, 140 rpm) and after 5 days, the cultures were filtered through glass wool and centrifuged (10 min, 3,000 rpm, 4°C). Obtained conidia were resuspended in sterile distilled water and sprayed onto CM agar plates containing appropriate antibiotics in the final concentration of 100 μg.mL−1.
Infection of barley plants with Fusarium
Fusarium grown in potato dextrose agar medium for 2 weeks at 28°C was washed with sterile distilled water containing 0.01% (v/v) Tween and filtered through sterile miracloth to obtain conidial spores (∼1 × 105 spores.mL−1) (Erayman et al., 2015). Five-day-old barley seedlings were infected by dipping in freshly prepared conidial suspension (sterile distilled water containing 0.01% (v/v) Tween was used as control) and put back to the media for incubation for further experiments. Microscopic, biochemical and proteomic analyses were performed on control and Fusarium-treated plants 24- and 48-h postinfection.
Barley leaf inoculation experiment was performed as described by Bedawy et al. (2018) with minor modifications. Fully developed second leaves from 12-day-old plants were briefly washed with 75% (v/v) ethanol for 5 s, and 3 times with sterile distilled water. The sterilized leaf pieces were then inoculated with F. graminearum conidial suspension (∼1 × 105 spores.mL−1) prepared in 0.01% Tween by dipping. Leaves treated in sterile 0.01% Tween served as control. After treatment the leaves were placed into Petri dish containing 1% (w/v) phytoagar. For incubation, Petri dishes containing inoculated and control leaves were placed in the phytotron with the same conditions as mentioned above for 4 days and documented using Nikon camera (Nikon D6500). For quantitative analysis, leaf areas showing necrosis (identified as dark-brown), chlorosis (identified as yellow), and healthy (identified as green) were selected and quantified using ImageJ software.
Phenotypic and microscopic analysis
For microscopic analysis, the roots of both WT and HvMPK3 KO plants 24 and 48 h after inoculation with GFP-labeled F. graminearum were used. Infected plants were grown in microscopic growth chambers (Nunc Lab-Tek II Chambered Coverglass [Thermo Fischer Scientific, Waltham, MA, USA]) and observed using confocal laser scanning microscope (CLSM) LSM710 (Carl Zeiss, Germany) using 488-nm laser at 2% of the laser power for GFP excitation and the collection bandwidth 517–527 nm for emission, with default gain settings. The images were color-coded in the Z-stacks for the analysis of mycelia spatial distribution and the penetration of the infection hyphae in root epidermal cells using appropriate Zen Blue software function. The numbers of appressoria-like structures were counted from the images and were compared among the lines by one-wayanalysis of variance (ANOVA) test at significance level at P < 0.05. Roots were stained with PI (1 mg.mL−1) for 10 min, washed with liquid Fåhraeus medium for 1 min, and observed under spinning disk microscope (Carl Zeiss) equipped with Plan-Apochromat 20×/0.8 NA (Carl Zeiss) at 488 nm (for GFP) and 561 nm (for PI) with emission filters BP525/50 (for GFP) and BP629/62 (for PI). Image postprocessing was done using ZEN 2014 software (Carl Zeiss), and the percentage of the dead cells were calculated from the processed images using the formulae (number of dead cells/total number of cells in the field × 100). To document root length, both treated and untreated plants were photographed by Nikon D6500 camera once per day for 10 days after the root inoculation with GFP-labeled F. graminearum spores. The experiment was performed in three biological replicates. Ten plants were used per replicate and treatment for each line. The statistical significance of treatment versus control was deemed by one-way ANOVA test at significance level at P < 0.05.
The degree of fungal growth directionality was quantitatively measured by analyzing the hyphae growth orientation with respect to the main root elongation axis. For this, maximum intensity projections of CLSM images of WT and HvMPK3 KO roots treated with GFP-labeled F. graminearum were analyzed using the Cytospectre freeware according to Krasylenko et al. (2021), which illustrates the angular distribution of fungal mycelium in selected regions plotted as circular graphs. The quantitative distribution of the fungal mycelial arrangement was measured using FibrilTool macro as described previously (Boudaoud et al., 2014). For this, the FibrilTool macro was applied on selected regions drawn using the Polygon tool of ImageJ carefully along the circumference of frontal view of the root with mycelial growth (avoiding root curvature at the edges of selected regions). In theory, the numerical value of complete isotropy (i.e. random distribution of fungal mycelium with no prevalent orientation) to perfect anisotropy (i.e. biased unidirectional arrangement of mycelial growth with respect to the cell axis) ranges between 0 and 1, respectively.
Skewness practically implies the fluorescence intensity of each given pixel in the image deviating from the mean value. When the sample is uniformly labeled (i.e. when there is uniform fluorescence distribution or in our case when fungal mycelium fully covers the root surface and is uniformly distributed), the skewness value is zero or close to zero. When fluorescence distribution is nonuniform (i.e. dark and fluorescent structures are alternating and unevenly distributed), then it is considered to be skewed and has values different (higher) than zero. Skewness was quantitatively analyzed by assessing the extent of fluorescence distribution of GFP-labeled F. graminearum on the CLSM images of selected areas of treated roots from both WT and HvMPK3 KO lines. Skewness is automatically extrapolated by histogram analysis by means of Zen Blue software (Carl Zeiss) (Krasylenko et al., 2021).
ROS staining
Control plants and treated plants from individual lines were used for ROS analysis 24-h postinoculation with GFP-labeled F. graminearum using the modified protocol of Kristiansen et al. (2009). Mock-treated and infected roots of WT and HvMPK3 KO plants were incubated in 30-µM 2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Thermo Fischer Scientific) and PI (1 mg.mL−1) diluted in Fåhraeus medium. Seedlings in microscopic chambers were stained by perfusion, followed by incubation in darkness for 15 min. After incubation the residual stain was washed using sterile Fåhraeus medium for 3 times using perfusion. After washing, the signal was recorded using confocal laser scanning microscope LSM710 (Carl Zeiss) with excitation at 488 nm for ROS/GFP and at 543 nm for PI at default gain settings. The relative power was set up to 2% and 0.6% for 488 and 543-nm laser, respectively. The emission was recorded at the emission range 517–527 nm for ROS/GFP and 610–625 nm for PI. The imaging of ROS accumulation in whole roots was performed after staining with CM-H2DCFDA using epifluorescence microscope Zeiss Axio Imager M2 (Carl Zeiss) with settings for GFP excitation and emission. ROS levels in roots were analyzed semi-quantitatively from the images with ZEN software (Carl Zeiss).
Histochemical detection of suberin and lignin
Root segments (first 25 mm from the root apex) of WT and HvMPK3 KO plants 24 h after inoculation with F. graminearum were used for suberin and lignin histochemical detection. Suberin and lignin staining was carried out according to a previously published protocol (Ursache et al., 2018; Sexauer et al., 2021). Freehand cross-sections of fixed and cleared root segments were made in the root region between 15 and 25 mm from the root apex and stained with 0.01% (v/v) Fluorol Yellow 088 in absolute ethanol (stock solution: 1% (w/v) Fluorol Yellow 088 in DMSO) for 30 min. For lignin detection root sections were stained with 0.2% (w/v) Basic Fuchsin in ClearSee solution for overnight. Stained cross-sections of roots were observed under confocal laser scanning microscope LSM710 (Carl Zeiss) using following settings for suberin: excitation with 488 nm laser at 2% of the laser power, default gain, and emission bandwidth detection at 500–550 nm. For lignin: excitation with 561 nm, at 0.6% of the laser power, default gain, and emission bandwidth detection at 600–650 nm. Measurement of fluorescence intensity and postprocessing were done using ZEN 2014 software (Carl Zeiss). Fluorescence mean intensities were calculated from at least two roots of each barley line in three biological replicates (n = 6), and the statistical significance was evaluated using Student’s t test (P < 0.05).
Western blot analysis
Samples were prepared and western blots performed as described before (Takáč et al., 2021). Polyvinylidene difluoride membranes were incubated with rabbit primary anti-L- cAPX antibody (#AS 08 368), diluted 1:2,000, or with rabbit primary anti-catalase antibody (#AS 09 501), diluted 1:2,000, or with rabbit primary anti-PR-1 antibody (#AS 10 687, all from Agrisera, Vännäs, Sweden), diluted 1:2,000, all in 1% (w/v) BSA in TBS-T at 4°C overnight. Membranes were washed thoroughly and subsequently incubated at RT with corresponding HRP-conjugated secondary antibody (Thermo Fisher Scientific), diluted 1:5,000 in 1% (w/v) BSA in TBS-T for 1.5 h. Following three washing steps, membranes were incubated with commercial Clarity Western ECL Substrate (BioRad, Hercules, CA, USA) and documented in a ChemiDoc MP imaging system (BioRad). Band optical densities were quantified using ImageLab software (BioRad). Experiments were performed in three biological replicates. Roots of four plants per line were pooled in one biological replicate. The statistical significance was evaluated using Student’s t test (P < 0.05).
Analysis of peroxidase activities and spectrophotometric measurement of ROS
Specific activities of PRXs were analyzed using staining on native polyacrylamide gel electrophoresis (PAGE) gels as published previously (Takáč et al., 2016). The optical densities of PRX isozymes were quantified using ImageJ software. The band density representing a PRX isozyme in a sample was normalized according to the sum of densities of the respective band in all analyzed samples within one biological replicate. ROS levels were estimated in water extracts using xylenol orange assay as described in Takáč et al. (2014). The analyses were carried out in three biological replicates on control and Fusarium-treated roots of WT and HvMPK3 KO seedlings 24-h postinoculation. Roots of four plants per line were pooled in one biological replicate. The statistical significance was evaluated using Student’s t test (P < 0.05).
Relative protein quantitation using nano-liquid chromatography–tandem mass spectrometry analysis
Barley WT, and HvMPK3 KO lines A, B, and D were used for proteomic study. Root parts from four plants of each line in control and treatment variants were pooled into one sample. Each sample was analyzed in two biological replicates, and the final differential proteomes of WT and HvMPK3 KO lines were obtained by comparing six biological replicates of Fusarium-treated lines (two replicates for each of the three HvMPK3 KO lines) versus corresponding mock controls (two replicates for each of the three WT lines). Proteins were extracted using phenol extraction and methanol/ammonium acetate precipitation, as described previously (Takáč et al., 2017). Total of 50 μg of proteins dissolved in 50 μL of 6-M urea were subjected to in-solution trypsin digestion.
The peptides were then cleaned on C18 cartridges (Bond Elut C18; Agilent Technologies, Santa Clara, CA, USA), dried using SpeedVac, and utilized for nano-liquid chromatography–tandem mass spectrometry analysis (Takáč et al., 2017).
Two micrograms of protein tryptic digest were analyzed as published previously (Takáč et al., 2021), using the Ultimate 3000 nano-LC system and LTQ-Ortbitrap Velos mass spectrometer (both Thermo Fisher Scientific). All raw and results files were deposited to a publicly accessible database (see “Data availability statement” for details). Protein identification and label-free quantification was performed by the Proteome Discoverer version 2.1 (Thermo Fisher Scientific), and an in-house script based on precursor ion intensities, as described before (Takáč et al., 2021). Statistically significant results were filtered with ANOVA P ≤ 0.05, applied to proteins exhibiting the fold change ≥1.5.
Proteins identified by single peptide were excluded from the results. Proteins present in all six replicates corresponding to the control proteome, and absent in all the treated replicates were considered unique for the control proteome, and vice versa.
The differential proteomes were evaluated by GO annotation analysis and screening of protein domains using OmixBox Functional analysis module, as specified previously (Takáč et al., 2021).
Supplementary Material
Acknowledgments
We would like to thank Lena Studt from Department of Applied Genetics and Cell Biology, University of Natural Resources and Life Sciences, Vienna for helping us with F. graminearum transformation. We also thank technicians Petra Trčková, Pavlína Floková, Katarína Takáčová and Monika Vadovičová for their expert technical help in all stages of the presented work.
Funding
This work was funded by ERDF project Plants as a tool for sustainable global development (CZ.02.1.01/0.0/0.0/16_019/0000827), and NIH MS-IDeA Network of Biomedical Research Excellence award 5P20GM103476-19. The mass spectrometry proteomics analysis was performed at the Institute for Genomics, Biocomputing and Biotechnology, Mississippi State University, with partial support from Mississippi Agricultural and Forestry Experiment Station.
Conflict of interest statement. Authors declare that they have no conflicts of interests.
Contributor Information
Jasim Basheer, Department of Biotechnology, Faculty of Science, Palacký University Olomouc, Olomouc, Czech Republic.
Pavol Vadovič, Department of Biotechnology, Faculty of Science, Palacký University Olomouc, Olomouc, Czech Republic.
Olga Šamajová, Department of Biotechnology, Faculty of Science, Palacký University Olomouc, Olomouc, Czech Republic.
Pavol Melicher, Department of Biotechnology, Faculty of Science, Palacký University Olomouc, Olomouc, Czech Republic.
George Komis, Department of Biotechnology, Faculty of Science, Palacký University Olomouc, Olomouc, Czech Republic.
Pavel Křenek, Department of Biotechnology, Faculty of Science, Palacký University Olomouc, Olomouc, Czech Republic.
Michaela Králová, Centre of the Region Haná for Biotechnological and Agricultural Research, Department of Molecular Biology, Faculty of Science, Palacký University Olomouc, Olomouc, Czech Republic.
Tibor Pechan, Institute for Genomics, Biocomputing and Biotechnology, Mississippi Agricultural and Forestry Experiment Station, Mississippi State University, Starkville, Mississippi, USA.
Miroslav Ovečka, Department of Biotechnology, Faculty of Science, Palacký University Olomouc, Olomouc, Czech Republic.
Tomáš Takáč, Department of Biotechnology, Faculty of Science, Palacký University Olomouc, Olomouc, Czech Republic.
Jozef Šamaj, Department of Biotechnology, Faculty of Science, Palacký University Olomouc, Olomouc, Czech Republic.
Data Availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD029964.
Accession numbers
Sequence data for the HvMPK3 from this article can be found in the Ensembl Plants data library (version IBSC_v2; http://plants.ensembl.org/index.html) under accession code HORVU4Hr1G057200.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Colonization of roots in barley plants of WT and HvMPK3 KO lines by F. graminearum mycelia 10 days after inoculation with spores.
Supplemental Figure S2. Characterization of distribution and growth pattern of F. graminearum hyphae in regard of the root longitudinal axis.
Supplemental Figure S3. Comparison of root phenotypes in barley WT and HvMPK3 KO lines between nontreated and F. graminearum-treated plants 10 days after inoculation with spores.
Supplemental Figure S4. Fluorescence visualization of F. graminearum hyphae distribution on root surface of barley WT and HvMPK3 KO lines 48 h after inoculation with spores.
Supplemental Figure S5. Concentration of hydrogen peroxide in roots of WT and HvMPK3 KO lines 24 h after inoculation with F. graminearum spores as examined by Xylenol orange assay.
Supplemental Figure S6. Comparison of gene ontology annotation according to biological process, carried out in differential proteomes of WT and HvMPK3 KO plants treated by F. graminearum for 24 h.
Supplemental Figure S7. Comparison of gene ontology annotation according to cellular compartments, carried out in differential proteomes of WT and HvMPK3 KO plants treated by F. graminearum for 24 h.
Supplemental Figure S8. Full scan of the entire original gel whose fragments are presented in Figure 8A.
Supplemental Figure S9. Full scan of the entire original blot and loading control whose fragments are presented in Figure 8C.
Supplemental Figure S10. Full scan of the entire original blot and loading control whose fragments are presented in Figure 8E.
Supplemental Figure S11. Full scan of the entire original blot and loading control whose fragments are presented in Figure 8G.
Supplemental Figure S12. Lignin staining of barley roots 24 h after inoculation with F. graminearum spores.
Supplemental Figure S13. Phenotype of WT and HvMPK3 KO plants.
Supplemental Figure S14. Leaf resistance test of young WT and HvMPK3 KO plants treated with F. graminearum spores and analyzed 4 days after inoculation.
Supplemental Table S1. Summary and quantification details of differentially regulated proteins found in roots of WT plants 24 h after the treatment with F. graminearum.
Supplemental Table S2. Summary and quantification details of differentially regulated proteins found in roots of HvMPK3 KO plants 24 h after the treatment with F. graminearum.
Supplemental Table S3. Proteins predicted to be localized in extracellular space.
J.B., P.V., and M.O. conducted phenotypic and microscopic documentation and analysis. P.V., T.T., and T.P. performed proteomics analysis. P.V. and P.M. conducted immunoblot analysis, native electrophoresis, and spectrophotometric measurements. O.Š. performed suberin and lignin staining and related microscopic observations and evaluation. P.K. selected HvMPK3 KO and WT barley lines. M.K. performed Fusarium transformation. J.B., P.V., M.O., T.T., G.K., and J.Š. drafted the manuscript with input from all co-authors. J.Š. conceived and supervised the project, provided infrastructure, and secured funding.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/General-Instructions) is Jozef Šamaj (jozef.samaj@upol.cz).
References
- Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros Barceló A, Pedreño MA (2009) Class III peroxidases in plant defence reactions. J Exp Bot 60: 377–390 [DOI] [PubMed] [Google Scholar]
- Barna B, Fodor J, Harrach BD, Pogány M, Király Z (2012) The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol Biochem 59: 37–43 [DOI] [PubMed] [Google Scholar]
- Bedawy IMA, Dehne HW, Léon J, Naz AA (2018) Mining the global diversity of barley for Fusarium resistance using leaf and spike inoculations. Euphytica 214: 18 [Google Scholar]
- Bernards MA, Summerhurst DK, Razem FA (2004) Oxidases, peroxidases and hydrogen peroxide: the suberin connection. Phytochem Rev 3: 113–126 [Google Scholar]
- Bigeard J, Colcombet J, Hirt H (2015) Signaling mechanisms in pattern-triggered immunity (PTI). Mol Plant 8: 521–539 [DOI] [PubMed] [Google Scholar]
- Boenisch MJ, Schäfer W (2011) Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudaoud A, Burian A, Borowska-Wykret D, Uyttewaal M, Wrzalik R, Kwiatkowska D, Hamant O (2014) Fibriltool, an ImageJ plug-in to quantify fibrillar structures in raw microscopic images. Nat Prot 9: 457–463 [DOI] [PubMed] [Google Scholar]
- Camagna M, Takemoto D (2018) Hypersensitive response in plants. eLS. John Wiley & Sons Ltd., Hoboken, NJ, pp 1–7 [Google Scholar]
- Camejo D, Guzmán-Cedeño Á, Moreno A (2016) Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol Biochem 103: 10–23 [DOI] [PubMed] [Google Scholar]
- Cenis JL (1992) Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res 20: 2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang L, Yuan M (2021) Update on the roles of rice MAPK cascades. Int J Mol Sci 22: 1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio C, Ranocha P, Francoz E, Burlat V, Zheng Y, Perry SE, Ripoll JJ, Yanofsky M, Dunand C (2017) The class III peroxidase PRX17 is a direct target of the MADS-box transcription factor AGAMOUS-LIKE15 (AGL15) and participates in lignified tissue formation. New Phytol 213: 250–263 [DOI] [PubMed] [Google Scholar]
- Cui H, Wang Y, Xue L, Chu J, Yan C, Fu J, Chen M, Innes RW, Zhou JM (2010) Pseudomonas syringae effector protein AvrB perturbs Arabidopsis hormone signaling by activating MAP kinase 4. Cell Host Microbe 7: 164–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Yang G, Yan J, Pan Y, Nie X (2019) Genome-wide identification, expression profiles and regulatory network of MAPK cascade gene family in barley. BMC Genom 20: 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond OJ, Manners JM, Stephens AE, Maclean DJ, Schenk PM, Gardiner DM, Gardiner DM, Munn AL, Kazan K (2008) The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol Plant Pathol 9: 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edqvist J, Blomqvist K, Nieuwland J, Salminen TA (2018) Plant lipid transfer proteins: are we finally closing in on the roles of these enigmatic proteins? J Lipid Res 59: 1374–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstam MM, Blomqvist K, Eklöf A, Wennergren U, Edqvist J (2013) Coexpression patterns indicate that GPI-anchored non-specific lipid transfer proteins are involved in accumulation of cuticular wax, suberin and sporopollenin. Plant Mol Biol 83: 625–649 [DOI] [PubMed] [Google Scholar]
- Erayman M, Turktas M, Akdogan G, Gurkok T, Inal B, Ishakoglu E, Ilhan E, Unver T (2015) Transcriptome analysis of wheat inoculated with Fusarium graminearum. Front Plant Sci 6: 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschen-Lippold L, Jiang X, Elmore JM, Mackey D, Shan L, Coaker G, Scheel D, Lee J (2016) Bacterial AvrRpt2-Like cysteine proteases block activation of the Arabidopsis mitogen-activated protein kinases, MPK4 and MPK11. Plant Physiol 171: 2223–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhraeus G (1957) The infection of clover root hairs by nodule bacteria studied by a simp le glass slide technique. J Gen Microbiol 16: 374–381 [DOI] [PubMed] [Google Scholar]
- Fry SC (2004) Oxidative coupling of tyrosine and ferulic acid residues: intra- and extra-protoplasmic occurrence, predominance of trimers and larger products, and possible role in inter-polymeric cross-linking. Phytochem Rev 3: 97–111 [Google Scholar]
- Hong L, Brown J, Segerson NA, Rose JKC, Roeder AHK (2017) CUTIN SYNTHASE 2 maintains progressively developing cuticular ridges in Arabidopsis sepals. Molec Plant 10: 560–574 [DOI] [PubMed] [Google Scholar]
- Johrde A, Schweizer P (2008) A class III peroxidase specifically expressed in pathogen-attacked barley epidermis contributes to basal resistance. Mol Plant Pathol 9: 687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kámán-Tóth E, Dankó T, Gullner G, Bozsó Z, Palkovics L, Pogány M (2019) Contribution of cell wall peroxidase- and NADPH oxidase-derived reactive oxygen species to Alternaria brassicicola-induced oxidative burst in Arabidopsis. Mol Plant Pathol 20: 485–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi-Kaboshi M, Okada K, Kurimoto L, Murakami S, Umezawa T, Shibuya N, Yamane H, Miyao A, Takatsuji H, Takahashi A, et al. (2010) A rice fungal MAMP-responsive MAPK cascade regulates metabolic flow to antimicrobial metabolite synthesis. Plant J 63: 599–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G, Illés P, Beck M, Šamaj J (2011) Microtubules and mitogen-activated protein kinase signalling. Curr Opin Plant Biol 14: 650–657 [DOI] [PubMed] [Google Scholar]
- Komis G, Šamajová O, Ovečka M, Šamaj J (2018) Cell and developmental biology of plant mitogen-activated protein kinases. Annu Rev Plant Biol 69: 237–265 [DOI] [PubMed] [Google Scholar]
- Krasylenko Y, Komis G, Hlynska S, Vavrdová T, Ovečka M, Pospíšil T, Šamaj J (2021) GR24, a synthetic strigolactone analog, and light affect the organization of cortical microtubules in Arabidopsis hypocotyl cells. Front Plant Sci 12: 675981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Křenek P, Chubar E, Vadovič P, Ohnoutková L, Vlčko T, Bergougnoux V, Cápal P, Ovečka M, Šamaj J (2021) CRISPR/Cas9-induced loss-of-function mutation in the barley mitogen-activated protein kinase 6 gene causes abnormal embryo development leading to severely reduced grain germination and seedling shootless phenotype. Front Plant Sci 12: 670302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Křenek P, Niks RE, Vels A, Vyplelová P, Šamaj J (2015) Genome-wide analysis of the barley MAPK gene family and its expression patterns in relation to Puccinia hordei infection. Acta Physiol Plant 37: 1–16 [Google Scholar]
- Kristiansen KA, Jensen PE, Møller IM, Schulz A (2009) Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy. Physiol Plant 136: 369–383 [DOI] [PubMed] [Google Scholar]
- Kumar J, Schäfer P, Hückelhoven R, Langen G, Baltruschat H, Stein E, Nagarajan S, Kogel KH (2002) Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control double dagger. Mol Plant Pathol 3: 185–195 [DOI] [PubMed] [Google Scholar]
- Lanoue A, Burlat V, Henkes GJ, Koch I, Schurr U, Röse USR (2010) De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol 185: 577–588 [DOI] [PubMed] [Google Scholar]
- Lee D, Lal NK, Lin ZJD, Ma S, Liu J, Castro B, Toruño T, Dinesh-Kumar SP, Coaker G (2020) Regulation of reactive oxygen species during plant immunity through phosphorylation and ubiquitination of RBOHD. Nat Commun 11: 1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ren D, Pike S, Pallardy S, Gassmann W, Zhang S (2007) Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J 51: 941–954 [DOI] [PubMed] [Google Scholar]
- Lozovaya VV, Lygin AV, Zernova OV, Li S, Widholm JM, Hartman GL (2006) Lignin degradation by Fusarium solani f. sp. glycines. Plant Dis 90: 77–82 [DOI] [PubMed] [Google Scholar]
- Ma L-J,, GeiserDM, , ProctorRH, , RooneyAP, , O'Donnell K,, Trail F,, Gardiner D,, MannersJM, , Kazan K (2013) Fusarium pathogenomics. Ann Rev Microbiol 67: 399–416 [DOI] [PubMed] [Google Scholar]
- Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S (2011) Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23: 1639–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher M, Gundlach H, Himmelbach A, Beier S, Twardziok SO, Wicker T, Radchuk V, Dockter C, Hedley PE, Russell J, et al. (2017) A chromosome conformation capture ordered sequence of the barley genome. Nature 544: 427–433 [DOI] [PubMed] [Google Scholar]
- Masuda D, Ishida M, Yamaguchi K, Yamaguchi I, Kimura M, Nishiuchi T (2007) Phytotoxic effects of trichothecenes on the growth and morphology of Arabidopsis thaliana. J Exp Bot 58: 1617–1626 [DOI] [PubMed] [Google Scholar]
- Meng X, Zhang S (2013) MAPK cascades in plant disease resistance signaling. Annu Rev Phytopathol 51: 245–266 [DOI] [PubMed] [Google Scholar]
- Misas-Villamil JC, van der Hoorn RAL, Doehlemann G (2016) Papain-like cysteine proteases as hubs in plant immunity. New Phytol 212: 902–907 [DOI] [PubMed] [Google Scholar]
- Mishler-Elmore JW, Zhou Y, Sukul A, Oblak M, Tan L, Faik A, Held MA (2021) Extensins: self-assembly, crosslinking, and the role of peroxidases. Front Plant Sci 12: 664738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller AN, Ziemann S, Treitschke S, Aßmann D, Doehlemann G (2013) Compatibility in the ustilago maydis–maize interaction requires inhibition of host cysteine proteases by the fungal effector Pit2. PLoS Pathol 9: e1003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Daudi A, Finch P, Butt VS, Whitelegge JP, Souda P, Ausubel FM, Bolwell GP (2012) A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol 158: 2013–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24: 255–265 [DOI] [PubMed] [Google Scholar]
- Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9: 534–540 [DOI] [PubMed] [Google Scholar]
- Pitzschke A, Datta S, Persak H (2014) Salt stress in Arabidopsis: lipid transfer protein AZI1 and its control by mitogen-activated protein klinase MPK3. Mol Plant 7: 722–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Wang J, Gong Z, Zhou JM (2017) Apoplastic ROS signaling in plant immunity. Curr Opin Plant Biol 38: 92–100 [DOI] [PubMed] [Google Scholar]
- Qiu JL, Fiil BK, Petersen K, Nielsen HB, Botanga CJ, Thorgrimsen S, Palma K, Suarez-Rodriguez MC, Sandbech-Clausen S, Lichota J, et al. (2008) Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J 27: 2214–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Zhao X, Fang W, Wu H, Abubakar YS, Lu G, Wang Z, Zheng W (2019) Spatiotemporal nature of Fusarium graminearum-wheat coleoptile interactions. Phytopathol Res 1: 26 [Google Scholar]
- Ralph J, Bunzel M, Marita JM, Hatfield RD, Lu F, Kim H, Schatz PF, Grabber JH, Steinhart H (2004) Peroxidase-dependent cross-linking reactions of p-hydroxycinnamates in plant cell walls. Phytochem Rev 3: 79–96 [Google Scholar]
- Ren D, Yang H, Zhang S (2002) Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem 277: 559–565 [DOI] [PubMed] [Google Scholar]
- Rózewicz M, Wyzinska M, Grabinski J (2021) The most important fungal diseases of cereals - problems and possible solutions. Agronomy 11: 714 [Google Scholar]
- Rudd JJ, Keon J, Hammond-Kosack KE (2008) The wheat mitogen-activated protein kinases TaMPK3 and TaMPK6 are differentially regulated at multiple levels during compatible disease interactions with Mycosphaerella graminicola. Plant Physiol 147: 802–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Iwai T, Hiraga S, Kuroda K, Seo S, Mitsuhara I, Miyasaka A, Iwano M, Ito H, Matsui H, et al. (2004) Ten rice peroxidases redundantly respond to multiple stresses including infection with rice blast fungus. Plant Cell Physiol 45: 1442–1452 [DOI] [PubMed] [Google Scholar]
- Shetty NP, Mehrabi R, Lütken H, Haldrup A, Kema GHJ, Collinge DB, Jørgensen HJL (2007) Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol 174: 637–647 [DOI] [PubMed] [Google Scholar]
- Schumacher J (2012) Tools for Botrytis cinerea: new expression vectors make the gray mold fungus more accessible to cell biology approaches. Fungal Genet Biol 49: 483–497 [DOI] [PubMed] [Google Scholar]
- Sewelam N, Kazan K, Schenk PM (2016) Global plant stress signaling: Reactive oxygen species at the cross-road. Front Plant Sci 7: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexauer M, Shen D, Schön M, Andersen TG, Markmann K (2021) Visualizing polymeric components that define distinct root barriers across plant lineages. Development 148: dev199820 doi:10.1242/dev.199820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler M, Serra O, Molinas M, Huguet G, Fluch S, Figueras M (2007) A genomic approach to suberin biosynthesis and cork differentiation. Plant Physiol 144: 419–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokan AV, Kuluev BR, Burkhanova G, Maksimov IV (2014) RNA silencing of the anionic peroxidase gene impairs potato plant resistance to Phytophthora infestans (Mont.) de Bary. Mol Biol 48: 709–717 [PubMed] [Google Scholar]
- Takáč T, Křenek P, Komis G, Vadovič P, Ovečka M, Ohnoutková L, Pechan T, Kašpárek P, Tichá T, Basheer J, et al. (2021) TALEN-based HvMPK3 knock-Out attenuates proteome and root hair phenotypic responses to flg22 in barley. Front Plant Sci 12: 666229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takáč T, Šamajová O, Luptovčiak I, Pechan T, Šamaj J (2017) Feedback microtubule control and microtubule-actin cross-talk in Arabidopsis revealed by integrative proteomic and cell biology analysis of KATANIN 1 mutants. Molec Cel Proteomics 16: 1591–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takáč T, Šamajová O, Vadovič P, Pechan T, Košútová P, Ovečka M, Husičková A, Komis G, Šamaj J (2014) Proteomic and biochemical analyses show a functional network of proteins involved in antioxidant defense of the Arabidopsis anp2anp3 double mutant. J Proteome Res 13: 5347–5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takáč T, Vadovič P, Pechan T, Luptovčiak I, Šamajová O, Šamaj J (2016) Comparative proteomic study of Arabidopsis mutants mpk4 and mpk6. Sci Rep 6: 28306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot NJ (2019) Appressoria. Curr Biol 29: R144–R146 [DOI] [PubMed] [Google Scholar]
- Trail F (2009) For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant Physiol 149: 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K,, Katagiri F (2010) Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol 13: 459–465 [DOI] [PubMed] [Google Scholar]
- Ursache R, Andersen TG, Marhavy P, Geldner N (2018) A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J 93: 399–412 [DOI] [PubMed] [Google Scholar]
- Walter S, Nicholson P, Doohan FM (2010) Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol 185: 54–66 [DOI] [PubMed] [Google Scholar]
- Wang Q, Buxa SV, Furch A, Friedt W, Gottwald S (2015) Insights into Triticum aestivum seedling root rot caused by Fusarium graminearum. Mol Plant Microbe Interact 28: 1288–1303 [DOI] [PubMed] [Google Scholar]
- Wang Q, Shao B, Shaikh FI, Friedt W, Gottwald S (2018) Wheat resistances to fusarium root rot and head blight are both associated with deoxynivalenol- and jasmonate-related gene expression. Phytopathology 108: 602–616 [DOI] [PubMed] [Google Scholar]
- Wojtasik W, Kulma A, Dymińska L, Hanuza J, Czemplik M, Szopa J (2016) Evaluation of the significance of cell wall polymers in flax infected with a pathogenic strain of Fusarium oxysporum. BMC Plant Biol 16: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Chen J, Wang Q, Yang Y (2014) Direct phosphorylation and activation of a mitogen-activated proteinkinase by a calcium-dependent protein kinase in rice. Plant Cell 26: 3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Yang Y (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid–inducible mitogen-activated protein kinase. Plant Cell 15: 745–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Pedersen C, Kwaaitaal M, Gregersen PL, Mørch SM, Hanisch S, Kristensen A, Fuglsang AT, Collinge DB, Thordal-Christensen H (2012) Interaction of barley powdery mildew effector candidate CSEP0055 with the defence protein PR17c. Mol Plant Pathol 13: 1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C (2009) Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol 12: 414–420 [DOI] [PubMed] [Google Scholar]
- Zipfel C (2014) Plant pattern-recognition receptors. Trends Immunol 35: 345–351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD029964.
Accession numbers
Sequence data for the HvMPK3 from this article can be found in the Ensembl Plants data library (version IBSC_v2; http://plants.ensembl.org/index.html) under accession code HORVU4Hr1G057200.