Environmental endotoxin is associated with a variety of respiratory diseases that arise in occupational, agricultural, and domestic settings. Endotoxin refers to lipopolysaccharide (LPS) or lipoöligosaccharide (LOS) molecules arising from the outer cell wall of Gram-negative bacteria (GNB) that function as microbial-associated molecular patterns (MAMPs) interacting with pattern recognition receptors to induce inflammation leading to asthma and asthma-like syndromes.
It has long been established that workers are exposed to dangerous concentrations of endotoxin in agricultural settings including concentrated animal feeding operations (CAFOs) housing swine, poultry and other livestock; grain handling facilities; seed and bulb processing facilities; during row crop harvesting, and during tasks such as grain bin cleanout.1 Workers in occupations such as wastewater treatment, cotton textile processing, industrial scale composting, and metals machining are also at risk. Geometric mean airborne endotoxin concentrations from a variety of environments measured in our research projects are shown in Figure 1A. Household endotoxin loads in reservoir dust are provided in Figure 1B. The high endotoxin exposures experienced in the environments shown in Figure 1A induce adverse respiratory effects including non-allergic asthma, asthma-like syndrome, organic dust toxic syndrome, mucous membrane irritation and chronic bronchitis.
Figure 1.
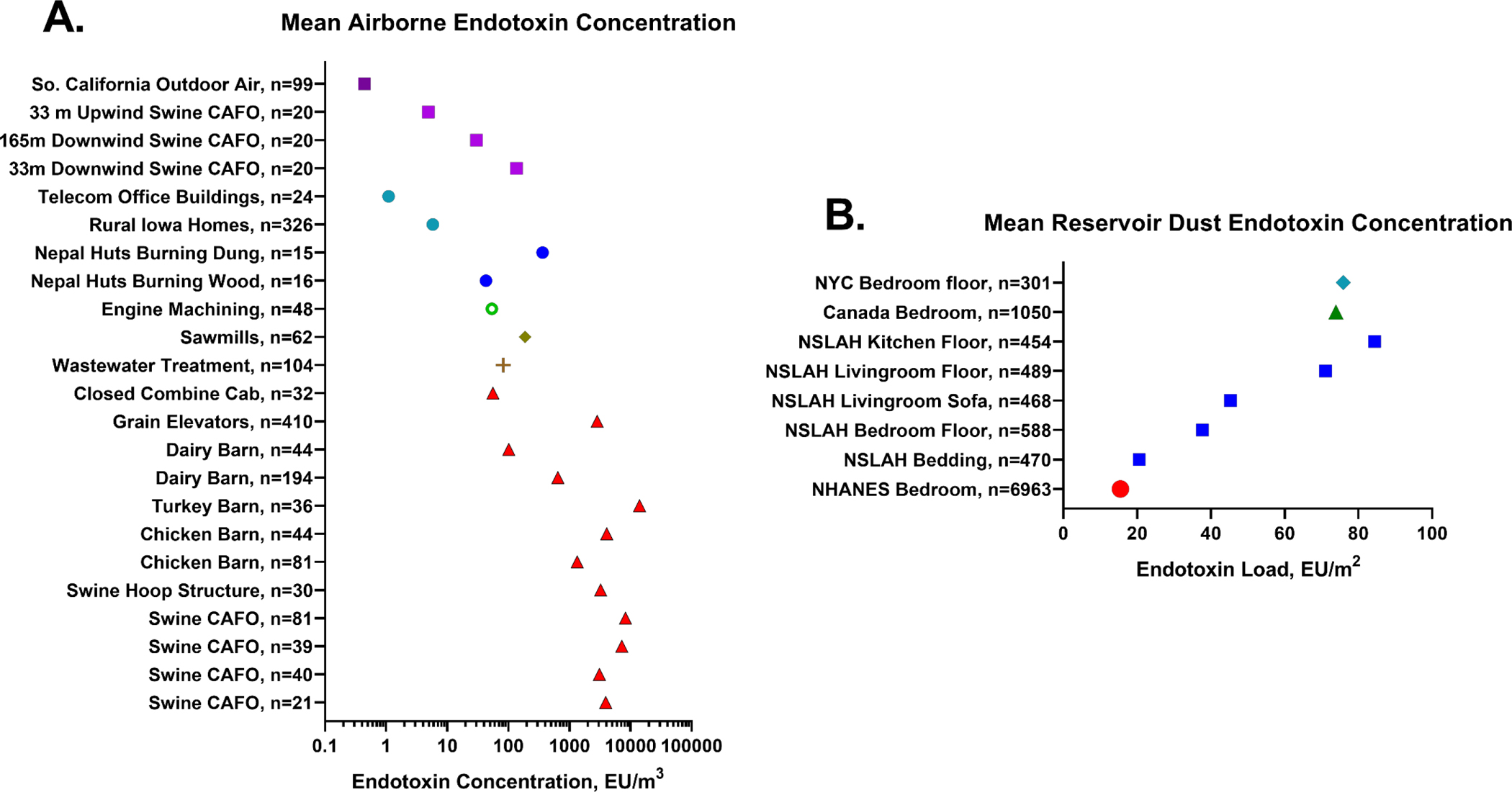
Geometric mean airborne endotoxin and reservoir dust endotoxin in a variety of settings. Figure 1A. Airborne endotoxin concentration (EU/m3) measured in 23 environments using the kinetic chromogenic Limulus amoebocyte lysate assay in our laboratory. It is noteworthy that endotoxin concentrations vary more than 10,000-fold across these locations and circumstances. Squares denote outdoor environments, circles denote indoor domestic and office locations, red triangles denote agricultural settings from multiple studies. Figure 1B. Reservoir dust endotoxin load (EU/m2) measured in residences using the kinetic chromogenic Limulus amoebocyte lysate assay. Squares denote data from the National Survey of Lead and Allergens in Housing (NSLAH), the circle denotes data from the National Health and Nutrition Examination Survey (NHANES). All assays were conducted in our University of Iowa Laboratory. CAFO: concentrated animal feeding operation; NYC: New York City.
Endotoxin Mechanism of Inflammation
Endotoxin in the environment exists as aggregates of hundreds of thousands of LPS molecules or as fragments of bacterial membranes or membrane blebs. In order to activate signal transduction leading to inflammation, these aggregates and membrane-bound endotoxin molecules need to be extracted and presented to TLR-4. Four cell surface and free extracellular host proteins are a requisite for maximum responsiveness to endotoxin. They are LPS binding protein (LBP), cluster determinant-14 (CD-14), lymphocyte antigen 96 (MD-2), and Toll-like receptor-4 (TLR-4).2 These host proteins interact sequentially with LBP catalyzing the transfer of endotoxin molecules from aggregates or GNB membranes to soluble or membrane-bound CD-14. Complexes of single endotoxin molecules and CD-14 then combine with extracellular MD-2 or with MD-2 associated with membrane bound TLR-4. Simultaneous engagement of MD-2 with endotoxin and TLR-4 results in cell activation and initiates intracellular signaling that releases proinflammatory mediators and recruits neutrophils to the lung. Murine endotoxin inhalation studies demonstrate a dose-dependent increase in TNF-α, MIP-1α, IL-6, G-CSF, KC, and IL-1β; peri-bronchiolar neutrophilia; release of LDH; and increased markers of oxidative stress.2 Figure 2 shows that these outcomes do not occur in TLR-4 mutant or CD-14 or MD-2 knock-out mice. If monomeric LOS:MD-2 is administered to MD-2−/− mice the inflammatory pathway is restored. The portion of the endotoxin molecule that triggers TLR-4 signaling is the phylogenetically conserved lipid A moiety that is embedded in the GNB cell outer membrane. Lipid A normally consists of six acyl chains attached to two linked glucosamine molecules. However, there are GNB endotoxins that contain only five acyl chains and these have been shown in murine pulmonary exposure studies to induce minimal inflammation.2
Figure 2.

Four strains of mice (C57Bl/6, TLR-3Lps-d, MD-2−/−, and CD-14−/−) were exposed to 300 EU of lipoöligosaccharide from Neisseria meningitidis alone (LOS) or complexed with MD-2 (LOS:MD-2) or received vehicle control (CNTL) by intranasal instillation. Neutrophils (Figure 2A) and cytokines (TNF-α, Figure 2B and IL-6, Figure 2C) in bronchoalveolar lavage were measured. Mice deficient in CD-14 did not mount an inflammatory response to the LOS alone nor to the monomeric LOS:MD-2 complex. Similarly, TLR-4 deficient mice had limited responses. The MD-2−/− mice did not respond to the LOS but demonstrated a robust response to the LOS:MD-2 showing that exogenously-administered MD-2 complexed with endotoxin could restore the pathway to inflammation.
Human inhalation exposure studies to endotoxin have validated findings from epidemiology and animal studies on the action of endotoxin as an innate immune activating agent.3 Analysis of induced sputum from human subjects exposed to 20,000 EU of inhaled endotoxin demonstrated increased induced sputum levels of neutrophils, eosinophils, IL-6 and TNF-α in both normal subjects and those with atopic asthma assessed six hours after exposure. Cell surface expression of CD-14 also increased significantly in both groups of subjects while expression of TLR-4 and TLR-2 increased only in the non-asthmatic subjects.3
Endotoxin Exposure Assessment and Exposure Control
Endotoxin exposure is commonly assessed from active air samples, passive electrostatic dust collector (EDC) samples, or vacuumed reservoir dust samples. Samples are then extracted into an aqueous medium comprised of pyrogen-free water (PFW), PFW with Tween 20, or TRIS-EDTA in PFW. Using [14C]-3-OH-fatty acids produced from metabolically labelled Neisseria meningitidis serogroup B strain and Escherichia coli CL99, we demonstrated that TRIS-EDTA provided the highest degree of release of endotoxin from whole GNB, GNB membrane blebs, and aggregates of endotoxin from environmental samples.4 TRIS-EDTA was particularly effective for samples in which the endotoxin was held in bacterial membranes. Two commercial assay methods are in common use for endotoxin quantification and both are based on the bioactivity of the hemolymph of the horseshoe crab, Limulus polyphemus: the kinetic chromogenic Limulus amebocyte lysate assay (kcLAL) and the recombinant Factor C (rFC) assay. We analyzed 912 paired samples from agricultural settings in both methods and showed they yielded comparable estimates of airborne endotoxin concentration (Pearson correlation=0.91, p<0.0001, slope=0.979±0.017).5
Endotoxin exposure control in occupational settings requires limiting exposure to tasks that generate high concentrations of inhalable bioaerosols such as high-pressure spraying; power washing; auguring, sifting, or mixing grain; or handling poultry and livestock, especially in confined spaces. Well-designed local and general exhaust systems and powered air purifying respirator use are essential. In residential, office and school settings source control, HEPA filtration and frequent cleaning with high-efficiency vacuum cleaners are the most effective measures to reduce endotoxin exposure.
Epidemiologic Studies of Endotoxin
Many epidemiologic studies have assessed the role of endotoxin in asthma and wheeze. Urban cohort studies have provided clear evidence that higher endotoxin in house dust is positively associated with higher prevalence of wheeze but lower allergy.6 Studies in children have shown that growing up on a family farm with higher exposure to endotoxin leaves one less likely to develop allergies and allergic asthma.7 This protective effect has received support from studies demonstrating a shift away from an allergic phenotype with early life exposure to endotoxin. However, studies from Australia, Europe, New Zealand, and the United States found the same or higher asthma rates (i.e., no protective effect) between farm and nonfarm children in one or more study groups.6
Children from two genetically similar agrarian founder populations were studied to assess the role of endotoxin and the environmental microbiome in innate and adaptive immunity.7 Amish and Hutterite populations both engage in dairy farming but in a different way. The Amish apply non-mechanized, 19th century farming methods and children enter the livestock barns from early life. Hutterites use modern farming practices and young children have less animal exposure. In the Amish, intense and sustained exposure to endotoxin and microbes activate innate immune pathways that shape and calibrate downstream immune responses. The result of this is very low prevalence of allergy and asthma in Amish children that is not seen in Hutterite children.7 Exposing mice to ovalbumin (OVA) in an asthma model along with airborne dust collected in Hutterite homes induced lung eosinophilia and bronchial hyperreactivity equal to or greater than OVA alone. However, mice exposed to Amish house dust with OVA had few eosinophils in the lung lavage and blunted airway reactivity. Importantly, the Hutterite EDC-collected house dust contained 648 EU/m2 of endotoxin while the Amish airborne dust was nearly 7 times higher at 4400 EU/m2 (p <0.001).7
Domestic epidemiologic studies of endotoxin and asthma often rely on measuring endotoxin in vacuum-sampled reservoir dust. Figure 1B shows geometric mean loads of endotoxin from several studies from our group including the National Health and Nutrition Examination Survey (NHANES) and the National Survey of Lead and Allergens in Housing (NSLAH). In NHANES, the largest national study of endotoxin ever conducted, the concentration of endotoxin in composite reservoir dust samples collected from bedding and bedroom floors was positively associated with wheeze in the past 12 months, wheeze during exercise, doctor and/or emergency room visits for wheeze, and use of prescription medications for wheeze.6 When associations between endotoxin exposure and outcomes were stratified by sensitization status, exercise-induced wheeze was associated primarily with non-sensitized participants, while wheeze and use of prescription medications for wheeze were more frequent in sensitized participants. Significant predictors of higher endotoxin exposures were lower family income; Hispanic ethnicity; participant age; dogs, cats, cockroaches, and/or smokers in the home; and having carpeted floors.6 Co-exposure to dog and cat allergens was found to increase the association of endotoxin with asthma and wheeze.8 Further studies in this cohort showed that predictors of endotoxin exposure and the association of endotoxin with asthma and wheeze differed across climate regions in the U.S.9 In very cold and cold regions of the country endotoxin exposure was positively associated with any wheeze, exercise-induced wheeze, prescription medication for wheeze, and doctor/ER visit for wheeze. Endotoxin exposure was positively associated with asthma and wheeze outcomes but was negatively associated with sensitization to inhalant allergens in hot-humid climates. These differences could explain why studies conducted in a single city or state often yield differing results. Analysis of geocoded NHANES data along with monitored and modelled air pollution data demonstrated that co-exposure to elevated concentrations of residential endotoxin and ambient PM2.5 in adults and children and NO2 in child participants was synergistically associated with increased visits to emergency departments for asthma.10
Evidence is overwhelming that endotoxin inhalation induces innate immune responses and lung inflammation.2–4,7 However, in most circumstances airborne endotoxin exposure is accompanied by varying amounts and types of bacteria, fungal spores and MAMPs. Thus, in some circumstances endotoxin exposure assessment may represent a proxy for other elements of the environmental microbiome that display a more complex array of immune active stimuli.6 This was evident in the study of Amish and Hutterite children in which the microbial composition of dust samples from Amish homes differed markedly from Hutterite homes and led to dramatic differences in the innate immune phenotypes found between the two groups of children.7 Intervention studies that target allergens and endotoxin in residences and schools also impact the concentrations and likely the diversity of the microbial environment in ways that may alter innate and adaptive immunity.
Footnotes
Conflict of Interest Statement: The research studies by the author that are described in this manuscript were funded by the U.S. National Institutes of Health, Centers for Disease Control and Prevention, and Housing and Urban Development. The author has no conflicts of interest to disclose.
References:
- 1.Sigsgaard T, Basinas I, Doekes G, de Blay F, Folletti I, Heederik D, et al. Respiratory diseases and allergy in farmers working with livestock: a EAACI position paper. Clin Transl Allergy. 2020;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hađina S, Weiss JP, McCray PB Jr., Kulhankova K, Thorne PS. MD-2-dependent pulmonary immune responses to inhaled lipooligosaccharide: Effect of acylation state of LOS. Am J Respir Cell Mol Biol. 2008;38:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez ML, Herbst M, Lay JC, Alexis NE, Brickey WJ, Ting JPY, et al. Atopic asthmatic patients have reduced airway inflammatory cell recruitment after inhaled endotoxin challenge compared with healthy volunteers. J Allergy Clin Immunol. 2012;130:869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoppe Parr KA, Hađina S, Kilburg-Basnyat B, Wang Y, Chavez D, Thorne PS, et al. Modification of sample processing for the Limulus amebocyte lysate assay enhances detection of inflammogenic endotoxin in intact bacteria and organic dust. Innate Immunity. 2017;23:307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorne PS, Perry SS, Saito R, O’Shaughnessy PT, Mehaffy J, Metwali N, et al. Evaluation of the Limulus amebocyte lysate and recombinant Factor C assays for assessment of airborne endotoxin. Appl Environ Microbiol. 2010. 76:4988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, et al. Endotoxin exposure: predictors and prevalence of associated asthma outcomes in the United States. Am J Respir Crit Care Med. 2015;192:1287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendy A, Wilkerson J, Salo PM, Cohn RD, Zeldin DC, Thorne PS. Exposure and sensitization to pets modify endotoxin association with asthma and wheeze. J Allergy Clin Immunol Pract. 2018;6:2006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendy A, Wilkerson J, Salo PM, Cohn RD, Zeldin DC, Thorne PS. Endotoxin predictors and associated respiratory outcomes differ with climate regions in the U.S. Environ Int. 2017;112:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendy A, Wilkerson J, Salo PM, Weir CH, Feinstein L, Zeldin DC, et al. Synergistic association of house endotoxin exposure and ambient air pollution with asthma outcomes. Am J Resp Crit Care Med. 2019;200:712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


