Abstract
Fibrosis, stiffening and scarring of an organ/tissue due to genetic abnormalities, environmental factors, infection, and/or injury, is responsible for > 40% of all deaths in the industrialized world, and to date, there is no cure for it despite extensive research and numerous clinical trials. Several biomarkers have been identified, but no effective therapeutic targets are available. Human galectin-3 is a chimeric gene product formed by the fusion of the internal domain of the collagen alpha gene [N-terminal domain (ND)] at the 5′-end of galectin-1 [C-terminal domain (CRD)] that appeared during evolution together with vertebrates. Due to the overlapping structural similarities between collagen and galectin-3 and their shared susceptibility to cleavage by matrix metalloproteases to generate circulating collagen-like peptides, this review will discuss present knowledge on the role of collagen and galectin-3 as biomarkers of fibrosis. We will also highlight the need for transformative approaches targeting both the ND and CRD domains of galectin-3, since glycoconjugate binding by the CRD is triggered by ND-mediated oligomerization and the therapies targeted only at the CRD have so far achieved limited success.
Keywords: galectin-3, collagens, fibroproliferative diseases, myocardial fibrosis, hepatic fibrosis, pulmonary fibrosis, biomarkers
Abbreviations: CITP, C-terminal fragment of collagen type I degradation; COPD, chronic obstructive pulmonary disease; ECM, extracellular matrix; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; MCP, modified citrus pectin; MMP, matrix metalloprotease; NASH, nonalcoholic steatohepatitis; ND, N-terminus domain; PAH, Pulmonary arterial hypertension; PICP, C-terminal propeptide of procollagen type I; PINP, procollagen type I N-terminal propeptide; SSc, systemic sclerosis; TGF-β, transforming growth factor-β
The extracellular matrix (ECM) is the noncellular scaffold structure present within all tissues and organs, composed mainly of proteoglycans and fibrous proteins. It is crucial for tissue morphogenesis, differentiation, and homeostasis. ECM can be divided into basement membrane and interstitial matrix. The basement membrane, which functions as a scaffold for epithelial and endothelial cells, is composed of collagen type IV, laminin, nidogen (enactin), and perlecan. The major component is collagen type IV, constituting about 50% of all basement membrane proteins. Laminin is the major noncollagenous component of the basement membrane. The interstitial matrix, mainly produced by fibroblasts and composed of collagen I, III, V, and VI, fibronectin, and proteoglycans makes up the majority of ECM in the body. These components together assemble in a highly cross-linked network, with great functional and compositional variations allowing rapid diffusion of certain small molecules (reviewed in (1)).
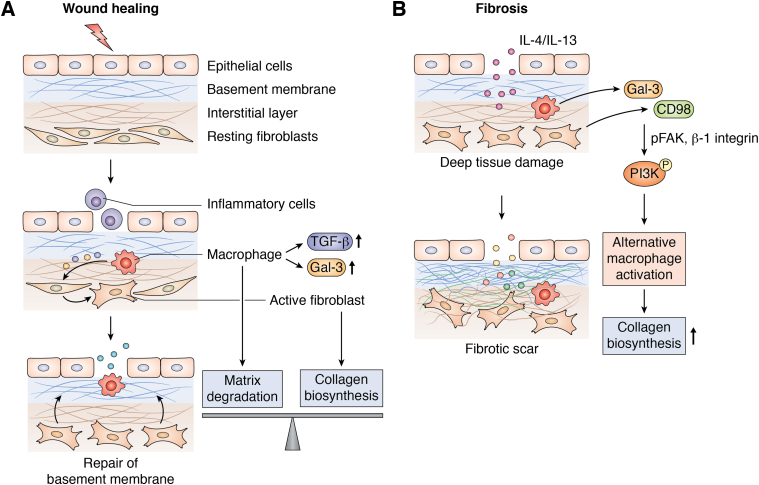
Tissue injury knocking out epithelial and endothelial cells and exposure of basement membrane results in an influx of inflammatory cells, such as macrophages and neutrophils into the damaged site. These cells secrete proteases to degrade the basement membrane and release the fragments of component proteins into circulation. To repair the basement membrane, the activated fibroblasts secrete new proteins to substitute the degraded proteins (Fig. 1A). This results in wound healing. In case of chronic inflammation, the deeper tissues including the interstitial layer of ECM are exposed and get damaged. Several inflammatory cytokines including the interleukins (2, 3) and members of transforming growth factor-β (TGF-β) (4, 5) secreted by platelets, endothelial cells, smooth muscle cells, and macrophages act on fibroblasts to induce proliferation and differentiation. The differentiated fibroblasts continue to secrete proteins leading to disproportionate accumulation of collagen in the interstitial space, causing scarring of the affected organ (1) (Fig. 1B). Galectin-3 is a profibrotic molecule regulating the functions of macrophages and fibroblasts in response to inflammation. It was shown that increased galectin-3 expression further activates myofibroblasts leading to wound scarring and is thus implicated in inflamed organ’s ‘fibrosis’(Fig. 1). On the other hand, its deficiency leads to reduced fibrotic response.
Figure 1.
Schematic presentation of wound healing and fibrosis: (A) Cell death resulting from tissue injury results in recruitment of inflammatory cells through the damaged epithelium. These cells secrete proteases, degrade basement membrane, and release the components into circulation. Galectin-3 and TGF-β secreted by macrophages activate the resting fibroblasts into myofibroblasts, which secrete fresh basement membrane resulting in wound healing. The synthetic and degradative processes are in balance. (B) Constant and repetitive injury induces damage to the deeper interstitial layer and results in overproduction of the basement membrane components in an unorganized way leading to fibrosis. Secretion of IL-4 and IL-13 by inflammatory cells activates alternative macrophages, activated macrophages secrete increased galectin-3 and overexpress its cell surface receptor CD98. Galectin-3 and CD98 binding stabilizes CD98 and activates PI3K via an association with phosphorylated FAK and β-1 integrin. A galectin-3 feedback loop drives alternative macrophage activation. (Adapted from Genovese and Karsdal, 2016, Expert Review of Proteomics, 13 (2), 213–225).
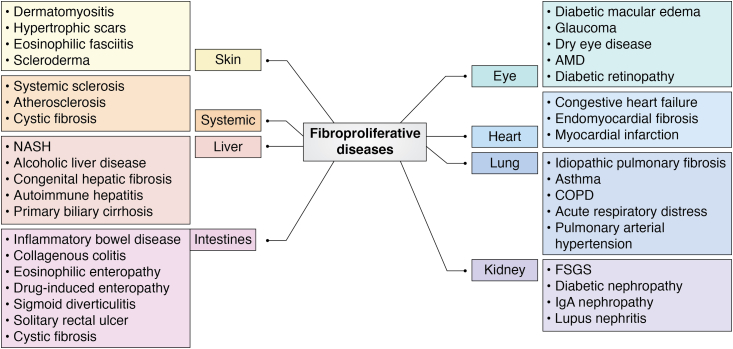
The abnormal remodeling of the ECM is related to a plethora of fibroproliferative diseases of various organs including heart, liver, lung, kidneys, skin, and some systemic disorders such as systemic sclerosis, atherosclerosis, and cystic fibrosis (Fig. 2) and is responsible for nearly 45% of all deaths (6). The mechanism of fibrosis is similar in various organs as all epithelial tissues such as skin, digestive tract, pulmonary organs, genitourinary tracts, and endothelial cells of blood vessels, as well as mesothelial cells in the body cavities, are lined by ECM (7, 8). In recent years, fibroproliferative process has been the focus of interest as a candidate for disease intervention. The noninvasive method of detecting fibrosis is by analyzing the levels of circulating biomarkers. Several biomarkers have been studied including collagen-related peptides, matrix metalloproteases (MMPs), tissue inhibitors of metalloproteases, selected miRNAs, galectin-3, and several noncollagen-related peptides. Among these, collagen peptides and galectin-3 appear to be most promising as reflecting combined effects of injury, inflammation, and fibrosis. In this review, we have selected the liver, heart, and lung as representative organs of fibroproliferative diseases, and we will discuss the current knowledge on these two proteins and their peptides as biomarkers of fibrosis and disease progression and value of galectin-3 as a therapeutic target.
Figure 2.
Fibroproliferative diseases: Various organs develop fibrotic scars as a result of constant tissue damage and insults. FSGS, focal segmental glomerlosclerosis; NASH, nonalcoholic steatohepatitis; AMD, age-related macular degeneration; COPD, chronic obstructive pulmonary disease. (Adapted from Karsdal et al, 2014 Alimentary Pharmacology and Therapeutics, 40: 233–249)
Biomarkers of fibrosis
Collagens
Collagens are a superfamily of 28 members comprising the most abundant proteins in humans. The common feature of all family members is a triple helix structure made up of three polypeptide chains, which can either be homotrimers (3 identical α chains) or heterotrimers (nonidentical α chains) and are of variable lengths in different members. Collagens are characterized by their capacity to form supramolecular assemblies (9). Based on the supramolecular structure, the collagens can be fibrils, beaded filaments, anchoring fibrils, and networks (10). Further diversity in the collagen family is due to the several molecular isoforms as well as alternative splicing and alternative promoters. Collagens are synthesized as procollagens and cleaved to mature form. The mature collagens are enzymatically cleaved and released as biologically active fragments (10). As collagens are an integral part of the ECM, their deregulated cleavage and reassembly play an important role in fibrosis. Circulating collagen fragments (neoepitopes) as biomarkers of the fibrogenic or fibrolytic events in various diseases have been studied extensively. Propeptides, which are released from procollagen as part of the maturing process, reflect the synthetic process, whereas the degradation epitopes, which are released as part of the degradation process reflect the fibrolytic process (9). Various collagen epitopes used as biomarkers have been summarized in Table 1.
Table 1.
Major collagen neo-epitopes used as serum biomarkers for fibroproliferative diseases
| Synthesis-related epitopes | |||
|---|---|---|---|
| Collagen type | Name | Description | Affected organ |
| Collagen type I | PINP | Amino-terminal peptide of procollagen type I | Heart |
| PICP | C-terminal peptide of procollagen type I | Heart | |
| Collagen type III | PIIINP | Amino-terminal peptide of procollagen Type III | Liver, heart, and lung |
| Pro C3 | A fragment of N-terminal type III collagen | Lung | |
| Collagen type IV | P4NP7 | 7S domain of type IV collagen | Liver |
| NC-1 | Carboxy-terminal region of alpha chain | Liver | |
| Collagen type VI | ProC6 | A fragment of C-terminal type VIa3 collagen | Lung |
| Degradation-related epitopes | |||
|---|---|---|---|
| Collagen type | Name | Description | Affected organ |
| Collagen type I | CIM | MMP degraded fragment of Collagen type I | Lung |
| CITP | Carboxy terminal telopeptide of collagen I | Heart | |
| Collagen type III | C3M | MMP degraded fragment of Type III collagen | Liver, Lung |
| C3A | ADAMTS degraded fragment of type III collagen | Lung | |
| Collagen type IV | C4M | MMP degraded fragment of type IV collagen | Liver |
| Collagen type V | C5M | MMP degraded fragment of type V collagen | Lung |
| Collagen type VI | C6M | MMP degraded fragment of type VI collagen | Lung |
Galectin-3
Human galectin-3 (LGALS3), a protein of 31Kda (11, 12), belongs to the galectin gene family of carbohydrate-binding proteins and is the only member that is expressed in vertebrates. All of the galectin family members contain a conserved carbohydrate-binding domain of ∼130 amino acids. Galectins have been divided into three subtypes based on their structure: prototype, tandem repeat, and chimera. Galectin-3 is the only chimera (fused) protein consisting of three distinct structural motifs: a short 12 amino acid N-terminal motif, followed by a long collagen α-like sequence (collagen α) and a C-terminal carbohydrate-binding domain (galectin-1).
The short N-terminal motif contains a site of serine6 phosphorylation for controlling the nuclear transport and ligand affinity (13). The long and intrinsically disorganized collagen α like proline-rich sequence of about 110 amino acids (ND) is cleavable by MMPs -2, -9, and membrane type 1 MMP at the Ala62-Tyr63 bond, resulting in the generation of a cleaved fragment of 22kD (14) (Fig. 3). The C-terminal domain (CRD), consisting of about 130 amino acids, is the common domain shared by all galectin family members and is responsible for their lectin activity. Galectin-3 has a preferential binding for N-acetyllactosamine residues on cell surface glycoconjugates. Galectin-3 interacts with several ligands both intracellularly and extracellularly and influences various pathways and processes via its CRD binding activity. It binds Bcl-2, CD95, Nucling, Alix/AIP1, synexin, and regulates apoptosis. Its interaction with activated K-Ras protein affects cell proliferation and survival. β-catenin is its binding partner in the Wnt signaling pathway. Galectin-3 binds to laminin, fibronectin, hensin, elastin, collagen IV, and tenascin-C and tenascin-R to modulate cell–ECM adhesion. In addition, it also binds α1β1, αvβ3, and αMβ1 integrins, the main proteins involved in cell adhesion (Table 2).
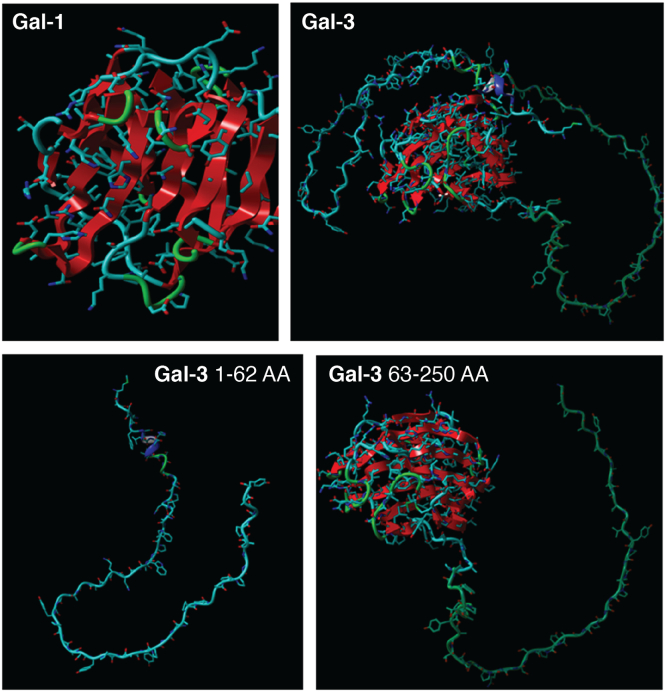
Figure 3.
Molecular structure of galectin-1(top left) and galectin-3 (top right). Lower panel: cleaved galectin-3 N-terminal and C-terminal. Visualization and analysis was done by YASARA model software.
Table 2.
Binding partners of galectin-3 C-terminal and N-terminal
| C-terminal | Function | Reference |
|---|---|---|
| Bcl2 | Apoptosis | Yang et al, 1996 (145) |
| CD95 | Apoptosis | Fukumori et al, 2004 (146) |
| Nucling | Apoptosis | Liu et al, 2004 (147) |
| Alix/AIP1 | Apoptosis | Liu et al, 2002 (148) |
| K-Ras | Cell proliferation | Eelad-sfadia et al, 2004 (149) |
| Akt | Cell Proliferation | Lee et al, 2003 (150), Oka et al, 2005 (151) |
| β-catenin | Wnt signaling | Shimura et al, 2004 (152) |
| Laminin | ECM adhesion | Massa et al, 1993 (153), |
| Fibronectin | ECM adhesion | Sato et al, 1992 (154) |
| Hensin | ECM adhesion | Hikita et al, 2000 (155) |
| Elastin | ECM adhesion | Ochieng et al, 1998 (156) |
| Collagen IV | ECM adhesion | Ochieng et al, 1998 (157) |
| Tenascin-C&-R | ECM adhesion | Probstmeier et al, 1995 (158)) |
| α1β1 integrin | Cell adhesion | Ochieng et al, 1998 (157) |
| CD11b/CD18 | Inflammatory macrophage | Dong et al, 1997 (28) |
| Lamp-1 and -2 | Inflammatory macrophage | Dong et al, 1997 (28) |
| IgE | Inflammation | Cherayil et al, 1989 (159) |
| CD44 | Cargo protein internalization | Lakshminarayan et al, 2014 (160) |
| CD98 | Membrane trafficking | Dalton et al, 2007 (161) |
| CD66 | Inflammatory neutrophils | Feuk-Lagersted et al,1999 (162) |
| N-terminal | Function | Reference |
| EGFR | Cross-linking & endocytosis | Partridge et al, 2004 (163); Liu 2012 (164) |
| TGFβR | Cross linking& endocytosis | Partridge et al, 2004 (163) |
| CD147 | Clustering &MMP9 induction | Mauris et al, 2014 (138) |
| Alix | HIV infection | Wang et al, 2014 (139) |
| Alix | T cell receptor (TCR) downregulation | Chen et al, 2009 (165) |
| Bacterial LPS | Inflammation | Lo et al, 2021 (142) |
Abbreviations: Alix, ALG2 interacting protein X; CEA, carcinoembryonic antigen.
A germ-line mutation at position 191 (rs4644) substituting amino acid proline64 to histidine makes this protein susceptible to MMP cleavage and enhances its migratory and angiogenic potential (15, 16). It was reported that cleaved galectin-3 had stronger affinity for glycoconjugates than the full-length protein (17), while some other interactions require both N-terminus domain and CRD motifs. Galectin-3 displays multivalency by the hydrophobic interactions of the N terminal with itself and with the CRD forming a fuzzy complex, which is the characteristic of intrinsically disordered proteins to achieve liquid–liquid phase separation (18). Additional functional oligomeric states exist due to the dynamic homodimerization of the N terminal. It is generally accepted that galectin-3 oligomerization gives rise to changes in activity, which are associated with and reflected in its diverse biological functions. Galectin-3 oligomer forms a lattice with T cell surface receptors that prevents their uncontrolled activation (19), and its cross-linking with either EGF and TGF-β receptors delays their internalization and degradation (20). Table 2 reflects the several biological processes regulated by C-terminal and N-terminal domains of galectin-3 in association with various binding partners.
Galectin-3 is a profibrotic molecule and implicated in modulation of fibroblasts and macrophage activity in chronically inflamed lung, liver, kidney, heart, skin, blood vessels, etc. affecting common fibroproliferative pathways leading to fibrosis (21, 22). It is a proinflammatory molecule (23). It regulates immune functions and mediates acute and chronic inflammation. It activates and is abundantly expressed in cells of myeloid origin, such as monocytes, macrophages, dendritic cells, and neutrophils (24). It interacts with inflammatory cytokines TGF-β and CD98 expressed by migrating inflammatory cells and plays a major role in the profibrotic response (5). In galectin-3–deficient mice, a dramatic reduction in fibrosis in response to TGF-β and bleomycin was observed accompanied with reduced epithelial to mesenchymal transition and myofibroblast activation (25, 26). Galectin-3 is instrumental in TGF-β1–induced fibroblasts differentiation via the MAPK/extracellular signal-regulated kinase (ERK)-ERK 1/2 signaling pathway (Fig. 1). It aids the extravasation of inflammatory cells and binds to specific cell surface receptors on macrophages (CD11b, CD98) and on neutrophils (CD66). It is upregulated in alternative macrophage activation by IL4 and IL13, and it activates PI3K via binding to CD98 and aids in increased collagen deposition (27, 28) by differentiation of resting fibroblasts into myofibroblasts leading to scar formation (29) (Fig. 1).
Hepatic fibrosis
Hepatitis B and Hepatitis C virus infections, innate immunity, and chronic inflammation play a role in the pathogenesis of liver metabolic disorders, nonalcoholic fatty liver disease, liver steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. Chronic liver disease and cirrhosis result in ∼35,000 deaths each year in the US and for approximately two million deaths per year worldwide.
The major histological components of the liver consist of (i) hepatocytes, constituting the parenchyma, (ii) stroma, (iii) sinusoids (the capillaries travelling between hepatocytes), and (iv) the spaces of Disse, which are located between the hepatocytes and the sinusoids. The liver sinusoids are a unique structure and are classified as discontinuous capillaries as they do not have a continuous endothelial lining but are endowed with fenestrated endothelium, which enables liver functions like ultrafiltration, endocytosis, and immunological activities. The spaces of Disse is enriched in collagen IV and perlecan but lacks laminin and nidogen (other components of the basement membrane). Sinusoidal capillarization is characterized by the formation of the basal lamina, loss of fenestrae, and transformation of the sinusoids into the continuous capillary. This interferes with hepatic microcirculation and leads to hepatic dysfunction (30). The capillarization of sinusoids is the basic remodeling in all chronic liver diseases, accompanied with increased collagen IV and laminin deposits in the spaces of Disse.
Collagen fragments as biomarkers of hepatic fibrosis
Collagen IV expression levels were used to distinguish between the early and late stages of fibrosis in hepatitis C-related fibrosis (31). A 14-fold increase in collagen IV was observed in liver cirrhosis (32), and the levels were higher in alcoholic than in nonalcoholic hepatitis and cirrhosis (33, 34). In addition, neoepitopes of collagen IV: 7S and NCI (noncollagenous C-terminal domain of collagen IV) domains have been studied as noninvasive biomarkers of chronic liver disease. A correlation between the serum 7S collagen fragment levels and liver fibrosis grade was observed in chronic hepatitis C (35), and these levels were much higher in chronic hepatitis C with cirrhosis (36, 37). A similar relationship was observed with NCI and progressive liver disease cirrhosis, when compared with chronic active hepatitis (38, 39). To date, the most used marker of liver fibrosis is the amino-terminal peptide of procollagen type III (PIIINP) (40, 41, 42) together with C3M, which is an MMP degraded fragment of type III collagen reported to be elevated in liver fibrosis, skin fibrosis, and ankylosing spondylitis (43, 44, 45).
Galectin-3 as a biomarker of hepatic fibrosis
Galectin-3–null mice fed high-fat diet exhibited all the symptoms of nonalcoholic fatty liver disease including increased liver weight, elevated triglycerides, hyperglycemia, and hepatic steatosis, as well as inflammation and fibrosis (44) subsequently developing liver nodules progressing to hepatocellular carcinoma (44, 45). However, some other investigators have documented protection from NASH with attenuation of fibrosis, inflammation, and hepatic injury and other symptoms related to high-fat diets such as hepatocyte degeneration and focal necrosis in galectin-3 KO mice (46, 47). In the carbon tetrachloride-induced cirrhosis mouse model, galectin-3 played a role in ECM production (48) and was responsible for diet-induced steatohepatitis through IL-33/ST2 axis (48).
The removal of advanced glycation end products and advanced lipidomic end products is performed by the liver and galectin-3 is reported to be directly involved with the endocytosis of these harmful byproducts by the liver's sinusoidal and endothelial cells. In galectin-3–null mice, the circulating levels of advanced glycation end products/advanced lipidomic end products were higher than the control mice (46, 47), though overexpression of galectin-3 is related to detoxification protection (48).
Myocardial fibrosis
Cardiac fibrosis is a significant worldwide health problem associated with nearly all forms of heart disease causing >650,000 deaths in the US. Formation of a fibrotic scar in the cardiac muscle is a fundamental part of several cardiovascular diseases, such as heart failure (HF), dilated cardiomyopathy, and hypertrophic cardiomyopathy and is characterized by activation and differentiation of cardiac fibroblasts into myofibroblasts leading to increased matrix stiffness and abnormalities in cardiac function (for review see (49)).
Collagen fragments as biomarkers of myocardial fibrosis
Both the synthesis and breakdown-related neoepitopes of collagen have been studied as biomarkers of cardiac fibrosis. The major component of the cardiac ECM is collagen type I, which makes fibrillar structures in combination with collagen type III. In addition, collagen type IV, V, and VI are also present in small amounts in the pericellular space and around the myocytes (50, 51, 52). Plasma levels of C-terminal propeptide of procollagen type I (PICP), which is released as a byproduct of the maturing process of procollagen type I, were shown to be correlated with the myocardial PICP content and collagen volume fraction as determined histologically in hypertrophic cardiomyopathy (53) and hypertension patients (54). However, in a study on the heart failure model in rats, no such correlation was observed (55) indicating the involvement of other confounding factors such as weight loss and a catabolic state. In a study including 111 patients with decompensated heart failure, serum PICP levels were found to be significantly increased in patients that underwent new hospitalizations or death (56). These authors concluded that PICP could be used as an independent predictive biomarker of heart failure, hospitalization, and death.
Another synthesis biomarker of collagen type I is the procollagen type I N-terminal propeptide (PINP), a cleavage product by the proteolytic activity of the ADAMTS family (57). A few studies have examined its validity as a biomarker for fibrosis. Zile et al. in a large case-control study reported that in patients of heart failure with reduced ejection fraction, levels of PINP and PIIINP were higher than the controls (58). In a rat model of ischemic cardiomyopathy, plasma PINP levels were much higher than the controls (59). Various studies have shown that serum PIIINP levels can be used as a biomarker of myocardial fibrosis. PIIINP levels in the serum were higher and could be correlated to the cardiac collagen III levels in patients suffering from idiopathic or ischemic dilated cardiomyopathy (60). Higher serum PIIINP levels could also be related to advanced heart diseases (61).
MMPs are the matrix metalloproteases that degrade mature collagen into smaller biologically active fragments. A C-terminal fragment of collagen type I degradation (CITP) has been of interest as a biomarker of fibrolysis. There are some controversial data on the relationship of CITP to heart disease. Lombardi et al. in a cross-sectional study demonstrated that levels of CITP were higher, while the levels of fibrogenic markers of collagen type I (PICP and PINP) were not affected indicating a shift toward the breakdown of collagen type I in hypertrophic cardiomyopathy patients (62). Similarly, elevated serum CITP levels were observed in patients with heart failure and atrial fibrillation (63, 64). Circulating levels of CITP were considered as an independent prognostic biomarker of acute myocardial infarction–related death (65). However, another study did not find a correlation of circulating CITP levels with either cardiac collagen type I and III expression levels or with other left ventricle remodeling parameters (66). Ding et al. (61) stated that CITP levels may have a diagnostic and prognostic value in myocardial fibrosis.
Galectin-3 as a biomarker of myocardial fibrosis
Several cardiovascular diseases, especially those that result from chronic inflammation, have been associated with increased serum galectin-3 levels. In 2017, the American Heart Association recommended that plasma levels of galectin-3 could be used as a risk factor and prognosis biomarker of heart failure (67). A pooled analysis of data from three cohorts (COACH, PRIDE, and UDM H-23258) showed that plasma galectin-3 concentration > 17.8 ng/ml was predictive of rehospitalizations in heart failure patients (68). Several other studies have shown a positive correlation between serum galectin-3 levels and prognosis for heart failure (69, 70, 71); however, some reports did not find such a correlation (72, 73). In their recent review, Blanda et al. concluded that galectin-3 has a prognostic value for HF patients, but its value in the prediction of early diagnosis of HF is not so certain (74).
Atherosclerosis, the plaque deposition in the arteries results from several risk factors including hyperlipidemia, hypertension, diabetes, and insulin resistance. It was shown that patients with unstable plaques had higher circulating galectin-3 levels than those with stable plaques (75). Moreover, a correlation was also shown between the number of compromised vessels and serum galectin-3 levels (75). Several other studies have shown higher levels of galectin-3 in advanced carotid atherosclerosis (76, 77) and demonstrated that galectin-3 is an independent biomarker of advanced atherosclerosis independent of age, sex, LDL cholesterol levels, and history of acute myocardial infarction (77). Galectin-3 was reported to directly affect the functioning of the three cell types involved in the development of atherosclerosis, i.e., the dysfunction of endothelial cells, differentiation of monocytes to macrophages and foam cells, and proliferation and migration of vascular smooth muscle cells (reviewed in (78)). Sharma et al. (79) demonstrated the conversion of fibroblasts to myofibroblasts by treating the cells with recombinant galectin-3, resulting in the expression of TGF-β, collagen, and cyclin D1. Activation of macrophages by galectin-3 resulted in increased production of IL-4 and IL-13 and ECM deposition (25). Galectin-3 also increases the collagen I synthesis in HL-I cardiomyocytes (80).
Ferreira et al. reported that galectin-3 is associated with the onset of left ventricular diastolic dysfunction in postmyocardial infarction patients (81). In a recent study, based on 5805 participants, Mortensen et al. (82) concluded that low galectin-3 was a useful independent negative predictor of cardiovascular risk. In a long-term follow-up study, patients with high plasma galectin-3 levels showed higher mortality rates (83, 84), while those without acute events showed decreased galectin-3 levels (85). High serum galectin-3 but not galectin-1 levels were associated with increased incidence of large atherosclerotic stroke (86) and postoperative stroke in patients undergoing carotid endarterectomy, postoperative cerebrovascular ischemic events (87). Galectin-3 distribution was altered in arteries from patients with the peripheral arterial disease; its expression was mainly localized in the middle media layer in peripheral arterial disease patients as compared to the outer adventitia layer in normal arteries (88).
Pulmonary fibrosis
Pulmonary fibrosis is a severe, lifelong lung disease that is nearly always fatal and affects ∼ 128,000 people per year in the US with recorded early mortality of ∼ 40,000 patients per year. It is manifested as lung scarring and results as a pathological response when the compensatory mechanisms for remodeling normal tissue integrity fail after constant and persistent abuses such as lung infection, cigarette smoking, drug, or radiation treatment. When fibrosis of the lung occurs in response to interstitial lung disease (ILD), it is more serious as it is progressive; the worst prognosis has been reported for idiopathic pulmonary fibrosis (IPF) with a median survival of about 3 years after diagnosis. Pulmonary arterial hypertension (PAH) is common in patients with ILD. It is characterized by thickening of the pulmonary wall resulting in high arterial blood pressure contributing to the failure of the right ventricle. In these patients, fibrosis occurs in both the lung blood vessels as well as in the right ventricle (89).
Collagen fragments as biomarkers of pulmonary fibrosis
The normal lung tissue contains twice as much type I collagen as type III collagen, with these two being the main collagen types (90, 91). It was reported that in early fibrosis, both collagen levels increase, but the ratio changes in favor of type III collagen. In long-standing fibrotic scars mainly collagen I remained (92, 93, 94), which is less elastic and contributes to fibrosis-related abnormalities. In addition to increased collagen I and its cross-linking, there is also increased elastin, fibronectin, hyaluronan, and proteoglycans all contributing to the inaccessibility of collagen cleavage sites by the proteolytic enzymes (95, 96, 97). Sand et al. (98) developed ELISA assays to assess the levels of circulating fragments of type IV collagen α1 and α3 chains as indicators of fibrosis and related their elevated levels to liver fibrosis and IPF or chronic obstructive pulmonary disease (COPD), respectively. Teles-Grilo et al. (99) demonstrated an increased expression of collagen type I and IV around the new pulmonary blood vessels of bleomycin-treated rats where pulmonary fibrosis was induced. The levels of neoepitope PIIINP increased significantly in broncho alveolar lavage fluid of IPF patients compared to controls (100). Tzortzaki et al. (101) showed expressions of collagen type XII and XIV in the lung’s fibrosis of IPF patients and overexpression of collagen type I in the fibrotic scar. Leeming et al. (102) were the first group to investigate the collagen turnover profiles in the serum of patients with lung fibrotic disease. They demonstrated that MMP-mediated degradation products of collagen type I, III, V, and VI (CIM, C3M, C5M, C6M) levels could differentiate between IPF and mild COPD patients and healthy controls. Elevated C4M and C3A (neo-epitope of ADAMTS-4, 5–mediated degradation of type III collagen) levels could be related to COPD.
Su et al. (103) showed that serum levels of PIIINP and three other ECM molecules (laminin, collagen type IV, and hyaluronic acid) could be used as biomarkers of disease progression from IPF to acute exacerbation of IPF and connective tissue disease–related ILD. Circulating levels of PIIINP were also reported to be higher in patients with progressive pulmonary fibrosis but not in those with stable fibrosis (104). Kubo et al. (105) analyzed nine serological biomarkers of collagen metabolism and organ involvement in 79 patients with systemic sclerosis (SSc) and concluded that increased turnover of collagen seen in patients of SSc may not be derived only from the skin. They reported that SSc patients with ILD or PAH showed increased type VI collagen metabolism as indicated by increased levels of C6M. In addition, ProC3 (a fragment of N-terminal type III collagen) and ProC6 (a fragment of C-terminal type VI a3 collagen) were also higher in SSc patients with PAH. In a prospective cohort study, 11 neoepitopes of MMP-degraded ECM proteins were tested in participants with idiopathic pulmonary fibrosis or idiopathic nonspecific interstitial pneumonia. In patients with progressive idiopathy pulmonary fibrosis, serum levels of C1M, C3A, C3M, C6M, CRPM (C-reactive protein metabolite), and VICM (a fragment of vimentin released by MMP) were significantly higher compared to healthy controls (106).
Lung tissues of the patients with IPF showed higher expression of collagen type VI α1 and collagen type VI α3 chains in the fibrotic foci containing a myofibroblasts core and procollagen I (107, 108). In addition, collagen type VI also interacts with ECM and cell surface proteins and forms a filamentous mesh around collagen I, II, III, and IV fibers (109). It was suggested that collagen type VI may also have a regulatory role in the early events of pulmonary fibrosis (110).
Galectin-3 as a biomarker in pulmonary fibrosis
Calvier et al. demonstrated a direct correlation between the onset of vascular hypertrophy, inflammation, and fibrosis with galectin-3 levels (111). Galectin-3 affects STAT 3 and MMP 9 signaling pathways in response to TGF-β induction and mediates vascular fibrosis (112). In a mouse model, TGF-β and bleomycin-induced lung fibrosis was blocked by TD139, a galectin-3 small molecule inhibitor with an affinity for carbohydrate-binding domain (26) via inhibiting β-catenin nuclear translocation. Similar effects were replicated in galectin-3 knockout mice (26).
In patients with PAH, serum galectin-3 levels were higher than the base levels (113) while other studies showed that serum galectin-3 could be a biomarker of the severity of PAH (114, 115). Feng et al. (116) demonstrated an increase in serum galectin-3 levels in patients with acute exacerbation of chronic obstructive pulmonary disease. Furthermore, the levels of circulating galectin-3 were higher in smokers compared to nonsmokers with acute exacerbation of chronic obstructive pulmonary disease. Idiopathic inflammatory myopathy patients with associated ILD showed elevated levels of serum galectin-3 compared to the healthy controls accompanied by increased galectin-3 expression in the inflammatory cells of interstitial fibrosis, myositis, and dermatitis (117). In a recent study, d’Alessandro et al. (118) reported a significant increase in serum galectin-1 and galectin-9 levels in fibrotic ILD patients compared to healthy controls.
Galectin-3 as a therapeutic target in fibroproliferative diseases
Galectin-3 secreted by macrophages, epithelial cells, and myofibroblasts regulates fibrosis, resulting in pathophysiological responses that include epithelial mesenchymal transition, apoptosis, activation, and proliferation of myofibroblasts resulting in increased production of ECM. Out of all the galectin family members, galectin-3 has shown the strongest involvement with fibrosis. Several preclinical and clinical studies have been conducted aiming at galectin-3 as an antifibrosis target; however, the results were not consistent.
Modified citrus pectin (MCP) was first reported as a functional inhibitor of galectin-3 (119, 120, 121). Later, other complex polysaccharides, e.g., belapectin, GCS-100, pectasol, and several small molecule inhibitors GM-CT-01, GR-MD-02, TD139, and GB1211, all focused on carbohydrate-binding domain, became commercially available as fibrosis inhibitors for preclinical and clinical studies. In galectin-3 null mice, hypoxia-induced right ventricle hypertrophy was ameliorated (122). Similar results were observed in N-Lac (a nonspecific galectin-3 inhibitor) ---treated mice (123). Barman et al. demonstrated a direct correlation between the galectin-3 levels and the pulmonary vascular remodeling using galectin-3 KO mice or using GM-CT-01 and GR-MD-02 inhibitors using an animal model of PAH (124).
Inhalation of a small molecule galectin-3 inhibitor TD139, having a strong affinity to the carbohydrate-binding domain, has been shown to reduce galectin-3 expression in the lungs of idiopathic pulmonary fibrosis patients along with decreased circulating levels of platelet-derived growth factor-BB, plasminogen activator inhibitor-1, galectin-3, CCL18, and YKL-40 (125). Another small oral molecule GB1211 is under development but has not been tested for fibrosis inhibition. GR-MD-02 attenuated the NASH-related hepatic fibrosis in a thioacetamide-induced mouse model of liver fibrosis and has undergone clinical trials for the treatment of NASH with advanced fibrosis (126, 127). In phase 2b clinical trials, however, no significant effect on NASH was observed in the patients treated with GR-MD-02 for 1 year compared to the placebo control (128).
In a mouse model of dilated cardiomyopathy, galectin-3 was upregulated by ∼40-fold in the heart, accompanied by dilation of cardiac chambers, reduced left ventricular ejection fraction, increased collagen content, and increased fibrosis. While galectin-3 gene knockout reduced these effects, MCP had no effect (129). In another study, however, MCP ameliorated cardiac dysfunction, reduced collagen deposition, and decreased myocardial injury, and galectin-3 expression in a rat model of heart failure (130). Belapectin was well tolerated by NASH patients but did not show anti-fibrotic effects in phase II clinical trial (131). Taken together, these studies imply that targeting just the carbohydrate-binding domain of galectin-3 is probably inadequate, as galectin-3 KO mice showed ameliorated fibrosis. Thus, most likely, both the N and C terminals of galectin-3 should simultaneously be targeted to combat fibrosis.
Concluding remarks
Chimeric galectin-3 is a biological marker of active inflammation and advanced disease that could be clinically useful alone or in combination with collagen to serve as marker(s) and therapeutic target(s), based on the fact that collagen fragments are being used as biomarkers for fibrosis and inflammation-related diseases as a noninvasive method to investigate the disease progression. Their abundance and diversity, as well as several synthesis-related and lysis-related neoepitopes of each collagen type make it a very complicated story to analyze. Moreover, as the neoepitopes are detected in serum, it is hard to predict the site of fibrosis unless other markers and symptoms of the disease are investigated. For example, serum levels of PIIINP are enhanced in progression of the liver, heart, and lung disease, while some other neoepitopes are specific for a particular organ, e.g., C6M and C3M are specific for liver fibrosis and PINP is specific for lung fibrosis (132). A detailed fingerprinting of various epitopes is required to be able to predict accurately the course of disease progression. Moreover, for accurate measurement of these markers, a specific antibody is crucial. Their levels can also be influenced by cellular uptake as some of the neoepitopes work as signaling molecules for cell surface receptors, e.g., integrin receptors (reviewed by (9)).
Galectin-3 on the other hand belongs to a small family. Specific antibodies are present for different members of the family. Moreover, galectin-3, galectin-1, and galectin-9 are the only galectins related to fibrosis, with galectin-3 being the most thoroughly investigated. Galectin-3 is of particular interest because it can be used as a biomarker as well as therapeutic target for fibrosis as it is one of the earlier proteins released during neutrophil activation. The secreted protein is cleaved by MMPs in the same manner as collagens, and its cleavage has been proposed to be used as a diagnostic marker of MMP activity (15). A comparison of the 3-dimensional structure of galectin-1 and galectin-3 full-length and cleaved forms has been shown in Fig. 3. Cleaved galectin-3 retains some part of the N-terminal domain, making it different from galectin-1, which is made up only of the CRD. Specific antibodies are able to distinguish between the full-length and cleaved galectin-3 (15).
However, targeting galectin-3 has not been as successful as antifibrosis treatment, compared to the studies in KO mice. This could be because all the inhibitors used so far are designed specifically to target CRD. As presented in Table 1, both N-terminal and C-terminal domains of galectin-3 play a significant role in ligand binding. N-terminal domain is important for full biological activity of galectin-3. It facilitates proper folding of the protein, its multivalency, secretion, and its carbohydrate-binding properties. Flores-Ibarra et al. (133) reported formation of hairpin by the amino acids spanning Tyr101 in the N-terminal to Leu 114 in CRD. Miller et al. demonstrated that binding of polysaccharide rhamnogalacturonan to galectin-3 utilized two epitopes within the carbohydrate-binding domain, and one novel epitope within the first 40 amino acids of the N-terminal domain (134). To neutralize the microbicidal properties of galectin-3, protozoan parasites Trypanosoma cruzi and Leishmania major have developed molecular mechanisms to proteolytically cleave galecin-3 at its N terminus. The cleaved galectin-3 retained the carbohydrate-binding capacity but failed to induce the immune response (135, 136). In a recent report, Zhao et al. (137) reported that replacing the prolines in the N-terminal domain impaired all the classical cellular functions attributed to galectin-3. Mauris et al. demonstrated the requirement of a full-length protein for interaction with and clustering of CD147, an MMP-9 inducer in migrating epithelia (138). N-terminal domain of galectin-3 secreted by T cells upon T cell receptor engagement interacts with the proline-rich region of ALG-2–interacting protein X (Alix) in HIV infected cells facilitating HIV-1 budding (139). Bocker et al. found that Δ1-62 and Δ1-116 showed a 3- to 6-fold increased binding efficiency to asialofetuin compared to native galectin-3 when tagged with SNAP (140). Although galectin-3 enhances neutrophil activation by binding to microbial lipopolysaccharides via its carbohydrate recognition domain, N-terminal domain is also required, indicating dependence upon multivalency of galectin-3 (141, 142).
Considering the above, it may be worthwhile to target both N-terminal and C-terminal domains of galectin-3 to control fibrosis by small molecule inhibitors and/or galectin-3 domains-specific antibodies. DX-52-1, a semisynthetic derivative of quinocarmycin, and HUK-921, a complex synthetic molecule related to naphthyridinomycin family, are two compounds that were reported to bind galectin-3 outside of its carbohydrate domain (143) and exhibited antimigration and antiproliferation effects on cells and showed no competition with the agonist lactose. In addition, several noncarbohydrate small molecules have been designed and tested (reviewed by (144)) in an attempt to obtain more maintainable, reproducible, and effective galectin-3 inhibition, however, with limited success. The potential for galectin-3 as a therapeutic target in fibrosis remains a challenge together with the need to further unveil the molecular mechanism that regulates MMPs activities in the circulation. It is expected that due to the deleterious effects of fibrosis on organs’ functions, a new class of drugs/antibodies selectively and explicitly directed against the intact and cleaved galectin-3 will be available as a novel therapeutic modality, not too far away in the future.
Conflict of interests
The authors declare that they have no conflict of interest with the contents of this article.
Acknowledgments
Author contributions
P. N.-M. and A. R. conceptualization; P. N.-M. and A. R. investigation; P. N.-M. writing-original draft; P. N.-M., V. H., and A. R. writing-reviewing and editing; V. B. visualization and analysis of galectin-1 and -3 structures.
Funding and additional information
This work was supported by the Paul Zuckerman Endowment (to A. R.) and by the NCI/NIH Cancer Center Support Grant (CA-22453).
Edited by Robert Haltiwanger
Contributor Information
Pratima Nangia-Makker, Email: ab4566@wayne.edu.
Avraham Raz, Email: araz@med.wayne.edu.
References
- 1.Genovese F., Karsdal M.A. Protein degradation fragments as diagnostic and prognostic biomarkers of connective tissue diseases: understanding the extracellular matrix message and implication for current and future serological biomarkers. Expert Rev. Proteomics. 2016;13:213–225. doi: 10.1586/14789450.2016.1134327. [DOI] [PubMed] [Google Scholar]
- 2.Nikolic-Paterson D.J., Main I.W., Tesch G.H., Lan H.Y., Atkins R.C. Interleukin-1 in renal fibrosis. Kidney Int. Suppl. 1996;54:S88–90. [PubMed] [Google Scholar]
- 3.O'Reilly S., Ciechomska M., Cant R., Hugle T., van Laar J.M. Interleukin-6, its role in fibrosing conditions. Cytokine Growth Factor Rev. 2012;23:99–107. doi: 10.1016/j.cytogfr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Wu N., Meng F., Invernizzi P., Bernuzzi F., Venter J., Standeford H., et al. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-beta1 biliary secretion in mice. Hepatology. 2016;64:865–879. doi: 10.1002/hep.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walton K.L., Johnson K.E., Harrison C.A. Targeting TGF-beta mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol. 2017;8:461. doi: 10.3389/fphar.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mak K.M., Mei R. Basement membrane type IV collagen and laminin: an overview of their biology and value as fibrosis biomarkers of liver disease. Anat. Rec. (Hoboken) 2017;300:1371–1390. doi: 10.1002/ar.23567. [DOI] [PubMed] [Google Scholar]
- 8.Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am. J. Pathol. 1974;77:314–346. [PMC free article] [PubMed] [Google Scholar]
- 9.Karsdal M.A., Daniels S.J., Holm Nielsen S., Bager C., Rasmussen D.G.K., Loomba R., et al. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int. 2020;40:736–750. doi: 10.1111/liv.14390. [DOI] [PubMed] [Google Scholar]
- 10.Ricard-Blum S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raz A., Pazerini G., Carmi P. Identification of the metastasis-associated, galactoside-binding lectin as a chimeric gene product with homology to an IgE-binding protein. Cancer Res. 1989;49:3489–3493. [PubMed] [Google Scholar]
- 12.Raz A., Carmi P., Raz T., Hogan V., Mohamed A., Wolman S.R. Molecular cloning and chromosomal mapping of a human galactoside-binding protein. Cancer Res. 1991;51:2173–2178. [PubMed] [Google Scholar]
- 13.Gong H.C., Honjo Y., Nangia-Makker P., Hogan V., Mazurak N., Bresalier R.S., et al. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59:6239–6245. [PubMed] [Google Scholar]
- 14.Ochieng J., Fridman R., Nangia-Makker P., Kleiner D.E., Liotta L.A., Stetler-Stevenson W.G., et al. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 15.Nangia-Makker P., Raz T., Tait L., Hogan V., Fridman R., Raz A. Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res. 2007;67:11760–11768. doi: 10.1158/0008-5472.CAN-07-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balan V., Nangia-Makker P., Schwartz A.G., Jung Y.S., Tait L., Hogan V., et al. Racial disparity in breast cancer and functional germ line mutation in galectin-3 (rs4644): a pilot study. Cancer Res. 2008;68:10045–10050. doi: 10.1158/0008-5472.CAN-08-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochieng J., Green B., Evans S., James O., Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim. Biophys. Acta. 1998;1379:97–106. doi: 10.1016/s0304-4165(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y.H., Qiu D.C., Chang W.H., Yeh Y.Q., Jeng U.S., Liu F.T., et al. The intrinsically disordered N-terminal domain of galectin-3 dynamically mediates multisite self-association of the protein through fuzzy interactions. J. Biol. Chem. 2017;292:17845–17856. doi: 10.1074/jbc.M117.802793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demetriou M., Granovsky M., Quaggin S., Dennis J.W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 20.Pugliese G., Iacobini C., Pesce C.M., Menini S. Galectin-3: an emerging all-out player in metabolic disorders and their complications. Glycobiology. 2015;25:136–150. doi: 10.1093/glycob/cwu111. [DOI] [PubMed] [Google Scholar]
- 21.Hara A., Niwa M., Noguchi K., Kanayama T., Niwa A., Matsuo M., et al. Galectin-3 as a next-generation biomarker for detecting early stage of various diseases. Biomolecules. 2020;10:389. doi: 10.3390/biom10030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miah A., S P., Tongue P., Roach K., Bradding P., Gooptu B. Ex vivo studies of the gal-3-fibrosome hypothesis in IPF and non-fibrotic control lung tissue and myofibroblasts. Thorax. 2019;74:A57. [Google Scholar]
- 23.Liu F.T., Hsu D.K. The role of galectin-3 in promotion of the inflammatory response. Drug News Perspect. 2007;20:455–460. doi: 10.1358/dnp.2007.20.7.1149628. [DOI] [PubMed] [Google Scholar]
- 24.Fulton D.J.R., Li X., Bordan Z., Wang Y., Mahboubi K., Rudic R.D., et al. Galectin-3: a harbinger of reactive oxygen species, fibrosis, and inflammation in pulmonary arterial hypertension. Antioxid. Redox Signal. 2019;31:1053–1069. doi: 10.1089/ars.2019.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacKinnon A.C., Farnworth S.L., Hodkinson P.S., Henderson N.C., Atkinson K.M., Leffler H., et al. Regulation of alternative macrophage activation by galectin-3. J. Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 26.Mackinnon A.C., Gibbons M.A., Farnworth S.L., Leffler H., Nilsson U.J., Delaine T., et al. Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012;185:537–546. doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato S., Ouellet N., Pelletier I., Simard M., Rancourt A., Bergeron M.G. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J. Immunol. 2002;168:1813–1822. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- 28.Dong S., Hughes R.C. Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen) Glycoconj J. 1997;14:267–274. doi: 10.1023/a:1018554124545. [DOI] [PubMed] [Google Scholar]
- 29.Slack R.J., Mills R., Mackinnon A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021;130:105881. doi: 10.1016/j.biocel.2020.105881. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Hernandez A., Martinez J. The role of capillarization in hepatic failure: studies in carbon tetrachloride-induced cirrhosis. Hepatology. 1991;14:864–874. doi: 10.1002/hep.1840140519. [DOI] [PubMed] [Google Scholar]
- 31.Chen W., Rock J.B., Yearsley M.M., Ferrell L.D., Frankel W.L. Different collagen types show distinct rates of increase from early to late stages of hepatitis C-related liver fibrosis. Hum. Pathol. 2014;45:160–165. doi: 10.1016/j.humpath.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Rojkind M., Ponce-Noyola P. The extracellular matrix of the liver. Coll. Relat. Res. 1982;2:151–175. doi: 10.1016/s0174-173x(82)80031-9. [DOI] [PubMed] [Google Scholar]
- 33.Ueno T., Inuzuka S., Torimura T., Oohira H., Ko H., Obata K., et al. Significance of serum type-IV collagen levels in various liver diseases. Measurement with a one-step sandwich enzyme immunoassay using monoclonal antibodies with specificity for pepsin-solubilized type-IV collagen. Scand. J. Gastroenterol. 1992;27:513–520. doi: 10.3109/00365529209000114. [DOI] [PubMed] [Google Scholar]
- 34.Hirayama C., Suzuki H., Takada A., Fujisawa K., Tanikawa K., Igarashi S. Serum type IV collagen in various liver diseases in comparison with serum 7S collagen, laminin, and type III procollagen peptide. J. Gastroenterol. 1996;31:242–248. doi: 10.1007/BF02389524. [DOI] [PubMed] [Google Scholar]
- 35.Murawaki Y., Ikuta Y., Koda M., Yamada S., Kawasaki H. Comparison of serum 7S fragment of type IV collagen and serum central triple-helix of type IV collagen for assessment of liver fibrosis in patients with chronic viral liver disease. J. Hepatol. 1996;24:148–154. doi: 10.1016/s0168-8278(96)80023-7. [DOI] [PubMed] [Google Scholar]
- 36.Sakugawa H., Nakayoshi T., Kobashigawa K., Yamashiro T., Maeshiro T., Miyagi S., et al. Clinical usefulness of biochemical markers of liver fibrosis in patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2005;11:255–259. doi: 10.3748/wjg.v11.i2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoneda M., Mawatari H., Fujita K., Yonemitsu K., Kato S., Takahashi H., et al. Type IV collagen 7s domain is an independent clinical marker of the severity of fibrosis in patients with nonalcoholic steatohepatitis before the cirrhotic stage. J. Gastroenterol. 2007;42:375–381. doi: 10.1007/s00535-007-2014-3. [DOI] [PubMed] [Google Scholar]
- 38.Hayasaka A., Schuppan D., Ohnishi K., Okuda K., Hahn E.G. Serum concentrations of the carboxyterminal cross-linking domain of procollagen type IV (NC1) and the aminoterminal propeptide of procollagen type III (PIIIP) in chronic liver disease. J. Hepatol. 1990;10:17–22. doi: 10.1016/0168-8278(90)90067-2. [DOI] [PubMed] [Google Scholar]
- 39.Babbs C., Haboubi N.Y., Mellor J.M., Smith A., Rowan B.P., Warnes T.W. Endothelial cell transformation in primary biliary cirrhosis: a morphological and biochemical study. Hepatology. 1990;11:723–729. doi: 10.1002/hep.1840110503. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui M.S., Yamada G., Vuppalanchi R., Van Natta M., Loomba R., Guy C., et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin. Gastroenterol. Hepatol. 2019;17:1877–1885.e1875. doi: 10.1016/j.cgh.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peleg N., Sneh Arbib O., Issachar A., Cohen-Naftaly M., Braun M., Shlomai A. Noninvasive scoring systems predict hepatic and extra-hepatic cancers in patients with nonalcoholic fatty liver disease. PLoS One. 2018;13:e0202393. doi: 10.1371/journal.pone.0202393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen M.J., Kazankov K., Leeming D.J., Karsdal M.A., Krag A., Barrera F., et al. Markers of collagen remodeling detect clinically significant fibrosis in chronic hepatitis C patients. PLoS One. 2015;10:e0137302. doi: 10.1371/journal.pone.0137302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trinchet J.C., Hartmann D.J., Pateron D., Munz-Gotheil C., Callard P., Ville G., et al. Serum type I collagen and N-terminal peptide of type III procollagen in patients with alcoholic liver disease: relationship to liver histology. Alcohol. Clin. Exp. Res. 1992;16:342–346. doi: 10.1111/j.1530-0277.1992.tb01388.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg W.M., Voelker M., Thiel R., Becka M., Burt A., Schuppan D., et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen M.J., Nedergaard A.F., Sun S., Veidal S.S., Larsen L., Zheng Q., et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am. J. Transl. Res. 2013;5:303–315. [PMC free article] [PubMed] [Google Scholar]
- 46.Iacobini C., Menini S., Ricci C., Blasetti Fantauzzi C., Scipioni A., Salvi L., et al. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J. Hepatol. 2011;54:975–983. doi: 10.1016/j.jhep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Negre-Salvayre A., Coatrieux C., Ingueneau C., Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butscheid M., Hauptvogel P., Fritz P., Klotz U., Alscher D.M. Hepatic expression of galectin-3 and receptor for advanced glycation end products in patients with liver disease. J. Clin. Pathol. 2007;60:415–418. doi: 10.1136/jcp.2005.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinderer S., Schenke-Layland K. Cardiac fibrosis - a short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019;146:77–82. doi: 10.1016/j.addr.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Eghbali M., Czaja M.J., Zeydel M., Weiner F.R., Zern M.A., Seifter S., et al. Collagen chain mRNAs in isolated heart cells from young and adult rats. J. Mol. Cell Cardiol. 1988;20:267–276. doi: 10.1016/s0022-2828(88)80059-2. [DOI] [PubMed] [Google Scholar]
- 51.Eghbali M., Blumenfeld O.O., Seifter S., Buttrick P.M., Leinwand L.A., Robinson T.F., et al. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J. Mol. Cell Cardiol. 1989;21:103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- 52.Engvall E., Hessle H., Klier G. Molecular assembly, secretion, and matrix deposition of type VI collagen. J. Cell Biol. 1986;102:703–710. doi: 10.1083/jcb.102.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang C., Qiao S., Song Y., Liu Y., Tang Y., Deng L., et al. Procollagen type I carboxy-terminal propeptide (PICP) and MMP-2 are potential biomarkers of myocardial fibrosis in patients with hypertrophic cardiomyopathy. Cardiovasc. Pathol. 2019;43:107150. doi: 10.1016/j.carpath.2019.107150. [DOI] [PubMed] [Google Scholar]
- 54.Ferreira J.P., Rossignol P., Pizard A., Machu J.L., Collier T., Girerd N., et al. Potential spironolactone effects on collagen metabolism biomarkers in patients with uncontrolled blood pressure. Heart. 2019;105:307–314. doi: 10.1136/heartjnl-2018-313182. [DOI] [PubMed] [Google Scholar]
- 55.Adamcova M., Baka T., Dolezelova E., Aziriova S., Krajcirovicova K., Karesova I., et al. Relations between markers of cardiac remodelling and left ventricular collagen in an isoproterenol-induced heart damage model. J. Physiol. Pharmacol. 2019;70 doi: 10.26402/jpp.2019.1.08. [DOI] [PubMed] [Google Scholar]
- 56.Ruiz-Ruiz F.J., Ruiz-Laiglesia F.J., Samperiz-Legarre P., Lasierra-Diaz P., Flamarique-Pascual A., Morales-Rull J.L., et al. Propeptide of procollagen type I (PIP) and outcomes in decompensated heart failure. Eur. J. Intern. Med. 2007;18:129–134. doi: 10.1016/j.ejim.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Colige A., Vandenberghe I., Thiry M., Lambert C.A., Van Beeumen J., Li S.W., et al. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J. Biol. Chem. 2002;277:5756–5766. doi: 10.1074/jbc.M105601200. [DOI] [PubMed] [Google Scholar]
- 58.Zile M.R., O'Meara E., Claggett B., Prescott M.F., Solomon S.D., Swedberg K., et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J. Am. Coll. Cardiol. 2019;73:795–806. doi: 10.1016/j.jacc.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 59.Zhang B., Li X., Chen C., Jiang W., Lu D., Liu Q., et al. Renal denervation effects on myocardial fibrosis and ventricular arrhythmias in rats with ischemic cardiomyopathy. Cell Physiol. Biochem. 2018;46:2471–2479. doi: 10.1159/000489653. [DOI] [PubMed] [Google Scholar]
- 60.Klappacher G., Franzen P., Haab D., Mehrabi M., Binder M., Plesch K., et al. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am. J. Cardiol. 1995;75:913–918. doi: 10.1016/s0002-9149(99)80686-9. [DOI] [PubMed] [Google Scholar]
- 61.Ding Y., Wang Y., Zhang W., Jia Q., Wang X., Li Y., et al. Roles of biomarkers in myocardial fibrosis. Aging Dis. 2020;11:1157–1174. doi: 10.14336/AD.2020.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lombardi R., Betocchi S., Losi M.A., Tocchetti C.G., Aversa M., Miranda M., et al. Myocardial collagen turnover in hypertrophic cardiomyopathy. Circulation. 2003;108:1455–1460. doi: 10.1161/01.CIR.0000090687.97972.10. [DOI] [PubMed] [Google Scholar]
- 63.Morine K.J., Paruchuri V., Qiao X., Mohammad N., McGraw A., Yunis A., et al. Circulating multimarker profile of patients with symptomatic heart failure supports enhanced fibrotic degradation and decreased angiogenesis. Biomarkers. 2016;21:91–97. doi: 10.3109/1354750X.2015.1118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kallergis E.M., Manios E.G., Kanoupakis E.M., Mavrakis H.E., Arfanakis D.A., Maliaraki N.E., et al. Extracellular matrix alterations in patients with paroxysmal and persistent atrial fibrillation: biochemical assessment of collagen type-I turnover. J. Am. Coll. Cardiol. 2008;52:211–215. doi: 10.1016/j.jacc.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 65.Manhenke C., Orn S., Squire I., Radauceanu A., Alla F., Zannad F., et al. The prognostic value of circulating markers of collagen turnover after acute myocardial infarction. Int. J. Cardiol. 2011;150:277–282. doi: 10.1016/j.ijcard.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 66.Nagao K., Inada T., Tamura A., Kajitani K., Shimamura K., Yukawa H., et al. Circulating markers of collagen types I, III, and IV in patients with dilated cardiomyopathy: relationships with myocardial collagen expression. ESC Heart Fail. 2018;5:1044–1051. doi: 10.1002/ehf2.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chow S.L., Maisel A.S., Anand I., Bozkurt B., de Boer R.A., Felker G.M., et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American heart association. Circulation. 2017;135:e1054–e1091. doi: 10.1161/CIR.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 68.Meijers W.C., Januzzi J.L., deFilippi C., Adourian A.S., Shah S.J., van Veldhuisen D.J., et al. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: a pooled analysis of 3 clinical trials. Am. Heart J. 2014;167:853–860.e854. doi: 10.1016/j.ahj.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Imran T.F., Shin H.J., Mathenge N., Wang F., Kim B., Joseph J., et al. Meta-analysis of the usefulness of plasma galectin-3 to predict the risk of mortality in patients with heart failure and in the general population. Am. J. Cardiol. 2017;119:57–64. doi: 10.1016/j.amjcard.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 70.Chen H., Chen C., Fang J., Wang R., Nie W. Circulating galectin-3 on admission and prognosis in acute heart failure patients: a meta-analysis. Heart Fail. Rev. 2020;25:331–341. doi: 10.1007/s10741-019-09858-2. [DOI] [PubMed] [Google Scholar]
- 71.Felker G.M., Fiuzat M., Shaw L.K., Clare R., Whellan D.J., Bettari L., et al. Galectin-3 in ambulatory patients with heart failure: results from the HF-action study. Circ. Heart Fail. 2012;5:72–78. doi: 10.1161/CIRCHEARTFAILURE.111.963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demissei B.G., Cotter G., Prescott M.F., Felker G.M., Filippatos G., Greenberg B.H., et al. A multimarker multi-time point-based risk stratification strategy in acute heart failure: results from the RELAX-AHF trial. Eur. J. Heart Fail. 2017;19:1001–1010. doi: 10.1002/ejhf.749. [DOI] [PubMed] [Google Scholar]
- 73.Tummalapalli S.L., Zelnick L.R., Andersen A.H., Christenson R.H., deFilippi C.R., Deo R., et al. Association of cardiac biomarkers with the Kansas city cardiomyopathy questionnaire in patients with chronic kidney disease without heart failure. J. Am. Heart Assoc. 2020;9:e014385. doi: 10.1161/JAHA.119.014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blanda V., Bracale U.M., Di Taranto M.D., Fortunato G. Galectin-3 in cardiovascular diseases. Int. J. Mol. Sci. 2020;21:9232. doi: 10.3390/ijms21239232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falcone C., Lucibello S., Mazzucchelli I., Bozzini S., D'Angelo A., Schirinzi S., et al. Galectin-3 plasma levels and coronary artery disease: a new possible biomarker of acute coronary syndrome. Int. J. Immunopathol. Pharmacol. 2011;24:905–913. doi: 10.1177/039463201102400409. [DOI] [PubMed] [Google Scholar]
- 76.Oyenuga A., Folsom A.R., Fashanu O., Aguilar D., Ballantyne C.M. Plasma galectin-3 and sonographic measures of carotid atherosclerosis in the atherosclerosis risk in communities study. Angiology. 2019;70:47–55. doi: 10.1177/0003319718780772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ciaccio M., Agnello L., Bracale U.M., Taranto M., Ciaccio M., Bracale U.M., et al. Galectin-3 and Lp(a) plasma concentrations and advanced carotid atherosclerotic plaques: correlation with plaque presence and features. Biochim. Cli. 2019;43:289–295. [Google Scholar]
- 78.Gao Z., Liu Z., Wang R., Zheng Y., Li H., Yang L. Galectin-3 is a potential mediator for atherosclerosis. J. Immunol. Res. 2020;2020:5284728. doi: 10.1155/2020/5284728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma U.C., Pokharel S., van Brakel T.J., van Berlo J.H., Cleutjens J.P., Schroen B., et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 80.Song X., Qian X., Shen M., Jiang R., Wagner M.B., Ding G., et al. Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim. Biophys. Acta. 2015;1853:513–521. doi: 10.1016/j.bbamcr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 81.Ferreira J.P., Bauters C., Eschalier R., Lamiral Z., Fay R., Huttin O., et al. Echocardiographic diastolic function evolution in patients with an anterior Q-wave myocardial infarction: insights from the REVE-2 study. ESC Heart Fail. 2019;6:70–79. doi: 10.1002/ehf2.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mortensen M.B., Fuster V., Muntendam P., Mehran R., Baber U., Sartori S., et al. Negative risk markers for cardiovascular events in the elderly. J. Am. Coll. Cardiol. 2019;74:1–11. doi: 10.1016/j.jacc.2019.04.049. [DOI] [PubMed] [Google Scholar]
- 83.Maiolino G., Rossitto G., Pedon L., Cesari M., Frigo A.C., Azzolini M., et al. Galectin-3 predicts long-term cardiovascular death in high-risk patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2015;35:725–732. doi: 10.1161/ATVBAHA.114.304964. [DOI] [PubMed] [Google Scholar]
- 84.Aksan G., Gedikli O., Keskin K., Nar G., Inci S., Yildiz S.S., et al. Is galectin-3 a biomarker, a player-or both-in the presence of coronary atherosclerosis? J. Investig. Med. 2016;64:764–770. doi: 10.1136/jim-2015-000041. [DOI] [PubMed] [Google Scholar]
- 85.Swiecki P., Sawicki R., Knapp M., Kaminski K.A., Ptaszynska-Kopczynska K., Sobkowicz B., et al. Galectin-3 as the prognostic factor of adverse cardiovascular events in long-term follow up in patients after myocardial infarction-A pilot study. J. Clin. Med. 2020;9:1640. doi: 10.3390/jcm9061640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dong H., Wang Z.H., Zhang N., Liu S.D., Zhao J.J., Liu S.Y. Serum Galectin-3 level, not Galectin-1, is associated with the clinical feature and outcome in patients with acute ischemic stroke. Oncotarget. 2017;8:109752–109761. doi: 10.18632/oncotarget.18211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Edsfeldt A., Bengtsson E., Asciutto G., Duner P., Bjorkbacka H., Fredrikson G.N., et al. High plasma levels of galectin-3 are associated with increased risk for stroke after carotid endarterectomy. Cerebrovasc. Dis. 2016;41:199–203. doi: 10.1159/000443022. [DOI] [PubMed] [Google Scholar]
- 88.Fort-Gallifa I., Hernandez-Aguilera A., Garcia-Heredia A., Cabre N., Luciano-Mateo F., Simo J.M., et al. Galectin-3 in peripheral artery disease. Relationships with markers of oxidative stress and inflammation. Int. J. Mol. Sci. 2017;18:973. doi: 10.3390/ijms18050973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bennett G.A., Smith F.J. Pulmonary hypertension in rats living under compressed air conditions. J. Exp. Med. 1934;59:181–193. doi: 10.1084/jem.59.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hance A.J., Crystal R.G. The connective tissue of lung. Am. Rev. Respir. Dis. 1975;112:657–711. doi: 10.1164/arrd.1975.112.5.657. [DOI] [PubMed] [Google Scholar]
- 91.Seyer J.M., Hutcheson E.T., Kang A.H. Collagen polymorphism in idiopathic chronic pulmonary fibrosis. J. Clin. Invest. 1976;57:1498–1507. doi: 10.1172/JCI108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bateman E.D., Turner-Warwick M., Adelmann-Grill B.C. Immunohistochemical study of collagen types in human foetal lung and fibrotic lung disease. Thorax. 1981;36:645–653. doi: 10.1136/thx.36.9.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Madri J.A., Furthmayr H. Collagen polymorphism in the lung. An immunochemical study of pulmonary fibrosis. Hum. Pathol. 1980;11:353–366. doi: 10.1016/s0046-8177(80)80031-1. [DOI] [PubMed] [Google Scholar]
- 94.Selman M., Montano M., Ramos C., Chapela R. Concentration, biosynthesis and degradation of collagen in idiopathic pulmonary fibrosis. Thorax. 1986;41:355–359. doi: 10.1136/thx.41.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bensadoun E.S., Burke A.K., Hogg J.C., Roberts C.R. Proteoglycan deposition in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1996;154:1819–1828. doi: 10.1164/ajrccm.154.6.8970376. [DOI] [PubMed] [Google Scholar]
- 96.Ebihara T., Venkatesan N., Tanaka R., Ludwig M.S. Changes in extracellular matrix and tissue viscoelasticity in bleomycin-induced lung fibrosis. Temporal aspects. Am. J. Respir. Crit. Care Med. 2000;162:1569–1576. doi: 10.1164/ajrccm.162.4.9912011. [DOI] [PubMed] [Google Scholar]
- 97.Kolb M., Margetts P.J., Sime P.J., Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L1327–1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 98.Sand J.M., Larsen L., Hogaboam C., Martinez F., Han M., Rossel Larsen M., et al. MMP mediated degradation of type IV collagen alpha 1 and alpha 3 chains reflects basement membrane remodeling in experimental and clinical fibrosis–validation of two novel biomarker assays. PLoS One. 2013;8:e84934. doi: 10.1371/journal.pone.0084934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Teles-Grilo M.L., Leite-Almeida H., Martins dos Santos J., Oliveira C., Boaventura P., Grande N.R. Differential expression of collagens type I and type IV in lymphangiogenesis during the angiogenic process associated with bleomycin-induced pulmonary fibrosis in rat. Lymphology. 2005;38:130–135. [PubMed] [Google Scholar]
- 100.Bjermer L., Lundgren R., Hallgren R. Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. Thorax. 1989;44:126–131. doi: 10.1136/thx.44.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tzortzaki E.G., Koutsopoulos A.V., Dambaki K.I., Lambiri I., Plataki M., Gordon M.K., et al. Active remodeling in idiopathic interstitial pneumonias: evaluation of collagen types XII and XIV. J. Histochem. Cytochem. 2006;54:693–700. doi: 10.1369/jhc.5A6835.2006. [DOI] [PubMed] [Google Scholar]
- 102.Leeming D.J., Sand J.M., Nielsen M.J., Genovese F., Martinez F.J., Hogaboam C.M., et al. Serological investigation of the collagen degradation profile of patients with chronic obstructive pulmonary disease or idiopathic pulmonary fibrosis. Biomark Insights. 2012;7:119–126. doi: 10.4137/BMI.S9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Su Y., Gu H., Weng D., Zhou Y., Li Q., Zhang F., et al. Association of serum levels of laminin, type IV collagen, procollagen III N-terminal peptide, and hyaluronic acid with the progression of interstitial lung disease. Medicine (Baltimore) 2017;96:e6617. doi: 10.1097/MD.0000000000006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Low R.B., Giancola M.S., King T.E., Jr., Chapitis J., Vacek P., Davis G.S. Serum and bronchoalveolar lavage of N-terminal type III procollagen peptides in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1992;146:701–706. doi: 10.1164/ajrccm/146.3.701. [DOI] [PubMed] [Google Scholar]
- 105.Kubo S., Siebuhr A.S., Bay-Jensen A.C., Juhl P., Karsdal M.A., Satoh Y., et al. Correlation between serological biomarkers of extracellular matrix turnover and lung fibrosis and pulmonary artery hypertension in patients with systemic sclerosis. Int. J. Rheum. Dis. 2020;23:532–539. doi: 10.1111/1756-185X.13804. [DOI] [PubMed] [Google Scholar]
- 106.Jenkins R.G., Simpson J.K., Saini G., Bentley J.H., Russell A.M., Braybrooke R., et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir. Med. 2015;3:462–472. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 107.Williams L.M., McCann F.E., Cabrita M.A., Layton T., Cribbs A., Knezevic B., et al. Identifying collagen VI as a target of fibrotic diseases regulated by CREBBP/EP300. Proc. Natl. Acad. Sci. U. S. A. 2020;117:20753–20763. doi: 10.1073/pnas.2004281117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herrera J., Forster C., Pengo T., Montero A., Swift J., Schwartz M.A., et al. Registration of the extracellular matrix components constituting the fibroblastic focus in idiopathic pulmonary fibrosis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.125185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Godwin A.R.F., Starborg T., Sherratt M.J., Roseman A.M., Baldock C. Defining the hierarchical organisation of collagen VI microfibrils at nanometre to micrometre length scales. Acta Biomater. 2017;52:21–32. doi: 10.1016/j.actbio.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Specks U., Nerlich A., Colby T.V., Wiest I., Timpl R. Increased expression of type VI collagen in lung fibrosis. Am. J. Respir. Crit. Care Med. 1995;151:1956–1964. doi: 10.1164/ajrccm.151.6.7767545. [DOI] [PubMed] [Google Scholar]
- 111.Calvier L., Miana M., Reboul P., Cachofeiro V., Martinez-Martinez E., de Boer R.A., et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013;33:67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 112.Wang X., Wang Y., Zhang J., Guan X., Chen M., Li Y., et al. Galectin-3 contributes to vascular fibrosis in monocrotaline-induced pulmonary arterial hypertension rat model. J. Biochem. Mol. Toxicol. 2017;31 doi: 10.1002/jbt.21879. [DOI] [PubMed] [Google Scholar]
- 113.Fenster B.E., Lasalvia L., Schroeder J.D., Smyser J., Silveira L.J., Buckner J.K., et al. Galectin-3 levels are associated with right ventricular functional and morphologic changes in pulmonary arterial hypertension. Heart Vessels. 2016;31:939–946. doi: 10.1007/s00380-015-0691-z. [DOI] [PubMed] [Google Scholar]
- 114.Calvier L., Legchenko E., Grimm L., Sallmon H., Hatch A., Plouffe B.D., et al. Galectin-3 and aldosterone as potential tandem biomarkers in pulmonary arterial hypertension. Heart. 2016;102:390–396. doi: 10.1136/heartjnl-2015-308365. [DOI] [PubMed] [Google Scholar]
- 115.Mazurek J.A., Horne B.D., Saeed W., Sardar M.R., Zolty R. Galectin-3 levels are elevated and predictive of mortality in pulmonary hypertension. Heart Lung Circ. 2017;26:1208–1215. doi: 10.1016/j.hlc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 116.Feng W., Wu X., Li S., Zhai C., Wang J., Shi W., et al. Association of serum galectin-3 with the acute exacerbation of chronic obstructive pulmonary disease. Med. Sci. Monit. 2017;23:4612–4618. doi: 10.12659/MSM.903472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Watanabe E., Kato K., Gono T., Chiba E., Terai C., Kotake S. Serum levels of galectin-3 in idiopathic inflammatory myopathies: a potential biomarker of disease activity. Rheumatology (Oxford) 2021;60:322–332. doi: 10.1093/rheumatology/keaa305. [DOI] [PubMed] [Google Scholar]
- 118.d'Alessandro M., De Vita E., Bergantini L., Mazzei M.A., di Valvasone S., Bonizzoli M., et al. Galactin-1, 3 and 9: potential biomarkers in idiopathic pulmonary fibrosis and other interstitial lung diseases. Respir. Physiol. Neurobiol. 2020;282:103546. doi: 10.1016/j.resp.2020.103546. [DOI] [PubMed] [Google Scholar]
- 119.Inohara H., Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj. J. 1994;11:527–532. doi: 10.1007/BF00731303. [DOI] [PubMed] [Google Scholar]
- 120.Nangia-Makker P., Hogan V., Honjo Y., Baccarini S., Tait L., Bresalier R., et al. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 121.Platt D., Raz A. Modulation of the lung colonization of B16-F1 melanoma cells by citrus pectin. J. Natl. Cancer Inst. 1992;84:438–442. doi: 10.1093/jnci/84.6.438. [DOI] [PubMed] [Google Scholar]
- 122.Hao M., Li M., Li W. Galectin-3 inhibition ameliorates hypoxia-induced pulmonary artery hypertension. Mol. Med. Rep. 2017;15:160–168. doi: 10.3892/mmr.2016.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luo H., Liu B., Zhao L., He J., Li T., Zha L., et al. Galectin-3 mediates pulmonary vascular remodeling in hypoxia-induced pulmonary arterial hypertension. J. Am. Soc. Hypertens. 2017;11:673–683.e673. doi: 10.1016/j.jash.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 124.Barman S.A., Chen F., Li X., Haigh S., Stepp D.W., Kondrikov D., et al. Galectin-3 promotes vascular remodeling and contributes to pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2018;197:1488–1492. doi: 10.1164/rccm.201711-2308LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hirani N., MacKinnon A.C., Nicol L., Ford P., Schambye H., Pedersen A., et al. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2021;57:2002559. doi: 10.1183/13993003.02559-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Traber P.G., Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8:e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harrison S.A., Marri S.R., Chalasani N., Kohli R., Aronstein W., Thompson G.A., et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment. Pharmacol. Ther. 2016;44:1183–1198. doi: 10.1111/apt.13816. [DOI] [PubMed] [Google Scholar]
- 128.Chalasani N., Abdelmalek M.F., Garcia-Tsao G., Vuppalanchi R., Alkhouri N., Rinella M., et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158:1334–1345.e1335. doi: 10.1053/j.gastro.2019.11.296. [DOI] [PubMed] [Google Scholar]
- 129.Nguyen M.N., Ziemann M., Kiriazis H., Su Y., Thomas Z., Lu Q., et al. Galectin-3 deficiency ameliorates fibrosis and remodeling in dilated cardiomyopathy mice with enhanced Mst1 signaling. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H45–H60. doi: 10.1152/ajpheart.00609.2018. [DOI] [PubMed] [Google Scholar]
- 130.Xu G.R., Zhang C., Yang H.X., Sun J.H., Zhang Y., Yao T.T., et al. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-kappaB signaling pathway. Biomed. Pharmacother. 2020;126:110071. doi: 10.1016/j.biopha.2020.110071. [DOI] [PubMed] [Google Scholar]
- 131.Harrison S.A., Dennis A., Fiore M.M., Kelly M.D., Kelly C.J., Paredes A.H., et al. Utility and variability of three non-invasive liver fibrosis imaging modalities to evaluate efficacy of GR-MD-02 in subjects with NASH and bridging fibrosis during a phase-2 randomized clinical trial. PLoS One. 2018;13:e0203054. doi: 10.1371/journal.pone.0203054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ricard-Blum S., Baffet G., Theret N. Molecular and tissue alterations of collagens in fibrosis. Matrix Biol. 2018;68-69:122–149. doi: 10.1016/j.matbio.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 133.Flores-Ibarra A., Vertesy S., Medrano F.J., Gabius H.J., Romero A. Crystallization of a human galectin-3 variant with two ordered segments in the shortened N-terminal tail. Sci. Rep. 2018;8:9835. doi: 10.1038/s41598-018-28235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller M.C., Zheng Y., Yan J., Zhou Y., Tai G., Mayo K.H. Novel polysaccharide binding to the N-terminal tail of galectin-3 is likely modulated by proline isomerization. Glycobiology. 2017;27:1038–1051. doi: 10.1093/glycob/cwx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pineda M., Corvo L., Callejas-Hernandez F., Fresno M., Bonay P. Trypanosoma cruzi cleaves galectin-3 N-terminal domain to suppress its innate microbicidal activity. Clin. Exp. Immunol. 2020;199:216–229. doi: 10.1111/cei.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pelletier I., Sato S. Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J. Biol. Chem. 2002;277:17663–17670. doi: 10.1074/jbc.M201562200. [DOI] [PubMed] [Google Scholar]
- 137.Zhao Z., Xu X., Cheng H., Miller M.C., He Z., Gu H., et al. Galectin-3 N-terminal tail prolines modulate cell activity and glycan-mediated oligomerization/phase separation. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2021074118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mauris J., Woodward A.M., Cao Z., Panjwani N., Argueso P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J. Cell Sci. 2014;127:3141–3148. doi: 10.1242/jcs.148510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang S.F., Tsao C.H., Lin Y.T., Hsu D.K., Chiang M.L., Lo C.H., et al. Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology. 2014;24:1022–1035. doi: 10.1093/glycob/cwu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bocker S., Elling L. Binding characteristics of galectin-3 fusion proteins. Glycobiology. 2017;27:457–468. doi: 10.1093/glycob/cwx007. [DOI] [PubMed] [Google Scholar]
- 141.Fermino M.L., Polli C.D., Toledo K.A., Liu F.T., Hsu D.K., Roque-Barreira M.C., et al. LPS-induced galectin-3 oligomerization results in enhancement of neutrophil activation. PLoS One. 2011;6:e26004. doi: 10.1371/journal.pone.0026004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lo T.H., Chen H.L., Yao C.I., Weng I.C., Li C.S., Huang C.C., et al. Galectin-3 promotes noncanonical inflammasome activation through intracellular binding to lipopolysaccharide glycans. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2026246118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kahsai A.W., Cui J., Kaniskan H.U., Garner P.P., Fenteany G. Analogs of tetrahydroisoquinoline natural products that inhibit cell migration and target galectin-3 outside of its carbohydrate-binding site. J. Biol. Chem. 2008;283:24534–24545. doi: 10.1074/jbc.M800006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sethi A., Sanam S., Alvala M. Non-carbohydrate strategies to inhibit lectin proteins with special emphasis on galectins. Eur. J. Med. Chem. 2021;222:113561. doi: 10.1016/j.ejmech.2021.113561. [DOI] [PubMed] [Google Scholar]
- 145.Yang R.Y., Hsu D.K., Liu F.T. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6737–6742. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fukumori T., Takenaka Y., Oka N., Yoshii T., Hogan V., Inohara H., et al. Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Res. 2004;64:3376–3379. doi: 10.1158/0008-5472.CAN-04-0336. [DOI] [PubMed] [Google Scholar]
- 147.Liu L., Sakai T., Sano N., Fukui K. Nucling mediates apoptosis by inhibiting expression of galectin-3 through interference with nuclear factor kappaB signalling. Biochem. J. 2004;380:31–41. doi: 10.1042/BJ20031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu F.T., Patterson R.J., Wang J.L. Intracellular functions of galectins. Biochim. Biophys. Acta. 2002;1572:263–273. doi: 10.1016/s0304-4165(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 149.Elad-Sfadia G., Haklai R., Balan E., Kloog Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J. Biol. Chem. 2004;279:34922–34930. doi: 10.1074/jbc.M312697200. [DOI] [PubMed] [Google Scholar]
- 150.Lee Y.J., Song Y.K., Song J.J., Siervo-Sassi R.R., Kim H.R., Li L., et al. Reconstitution of galectin-3 alters glutathione content and potentiates TRAIL-induced cytotoxicity by dephosphorylation of Akt. Exp. Cell Res. 2003;288:21–34. doi: 10.1016/s0014-4827(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 151.Oka N., Nakahara S., Takenaka Y., Fukumori T., Hogan V., Kanayama H.O., et al. Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Res. 2005;65:7546–7553. doi: 10.1158/0008-5472.CAN-05-1197. [DOI] [PubMed] [Google Scholar]
- 152.Shimura T., Takenaka Y., Tsutsumi S., Hogan V., Kikuchi A., Raz A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004;64:6363–6367. doi: 10.1158/0008-5472.CAN-04-1816. [DOI] [PubMed] [Google Scholar]
- 153.Massa S.M., Cooper D.N., Leffler H., Barondes S.H. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry. 1993;32:260–267. doi: 10.1021/bi00052a033. [DOI] [PubMed] [Google Scholar]
- 154.Sato S., Hughes R.C. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J. Biol. Chem. 1992;267:6983–6990. [PubMed] [Google Scholar]
- 155.Hikita C., Vijayakumar S., Takito J., Erdjument-Bromage H., Tempst P., Al-Awqati Q. Induction of terminal differentiation in epithelial cells requires polymerization of hensin by galectin 3. J. Cell Biol. 2000;151:1235–1246. doi: 10.1083/jcb.151.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ochieng J., Warfield P., Green-Jarvis B., Fentie I. Galectin-3 regulates the adhesive interaction between breast carcinoma cells and elastin. J. Cell Biochem. 1999;75:505–514. doi: 10.1002/(sici)1097-4644(19991201)75:3<505::aid-jcb14>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 157.Ochieng J., Leite-Browning M.L., Warfield P. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem. Biophys. Res. Commun. 1998;246:788–791. doi: 10.1006/bbrc.1998.8708. [DOI] [PubMed] [Google Scholar]
- 158.Probstmeier R., Montag D., Schachner M. Galectin-3, a beta-galactoside-binding animal lectin, binds to neural recognition molecules. J. Neurochem. 1995;64:2465–2472. doi: 10.1046/j.1471-4159.1995.64062465.x. [DOI] [PubMed] [Google Scholar]
- 159.Cherayil B.J., Weiner S.J., Pillai S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J. Exp. Med. 1989;170:1959–1972. doi: 10.1084/jem.170.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lakshminarayan R., Wunder C., Becken U., Howes M.T., Benzing C., Arumugam S., et al. Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nat. Cell Biol. 2014;16:595–606. doi: 10.1038/ncb2970. [DOI] [PubMed] [Google Scholar]
- 161.Dalton P., Christian H.C., Redman C.W., Sargent I.L., Boyd C.A. Membrane trafficking of CD98 and its ligand galectin 3 in BeWo cells–implication for placental cell fusion. FEBS J. 2007;274:2715–2727. doi: 10.1111/j.1742-4658.2007.05806.x. [DOI] [PubMed] [Google Scholar]
- 162.Feuk-Lagerstedt E., Movitz C., Pellme S., Dahlgren C., Karlsson A. Lipid raft proteome of the human neutrophil azurophil granule. Proteomics. 2007;7:194–205. doi: 10.1002/pmic.200600482. [DOI] [PubMed] [Google Scholar]
- 163.Partridge E.A., Le Roy C., Di Guglielmo G.M., Pawling J., Cheung P., Granovsky M., et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 164.Liu W., Hsu D.K., Chen H.Y., Yang R.Y., Carraway K.L., 3rd, Isseroff R.R., et al. Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration. J. Invest. Dermatol. 2012;132:2828–2837. doi: 10.1038/jid.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen H.Y., Fermin A., Vardhana S., Weng I.C., Lo K.F., Chang E.Y., et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14496–14501. doi: 10.1073/pnas.0903497106. [DOI] [PMC free article] [PubMed] [Google Scholar]