Abstract
One of the defects commonly found in cooked marinated chicken breast products is a red blood spot (RBS), which is caused by undercooked blood in vessels. This problem was alleviated by microwave (MW) pre-heating for 6 to 7 min, followed by steaming. RBS formation decreased when samples were heated to a core temperature of 80°C and were completely eliminated at a core temperature of 82°C and 85°C when a MW pre-heating step was applied for 7 min. Based on synchrotron-based Fourier transform infrared spectroscopy (SR-FTIR), blood remaining in the blood vessel had a lower α-helical content when samples were cooked by the combination of MW heating and steaming as compared with those prepared by steaming alone (P < 0.05). MW pre-heating decreased cooking time by 28 to 48% as compared with steaming alone. Heating regimes had no effect on cooking loss, pH, water-holding capacity, and shear force. MW pre-heating for 7 min followed by steaming to a core temperature of 82°C appeared to be an effective heating regime to reduce the occurrence of RBS, with acceptable cooking loss.
Key words: red blood spot, microwave heating, steaming, blood denaturation, synchrotron radiation Fourier transform infrared spectroscopy
INTRODUCTION
The blood circulatory system in the skeletal muscle plays a vital role in the efficient provision of oxygen and nutrients required for contraction. Blood vessels are closely intertwined with skeletal muscle tissues lying between the bundles of muscle fibers (Hoving-Bolink et al., 2000). A chicken is slaughtered by cutting the jugular veins and carotid arteries. Bleeding out takes place rapidly after slaughtering owing to the passive process of cardiac pumping (Lambooij et al., 1999). Blood loss gradually decreases with the incidence of cardiac arrest (Ali et al., 2007). Blood retained in the blood vessels results in the formation of red blood spots (RBS) upon cooking (Jantaranikorn and Yongsawatdigul, 2020). This incidence is problematic for cooked chicken breast products. Red spots observed after transverse cutting are due to the incomplete denaturation of blood residues. The presence of RBS in commercial cooked products is a sporadic incidence, which is not acceptable by most consumers as they are deemed to be undercooked meat (Smith and Northcutt, 2003; Bae, et al., 2018).
Thermal processing applied to chicken meat in industry includes steaming, roasting, grilling, or frying. Heat is typically transferred from the heating medium or the heat source to the chicken breast meat by convection, radiation, and/or conduction (Rincon et al., 2015). Limited heat transfer may be a reason for the incomplete denaturation of blood residues in blood vessels, particularly those located in the middle of the breast muscle (Sturkie, 2015). Although high heating temperatures and/or prolonged heating time can induce the complete denaturation of blood in blood vessels, it causes overcooking and significantly reduces yield (Pathare and Roskilly, 2016). Therefore, thermal processes that can assure the denaturation of blood residues in breast muscle without a significant loss of yield must be identified.
Microwave (MW) heating generates heat internally by dipole rotation and ionic conduction. This induces molecular friction and generates heat within meat products in an alternating electromagnetic field (Hebbar and Rastogi, 2012). For industrial, scientific, and medical heating applications, the frequency range used for MW heating is 915 to 2,450 MHz (Piyasena et al., 2003). MW heating has attracted much interest in food industry owing to its rapid heating rate and low energy consumption (McKenna et al., 2006; Jouquand et al., 2015). However, MW heating can lead to the non-uniform heating of marinated meat owing to heterogeneity and the varied thickness and geometry of meat pieces (Vadivambal and Jayas, 2010). Goksoy et al. (1999) reported that MW heating of whole chicken carcass at 2,450 MHz and 500 W for 5 min resulted in temperature differences of 15°C to 45°C between the breast and the leg. In addition, Jeong et al. (2007) demonstrated that ground pork patties with salt cooked by MW at 2,450 MHz exhibited a temperature differential of approximately 13°C between the edge and the center of the sample. MW heating did not increase lipid oxidation of restructured beef steak containing 13.0% fat as measured by thiobarbituric reactive substances (TBARS) value when compared to the raw sample (Serrano et al., 2007). In addition, MW heating did not alter fatty acid profile of chicken patties, but appeared to increase cholesterol oxidation (Echarte et al., 2003). However, when MW heating is combined with a conventional method, heating uniformity, energy efficiency, and lipid oxidation have been improved (Geedipalli et al., 2008; Hebber and Rastogi, 2012; Datta and Rakesh, 2013; Pathera et al., 2016). Typically, the commercial heating process of chicken breast relies mainly on a steaming process, which requires a relatively long processing time of 30 to 50 min to reach a core temperature of 80°C to 82°C (Pathare and Roskilly, 2016). The combination of MW heating and steaming can be explored to evaluate the feasibility of RBS reduction.
Thermal processes greatly affect the structural changes in proteins, resulting in protein unfolding and aggregation (Bertram et al., 2006). These can be described by changes in the secondary structure, namely α-helix, β-sheet, and β-turn. Fourier transform infrared (FTIR) spectroscopy is normally used to elucidate the secondary structure of proteins. Synchrotron radiation based-FTIR (SR-FTIR) utilizes synchrotron a light source, which is usually 100 to 1,000 times brighter than a conventional globar source, allowing the selective measurement of a region of interest of a sample as small as 3 µm (Pascolo et al., 2014; Guo et al., 2020), whereas a typical FTIR measures the composition within 10 μm (Petibois et al., 2010). Ellis et al. (2010) reported that SR-FTIR spectra were a higher quality than those of conventional FTIR with a small aperture of 3 × 3 μm2. SR-FTIR could directly target proteins in blood vessels with a size of at least 8 µm (Sturkie, 2015), leading to higher quality spectra than those obtained from conventional FTIR. Therefore, SR-FTIR would likely be able to probe the changes in blood residues remaining in chicken breast veins. The objectives of this study were to reduce RBS in cooked marinated chicken breast through the use of a combined MW heating and steaming process. The quality of marinated chicken breast cooked by the combined heating processes was assessed. Furthermore, the extent of denaturation of the blood remaining in the blood vessels of cooked marinated chicken breast was elucidated by SR-FTIR.
MATERIALS AND METHODS
Marinated Chicken Breast Preparation
Ten kilograms of boneless skinless chicken breast (Pectoralis major) were obtained from a commercial chicken processing plant (Charoen Pokphand Foods PCL., Nakhon Ratchasima, Thailand); the pieces were 250 to 280 g in size and had a maximum thickness of 4 to 5 cm. Three independent lots of chicken breast meat were prepared. The samples were collected immediately after slaughtering and refrigerated at 4°C for 24 h. Chicken breast meat was vacuum tumbled (DVTS-50, Davison's Butcher Supply, Los Angeles, CA) at temperature less than 8°C for 65 min. Marinated solution containing 5% NaCl (Pimai Salt Co., Ltd, Nakorn Ratchasima, Thailand), 3% glucose (Kornthai Co., Ltd, Ratchaburi, Thailand), and 2% sodium tripolyphosphate (STPP) (Aditya Birla Chemicals Ltd, Samutprakarn, Thailand) was added at 16.5% (w/w) to the chicken breast sample. The samples were stored at 4°C for 20 h after tumbling. The marinated samples were divided into 3 groups, which were subjected to different heating regimes as detailed below.
Cooking
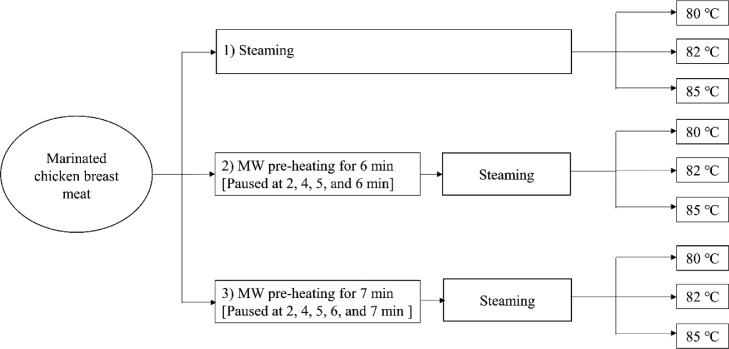
An industrial MW oven (NE 1756, 18L, Panasonic Corp., Osaka, Japan) with a frequency of 2,450 MHz and power output of 1.7 kW was used. Based on preliminary studies, a longer MW pre-heating step could rapidly increase the internal temperature, but caused burning and/or tissue explosion. Thus, MW pre-heating was applied for 6 and 7 min, contributing to an internal temperature of approximately 50°C to 70°C without defects. Three pieces of marinated chicken breast samples (300 ± 20 g/piece) were placed in a polypropylene box, which was then placed at the middle of the MW chamber and heated for 6 or 7 min. To obtain uniform heating, the MW was paused at 2, 4, 5, and 6 min of the 6-min heating treatment and at 2, 4, 5, 6, and 7 min of the 7-min treatment. The core temperature was immediately measured at each paused interval using a type K thermocouple (54 II, 80PT-5A, Fluke Corp., Moorpark, CA). Three pieces of the pre-heated samples were immediately placed in a steam oven (Model 101, Rational, Landsberg am Lech, Bavaria, Germany) set at 92°C, a dew point of 100%, and fan speed of level 3. The internal temperature of the sample during steaming was continuously monitored using an oven equipped with a thermocouple and steaming was continued until core temperatures of 80°C, 82°C, and 85°C (as commercial control) were attained as shown in Figure 1. The experiment was repeated in triplicate for each core temperature. The steamed alone process was used as a control.
Figure 1.
Diagram of heating regime of microwave heating and steaming.
SR-FTIR Spectroscopy
Both the raw and cooked samples were prepared for IR spectroscopy as follows. The samples were transversely cut at the area of blood retained in the veins, embedded in OCT (Bio-optica, Milano, Italy), and immediately frozen in liquid nitrogen and stored at –80°C prior to sectioning. Sectioning was conducted at –20°C using a cryostat microtome (AST500, AMOS Scientific, Clayton South Victoria, Australia) to obtain a thickness of 8 µm. The cut sample was attached on infrared-transparent, 2-mm thick barium fluoride (BaF2) slides and dried in a vacuum chamber overnight. The infrared spectra were obtained with a Vortex 70 FTIR spectrometer (Bruker Optics, Ettlingen, Germany) coupled with a 36× objective microscope (Hyperion 2000, Bruker, Ettlingen, Germany) with an MCT D315 detector cooled with liquid nitrogen. The SR-FTIR at beamline BL4.1 of the Synchrotron Light Research Institute (Nakhon Ratchasima, Thailand) was used, which connected to synchrotron radiation entering the interferometer via an instrument port designed for IR emission. The measurement was performed in mapping mode over the wavenumber range of 4,000 to 600 cm−1. The most sensitive spectral region of the secondary structure, namely amide I at 1,700 to 1,600 cm−1, was measured at 6 cm−1 resolution and 64 scans. The changes in the secondary structure were acquired from curve fitting using OPUS software (version 7.0, Bruker Optics Inc, Billerica, MA). The IR difference in the spectra was determined. The spectra were processed by subtracting spectra of cooked sample from those of raw samples. The net results reflected the effect of heating method on blood denaturation.
Qualities of Cooked Chicken Breast
The quality of cooked marinated chicken breast samples, namely cooking loss, moisture content, pH, water-holding capacity (WHC), texture, and color, was analyzed within 24 h of cooking.
Cooking loss was determined in 3 replicates per lot of chicken based on the weight after tumbling, as follows:
For pH determination, marinated chicken breast samples were homogenized with deionized water at a ratio of 1:5 (w/v) for 1 min using a homogenizer (T25 digital Ultra-Turrax, IKA Works Inc., Wilmington, NC). The pH of homogenized samples was measured in triplicate per lot using a glass electrode pH meter (Mettler Toledo S220 SevenCompact, Schwerzenbach, Switzerland) according to the method described by Wattanachant et al. (2004), which was calibrated against pH 4.00 and pH 7.00 buffers before use.
The moisture content of cooked samples was determined in accordance with the Association of Official Analytical Chemists (AOAC, 2010).
The WHC of cooked marinated chicken breast was determined in 3 replications per lot by the centrifugal method in accordance with Laycock et al. (2003), with slight modifications. Minced samples (2 g) were placed on filter paper (Whatman No.1) and centrifuged (Sorvall Legend MACH 1.6R, Thermo Electron LED GmbH, Lengensellbold, Germany) at 6,000 × g for 15 min at 4°C. The samples after centrifugation were weighed and analyzed with respect to the moisture content of the original chicken meat sample. The WHC was calculated as follows:
The Warner–Bratzler shear force was determined using a Texture Analyzer (TA.XT. Plus, Stable Micro Systems, Surrey, UK). The samples were measured using a 25 kg load cell at a crosshead speed of 10 mm/s. The cooked samples were cut to 1.9 cm wide, 2.5 cm long, and 2.0 cm thick. The Warner–Bratzler shear force expressed as kg was measured from 5 cooked samples from each cooking condition.
The color of a cross-section of cooked chicken breast meat was evaluated using a Hunter lab/Color Quest XE (Hunter Associates Laboratory Inc., Reston, VA) based on D65 illuminant and 10° observer angle. Lightness (L*), redness (a*), and yellowness (b*) values were recorded. The color values of 5 chicken breast strips per lot were measured for each treatment.
Statistical Analysis
Three independent lots of chicken breast were tumbled and cooked. All experimental measurements otherwise stated were performed on at least 3 replicates per lot. The data were analyzed by one-way analysis of variance using SPSS (Version 23.0, SPSS Inc., Chicago, IL). The mean comparisons were performed using Duncan's test to evaluate the significance of differences among mean values with P < 0.05. The mean values ± standard deviation were reported.
RESULTS AND DISCUSSION
Time-Temperature Profiles
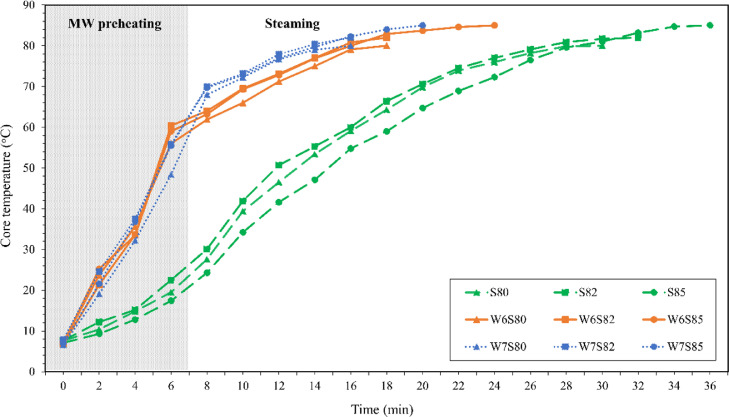
The temperature increased sharply during MW heating and then gradually increased during steaming (Figure 2). The heat generation by MW is caused by the dipolar rotation of polar molecules and the conductive migration of dissolved ions, which result in molecular friction. Thus, the internal heat generation led to a rapid temperature increase. In contrast, the heat transfer of conventional steaming relies on convection and conduction, resulting in a slower heating rate (Awuah et al., 2015; Li et al., 2019). The heating rate of MW alone was approximately 7.6 to 8.3°C/min, whereas that of steaming was 2.4 to 2.7°C/min. Pérez-Juan et al. (2012) reported that heating rate of cooked marinated beef by MW was 15.6 to 16.8°C/min. The heating rate of marinated meat cooked by MW also depended on the concentration of ionic ingredients added to the marinade as these also increase the dielectric properties (Lyng et al, 2005). However, MW heating alone caused non-uniform heating by thermal runaway, which could eventually lead to burning. In contrast, the steaming of marinated chicken breast alone required 26.2 to 32.1 min to reach a core temperature of 80°C to 85°C. When the 2 processes were combined, the process time was reduced to 13.6 to 23.2 min, which accounted for a 28 to 48% reduction in process time (P < 0.05, Table 1). The reduction of cooking time contributes to higher nutrient retention and yield, as well as saving energy (Yarmand and Homayouni, 2011; Joseph, 2017). Yarmand and Homayouni (2011) reported that MW heating shortened cooking time, resulting in thiamin retention of 98%, whereas that in conventionally cooked chicken was only 77%. Moreover, Jouquand et al. (2015) reported that the MW cooking of beef burgundy (4.6 kWh) had a lower energy consumption than conventional cooking (6.5 kWh), which was attributed to a 56% reduction in cooking time. Our results suggested that the combined process of MW heating and steaming reduced the heating time required for marinated chicken breast compared with steaming alone.
Figure 2.
Temperature profiles of chicken breast subjected to steaming alone and the combination of microwave and steaming at various core temperatures. S80, S82, and S85 indicate steaming to core temperatures of 80°C, 82°C, and 85°C, respectively. W6S80, W6S82, and W6S85 denote microwave pre-heating for 6 min followed by steaming to core temperatures of 80°C, 82°C, and 85°C, respectively. W7S80, W7S82, and W7S85 denote microwave pre-heating for 7 min followed by steaming to core temperatures of 80°C, 82°C, and 85°C, respectively.
Table 1.
Effect of microwave pre-heating (core temperature ∼ 50–70°C) followed by steaming to various core temperatures on cooking qualities and RBS formation of marinated chicken breast (n = 9).
| Core temperature after steaming (°C) | Time of MW heating (min) | Treatment | Cooking time (min) | Cooking loss (%) | RBS (%) |
|---|---|---|---|---|---|
| 80 | 0 | S80 | 26.2 ± 1.8c | 26.5 ± 2.0b | 44.4 |
| 6 | W6S80 | 17.0± 1.5ef | 26.3 ± 2.8b | 33.3 | |
| 7 | W7S80 | 13.6 ± 1.6g | 26.9 ± 2.3ab | 22.2 | |
| 82 | 0 | S82 | 28.8 ± 2.4b | 28.1 ± 1.8ab | 33.3 |
| 6 | W6S82 | 17.7 ± 0.8e | 27.6 ± 0.3ab | 11.1 | |
| 7 | W7S82 | 15.1 ± 1.6fg | 27.8 ± 1.1ab | 0 | |
| 85 | 0 | S85 | 32.1 ± 2.4a | 28.5 ± 1.1ab | 0 |
| 6 | W6S85 | 23.2 ± 1.2d | 28.4 ± 0.9ab | 0 | |
| 7 | W7S85 | 19.5 ± 1.0e | 29.7 ± 0.1a | 0 |
Different letters in the same column indicate significant difference (P < 0.05).
Quality of Cooked Chicken Meat
The combined process of MW heating and steaming had no effect on cooking loss as compared with steaming alone at the same core temperature (P > 0.05, Table 1). Cooking loss appeared to increase as the final core temperature increased. It has been reported that MW heating of meat resulted in higher cooking losses when compared with conventional methods (Yarmand and Homayouni, 2011; Domínguez et al., 2014; Li et al., 2019). MW heating resulted in a higher heating rate, greater muscle destruction, and increased released of water upon cooking (Nikmaram et al., 2011). These results indicated that the combined process of MW heating and steaming could increase blood denaturation in meat without significant cooking loss.
The values of pH, WHC, and shear force were comparable, regardless of the heating regime applied (P > 0.05, Table 2). The average pH value of all samples was between 6.30 and 6.36 (P > 0.05, Table 2). Based on these results, the combined process of MW heating and steaming did not increase water expulsion in the cooked meat. The thermal denaturation of myofibrillar proteins leads to meat toughness (Laakkonen et al., 1972; Pathare and Roskilly, 2016). Nikmaram et al. (2011) reported that the shear force of veal muscle cooked by MW heating, roasting, and braizing were comparable. This probably illustrates that the extent of myofibrillar protein denaturation was similar between cooking methods. However, MW cooking of yak meat at a high power (700 W) resulted in lower shear values than boiling. This was attributed to the rapid thermal shrinkage of muscles (Li et al., 2019). Our study indicated that the combined heating from MW cooking and steaming did not negatively affect the quality of cooked chicken breast when compared with the traditional steaming process.
Table 2.
Physicochemical and textural properties of marinated chicken breast cooked by various regimes of combined MW heating and steaming.
| Core temperature (°C) | Time of MW heating (min) | Treatment | pH | Moisture content (%) | WHC (%) | Shear force (kg) | L* | a* | b* |
|---|---|---|---|---|---|---|---|---|---|
| 0 | S80 | 6.36 ± 0.04 | 72.03 ± 0.47a | 53.45 ± 2.82 | 3.18 ± 0.23 | 82.6 ± 0.4a | 2.5 ± 0.3ab | 14.1 ± 0.3bc | |
| 80 | 6 | W6S80 | 6.33 ± 0.02 | 70.14 ± 0.46c | 56.33 ± 1.80 | 3.19 ± 0.15 | 82.2 ± 0.7ab | 2.2 ± 0.2cd | 13.7 ± 0.3d |
| 7 | W7S80 | 6.37 ± 0.01 | 70.24 ± 0.35c | 56.00 ± 1.08 | 3.20 ± 0.32 | 82.4 ± 0.8ab | 2.3 ± 0.2bc | 14.0 ± 0.2bcd | |
| 0 | S82 | 6.30 ± 0.07 | 71.35 ± 0.45ab | 54.20 ± 1.60 | 3.03 ± 0.09 | 80.8 ± 0.2c | 2.6 ± 0.3a | 15.3 ± 0.1a | |
| 82 | 6 | W6S82 | 6.31 ± 0.03 | 70.85 ± 0.45bc | 55.95 ± 3.14 | 3.12 ± 0.12 | 81.7 ± 0.5ab | 2.0 ± 0.1d | 13.9 ± 0.1cd |
| 7 | W7S82 | 6.35 ± 0.08 | 70.17 ± 0.83c | 55.78 ± 3.09 | 3.17 ± 0.14 | 82.2 ± 0.6ab | 2.1 ± 0.2cd | 14.0 ± 0.1bcd | |
| 0 | S85 | 6.30 ± 0.11 | 71.19 ± 0.78b | 54.36 ± 1.33 | 3.02 ± 0.21 | 82.0 ± 1.1ab | 2.2 ± 0.3cd | 14.0 ± 0.4bcd | |
| 85 | 6 | W6S85 | 6.33 ± 0.02 | 71.08 ± 0.29b | 55.68 ± 3.16 | 3.10 ± 0.15 | 82.4 ± 0.7ab | 2.0 ± 0.1d | 14.3 ± 0.3b |
| 7 | W7S85 | 6.37 ± 0.01 | 70.21 ± 1.15c | 55.90 ± 3.50 | 3.12 ± 0.12 | 81.4 ± 0.6bc | 2.0 ± 0.1cd | 14.1 ± 0.2bc |
Different letters in the same column show significant difference (P < 0.05).
It should be noted that the samples cooked by the combined heating process had different moisture contents and color values than those cooked by steaming alone (P < 0.05, Table 2). The moisture content of samples cooked for a longer MW heating time was lower than that of steamed samples (P < 0.05). MW heating caused greater moisture evaporation from samples (Datta and Rakesh, 2013). In addition, the marinated samples cooked by the combined heating process had higher lightness (L*) but lower redness (a*) and yellowness (b*) than the steamed samples with a core temperature of 82°C (P < 0.05, Table 2). These results were in agreement with Ergonul (2017), who reported that lower a* and b* values were observed in MW-cooked chicken breast as compared with samples cooked by blanching. It has been reported that a decrease in the a* value of cooked meat was due to oxidation and the thermal denaturation of myoglobin, leading to the formation of ferrihemochrome, which has a brown color (Moya et al., 2021). When the core temperature increased to 85°C, the a* value was comparable between the 2 cooking methods. This was likely due to the complete denaturation of hemoglobin at 85°C. The results suggested that the combined MW and steaming process decreased redness of cooked marinated chicken breast with a core temperature of 82°C.
RBS Formation
The incidence of RBS in samples cooked by the combined heating was lower than that of samples cooked by steaming alone at all core temperatures studied (Table 1). A longer microwave pretreatment time showed a greater reduction in RBS. At core temperature of 82°C, RBS were not found with a MW pre-heating of 7 min. Jantaranikorn and Yongsawatdigul (2020) reported that RBS did not occur at core temperature of 85°C in water bath heating. Although cooking loss was comparable between samples cooked to core temperatures of 82°C and 85°C, cooking to a core temperature of 82°C resulted in a larger energy saving. Generally, cooking to a core temperature of 82°C is applied in commercial steaming processes alone, which leads to a sporadic incidence of 2 to 3% for RBS (Smith and Northcutt, 2003). This study illustrated that MW pre-heating for 7 min followed by steaming to 82°C (W7S82) could effectively eliminate the occurrence of RBS without a significant loss in yield. MW heating rapidly increased the internal temperature of the meat sample, leading to greater denaturation of the blood remaining in the blood vessels and the conversion of bloody spots to brown (Gowen et al., 2006; Datta and Rakesh, 2013; Suman et al., 2016), which corresponded with a reduction of redness (a* value) as shown in Table 2. Therefore, the combined heating process appeared to be an alternative process for the reduction of RBS and required lower energy consumption.
SR-FTIR Spectroscopy
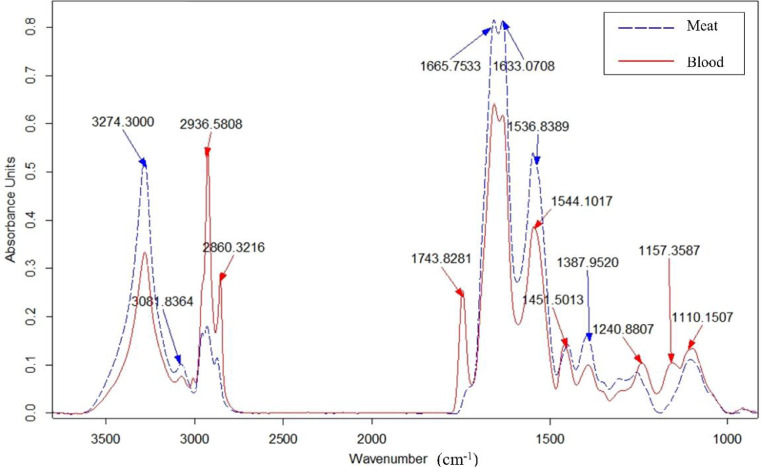
The area of the blood remaining in the blood vessels selected for SR-FTIR measurement is shown in Figure 3. The spectra of the steamed blood remaining in the blood vessels in situ showed a different profile from the steamed meat, with higher absorption at 2,936 and 2,860 cm−1 (Figure 4), corresponding to the C–H stretching vibration of hemoglobin (Kuenstner et al., 2000; Yarrow and Rice, 2011). In addition, higher absorbance at 1,743 cm−1, which was indicative of cellular proteins, was also obvious in the spectrum of the blood (Wolkers and Oldenhof, 2010). The band at 1,100 cm−1, which is characteristic of hemoglobin (Kim, 2006), indicated higher absorbance in the blood sample. While higher absorbance at 3,274 cm−1 is associated with the stretching vibration of N-H bonds, which are typical for proteins (Lozano et al., 2017). The cooked samples had a concomitantly lower α-helical content with a higher β-sheet content when compared with the raw sample (P < 0.05, Table 3). The combined MW heating and steaming process resulted in lower α-helical content than steaming alone (P < 0.05), suggesting a greater degree of protein denaturation in the samples subjected to the combined heating process. The quantity of β-turns and aggregated β-sheets was comparable in the different cooking treatments. In the combined heating process, the β-sheet content was slightly increased as the core temperature increased, indicating that the degree of protein denaturation increased as the core temperature increased.
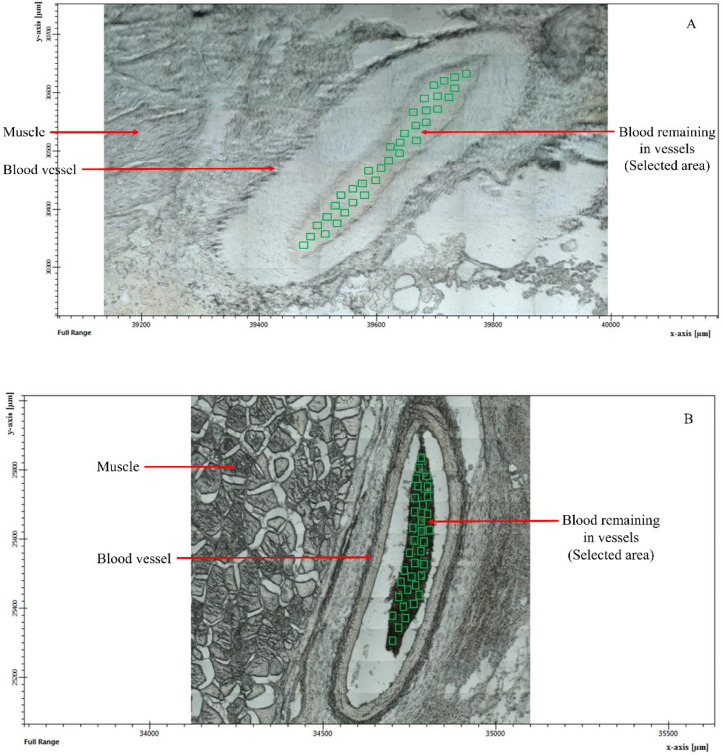
Figure 3.
Representative micrographs of blood remaining in the blood vessels of raw meat (A) and samples cooked by the combined heating process to 82°C (B) obtained from a 36× objective microscope connected to a Synchrotron IR.
Figure 4.
SR-FTIR spectra of cooked blood remaining in the blood vessels in situ and marinated chicken breast cooked by steaming alone to a core temperature of 85°C. Abbreviation: SR-FTIR, synchrotron-based Fourier transform infrared spectroscopy.
Table 3.
Relative content (%) of secondary structures of blood remained in the vessel of marinated chicken breast cooked by the combined MW heating and steaming at various regimes.
| Core temperature (°C) | Time of MW heating (min) | Treatment | α-Helix (1,654, 1,660 cm−1) | β-Sheet (1,620, 1,630 cm−1) | β-Turn (1,670, 1,680 cm−1) | Aggregated β-sheet (1,610, 1,690 cm−1) |
|---|---|---|---|---|---|---|
| Raw | 0 | - | 50.94 ± 1.29a | 24.11 ± 1.54f | 19.16 ± 0.69d | 5.79 ± 0.62ab |
| 0 | S80 | 38.74 ± 0.68b | 33.61 ± 0.50e | 22.04 ± 0.60ab | 5.61 ± 0.25ab | |
| 80 | 6 | W6S80 | 37.20 ± 1.04c | 35.75 ± 0.41cd | 21.50 ± 0.49abc | 5.54 ± 0.72a |
| 7 | W7S80 | 35.42 ± 0.32e | 35.98 ± 0.49bcd | 22.35 ± 0.77ab | 6.25 ± 0.52a | |
| 0 | S82 | 38.87 ± 0.73b | 34.40 ± 0.35de | 21.31 ± 0.97bc | 5.42 ± 0.55ab | |
| 82 | 6 | W6S82 | 36.31 ± 0.89cde | 36.18 ± 1.48abc | 21.22 ± 0.69bc | 6.30 ± 0.53ab |
| 7 | W7S82 | 35.42 ± 0.40e | 36.35 ± 0.82abc | 22.57 ± 0.22a | 5.66 ± 0.38ab | |
| 0 | S85 | 38.85 ± 0.43b | 35.34 ± 0.32cd | 21.44 ± 0.31abc | 4.36 ± 0.45c | |
| 85 | 6 | W6S85 | 36.90 ± 0.69cd | 37.51 ± 1.38ab | 20.71 ± 0.57b | 4.88 ± 0.44bc |
| 7 | W7S85 | 35.67 ± 1.04de | 37.76 ± 0.84a | 21.60 ± 1.28abc | 4.96 ± 0.57bc |
Different letters in the same column show significant difference (P < 0.05).
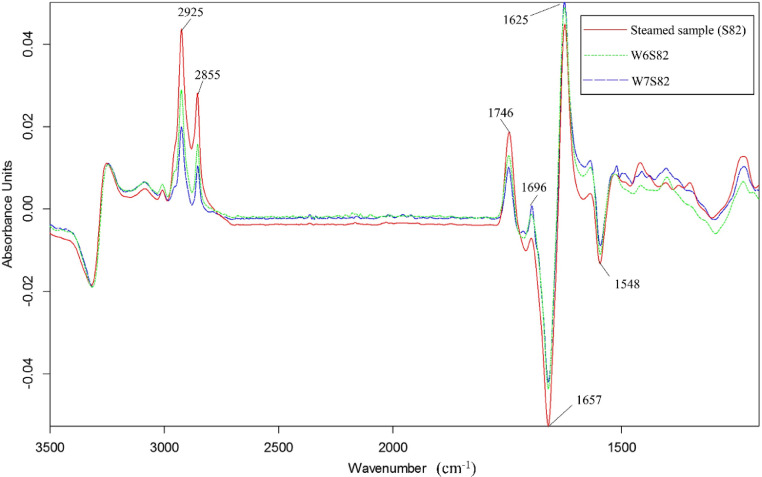
When compared among samples cooked to core temperature of 82°C, samples cooked by the heating regime of W7S82 did not encounter RBS problem (Table 1), corresponding to the lowest α-helix and highest β-sheet content (Table 3). This can also be seen from the FTIR spectra at 1,657 and 1,625 cm−1, which W7S82 exhibited predominant β-sheet structure when compared to samples subjected to steaming alone (S82, Figure 5). This suggested that the combined MW heating and steaming process induced greater denaturation of the blood proteins than steaming alone, even when the same core temperature was reached.
Figure 5.
SR-FTIR spectra of blood remaining in vessels of chicken breast samples cooked to core temperature of 82°C by steaming and the combined microwave heating regimes. Spectra of cooked samples were subtracted from those of raw samples after baseline correction and vector normalization. Abbreviations of heating regimes are shown in Figure 2. Abbreviation: SR-FTIR, synchrotron-based Fourier transform infrared spectroscopy.
CONCLUSIONS
The combined MW heating and steaming process reduced the incidence of RBS in a more effective fashion than steaming alone. Pre-heating by MW for 7 min followed by steaming to reach a core temperature of 82°C completely eliminated RBS. SR-FTIR spectroscopy of the blood remaining in the blood vessels showed the lower α-helix and higher β-sheet structure of the blood proteins, suggesting the greater extent of protein denaturation induced by the combined heating process. The MW pre-heating step reduced cooking time by 28 to 48% as compared with steaming alone. Therefore, the combined MW heating and steaming process could be a promising method to alleviate the problem of RBS in cooked marinated chicken breast products.
ACKNOWLEDGMENTS
This work was financially supported in part by Charoen Pokphand Foods PLC (Thailand). Partial funding was also obtained from the Food Innovation for Safety and Value Creation of Nakhonchaiburin Project, Suranaree University of Technology (SUT3-305-61-12-06).
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Ali S.A., Lawson A.M., Tauson H.A., Jensen F.J., Chwalibog A. Influence of electrical stunning voltages on bleed out and carcass quality in slaughtered broiler chickens. Arch. Geflugelkd. 2007;71:35–40. [Google Scholar]
- AOAC . 18th ed. Association of Official Analytical Chemists; Washington, DC: 2010. Official Methods of Analysis. [Google Scholar]
- Awuah B.G., Ramaswamy S.H., Tang J. CRC. Press; New York, NY: 2015. Radio-Frequency Heating in Food Processing. [Google Scholar]
- Bae M.S., Cho G.M., Hong T.G., Jeong Y.J. Effect of NaCl concentration and cooking temperature on the color and pigment characteristics of presalted ground chicken breasts. Korean J. Food Sci. Anim. Resour. 2018;8:417–430. doi: 10.5851/kosfa.2018.38.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram C.H., Kohler A., Bocker U., Ofstad R., Andersen J.A. Heat-induced changes in myofibrillar protein structures and myowater of two pork qualities: A combined FT-IR spectroscopy and low-field NMR relaxometry study. J. Agric. Food Chem. 2006;54:1740–1746. doi: 10.1021/jf0514726. [DOI] [PubMed] [Google Scholar]
- Datta K.A., Rakesh V. Principles of microwave combination heating. Compr. Rev. Food Sci. Food Saf. 2013;12:24–39. [Google Scholar]
- Domínguez R., Gómez M., Fonseca S., Lorenzo M.J. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014;97:223–230. doi: 10.1016/j.meatsci.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Echarte M., Ansorena D., Astiasarán I. Consequences of microwave heating and frying on the lipid fraction of chicken and beef patties. J. Agric. Food Chem. 2003;51:5941–5945. doi: 10.1021/jf0345245. [DOI] [PubMed] [Google Scholar]
- Ellis G., Santoro G., Gómez M.A., Marco C. Synchrotron IR microspectroscopy: opportunities in polymer science. IOP Conf. Ser. Mater Sci. Eng. 2010;14:1–9. [Google Scholar]
- Ergonul B. Influence of different cooking methods on quality attributes of chicken breast meat. Celal Bayar Univ. J. Sci. 2017;13:883–885. [Google Scholar]
- Geedipalli S., Datta K.A., Rakesh V. Heat transfer in a combination microwave–jet impingement oven. Food Bioprod. Process. 2008;86:53–63. [Google Scholar]
- Goksoy E.O., James C., James S.J. Non-uniformity of surface temperatures after microwave heating of poultry meat. J. Microw. Power Electromagn. Energy. 1999;34:149–160. doi: 10.1080/08327823.1999.11688400. [DOI] [PubMed] [Google Scholar]
- Gowen A., Abu-Ghannam N., Frias J., Oliveira J. Optimisation of dehydration and rehydration properties of cooked chickpeas (Cicer arietinum L.) undergoing microwave-hot air combination drying. Trends Food Sci. Technol. 2006;17:177–183. [Google Scholar]
- Guo Y., Chen T., Wang S., Zhou X., Zhang H., Li D., Mu N., Tang M., Hu M., Tang D., Yang Z., Zhong J., Tang Y., Feng H., Zhang X., Wang H. Synchrotron radiation-based FTIR microspectroscopic imaging of traumatically injured mouse brain tissue slices. ACS. Omega. 2020;5:29698–29705. doi: 10.1021/acsomega.0c03285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbar U.H., Rastogi K.N. Elsevier; London, UK: 2012. Novel Thermal and Non-Thermal Technologies for Fluid Foods. [Google Scholar]
- Hoving-Bolink A.H., Kranen R.W., Klont R.E., Gerritsen C.L.M., Greef K.H. Fibre area and capillary supply in broiler breast muscle in relation to productivity and ascites. Meat sci. 2000;56:397–402. doi: 10.1016/s0309-1740(00)00071-1. [DOI] [PubMed] [Google Scholar]
- Jantaranikorn M., Yongsawatdigul J. Effect of marinating ingredients on temperature-induced denaturation of hemoglobin and its relation to red blood spot formation in cooked chicken breast. J. Food Sci. 2020;85:2398–2405. doi: 10.1111/1750-3841.15308. [DOI] [PubMed] [Google Scholar]
- Jeong J.Y., Lee E.S., Choi J.H., Lee J.Y., Kim J.M., Min S.G., Chae Y.C., Kim C.J. Variability in temperature distribution and cooking properties of ground pork patties containing different fat level and with/without salt cooked by microwave energy. Meat Sci. 2007;75:415–422. doi: 10.1016/j.meatsci.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Joseph I.O. Microwave heating in food processing. BAOJ. Nutr. 2017;3:1–9. [Google Scholar]
- Jouquand C., Frederic J.T., Bernard J., Marier D., Woodward K., Jacolot P., Gadonna-Widehem P., Laguerre C.J. Optimization of microwave cooking of beef burgundy in terms of nutritional and organoleptic properties. Food Sci. Technol. 2015;60:271–276. [Google Scholar]
- Kim J.Y. Prediction of glucose in whole blood by near-infrared spectroscopy: influence of wavelength region, preprocessing, and hemoglobin concentration. J. Biomed. Opt. 2006;11 doi: 10.1117/1.2342076. 041128-1-7. [DOI] [PubMed] [Google Scholar]
- Kuenstner J.T., Norris K., Kalasinsky V.F. Spectrophotometry of human hemoglobin in the midinfrared region. Biospectroscopy. 2000;3:225–232. [Google Scholar]
- Laakkonen E., Wellintong G.H., Sherbon J.W. Low temperature, long-time heating of bovine muscle: changes in tenderness, water-binding capacity, pH and amount of water-soluble components. J. Food Sci. 1972;35:175–177. [Google Scholar]
- Lambooij E., Pieterse C., Hillebrand S.J.W., Dijksterhuis G.B. The effects of captive bolt and electrical stunning, and restraining methods on broiler meat quality. Poult. Sci. 1999;78:600–607. doi: 10.1093/ps/78.4.600. [DOI] [PubMed] [Google Scholar]
- Laycock L., Piyasena P., Mittal S.G. Radio frequency cooking of ground, comminuted and muscle meat products. Meat Sci. 2003;65:959–965. doi: 10.1016/S0309-1740(02)00311-X. [DOI] [PubMed] [Google Scholar]
- Li S., Tang S., Yan L., Li R. Effects of microwave heating on physicochemical properties, microstructure and volatile profiles of yak meat. J. Appl. Anim. Res. 2019;47:262–272. [Google Scholar]
- Lozano M., Rodriguez-Ulibarri P., Echeverria J.C., Beruete M., Sorolla M., Beriain M.J. Mid-infrared spectroscopy (MIR) for simultaneous determination of fat and protein content in meat of several animal species. Food Anal. Methods. 2017;10:3462–3470. [Google Scholar]
- Lyng J.G., Zhang L., Brunton N.P. A survey of the dielectric properties of meats and ingredients used in meat product manufacture. Meat Sci. 2005;69:589–602. doi: 10.1016/j.meatsci.2004.09.011. [DOI] [PubMed] [Google Scholar]
- McKenna M.B., Lyng J., Brunton N., Shirsat N. Advances in radio frequency and ohmic heating of meats. J. Food Eng. 2006;77:215–229. [Google Scholar]
- Moya J., LorenteBailo S., FerrerMairal A., Martínez A.M., Calvo B., Grasa J., Salvador L.M. Color changes in beef meat during pan cooking: kinetics, modeling and application to predict turn over time. Eur. Food Res. Technol. 2021;247:2751–2764. [Google Scholar]
- Nikmaram P., Yarmand S.M., Emamjomeh Z., Darehabi K.H. The effect of cooking methods on textural and microstructure properties of veal muscle (Longissimus dorsi) Glob. Vet. 2011;6:201–207. [Google Scholar]
- Pascolo L., Bortot B., Benseny-Cases N., Gianoncelli A., Tosi G., Ruozi B., Rizzardj C., De Martino E., Vandelli M.A., Severini G.M. Detection of PLGA-based nanoparticles at a single-cell level by synchrotron radiation FTIR spectromicroscopy and correlation with X-ray fluorescence microscopy. Int. J. Nanomed. 2014;9:2791–2801. doi: 10.2147/IJN.S58685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathare B.P., Roskilly P.A. Quality and energy evaluation in meat cooking. Food Eng. Rev. 2016;8:435–447. [Google Scholar]
- Pathera K.A., Riar S.C., Yadav S., Singh K.P. Effect of cooking methods on lipid oxidation, microbiological and sensory quality of chicken nuggets under refrigerated storage. Cogent Food Agric. 2016;2:1–8. [Google Scholar]
- Pérez-Juan M., Kondjoyan A., Picouet P., Realini E.C. Effect of marination and microwave heating on the quality of Semimembranosus and Semitendinosus muscles from Friesian mature cows. Meat Sci. 2012;92:107–114. doi: 10.1016/j.meatsci.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Petibois C., Cestelli-Guidi M., Piccinini M., Moenner M., Marcelli A. Synchrotron radiation FTIR imaging in minutes: a first step towards real-time cell imaging. Anal. Bioanal. Chem. 2010;397:2123–2129. doi: 10.1007/s00216-010-3817-2. [DOI] [PubMed] [Google Scholar]
- Piyasena P., Dussault C., Koutchma T., Ramaswamy S.H., Awuah B.G. Radio frequency heating of foods: principles, applications and related properties. Crit. Rev. Food Sci. Nutr. 2003;43:587–606. doi: 10.1080/10408690390251129. [DOI] [PubMed] [Google Scholar]
- Rincon M.A., Singh K.R., Stelzleni M.A. Effects of endpoint temperature and thickness on quality of whole muscle non-intact steaks cooked in a radio frequency oven. Food Sci. Technol. 2015;64:1323–1328. [Google Scholar]
- Serrano A., Librelotto J., Cofrades S., Saánchez-Muniz F.J., Jiménez-Colmenero F. Composition and physicochemical characteristics of restructured beef steaks containing walnuts as affected by cooking method. Meat Sci. 2007;77:304–313. doi: 10.1016/j.meatsci.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Smith P.D., Northcutt K.J. Red discoloration of fully cooked chicken products. J. Appl. Poult. Res. 2003;12:515–521. [Google Scholar]
- Sturkie P.D. Elsevier; New York, NY: 2015. Avian Physiology. [Google Scholar]
- Suman P.S., Nair N.M., Joseph P., Hunt C.M. Factors influencing internal color of cooked meats. Meat Sci. 2016;120:133–144. doi: 10.1016/j.meatsci.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Vadivambal R., Jayas S.D. Non-uniform temperature distribution during microwave heating of food materials–a review. Food Bioproc. Tech. 2010;3:161–171. [Google Scholar]
- Wattanachant S., Benjakul S., Ledward D.A. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poult. Sci. 2004;83:123–128. doi: 10.1093/ps/83.1.123. [DOI] [PubMed] [Google Scholar]
- Wolkers F.W., Oldenhof H. In situ FTIR studies on mammalian cells. J. Spectrosc. 2010;24 Article ID 576151. [Google Scholar]
- Yarmand S.M., Homayouni A. Rijeka, Intechweb; Croatia: 2011. Microwave Processing of Meat. [Google Scholar]
- Yarrow F., Rice H.J. Localised IR spectroscopy of hemoglobin. Eur. Biophys. J. 2011;40:217–219. doi: 10.1007/s00249-010-0634-7. [DOI] [PubMed] [Google Scholar]