Abstract
Background and aims
It has been indicated that Melatonin receptor 1B (MTNR1B) gene rs10830963 C/G polymorphism was associated with the increased type 2 diabetes mellitus (T2DM) risk. Nevertheless, due to the inconsistent results among the individual studies on this topic, no consensus has been reached now. Hence, the present meta-analysis was conducted to illuminate the potential association of human MTNR1B gene rs10830963 C/G polymorphism and T2DM.
Methods and results
There were 13,752 participants from 7 studies in the present meta-analysis. The pooled odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were assessed by using the fixed or random effects models. A significant association between MTNR1B gene rs10830963 C/G polymorphism and T2DM was found under recessive (OR: 1.148, 95% CI: 1.052–1.253, P = 0.002), homozygous (OR: 1.197, 95% CI: 1.023–1.401, P = 0.025), and additive (OR: 1.067, 95% CI: 1.017–1.120, P = 0.008) genetic models.
Conclusions
MTNR1B gene rs10830963 C/G polymorphism was significantly related to T2DM in the Chinese population. The persons with G allele of the MTNRB1 gene rs10830963 C/G polymorphism might be the T2DM susceptible population.
Keywords: MTNR1B, rs10830963, Polymorphism, Type 2 diabetes mellitus, Genetic
MTNR1B; rs10830963; Polymorphism; Type 2 diabetes mellitus; Genetic.
1. Introduction
T2DM is a metabolic disorder syndrome mainly characterized by hyperglycemia, which is formed by insulin resistance and pancreatic beta cell defect. T2DM is the third chronic disease that seriously threatens human life and health after cardiovascular disease and tumor, and its morbidity and fatality rate are increasing. The diabetes revalence in Chinese adults increased from 0.67% in 1980 to 10.9% in 2013 to12.8% in 2017 [1]. The exact pathogenesis of T2DM has not been clarified yet. T2DM has obvious genetic tendency and significant genetic heterogeneity.
Melatonin (MT) is a kind of indole hormone commonly found in living organisms. It is mainly secreted by the pineal gland in human body, and it regulates many important physiological functions such as circadian rhythm, sleep, endocrine, immune and anti-aging [2]. The action of MT is mediated by melatonin receptor 1 (Mella, MTI) and melatonin receptor 2 (Mellb, MT2), both of which are G protein coupled receptors [3]. MT1 is encoded by MTNR1A gene and MT2 is encoded by MTNR1B gene. MTNR1B gene might mediate the inhibitory effect of MT on insulin secretion through MT signaling pathway, thus participate in the T2DM pathogenetic process. It was reported that MTNR1B gene polymorphisms was associated with the fasting blood glucose (FBG) level and T2DM pathogenesis in the European population for the first time in 2008 [4]. The human MTNR1B gene, located in 11q21-q22, contains 2 exons and 1 intron, and encodes 362 amino acids, of which the full length is 13159bp. The MTNR1Bgene was mainly expressed in the retina, brain, and pancreas islet. MTNR1B belongs to G-protein coupled receptors and is structurally an intramembrane protein. Melatonin is a neuroendocrine hormone that acts on pancreatic islet cells, resulting in a decrease in cAMP concentration, which is contrary to the pancreas’ function, and the insulin secretion is inhibited. After melatonin binds to the receptor, the secretion cycle occurs synchronously with the circadian rhythm. It is speculated that the inhibition of secretion of pancreatic islet cells by these endocrine neurons may lead to the circadian transmission of insulin release. There is abundant evidence that the disruption of the insulin release circadian rhythm in the rodent and human islet cells is associated with metabolic disease [5, 6, 7, 8].
The MTNR1B gene has a conserved sequence at the splicing site. In this case, the intron changes during splicing, leading to potential functional and structural changes in the MTNR1B gene. The MTNR1B gene rs10830963 C/G polymorphism is located in the intron [9]. The risk allele G of MTNR1B gene rs10830963 C/G polymorphism leads to the MTNR1B gene overexpression in the pancreatic islet cells, which contributes to the decreased insulin secretion, elevated FBG level and results in the T2DM occurrence.
Multiple studies on the correlation between MTNR1B gene rs10830963 C/G polymorphism and T2DM in the Chinese population have been performed, but no consensus has been reached up to now. In 2009, Rönn et al. reported that the MTNR1B gene rs10830963 C/G polymorphism increased the T2DM risk in a Shanghai population and the G allele was the risk allele [10]. Analogously, in 2010, Kan et al. found that the common variant of the MTNR1B gene rs10830963 C/G polymorphism confers the T2DM risk in another Shanghai Chinese population and the G allele was associated with the increased T2DM risk [11]. Discriminatively, in 2011, Ling et al. reported that there was no significant association between rs10830963 polymorphism and T2DM risk in another Shanghai Han population [12]. In 2015, Shi et al. replicated the same conclusion in a Gansu Baoan population [13].
For the sake of determining whether the MTNR1B gene rs10830963 C/G polymorphism was correlated with T2DM susceptibility, the current meta-analysis including 13,752 participants from 7 researches was carried out (Supplements S1).
2. Materials and methods
2.1. Publication search and inclusion criteria
The words as “type 2 diabetes mellitus”, “Melatonin receptor 1B″, “MTNR1B″, “rs10830963”, “polymorphism”, and “gene” were adopted to search the papers. The studies were retrieved from such electronic databases as the Weipu (VIP) database, WanFang database, the China National Knowledge Infrastructure, the Web of Science, and PubMed. The last search was updated on Febuary 22, 2022. The selected papers were published between 2009 and 2016.
The subsequent recruitment standard should be followed by the selected studies in this meta-analysis. The studies selected should: a) evaluate the association of T2DM with MTNR1B gene rs10830963 C/G polymorphism; b) In regard to the T2DM diagnosis criteria: typical diabetic symptoms (polyuria, polydipsia, polyphagia, unexplained weight loss) plus random blood glucose ≥11.1mmol/L, or plus blood glucose ≥11.1 mmol/L 2h after glucose load or plus fasting blood glucose ≥7.0mmol/L, or plus glycated hemoglobin A1c (HbA1c) ≥6.5%. If the patients have no typical diabetic symptoms, they should be retested in another day. c) The selected researches ought to be the case-control or cohort studies. The exclusive criteria included the subsequent items: (1) the repeated literatures published by the same research group could only be used once. For the repeated studies, only the studies with large sample size were selected; (2) the literature in which the original research data were not available and the authors were not available by contact.
2.2. Data extraction
According to standard operating procedures, three investigators were involved in the data collection process. The data was parallelly collected by two of them. They collected the data independently. In the data collection process, if there was dispute between them, they would consult with the third investigator and resolve the disagreement together. The collected data should include these items as the name of the first author, year of publication, region of study, ethnicity of the study populations, the CC, CG, GG genotype counts of the MTNR1B gene rs10830963 C/G polymorphism in the case group and control group, the methods for genotyping, sample size in the two groups, and the P value for judging whether the genotype distribution of the control group conformed to the Hardy Weinberg equilibrium (HWE) or not.
2.3. Statistical analyses
In the current research, the software as the Stata 12.0 and Review Manager 5.3 were used to perform the statistical analyses. The relationship of MTNR1B gene rs10830963 C/G polymorphism with T2DM was assessed by using the pooled odds ratios (ORs) and their corresponding 95% confidence intervals (CIs). Six genetic models including allelic (G allele vs. G allele + C allele), recessive (GG vs. CC + CG), dominant (CC vs. GG + CG), heterozygous (CG vs. CC), homozygous (GG vs. CC), and additive (G allele vs. C allele) were used in the statistical analyses process.
The heterogeneity from these selected studies was assessed in terms of the Pheterogeneity and I2 results [14]. Under the condition of Pheterogeneity> 0.05, the data would be analyzed by using the fixed effects models. If not, the data would be analyzed by utilizing the random effects models [15].
The combined ORs were assessed through the Z test. In order to evaluate the HWE, the Fisher's exact test was performed by using the open source software R [16]. The publication bias was diagnosed by using the funnel plot under the recessive genetic model. The symmetry of the funnel plot was evaluated by the Egger linear regression and Begg's test under the additive genetic model [17]. P < 0.05 means the significant difference in all these tests items.
3. Results
3.1. Studies and populations
The included data consist of 6,576 T2DM cases and 7,176 controls which were extracted from 7 studies (Table 1) [[10], [11], [12], [13],[18], [19], [20]]. After an initiatory investigation, 16 papers were found out. Among these papers, 7 papers met the conditions of this meta-analysis. A manuscript deviating from HWE was not ruled out [12]. Four eliminated studies were not correlated with the MTNR1B rs10830963 C/G mutation or T2DM. Five manuscripts were eliminated for their reviews character (Supplement S2).
Table 1.
The studies characteristics included in this meta-analysis.
| Author | Year | Region | Ethnicity | T2DM |
Control |
Matching criteria | Genotyping method | sample size (T2DM/control) | PHWE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CG | GG | CC | CG | GG | ||||||||
| Ling Y [12] | 2011 | Shanghai | Han | 403 | 538 | 177 | 404 | 590 | 167 | Sex, ethnicity | Spectroscopy | 1118/1161 | 0.039∗ |
| Kan MY [11] | 2010 | Shanghai | Han | 585 | 960 | 367 | 675 | 989 | 350 | Sex, BMI,WHR,DBP,LDL-C,ethnicity, | PCR-RFLP | 1912/2041 | 0.707 |
| Gao KP [18] | 2016 | Guangdong | Han | 243 | 347 | 134 | 280 | 350 | 129 | Smoking, ethnicity | Taqman | 724/759 | 0.274 |
| Ronn T [10] | 2009 | Shanghai | Han | 371 | 553 | 241 | 374 | 558 | 173 | Age, sex, BMI, ethnicity | iPLEX | 1165/1105 | 0.139 |
| Shi XE [13] | 2015 | Gansu | Baoan | 80 | 100 | 44 | 144 | 208 | 52 | Age, sex, BMI,height, ethnicity | MALDI-TOF-MS | 224/404 | 0.084 |
| Liang SQ [19] | 2010 | Jilin | Han | 15 | 47 | 29 | 25 | 46 | 18 | Age, sex, DBP, TC, TG, BMI, ethnicity | PCR-RFLP | 91/89 | 0.705 |
| Tam CH [20] | 2010 | Hongkong | Han | 448 | 633 | 261 | 523 | 789 | 332 | Ethnicity | PCR-RFLP | 1342/1644 | 0.273 |
∗P < 0.05.
Abbreviations: MTNR1B: melatonin receptor 1B; T2DM: type 2 diabetes mellitus; sample size (T2DM/control) size: the total number of T2DM or control group; WHR: waist hip rate; BMI: body mass index; TC: total cholesterol; TG: triglyceride; DBP: diastolic blood pressure; PCR-RFLP: Polymerase chain reaction-restricted fragment length polymorphism; iPLEX: mult iplex PCR; MALDI-TOF-MS: Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry.
3.2. Pooled analyses
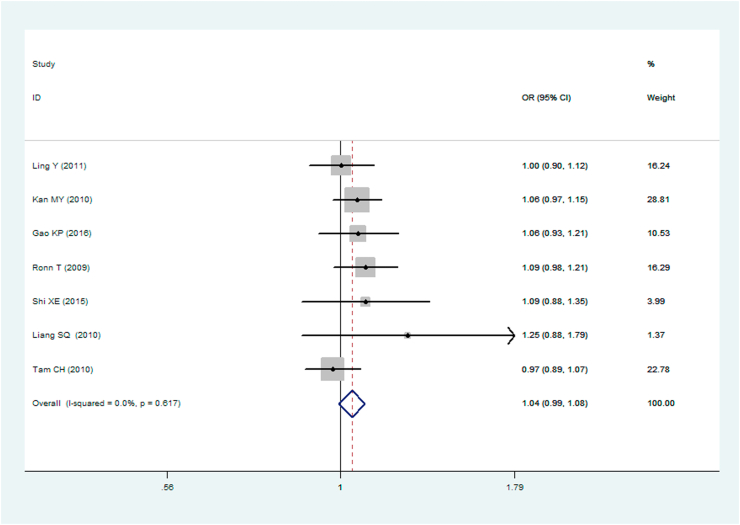
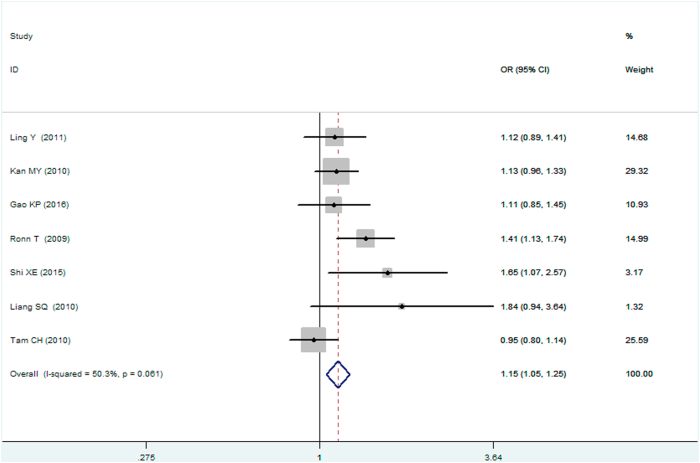
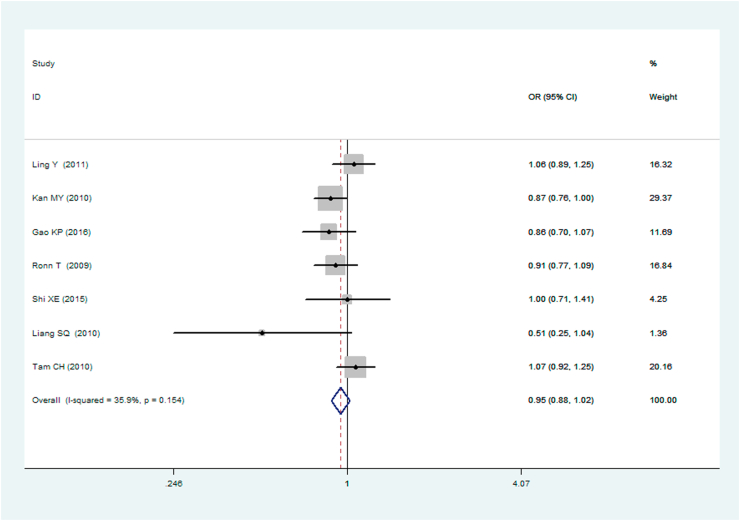
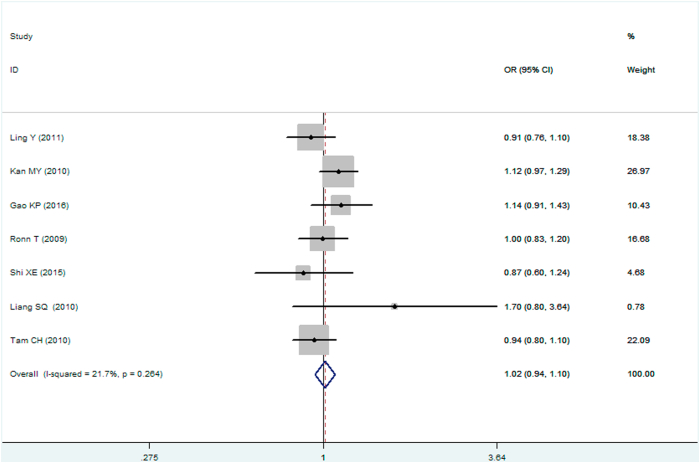
A significant association between MTNR1B gene rs10830963 C/G polymorphism and T2DM was found under recessive (OR: 1.148, 95% CI: 1.052–1.253, P = 0.002), homozygous (OR: 1.197, 95% CI: 1.023–1.401, P = 0.025), and additive (OR: 1.067, 95% CI: 1.017–1.120, P = 0.008) genetic models. No significant association between MTNR1B gene rs10830963 C/G polymorphism and T2DM was found under the allelic (OR: 1.038, 95% CI: 0.994–1.084, P = 0.093), dominant (OR: 0.950, 95% CI: 0.885–1.020, P = 0.160), or heterozygous (OR: 1.016, 95% CI: 0.943–1.096, P = 0.672) genetic models (Table 2, Figures 1, 2, 3, 4, 5, and 6).
Table 2.
The current meta-analysis results under the six genetic models.
| Genetic models | Pooled OR (95% CI) | Z | P | Studies number | T2D size | control size | Pheterogeneity(I2%) |
|---|---|---|---|---|---|---|---|
| Allelic genetic model▽ | 1.038 (0.994–1.084) | 1.68 | 0.093 | 7 | 6576 | 7176 | 0.617 (0%) |
| Recessive genetic model▽ | 1.148 (1.052–1.253) | 3.10 | 0.002∗ | 7 | 6576 | 7176 | 0.061 (50.3%) |
| Dominant genetic model▽ | 0.950 (0.885–1.020) | 1.41 | 0.160 | 7 | 6576 | 7176 | 0.154 (35.9%) |
| Heterozygous genetic model▽ | 1.016 (0.943–1.096) | 0.42 | 0.672 | 7 | 6576 | 7176 | 0.264 (21.7%) |
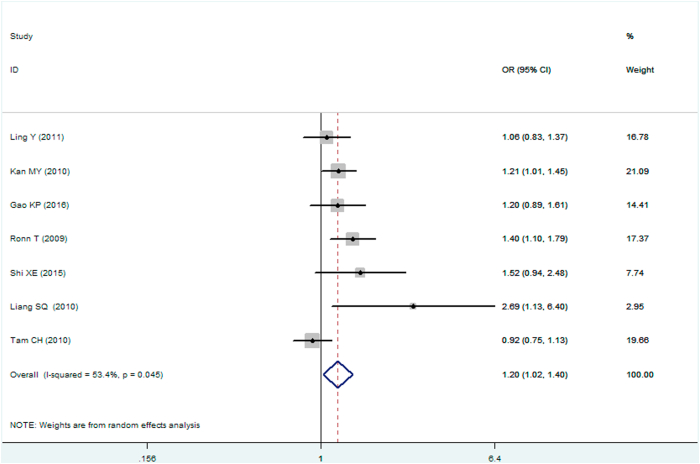
| Homozygous genetic model△ | 1.197 (1.023–1.401) | 2.24 | 0.025∗ | 7 | 6576 | 7176 | 0.045∗ (53.4%) |
| Additive genetic model▽ | 1.067 (1.017–1.120) | 2.65 | 0.008∗ | 7 | 6576 | 7176 | 0.063 (49.9%) |
∗P < 0.05.
▽: the “fixed effects model” was applied to compute the pooled OR under the allelic, recessive, dominant, heterozygous and additive genetic models.
△: the “random effects model” was applied to compute the pooled OR under the homozygous genetic models.
Abbreviations: CI: confidence interval; OR: odds ratio; T2D size: the total number of T2DM cases; control size: the total number of control group; Allelic genetic model: G allele vs. G allele + C allele; Recessive genetic model: GG vs. CC + CG; Dominant genetic model: CC vs. GG + CG; Heterozygous genetic model: CG vs. CC; Homozygous genetic model: GG vs. CC; Additive genetic model: G allele vs. C allele.
Figure 1.
Forest plot of T2DM associated with MTNR1B gene rs10830963 C/G polymorphism under an allelic genetic model (i.e. G allele vs. G allele + C allele of MTNR1B gene rs10830963 C/G polymorphism).
Figure 2.
Forest plot of T2DM associated with MTNR1B gene rs10830963 C/G polymorphism under a recessive genetic model (i.e. GG VS. CC + CG of MTNR1B gene rs10830963 C/G polymorphism).
Figure 3.
Forest plot of T2DM associated with MTNR1B gene rs10830963 C/G polymorphism under a dominant genetic model (i.e. CC VS. GG + CG of MTNR1B gene rs10830963 C/G polymorphism).
Figure 4.
Forest plot of T2DM associated with MTNR1B gene rs10830963 C/G polymorphism under a heterozygous genetic model (i.e., CG vs. CC of MTNR1B gene rs10830963 C/G polymorphism).
Figure 5.
Forest plot of T2DM associated with MTNR1B gene rs10830963 C/G polymorphism under a homozygous genetic model (i.e., GG vs. CC of MTNR1B gene rs10830963 C/G polymorphism).
Figure 6.
Forest plot of T2DM associated with MTNR1B gene rs10830963 C/G polymorphism under an additive genetic model (i.e., G allele vs. C allele of MTNR1B gene rs10830963 C/G polymorphism).
4. Publication bias diagnostics
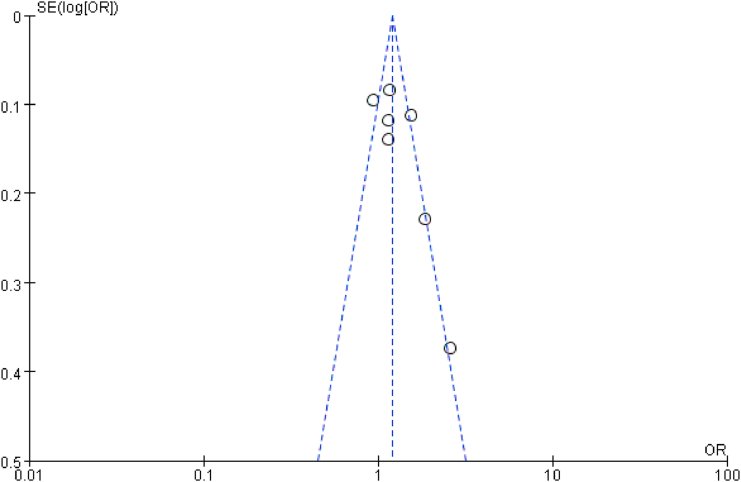
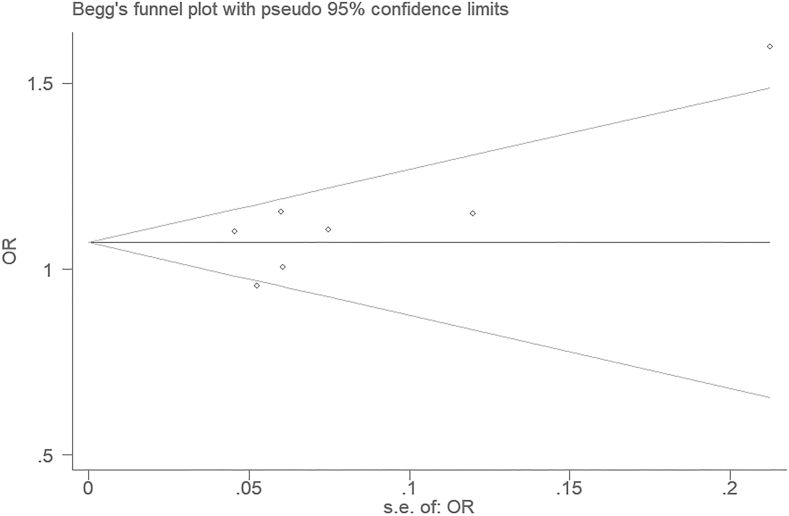
Since the studies with negative results might not be published or retrieved, the publication bias might occur and affect the research results, the publication bias analysis is then conducted. On the funnel plot for the recessive genetic model, the publication bias was not significant (Figure 7). Furthermore, for the additive genetic model, no significant publication bias was uncovered through the Egger's test (T = 1.72, P = 0.146) (Figure 8).
Figure 7.
The funnel plot for studies of the association of T2DM and MTNR1B gene rs10830963 C/G polymorphism under a recessive genetic model (i.e. GG VS.CC + CG of MTNR1B gene rs10830963 C/G polymorphism). The horizontal and vertical axes correspond to the OR on log scale and SE (logOR), respectively. OR: odds ratio; SE: standard error.
Figure 8.
The Begg's funnel plot for studies of the association of T2DM and MTNR1B gene rs10830963 C/G polymorphism under an additive genetic model (i.e., G allele vs. C allele of MTNR1B gene rs10830963 C/G polymorphism). The horizontal and vertical axes correspond to the OR on log scale and SE (logOR), respectively. OR: odds ratio; SE: standard error.
4.1. Sensitivity analysis
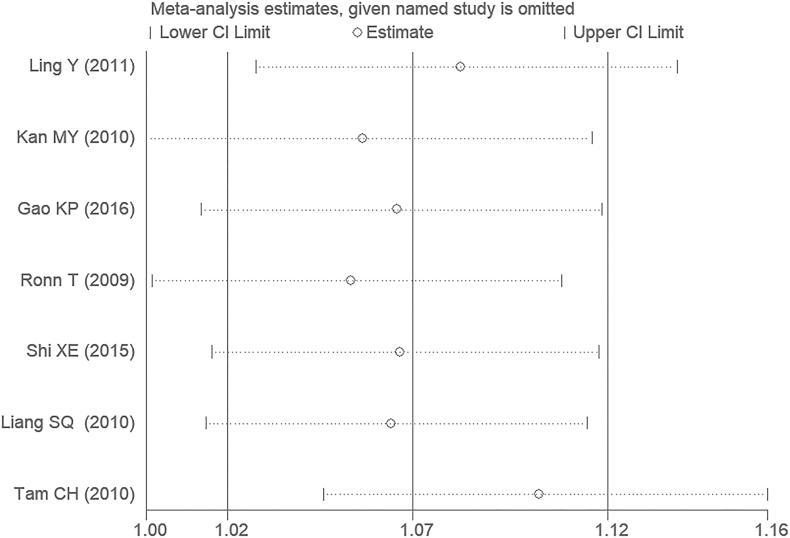
After removing any of the individual studies from the meta-analysis, the primary outcome was not influenced under the additive genetic model. It was suggested that the outcome from the current meta-analysis was relatively stable (Figure 9).
Figure 9.
The sensitivity analysis on the association of T2DM and MTNR1B gene rs10830963 C/G polymorphism under an additive genetic model (i.e., G allele vs. C allele of MTNR1B gene rs10830963 C/G polymorphism).
5. Discussion
In the present meta-analysis, MTNR1B gene rs10830963 C/G polymorphism was found to be significantly associated T2DM risk under the recessive (OR: 1.148), homozygous (OR: 1.197), and additive (OR: 1.067) genetic models.
T2DM is a polygenic, multi-factorial disease with high genetic heterogeneity. Insulin resistance and pancreatic beta cell dysfunction are the main characteristics of T2DM. MT is a neuroendocrine hormone secreted by the pineal gland, which plays a regulatory role in biological circadian rhythm, sexual maturation and reproductive immune response, tumor, aging, and so on. Hence, it has a wide range of physiological and pharmacological effects. MT not only regulates sleep, but also insulin secretion. Shorter or less quality sleep can lead to reduced glucose tolerance, increasing the risk of type 2 diabetes. The level of MT secretion is just opposite to the rule of insulin secretion, indicating that MT may have an inhibitory effect on insulin release [21]. MTNR1B is conjugated to inhibitory G protein, and MT activation of MTNR1B will block the activation of adenylate cyclase (AC). Activation of AC is the primary activation mechanism of endocrine hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulin-promoting polypeptide (GIP). GLP-1 and GIP are incretin which can promote the islet β cells to secret the insulin and inhibit the islet α cells to secret the glucagon. Furthermore, MT might inhibit the release of insulin through the MTNR1B mediated cyclic guanine nucleotide pathway [22]. Therefore, the risk allele G of rs10830963 increases the overexpression of MTNR1B mRNA in islet cells, which results in the elevated fasting glucose and leads to the T2DM occurrence. In 2009, Lyssenko et al. found that each G allele of rs10830963 polymorphism increased FBG by 0.07 mmol/L and was associated with reduced β-cell function which increased the T2DM risk [23]. The out of balance of glycometabolism steady state and the elevated FBG level are the symbols of T2DM occurrence as they could continuously affect the pancreatic islet β cells function and reduce the insulin secretion. In short, the correlation of rs10830963 C/G polymorphism and T2DM is probably due to the MT overexpression or the increased MT activity which leads to the decreased insulin level, increased FBG level and the T2DM occurrence [4].
The potential therapy significance of MTNR1B gene rs10830963 C/G polymorphism on the T2DM was presented as the follows. First, as the MT has the negative impact on the T2DM development, the antagonist targeting on the MT2 in the β cells might be effective. Secondly, the individuals carrying the G risk allele of MTNR1B gene rs10830963 C/G polymorphism might have the lower curative effects on the GLP-1 analogues and the inhibitors of the substance promoting the GLP-1 degradation. Hence, recognizing these individuals would be like to provide a more accurate T2DM diagnosis and treatment methods [23].
Similar meta-analyses on the relevance of T2DM risk with MTNR1B gene rs10830963 C/G polymorphism were performed previously. In 2019, Shen et al. carried out a meta-analysis to evaluate whether there was a significant association between MTNR1B gene rs10830963 C/G polymorphism and T2DM risk [24]. They found that the MTNR1B gene rs10830963 C/G variant was significantly correlated with T2DM susceptibility in South Asians, but not in East Asians. However, they mixed the Chinese population with other population and only 5 Chinese studies were involved to assess this relationship. Consequently, Shen's meta-analysis might not be as objective as the present research because they neglected the ethnicity difference. In 2021, Feng et al. also performed a meta-analysis to assess the correlation of MTNR1B rs10830963 C/G mutation and T2DM risk in a Chinese population [25]. Although they found a significant association between them, only 6 individual studies were contained in their meta-analysis. Hence, the conclusion deduced from their meta-analysis might not be as entire as that from the current meta-analysis.
Some limitations still existed inevitably in this current meta-analysis. There were not sufficient large-scale researches to estimate the correlation between the MTNR1B gene rs10830963 C/G polymorphism and T2DM yet. Moreover, environmental factors such as too much diet intake, too little physical exercise, and chronic tension and anxiety were closely linked to the T2DM onset. Other genes polymorphism as NEUROD1 gene Ala45Thr polymorphism, GHRL gene Leu72Met polymorphism and SUM O 4 gene M55V polymorphism might influence the T2DM development [26,27,28]. In the present research, the correlation of the MTNR1B rs10830963 C/G mutation and T2DM was assessed. Otherwise, there's another mutation, i.e., MTNR1B gene rs1387153 C/T polymorphism which was associated with the T2DM risk [29].
In all, MTNR1B gene rs10830963 C/G mutant was significantly associated with T2DM risk in the Chinese population. The persons carrying the G allele of MTNR1B gene rs10830963 C/G polymorphism might be easier to suffer from T2DM than the persons carrying the C allele. Larger scale researches on the correlation of MTNR1B gene rs10830963 C/G polymorphism and T2DM should be performed to further elucidate this correlation.
Declarations
Author contribution statement
Yan-yan Li: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hui Wang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Yang-yang Zhang: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Dr Yanyan Li was supported by National Natural Science Foundation of China [81100073].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Li Y., Teng D., Shi X., Qin G., Qin Y., Quan H., Shi B., Sun H., Ba J., Chen B., Du J., He L., Lai X., Li Y., Chi H., Liao E., Liu C., Liu L., Tang X., Tong N., Wang G., Zhang J.A., Wang Y., Xue Y., Yan L., Yang J., Yang L., Yao Y., Ye Z., Zhang Q., Zhang L., Zhu J., Zhu M., Ning G., Mu Y., Zhao J., Teng W., Shan Z. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. doi: 10.1136/bmj.m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kvetnoy I.M. Extrapineal melatonin: location and role within diffuse neuroendocrine system. Histochem. J. 1999;31:1–12. doi: 10.1023/a:1003431122334. [DOI] [PubMed] [Google Scholar]
- 3.Reppert S.M., Godson C., Mahle C.D., Weaver D.R., Slaugenhaupt S.A., Gusella J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl AcadSci U S A. 1995;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prokopenko I., Langenberg C., Florez J.C., Saxena R., Soranzo N., Thorleifsson G., Loos R.J., Manning A.K., Jackson A.U., Aulchenko Y., Potter S.C., Erdos M.R., Sanna S., Hottenga J.J., Wheeler E., Kaakinen M., Lyssenko V., Chen W.M., Ahmadi K., Beckmann J.S., Bergman R.N., Bochud M., Bonnycastle L.L., Buchanan T.A., Cao A., Cervino A., Coin L., Collins F.S., Crisponi L., de Geus E.J., Dehghan A., Deloukas P., Doney A.S., Elliott P., Freimer N., Gateva V., Herder C., Hofman A., Hughes T.E., Hunt S., Illig T., Inouye M., Isomaa B., Johnson T., Kong A., Krestyaninova M., Kuusisto J., Laakso M., Lim N., Lindblad U., Lindgren C.M., McCann O.T., Mohlke K.L., Morris A.D., Naitza S., Orrù M., Palmer C.N., Pouta A., Randall J., Rathmann W., Saramies J., Scheet P., Scott L.J., Scuteri A., Sharp S., Sijbrands E., Smit J.H., Song K., Steinthorsdottir V., Stringham H.M., Tuomi T., Tuomilehto J., Uitterlinden A.G., Voight B.F., Waterworth D., Wichmann H.E., Willemsen G., Witteman J.C., Yuan X., Zhao J.H., Zeggini E., Schlessinger D., Sandhu M., Boomsma D.I., Uda M., Spector T.D., Penninx B.W., Altshuler D., Vollenweider P., Jarvelin M.R., Lakatta E., Waeber G., Fox C.S., Peltonen L., Groop L.C., Mooser V., Cupples L.A., Thorsteinsdottir U., Boehnke M., Barroso I., Van Duijn C., Dupuis J., Watanabe R.M., Stefansson K., McCarthy M.I., Wareham N.J., Meigs J.B., Abecasis G.R. Variants in MTNR1B influence fasting glucose levels. Nat. Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stumpf I., Mühlbauer E., Peschke E. Involvement of the cGMP pathway in mediating the insulin-inhibitory effect of melatonin in pancreatic beta-cells. J. Pineal Res. 2008;45:318–327. doi: 10.1111/j.1600-079X.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 6.Boden G., Ruiz J., Urbain J.L., Chen X. Evidence for a circadian rhythm of insulin secretion. Am. J. Physiol. 1996;271:E246–E252. doi: 10.1152/ajpendo.1996.271.2.E246. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 8.Turek F.W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D.R., Eckel R.H., Takahashi J.S., Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y. Ningxia Medical University; 2011. The relationship between polymorphism in MTNR1B gene and type 2 diabetes mellitus in Han people in Ningxia. Master Thesis; p. 11. [Google Scholar]
- 10.Rönn T., Wen J., Yang Z., Lu B., Du Y., Groop L., Hu R., Ling C. A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia. 2009;52:830–833. doi: 10.1007/s00125-009-1297-8. [DOI] [PubMed] [Google Scholar]
- 11.Kan M.Y., Zhou D.Z., Zhang D., Zhang Z., Chen Z., Yang Y.F., Guo X.Z., Xu H., He L., Liu Y. Two susceptible diabetogenic variants near/in MTNR1B are associated with fasting plasma glucose in a Han Chinese cohort. Diabet. Med. 2010;27:598–602. doi: 10.1111/j.1464-5491.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- 12.Ling Y., Li X., Gu Q., Chen H., Lu D., Gao X. A common polymorphism rs3781637 in MTNR1B is associated with type 2 diabetes and lipids levels in Han Chinese individuals. Cardiovasc. Diabetol. 2011;10:27. doi: 10.1186/1475-2840-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi X.E., Peng X.J., Wang Z.T., Ma J.L. Correlation between MTNR1B gene rs10830963 polymorphism and type 2 diabetes mellitus in Gansu Bao-an population. Clinical Focus. 2015;30:544–547. [Google Scholar]
- 14.UmansDS, HallenslebenND, VerdonkRC, BouwenseSAW, FockensP, vanSantvoort H.C., et al. Recurrence of idiopathic acute pancreatitis after cholecystectomy: systematic review and meta-analysis. Br. J. Surg. 2020;107:191–199. doi: 10.1002/bjs.11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgado-Rodríguez M., Sillero-Arenas M. Systematic review and meta-analysis. Med. Intensiva. 2018;42:444–453. doi: 10.1016/j.medin.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 17.Kassardjian V., Varma S., Andiappan M., Creugers N.H.J., Bartlett D. A systematic review and meta analysis of the longevity of anterior and posterior all-ceramic crowns. J. Dent. 2016;55:1–6. doi: 10.1016/j.jdent.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Gao K., Wang J., Li L., Zhai Y., Ren Y., You H., Wang B., Wu X., Li J., Liu Z., Li X., Huang Y., Luo X.P., Hu D., Ohno K., Wang C. Polymorphisms in four genes (KCNQ1rs151290, KLF14 rs972283, GCKR rs780094 and MTNR1B rs10830963) and TheirCorrelation with type 2 diabetes mellitus in han Chinese in henan province, China. Int. J. Environ. Res. Publ. Health. 2016;13:260. doi: 10.3390/ijerph13030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang S.Q. Norman Bethune Health Science Center of Jilin University; 2010. Association of MTNR1B gene with type II diabetes in the peope of the Han nationality of Jilin region. Master Thesis; p. p25. [Google Scholar]
- 20.Tam C.H., Ho J.S., Wang Y., Lee H.M., Lam V.K., Germer S., Martin M., So W.Y., Ma R.C., Chan J.C., Ng M.C. Common polymorphisms in MTNR1B, G6PC2 and GCK are associated with increased fasting plasma glucose and impaired beta-cell function in Chinese subjects. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma G.G., Zhang J.X. Melatonin receptor gene variation and diabetes mellitus. Shandong Med. J. 2011;51:103–104. [Google Scholar]
- 22.Peschke E. Melatonin, endocrine pancreas and diabetes. J. Pineal Res. 2008;44:26–40. doi: 10.1111/j.1600-079X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 23.Lyssenko V., Nagorny C.L., Erdos M.R., et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen L.L., Jin Y. Effects of MTNR1B genetic variants on the risk of type 2 diabetes mellitus: a meta-analysis. Mol Genet Genomic Med. 2019;7:e611. doi: 10.1002/mgg3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng S.S., Wei S.Y., He S., Ma F.F., Ha X.Q. Meta-analysis of association between MTNR1B rs10830963 gene polymorphism and type 2 diabetes mellitus in Chinese population. Chin Med Biotechnol. 2021;16:78–81. [Google Scholar]
- 26.Li Y.Y., Wang H., Zhang Y.Y. Neuronal Differentiation 1 gene Ala45Thr polymorphism and type 2 diabetes mellitus: a meta-analysis of 7,940 subjects. Nutr. Metabol. Cardiovasc. Dis. 2021;31:1809–1821. doi: 10.1016/j.numecd.2021.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.Y., Lu X.Z., Yang X.X., Wang H., Geng H.Y., Gong G., et al. GHRL gene Leu72Met polymorphism and type 2 diabetes mellitus: a meta-analysis involving 8,194 participants. Front. Endocrinol. 2019;10:559. doi: 10.3389/fendo.2019.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y.Y., Wang H., Yang X.X., Geng H.Y., Gong G., Kim H.J., et al. Small ubiquitin-like modifier 4 (SUMO4) gene M55V polymorphism and type 2 diabetes mellitus: a meta-analysis including 6,823 subjects. Front. Endocrinol. 2017;8:303. doi: 10.3389/fendo.2017.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouatia-NajiN N., Bonnefond A., et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat. Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.