Abstract
Hydrogen peroxide generated by viridans group streptococci has an antagonistic effect on many bacterial species, including a number of pathogens, in the oral environment. This study examines the influence of a variety of environmental conditions on rates of hydrogen peroxide synthesis by Streptococcus gordonii. Hydrogen peroxide was synthesized at every concentration of glucose and sucrose tested from 10 μM to 1 M, with the highest rates occurring at 0.1 mM sucrose and 1 mM glucose. S. gordonii appeared to have an intracellular store of polysaccharide which supported hydrogen peroxide formation even when the assay buffer contained no carbohydrate. Most heavy metal ions inhibited peroxidogenesis, and anaerobic conditions induced adaptive down-regulation of hydrogen peroxide synthesis; however, peroxidogenesis was generally insensitive to moderate increases in salt concentration, alteration of the mineral content of the assay solution, and changes in pH between 5.0 and 7.5. In contrast, stimulation of peroxidogenesis occurred in 1 mM Mg2+ and 10 to 50 mM potassium l-lactate. Maximum peroxidogenesis occurred during the mid-logarithmic and late-logarithmic phases of bacterial growth. These bacterial responses may have significant implications for oral ecology and oral health.
Clinical studies which have identified the microflora associated with dental caries have also revealed that certain species of streptococci are associated with oral health. De Stoppelaar et al. found an inverse correlation between levels of Streptococcus sanguis and S. mutans in dental plaque and a trend toward decreasing caries as S. sanguis levels increase (8, 9). Likewise, Svanberg and Loesche noted that a high ratio of S. sanguis to S. mutans correlates with a low decayed-missing-filled tooth score (32). Nyvad and Kilian confirmed these observations, finding that significantly higher levels of S. sanguis are present in the dental plaque of caries-inactive individuals (25). This protective effect has also been observed in studies of periodontal disease, which have shown that S. sanguis, S. mitis, and S. oralis appear more frequently at sites that are free of disease (1, 16, 33).
These protective species synthesize hydrogen peroxide, the viridans alpha-hemolysin (2), which provides a possible explanation for the clinical observations. In vitro, hydrogen peroxide generated by S. sanguis and other streptococci kills or inhibits the growth of such putative cariogenic and periodontal pathogens as Streptococcus mutans, Bacteroides forsythus, Capnocytophaga sputigena, Eikenella corrodens, Fusobacterium nucleatum, Porphyromonas gingivalis, Prevotella intermedia, and Wolinella recta (16, 17). Inhibition of S. mutans growth by peroxidogenic streptococci has also been observed in direct competition experiments in liquid coculture (35). In these studies, controls using catalase, peroxidase, or anaerobic conditions to eliminate hydrogen peroxide have confirmed that bacterial antagonism is due to hydrogen peroxide rather than to a bacteriocin (16, 17, 35). S. mutans and most of the other pathogens listed above are also susceptible to hypothiocyanite ion (OSCN−) (7, 35). This antimicrobial agent is generated by salivary peroxidase using streptococcal hydrogen peroxide and thiocyanate ion (SCN−), a common component of saliva (27).
Although streptococci are known to have an unusual glycolytic pathway that converts oxygen into hydrogen peroxide (4, 14, 31, 39), the influence of the oral environment on peroxidogenesis has not been thoroughly investigated. Several studies have detected hydrogen peroxide synthesis from various carbohydrates by S. sanguis, S. mitis, S. gordonii, and S. oralis but only at one or two carbohydrate concentrations (2, 5, 12, 29). Germaine and Tellefson investigated the influence of a wide range of glucose concentrations on peroxidogenesis, but their data primarily reflect the extent of peroxide accumulation rather than initial rates of peroxide synthesis (13).
Since hydrogen peroxide generated by streptococci may play a prominent role in the prevention of oral disease, it is essential to determine how the widely fluctuating conditions in the oral cavity alter peroxidogenesis. This study examines the influence of a broad range of environmental conditions on initial rates of hydrogen peroxide synthesis by S. gordonii (formerly S. sanguis genotype I). Initial rates of peroxidogenesis were determined because this is the most accurate measure of the capacity of streptococci to inhibit competing bacteria. In the oral biofilm, hydrogen peroxide diffuses away from the streptococci as soon as it is made. Consequently, high, maximally inhibitory concentrations of hydrogen peroxide can be attained only if streptococci synthesize it at a high rate. To obtain results that best describe the situation in vivo, initial rates of peroxidogenesis were determined under conditions which emulate those experienced by streptococci in the oral cavity (37).
MATERIALS AND METHODS
Materials.
Dehydrated tryptic soy broth (TSB) and yeast extract were from Becton Dickinson Microbiology Systems, Cockeysville, Md., and Difco Laboratories, Detroit, Mich., respectively. Hydrophilic Durapore filters (type GVWP; 25-mm diameter, 0.22-μm pore size) were from Millipore Corp., Bedford, Mass.; the Bio-Rad Detergent Compatible (DC) protein assay was from Bio-Rad Laboratories, Richmond, Calif.; and GasPak anaerobic systems were from BBL Microbiology Systems. Glucose, sucrose, Pb(NO3)2, CrK(SO4)2, and HgCl2 were from Fisher Scientific, Pittsburgh, Pa. Fluorophosphoric acid and NiCl2 were from Aldrich Fine Chemicals, Milwaukee, Wis. Potassium fluoride, CuSO4, ZnSO4, and CaCl2 were from J. T. Baker Chemical Co., Phillipsburg, N.J. Bovine liver catalase (20,000 to 25,000 U/mg), l-lactic acid, d-lactic acid, l-lactic acid oxidase, chemically stabilized horseradish peroxidase (HRP), chicken egg white lysozyme (3× crystallized and dialyzed), AgNO3, CoCl2, CdCl2, MnSO4, piperazine, bis-Tris, and ABTS [2,2′-azinobis-(3-ethylbenzthiazoline)-6-sulfonic acid] were from Sigma Chemical Co., St. Louis, Mo.
Cultivation of bacteria.
S. gordonii Challis-1 (CH1) was obtained from D. Clewell (University of Michigan, Ann Arbor). Stock cultures were passaged weekly by growth, without shaking, at 37°C in 3% TSB–0.5% yeast extract (TSBY) and then stored at 4°C. For determination of the rates of peroxidogenesis, stock cultures were diluted 40-fold in 12 ml of TSBY–10 U of catalase per ml and grown at 37°C with vigorous aeration achieved by shaking at 225 to 250 rpm in a 125-ml flask. Catalase was included to prevent autotoxicity, which can occur if hydrogen peroxide accumulates in the cultures during growth (11, 28). When the cultures reached the mid-logarithmic phase of growth (optical density at 600 nm [OD600], 0.6 to 0.9), streptococci were sedimented to a pellet by centrifugation for 5 min at 33,000 × g, resuspended in fresh TSBY to an OD600 of 1.05 (109 cells/ml), and held on ice. Streptococci grown under these conditions contained 41.9 ± 6.8 μg of protein/109 cells [39.9 ± 6.5 μg of protein/(OD600 × 1 ml)] (mean ± standard error of the mean of six independent cultures).
Protein assays.
Total protein concentrations were determined by the Bio-Rad DC protein assay with the following modifications: the total sample volume was 0.2 ml, the sample was adjusted to 1% sodium dodecyl sulfate (SDS) by addition of 10% SDS to ensure complete lysis of streptococci (3), and only 0.1 ml of working reagent A′ and 0.8 ml of reagent B were added. This procedure gave a linear response for protein concentrations in the 0.5- to 10-μg/sample range with lysozyme as the standard.
Determination of rates of hydrogen peroxide formation.
For each rate measurement, a 0.2-ml aliquot of streptococcal suspension in TSBY was equilibrated to room temperature for 10 min (to avoid heat shock effects), diluted to 1.2 ml with assay solution, and washed three times by sedimentation to a pellet for 4 min at 12,000 × g, removal of the supernatant, and resuspension in 1.2 ml of assay solution. For certain experiments, streptococci were washed into 37°C assay solution by a rapid filtration technique. Durapore filters were placed on a sintered glass filtration manifold under the strongest vacuum that could be obtained with a sink trap aspirator. Filters were prewetted by filtration of 1 ml of assay solution, and then an aliquot of cell suspension (0.3 to 0.75 ml, depending on the cell density) was filtered dropwise, leaving the bacteria on the filter. The cells were then washed dropwise with 4 ml of assay solution, the vacuum was shut off, and the cells were resuspended by gently pipetting and scraping them off the filter and into 1.4 ml of assay solution. The assay solution was prewarmed to 37°C for the prewetting, washing, and resuspension steps. Both the centrifugation and rapid filtration methods of washing streptococci reduced the catalase present in the growth medium to undetectable levels (data not shown). Unless specified otherwise, the assay solution was 25 mM KH2PO4-K2HPO4 (pH 7.0)–1 mM MgCl2, containing the concentrations of carbohydrate and other substances indicated in the figures and tables. Aliquots (0.1 ml) of washed cells were placed in prewarmed microcentrifuge tubes in a 37°C water bath to allow rapid equilibration of the temperature of the cell suspension. The cell suspension was separated into small, well-aerated aliquots at the beginning of the assay to avoid any interruption of the supply of oxygen, which is the substrate for H2O2 synthesis. After 0, 7.5, 15, and 22.5 min at 37°C, two of the aliquots were centrifuged for 2 min at 14,000 × g. A 50-μl aliquot of each supernatant was immediately assayed spectrophotometrically for hydrogen peroxide, as previously described (2), by mixing with 2 U of HRP and 0.1 μmol of ABTS in a total volume of 1 ml of NaH2PO4-Na2HPO4 (pH 6.0). The colorimetric reaction was allowed to go to completion, which required no more than 2 min, and then the A414 was read immediately versus a no-peroxide blank. Under these conditions, the assay was linearly proportional to 72 absorbance units/(mM · cm) for concentrations of up to 8 nmol of H2O2 per assay. Initial rates of peroxidogenesis were determined by linear regression analysis of the increase in hydrogen peroxide concentration over time. To adjust for variable cell losses during washing into assay solution, after each time course the cell concentration in the washed cell suspension was determined by measuring the OD600. The OD600 was corrected for any absorbance contributed by inhibitors such as CoCl2 and CuSO4. Based on microscopic enumeration in a Petroff-Hausser chamber, an OD600 of 1 corresponded to 9.53 × 108 cells/ml. All rates reported are means ± standard deviations (SD) of at least three independent determinations.
Determination of rates of lactic acid production.
The assay for lactic acid synthesis was identical to the assay for hydrogen peroxide synthesis except for the addition of one step. After measurement of the hydrogen peroxide concentration at each time point, lactic acid was quantitated spectrophotometrically by conversion to hydrogen peroxide upon addition of 0.33 units of l-lactic acid oxidase to each HRP-ABTS assay cuvette. The lactic acid oxidase reaction was allowed to proceed for 15 min at room temperature before readings were taken. As described above, after each time course, data were adjusted based on the cell density, which was quantitated by determination of the OD600, and initial rates of lactic acid production were deduced by linear regression analysis of the increase in lactic acid concentration over time.
Statistical analysis.
All P values were determined by unpaired two-tailed t tests performed with the StatView 512+ Statistical Analysis Package (version 1.1) from Brain Power, Inc., Calabasas, Calif. This software package was also used for all linear regression analyses.
RESULTS
Influence of carbohydrate concentration on rates of hydrogen peroxide and lactic acid synthesis.
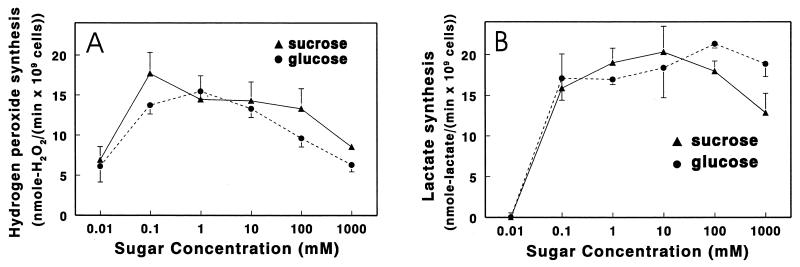
S. gordonii produced hydrogen peroxide at a maximal rate in 0.1 mM sucrose, as shown in Fig. 1A. The maximal rate of peroxidogenesis obtained with glucose was similar, but 10-fold more sugar was required (Fig. 1A). Decreases in rates of peroxidogenesis were observed at lower glucose and sucrose concentrations, presumably due to failure to keep the glycolytic and peroxidogenic enzymes saturated with substrate. Rates of lactic acid synthesis also declined at carbohydrate concentrations below 0.1 mM, and no acidogenesis was detected in 10 μM glucose or 10 μM sucrose (Fig. 1B). In contrast, peroxidogenesis in 10 μM glucose or 10 μM sucrose proceeded at over one-third of the maximal rates (Fig. 1A). Moreover, in the complete absence of extraneous glucose, S. gordonii produced 1.1 ± 0.3 nmol of H2O2/(min · 109 cells) (mean ± SD of three determinations), apparently by consuming stored intracellular polysaccharide (23).
FIG. 1.
Initial rates of hydrogen peroxide and l-lactic acid formation by S. gordonii CH1 in selected concentrations of glucose or sucrose. Cultures were grown to mid-logarithmic phase in TSBY–10 U of catalase per ml with vigorous shaking in air to obtain highly peroxidogenic cells. Cells were washed by repeated centrifugation and resuspension in 25 mM KH2PO4-K2HPO4 (pH 7.0)–1 mM MgCl2 containing the indicated concentration of glucose or sucrose. The accumulation of hydrogen peroxide or l-lactic acid was measured at 37°C over time to determine initial rates of hydrogen peroxide formation (A) and l-lactic acid formation (B). In assay buffer which contained no carbohydrate, the rate of peroxidogenesis was 1.1 ± 0.3 nmol/(min · 109 cells). Results are means ± SD of three rate determinations at each concentration point.
Peroxidogenesis also decreased at high concentrations of carbohydrate, especially at 100 mM and above (Fig. 1A). In the case of glucose, this decrease in peroxidogenesis did not seem to be due to a general decline in streptococcal metabolism, since rates of lactic acid synthesis did not decrease under the same conditions (Fig. 1B). In fact, in 100 mM versus 1 mM glucose, the rate of lactic acid formation increased by 26% ± 3% (P = 0.0007), suggesting that streptococcal metabolism shifted to produce more lactic acid and less hydrogen peroxide. Consistent with this conclusion, the glycolytic pathway in peroxidogenic streptococci branches to yield either hydrogen peroxide or lactic acid as the end product (5). To rule out the possibility that the observed decreases in peroxidogenesis were due to direct decomposition of hydrogen peroxide by carbohydrate, 1 M glucose and 1 M sucrose were incubated at 37°C with concentrations of hydrogen peroxide similar to those generated by the streptococci during the assay. These controls indicated that hydrogen peroxide was completely stable at the highest sugar concentrations tested (data not shown).
To determine if hydrogen peroxide is derived from glucose and sucrose by the same pathway, S. gordonii was incubated with a mixture of 1 mM glucose and 0.1 mM sucrose, the carbohydrate concentrations which gave optimal peroxidogenesis individually (Fig. 1A). The rate of peroxidogenesis with both sugars was not significantly different from the rate obtained with 1 mM glucose or 0.1 mM sucrose alone (P > 0.05) (data not shown), indicating that glucose and sucrose are converted to hydrogen peroxide via the same pathway. For this and all subsequent experiments in this study, glucose was used at the optimal concentration of 1 mM (Fig. 1A) to ensure that stimulatory and inhibitory effects were not exaggerated by use of a compromised system.
Influence of minerals and ionic strength on peroxidogenesis by S. gordonii.
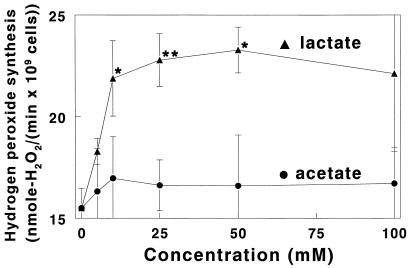
During consumption of food, the ionic strength and mineral content of saliva can change dramatically. As shown in Fig. 2, when S. gordonii was incubated with 5 to 100 mM potassium acetate, hydrogen peroxide production was virtually unchanged. Essentially identical results were obtained in the presence 5 to 100 mM KCl (Table 1). Likewise, neither inclusion of 100 mM NaCl in the 25 mM potassium phosphate assay buffer nor replacement of all or half of the potassium phosphate with sodium phosphate caused any significant alteration of hydrogen peroxide synthesis (Table 1). In phosphate-free assay buffer with chloride as the only anion (bis-Tris–HCl buffer), peroxidogenesis decreased by 10% ± 4% (P = 0.012). However, no statistically significant change in peroxidogenesis occurred when chloride and phosphate were replaced by nitrate (Table 1).
FIG. 2.
Initial rates of hydrogen peroxide production by S. gordonii CH1 in 1 mM glucose and selected concentrations of potassium l-lactate and potassium acetate. Cells were grown and initial rates of hydrogen peroxide synthesis were measured as described in Materials and Methods and in the legend to Fig. 1. Results are means ± SD of three rate determinations at each concentration point, and asterisks denote the level of statistical significance between data for equal concentrations of potassium l-lactate and potassium acetate (∗, P < 0.05; ∗∗, P < 0.005).
TABLE 1.
Influence of selected ions on peroxidogenesis
| Assay
conditions
|
H2O2 synthesisa [nmol/(min · 109 cells)] | |||
|---|---|---|---|---|
| Bufferb | Mg2+ (mM) | Additive | Concn of additive (mM) | |
| KP | 1 | 15.5 ± 1.0 | ||
| KP | 1 | KCl | 5 | 15.8 ± 0.2 (102 ± 1) |
| KP | 1 | KCl | 10 | 16.8 ± 2.5 (108 ± 16) |
| KP | 1 | KCl | 25 | 17.8 ± 0.9 (115 ± 6) |
| KP | 1 | KCl | 50 | 14.3 ± 1.3 (92 ± 8) |
| KP | 1 | KCl | 100 | 16.3 ± 0.9 (105 ± 6) |
| KP | 1 | NaCl | 100 | 15.6 ± 0.1 (101 ± 1) |
| KP:NaP (1:1) | 1 | 16.3 ± 1.4 (105 ± 9) | ||
| NaP | 1 | 14.2 ± 0.5 (92 ± 3) | ||
| KP | 0 | 10.3 ± 2.3 (66 ± 15) | ||
| KP | 0.5 | 17.8 ± 0.8 (115 ± 5) | ||
| KP | 5 | 17.2 ± 0.3 (111 ± 2) | ||
| KP | 10 | 18.2 ± 1.1 (117 ± 7) | ||
| KP | 50 | 17.5 ± 1.6 (113 ± 10) | ||
| KP | 1 | potassium citrate | 25 | 11.8 ± 2.4 (76 ± 15) |
| KP | 1 | potassium D-lactate | 50 | 16.8 ± 1.8 (108 ± 12) |
| BT-P | 1 | KCl | 25 | 14.8 ± 0.1 |
| BT-Cl | 1 | KCl | 25 | 13.3 ± 0.6 (90 ± 4) |
| BT-NO3 | 1 | KNO3 | 25 | 13.7 ± 0.8 (93 ± 5) |
Initial rates of hydrogen peroxide formation by S. gordonii were determined as described in Materials and Methods. The glucose concentration for all determinations was 1 mM, and results are means ± SD of at least three independent determinations under each condition. Values in parentheses are percentages of activity relative to the appropriate control.
Buffer abbreviations are as follows: KP, 25 mM KH2PO4-K2HPO4 (pH 7.0); NaP, 25 mM NaH2PO4-Na2HPO4 (pH 7.0); BT-P, 25 mM bis-Tris–H3PO4 (pH 6.5); BT-Cl, 25 mM bis-Tris–HCl (pH 6.5); BT-NO3, 25 mM bis-Tris–HNO3 (pH 6.5).
Although the rate of peroxidogenesis by S. gordonii was relatively insensitive to changes in the Mg2+ concentration, complete removal of Mg2+ caused a 34% ± 15% decrease in peroxidogenesis (P = 0.023) (Table 1). Potassium citrate, a Mg2+ chelator, inhibited peroxidogenesis by 24% ± 15% (P = 0.039) at a concentration of 25 mM (Table 1).
Metal ions other than Mg2+ had diverse effects on streptococcal hydrogen peroxide formation, ranging from potent inhibition by Ag+ and Hg2+ to no effect by 10 mM Mn2+ (Table 2). Every agent listed in Table 2 had an inhibitory or neutral effect on peroxidogenesis except for 10 mM Ca2+, which caused a 17% ± 6% stimulation (P = 0.024). These experiments were performed in 25 mM bis-Tris buffer (pH 6.5)–1 mM MgCl2–1 mM glucose rather than phosphate buffer since many of the metals tested form insoluble salts with phosphate. As noted in Table 2, footnote c, in some cases it was also necessary to replace Cl− with NO3− ions to prevent the formation of precipitates. As shown by the last three entries of Table 1, this anion substitution had little effect on rates of peroxidogenesis. Moreover, levels of inhibition were calculated relative to controls with the same buffer composition but without the inhibitory metal ions.
TABLE 2.
Inhibition of peroxidogenesis by heavy metals and other agents
| Inhibitora | IC50b |
|---|---|
| Ag+ | 446 ± 36 nMc |
| Hg2+ | 568 ± 15 nM |
| Zn2+ | 185 ± 7 μM |
| Cd2+ | 452 ± 155 μM |
| Cr3+ | 515 ± 182 μM |
| Pb2+ | 890 ± 157 μMc |
| Cu2+ | 2.2 ± 1.1 mM |
| Co2+ | 5.4 ± 0.9 mM |
| Ni2+ | 5.9 ± 1.5 mM |
| F− | 9.8 ± 1.5 mM |
| FHPO3− | >10 mM |
| Mn2+ | >10 mM |
| Ca2+ | >10 mM |
In every case in which inhibition was observed, controls were performed to confirm that the agents were inhibiting streptococcal peroxidogenesis rather than directly decomposing H2O2 or inhibiting the enzymatic assay for detection of H2O2. Agents which failed these tests were excluded from analysis.
All values reported are means ± SD of three independent determinations.
To avoid precipitation with Cl−, the IC50 for this metal was determined in a medium in which NO3− was substituted for Cl−.
Alteration of S. gordonii peroxidogenesis by potassium lactate.
Many oral bacteria synthesize large quantities of l-lactic acid and release it into the environment. In the presence of 1 mM glucose, 10 to 50 mM potassium l-lactate caused a statistically significant, 29 to 40%, increase in peroxidogenesis by S. gordonii (Fig. 2). To determine if potassium l-lactate stimulated peroxidogenesis or was metabolized to generate additional hydrogen peroxide, peroxide formation was measured in glucose-free assay buffer containing 100 mM potassium l-lactate. The rate obtained was 1.75 ± 0.13 nmol of H2O2/(min · 109 cells), slightly more than the rate of 1.1 ± 0.3 nmol of H2O2/(min · 109 cells) obtained in medium devoid of both glucose and potassium l-lactate. The difference between these rates [0.65 nmol of H2O2/(min · 109 cells)] could be due to conversion of 100 mM potassium l-lactate into hydrogen peroxide or to accelerated conversion of intracellular polysaccharide into hydrogen peroxide. The latter explanation, stimulation of peroxidogenesis by l-lactate, is more likely, since 0.65 nmol of H2O2/(min · 109 cells) is 8.3-fold too slow to account for the 5.42-nmol of H2O2/(min · 109 cells) rate increase that occurred when 100 mM potassium l-lactate was mixed with 1 mM glucose (Fig. 2). As a control, potassium acetate was used instead of potassium l-lactate and was found to have no measurable effect on streptococcal peroxidogenesis (Fig. 2). The stimulation of peroxidogenesis by potassium lactate was also stereospecific; 50 mM potassium d-lactate did not cause any statistically significant increase in the rate of peroxidogenesis (Table 1).
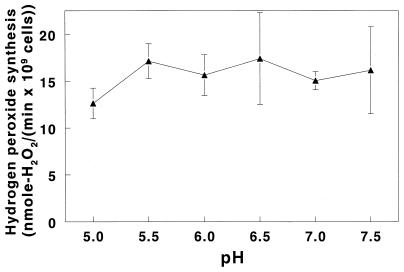
Influence of pH on hydrogen peroxide formation by S. gordonii.
Another fluctuating property of the oral environment is pH. Carbohydrate fermentation by oral bacteria yields lactic acid which can depress plaque pH to a value of 5.0, from which the pH slowly returns to neutrality (10). Initial rates of hydrogen peroxide formation were measured in a 20 mM bis-Tris–20 mM piperazine–25 mM KH2PO4–1 mM MgCl2–1 mM glucose solution designed to provide stable buffering at each pH selected. The rate of hydrogen peroxide synthesis in this assay buffer at pH 7.0 (Fig. 3) was essentially identical to that obtained without bis-Tris and piperazine (Fig. 1A), indicating that these buffers did not interfere with either the synthesis or detection of hydrogen peroxide. As seen in Fig. 3, peroxidogenesis did not undergo any statistically significant changes over the pH range tested except for a 26% ± 10% decrease between pH 5.5 and 5.0 (P = 0.035).
FIG. 3.
Influence of pH on initial rates of hydrogen peroxide formation by S. gordonii CH1. Rates of hydrogen peroxide synthesis were measured as described in Materials and Methods and in the legend to Fig. 1, except that the assay solution was supplemented with 20 mM piperazine and 20 mM bis-Tris buffer. The glucose concentration for all determinations was 1 mM. Results are means ± SD of three rate determinations at each pH.
Effect of growth conditions on streptococcal metabolism.
The experiments described above explored the responses of streptococci to rapid fluctuations in their environment, but streptococci must also adapt to gradual changes, such as prolonged periods of reduced oxygen tension. To study adaptation to microaerophilic conditions, S. gordonii was grown without shaking in 10 ml of TSBY in a 15- by 100-mm culture tube and then washed into fully aerated assay buffer containing 1 mM glucose. These statically grown cells synthesized hydrogen peroxide at a 36% ± 5% lower rate than cells which were aerated by shaking during growth (Table 3) (P = 0.0016). Streptococci that were grown anaerobically with shaking and then assayed with fully aerated buffer containing 1 mM glucose produced hydrogen peroxide 65% ± 3% more slowly than shaken, aerobically grown cells (Table 3) (P < 0.0001). The cells grown without oxygen were shaken to confirm that anaerobiosis rather than lack of agitation caused the decrease in peroxidogenesis. For this experiment, care was taken to remove oxygen from the medium by shaking it overnight in the anaerobic chamber before it was inoculated with bacteria.
TABLE 3.
Influence of growth conditions on streptococcal metabolism
| Growth condition | Synthesis [nmol/(min · 109
cells)] ofa:
|
|
|---|---|---|
| H2O2 | Lactic acid | |
| Shaken in air | 15.5 ± 1.0 | 17.0 ± 0.6 |
| Static in air | 9.9 ± 0.8 | 30.5 ± 1.8 |
| Shaken −O2b | 5.4 ± 0.4 | 32.6 ± 2.9 |
After growth to mid-logarithmic phase under the indicated conditions, initial rates of hydrogen peroxide and lactic acid formation by S. gordonii CH1 were determined with fully aerated assay buffer, as described in Materials and Methods and in the legend to Fig. 1. The glucose concentration for all determinations was 1 mM, and results are means ± SD of three rate determinations under each growth condition.
Culture medium was deoxygenated by shaking overnight at 37°C in GasPak anaerobic canisters; the medium was then inoculated with bacteria under a stream of nitrogen gas, and the bacteria were grown in canisters containing fresh H2 and CO2 generator envelopes.
Although statically and anaerobically grown cells synthesized hydrogen peroxide more slowly, they synthesized lactic acid much more rapidly (Table 3). This demonstrates that microaerophilic and anaerobic conditions did not cause a general suppression of streptococcal metabolism. Instead, metabolism was shifted away from hydrogen peroxide formation and into lactic acid formation. It should be noted that after growth under each condition (aerobic, microaerophilic, and anaerobic), streptococci were assayed for hydrogen peroxide and lactic acid synthesis with air-saturated buffers. Thus, the changes in lactic acid and hydrogen peroxide production were not due to the absence of oxygen in the assay buffer but rather reflected a fundamental shift in metabolism, such as down-regulation of the peroxidogenic enzymes in response to anaerobic growth conditions.
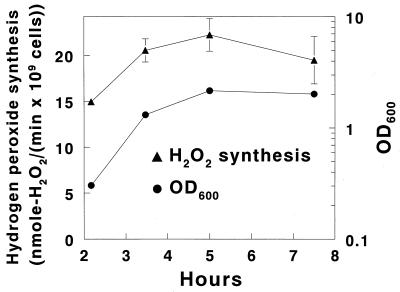
Peroxidogenesis during the phases of growth.
The capacity of S. gordonii to synthesize hydrogen peroxide increased during growth, reaching a peak rate in late-logarithmic phase that was 49% ± 12% higher than the rate attained by cells in early-logarithmic phase (Fig. 4).
FIG. 4.
Initial rates of hydrogen peroxide production by S. gordonii CH1 harvested at various stages of growth. Streptococci were grown and rates of hydrogen peroxide synthesis were measured as described in Materials and Methods and in the legend to Fig. 1, except that a rapid filtration wash technique was used and cells were harvested at the early-logarithmic, mid-logarithmic, late-logarithmic, and stationary phases of growth. Turbidity of the cultures at time zero, as determined by measuring the OD600, was 0.043 ± 0.006. The glucose concentration for all determinations of hydrogen peroxide synthesis was 1 mM, and results are means ± SD of three rate determinations at each stage of growth. For each data point, error bars are shown only if they fall outside the plot symbol.
DISCUSSION
We have examined how certain conditions which emulate those in the oral environment influence hydrogen peroxide synthesis by S. gordonii. Maximal peroxidogenesis is desirable since it seems to be one of the factors in the oral cavity that suppresses the growth of cohabiting cariogenic and periodontal pathogens (16, 17, 35). Numerous clinical studies have shown that the presence of peroxidogenic streptococci is correlated with the absence of tooth decay and periodontal disease (1, 8, 9, 16, 25, 32, 33). These studies have been corroborated by in vitro studies of the sensitivity of cariogenic and putative periodontal pathogens to streptococcal hydrogen peroxide or to hypothiocyanite, which salivary peroxidase generates with streptococcal hydrogen peroxide (7, 16–18, 24, 35). Since controls in several of these studies indicate that bacterial antagonism is due to hydrogen peroxide rather than to a streptococcal bacteriocin (16, 17, 35), it is logical to conclude that streptococcal peroxidogenesis contributes to oral health.
Although dental plaque is a complex system, a consideration of the general ecological succession which takes place in plaque suggests two ways in which streptococcal hydrogen peroxide could contribute to oral health. Peroxidogenic streptococci are among the first species to colonize freshly cleaned tooth surfaces (25). As plaque matures, it thickens and becomes able to support the growth of a much larger set of species, including species which cause caries and periodontal disease (38). Heavy early colonization by peroxidogenic streptococci would create a plaque with a high potential to produce hydrogen peroxide; this might slow the ecological succession, delaying or preventing the proliferation of pathogens in dental plaque.
A complicating factor in this scenario is that, as plaque thickens, the deeper layers tend to become anaerobic. Oxygen is required for streptococcal hydrogen peroxide synthesis, so peroxidogenic streptococci may have to proliferate and invade the upper, aerobic layers of dental plaque in order to inhibit the growth of oral pathogens. Another possibility is that both of the mechanisms described here are required to explain the clinical observations that peroxidogenic streptococci are associated with “healthy plaque.”
This study has examined peroxidogenesis by S. gordonii, which is only one of several streptococcal species that generate hydrogen peroxide in the oral cavity; however, all of these species seem to use the enzymes NADH oxidase and pyruvate oxidase to synthesize hydrogen peroxide (4, 15, 31, 39). Thus, the responses of S. gordonii that are described here may be parallel or identical to those of the other peroxidogenic streptococci that reside in the oral cavity.
To obtain the most relevant measurement of the capacity of streptococcal hydrogen peroxide to inhibit competing bacteria, all determinations of peroxidogenesis reported here were initial rates of formation rather than final endpoints of production. When streptococci release hydrogen peroxide into the oral biofilm, there is no barrier to prevent it from diffusing away. Consequently, streptococci can generate high, maximally inhibitory levels of hydrogen peroxide only by rapidly and continuously excreting it into their surroundings.
A particularly interesting finding in this regard is the stimulation of peroxidogenesis by potassium lactate (Fig. 2). Many bacterial species which inhabit oral biofilms, such as the cariogenic pathogen S. mutans, ferment carbohydrate and release large quantities of lactic acid. This acid causes decreases in plaque pH which lead to demineralization of tooth enamel and dental caries (38). Studies of lactic acid production in human dental plaque have shown that shortly after consumption of fruit, bread, or a 0.5 to 10% sucrose solution, the concentration of lactic acid jumps from less than 6 mM to between 8.6 and 56.1 mM (20–22, 26). Remarkably, the concentrations of lactic acid attained shortly after eating are almost identical to the potassium lactate concentrations that optimally stimulate S. gordonii hydrogen peroxide synthesis in vitro (Fig. 2). The stimulation of peroxidogenesis by potassium lactate suggests that S. gordonii uses lactic acid as an environmental cue. In the oral biofilm, a sudden increase in the lactic acid concentration indicates that fermentable carbohydrate is available and that bacteria are consuming it. In response to this signal, S. gordonii accelerates its peroxide synthesis (Fig. 2), presumably in an attempt to inhibit the metabolism of competing bacteria and gain preferential access to nutrients in the oral cavity.
The decline in peroxidogenesis observed at high glucose and sucrose concentrations (Fig. 1A) may also reflect an optimized strategy for suppressing competing bacteria. When carbohydrate is abundant, competition is less important; thus, streptococci may limit synthesis of hydrogen peroxide in order to devote their metabolism to anabolic processes.
Peroxidogenesis was also found to decline at very low sugar concentrations, but conversion of carbohydrate to hydrogen peroxide was still extremely efficient. In 50 μM glucose, the concentration found in unstimulated, fasting saliva (37), hydrogen peroxide synthesis by S. gordonii was 64% of the rate attained under optimal conditions of 1 mM glucose (Fig. 1A). Although peroxidogenesis is slower in these low, fasting-state glucose concentrations, most of the hydrogen peroxide synthesized in the oral cavity may be generated under these conditions since humans usually spend more time fasting than feeding. S. gordonii was able to generate hydrogen peroxide even when no glucose or sucrose was available (see the legend to Fig. 1), apparently by consuming intracellular stores of polysaccharide. This observation is consistent with results obtained by Minah and Loesche which show that the peroxidogenic species S. sanguis and S. mitis convert up to 14% of the carbohydrate they consume into intracellular polysaccharide (23).
A remarkable feature of streptococcal metabolism, which again seems to pertain to an optimized strategy for inhibition of competing bacteria, is the differential synthesis of hydrogen peroxide versus lactic acid in 10 μM glucose or 10 μM sucrose. The observations presented in Fig. 1 suggest that when fermentable carbohydrate is scarce and competition for it most intense, peroxidogenic streptococci change their metabolism. They stop producing approximately equal amounts of hydrogen peroxide and lactic acid and start producing only hydrogen peroxide, which may maximize their ability to inhibit competing bacteria (Fig. 1).
The influence of anaerobic growth conditions on S. gordonii reinforces the hypothesis that hydrogen peroxide is a critical antagonist of competing bacteria. Oxygen is the substrate streptococci reduce to form H2O2. However, when S. gordonii was grown in the complete absence of oxygen, it retained 35% of its capacity to synthesize hydrogen peroxide (Table 3). This observation is consistent with reports that, during anaerobic growth, streptococci have low, constitutive levels of the peroxidogenic enzymes NADH oxidase and pyruvate oxidase (4, 14). Maintenance of the capacity to synthesize hydrogen peroxide even when no oxygen is available to support that synthesis suggests that hydrogen peroxide is an extremely valuable antagonist of competing bacteria, so valuable that peroxidogenic streptococci are always prepared to make it in case oxygen suddenly becomes available.
The increase in peroxidogenic capacity during growth (Fig. 4) provides one more indication that peroxidogenic streptococci, like S. gordonii, rely on hydrogen peroxide to antagonize competing bacteria. Actively growing cells have the greatest need to suppress competing bacteria because they have highest requirement for carbohydrate and other nutrients. Consistent with this premise, the longer S. gordonii grew at a logarithmic rate, the faster it produced hydrogen peroxide (Fig. 4).
Since many cariogenic and periodontal pathogens are sensitive to the levels of hydrogen peroxide released by streptococci (16–18, 24, 35), streptococcal peroxidogenesis may represent an innate defense system that helps protect hard and soft oral tissues from infectious diseases. Consequently, it is worthwhile to discover any inhibitors of peroxidogenesis that may appear in food or water and compromise the protection afforded by this system. Hydrogen peroxide synthesis decreased upon removal of Mg2+ (Table 1), but this is a nonphysiological state since Mg2+ is normally present in saliva (37). However, consumption of foods and beverages containing citric acid may induce periods of starvation for Mg2+, since 25 mM potassium citrate inhibited peroxidogenesis in vitro (Table 1), presumably by chelating Mg2+.
Most heavy metal ions inhibited hydrogen peroxide synthesis by S. gordonii, with Ag+ and Hg2+ acting at the lowest concentrations (Table 2). While it is tempting to speculate that these inhibitors poison peroxidogenesis and make the oral environment more hospitable for pathogens, mercury released from dental amalgam has an antibacterial effect that may confer protection against oral disease despite its effect on peroxidogenesis (19). A similar conclusion can be reached regarding fluoride, a widely used anticariogenic agent which inhibits acid production by S. mutans (36). Fluoride also inhibited hydrogen peroxide synthesis, but the 50% inhibitory concentration (IC50) for peroxidogenesis by S. gordonii (Table 2) was found to be nearly identical to the IC50 for acidogenesis by S. mutans (36). Thus, although fluoride can compromise the protection afforded by streptococcal hydrogen peroxide, its direct effect on S. mutans seems to be sufficient to protect teeth from decay.
Peroxidogenesis was also adversely affected by low pH, decreasing by 26% between pH 5.5 and 5.0 (Fig. 3). On the surface, this would appear to compromise the ability of peroxidogenic streptococci, such as S. gordonii, to antagonize the colonization and growth of oral pathogens; however, virtually all the hydrogen peroxide synthesized in the oral cavity is used by salivary peroxidase to convert salivary thiocyanate ion (SCN−) to the antimicrobial oxidizing agent hypothiocyanate ion (OSCN−) (27). This peroxidase system has a compensatory effect as acidogenesis of plaque drives pH values below pH 5.5. Hypothiocyanite ion is protonated below pH 5.3, neutralizing its charge and increasing its ability to diffuse into and damage bacteria (34). Thus, at pH 5.0, enhanced permeability of hypothiocyanite may more than counteract decreased streptococcal peroxidogenesis so as to maintain a uniform antimicrobial effect with decreasing plaque pH. It should be emphasized that peroxidogenic streptococci have a hypothiocyanite reductase which protects them from the hypothiocyanite generated from their hydrogen peroxide (5, 6).
This study has not resolved the question of how potassium l-lactate stimulates peroxidogenesis. One possibility is that elevated concentrations of l-lactate enter the streptococci to cause product inhibition of l-lactate dehydrogenase (LDH). This would decrease the amount of pyruvate being reduced to lactate, increasing the amount of pyruvate oxidized by the H2O2-forming enzyme pyruvate oxidase. A similar mechanism would explain why peroxidogenesis continues while lactic acid synthesis ceases in 10 μM glucose (Fig. 1). In this case, inhibition of LDH would most likely occur due to decreases in the levels of glycolytic intermediates and, in particular, fructose-1,6-bis-phosphate, which is an allosteric activator of LDH (30).
ACKNOWLEDGMENT
This project was supported by Public Health Service grant R01-DE05696 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Ali R W, Johannessen A C, Dahlen G, Socransky S S, Skaug N. Comparison of the subgingival microbiota of periodontally healthy and diseased adults in northern Cameroon. J Clin Periodontol. 1997;24:830–835. doi: 10.1111/j.1600-051x.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 2.Barnard J P, Stinson M W. The alpha-hemolysin of Streptococcus gordoniiis hydrogen peroxide. Infect Immun. 1996;64:3853–3857. doi: 10.1128/iai.64.9.3853-3857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaduri S, Demchick P H. Simple and rapid method for disruption of bacteria for protein studies. Appl Environ Microbiol. 1983;46:941–943. doi: 10.1128/aem.46.4.941-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson J, Edlund M B. Pyruvate oxidase in Streptococcus sanguisunder various growth conditions. Oral Microbiol Immunol. 1987;2:10–14. doi: 10.1111/j.1399-302x.1987.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson J, Iwami Y, Yamada T. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun. 1983;40:70–80. doi: 10.1128/iai.40.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtois P H, Pourtois M. Purification of NADH: hypothiocyanite oxidoreductase in Streptococcus sanguis. Biochem Mol Med. 1996;57:134–138. doi: 10.1006/bmme.1996.0019. [DOI] [PubMed] [Google Scholar]
- 7.Courtois P, Majerus P, Labbe M, Vanden Abbeele A, Yourassowsky E, Pourtois M. Susceptibility of anaerobic microorganisms to hypothiocyanite produced by lactoperoxidase. Acta Stomatol Belg. 1992;89:155–162. [PubMed] [Google Scholar]
- 8.De Stoppelaar J D, Van Houte J, Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3:190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- 9.De Stoppelaar J D, Van Houte J, Backer Dirks O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguisand iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4:114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- 10.Edgar M, Higham S M. Saliva and the control of plaque pH. In: Edgar W M, O'Mullane D M, editors. Saliva and oral health. London, England: British Dental Association; 1996. pp. 81–94. [Google Scholar]
- 11.Eisenberg R J. Induction of unbalanced growth and death of Streptococcus sanguisby oxygen. J Bacteriol. 1973;116:183–191. doi: 10.1128/jb.116.1.183-191.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Mendoza A, Liebana J, Castillo A M, de la Higuera A, Piedrola G. Evaluation of the capacity of oral streptococci to produce hydrogen peroxide. J Med Microbiol. 1993;39:434–439. doi: 10.1099/00222615-39-6-434. [DOI] [PubMed] [Google Scholar]
- 13.Germaine G R, Tellefson L M. Glucose uptake by Streptococcus mutans, Streptococcus mitis, and Actinomyces viscosusin the presence of human saliva. Infect Immun. 1982;38:1060–1067. doi: 10.1128/iai.38.3.1060-1067.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higuchi M. The effect of oxygen on the growth and mannitol fermentation of Streptococcus mutans. J Gen Microbiol. 1984;130:1819–1826. doi: 10.1099/00221287-130-7-1819. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi M, Shimada M, Yamamoto Y, Hayashi T, Koga T, Kamio Y. Identification of two distinct NADH oxidases corresponding to H2O2-forming oxidase and H2O-forming oxidase induced in Streptococcus mutans. J Gen Microbiol. 1993;139:2343–2351. doi: 10.1099/00221287-139-10-2343. [DOI] [PubMed] [Google Scholar]
- 16.Hillman J D, Socransky S S, Shivers M. The relationships between streptococcal species and periodontopathic bacteria in human dental plaque. Arch Oral Biol. 1985;30:791–795. doi: 10.1016/0003-9969(85)90133-5. [DOI] [PubMed] [Google Scholar]
- 17.Holmberg K, Hallander H O. Production of bactericidal concentrations of hydrogen peroxide by Streptococcus sanguis. Arch Oral Biol. 1973;18:423–434. doi: 10.1016/0003-9969(73)90167-2. [DOI] [PubMed] [Google Scholar]
- 18.LeBien T W, Bromel M C. Antibacterial properties of a peroxidogenic strain of Streptococcus mitior (mitis) Can J Microbiol. 1975;21:101–103. doi: 10.1139/m75-015. [DOI] [PubMed] [Google Scholar]
- 19.Lyttle H A, Bowden G H. The level of mercury in human dental plaque and interaction in vitro between biofilms of Streptococcus mutansand dental amalgam. J Dent Res. 1993;72:1320–1324. doi: 10.1177/00220345930720091101. [DOI] [PubMed] [Google Scholar]
- 20.Margolis H C, Moreno E C. Composition of pooled plaque fluid from caries-free and caries-positive individuals following sucrose exposure. J Dent Res. 1992;71:1776–1784. doi: 10.1177/00220345920710110301. [DOI] [PubMed] [Google Scholar]
- 21.Margolis H C, Zhang Y P, Gewirtz A, Van Houte J, Moreno E C. Cariogenic potential of pooled plaque fluid from exposed root surfaces in humans. Arch Oral Biol. 1993;38:131–138. doi: 10.1016/0003-9969(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 22.Margolis H C, Zhang Y P, Van Houte J, Moreno E C. Effect of sucrose concentration on the cariogenic potential of pooled plaque fluid from caries-free and caries-positive individuals. Caries Res. 1993;27:467–473. doi: 10.1159/000261582. [DOI] [PubMed] [Google Scholar]
- 23.Minah G E, Loesche W J. Sucrose metabolism by prominent members of the flora isolated from cariogenic and non-cariogenic dental plaques. Infect Immun. 1977;17:55–61. doi: 10.1128/iai.17.1.55-61.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyasaki K T, Wilson M E, Zambon J J, Genco R J. Influence of endogenous catalase activity on the sensitivity of the oral bacterium Actinobacillus actinomycetemcomitansand the oral haemophili to the bactericidal properties of hydrogen peroxide. Arch Oral Biol. 1985;30:843–848. doi: 10.1016/0003-9969(85)90141-4. [DOI] [PubMed] [Google Scholar]
- 25.Nyvad B, Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990;24:267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- 26.Pollard M A, Higham S M, Curzon M E, Edgar W M. Acid anion profiles in dental plaque following consumption of cereal-based foods and fruits. Eur J Oral Sci. 1996;104:535–539. doi: 10.1111/j.1600-0722.1996.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 27.Pruitt K M, Mansson-Rahemtulla B, Baldone D C, Rahemtulla F. Steady-state kinetics of thiocyanate oxidation catalyzed by human salivary peroxidase. Biochemistry. 1988;27:240–245. doi: 10.1021/bi00401a036. [DOI] [PubMed] [Google Scholar]
- 28.Rosan B, Eisenberg R J. Morphological changes in Streptococcus sanguisassociated with growth in the presence of oxygen. Arch Oral Biol. 1973;18:1441–1444. doi: 10.1016/0003-9969(73)90118-0. [DOI] [PubMed] [Google Scholar]
- 29.Ryan C S, Kleinberg I. Bacteria in human mouths involved in the production and utilization of hydrogen peroxide. Arch Oral Biol. 1995;40:753–763. doi: 10.1016/0003-9969(95)00029-o. [DOI] [PubMed] [Google Scholar]
- 30.Sommer P, Klein J P, Scholler M, Frank R M. Lactate dehydrogenase from Streptococcus mutans: purification, characterization, and crossed antigenicity with lactate dehydrogenases from Lactobacillus casei, Actinomyces viscosus, and Streptococcus sanguis. Infect Immun. 1985;47:489–495. doi: 10.1128/iai.47.2.489-495.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spellerberg B, Cundell D R, Sandros J, Pearce B J, Idanpaan-Heikkila I, Rosenow C, Masure H R. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996;19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- 32.Svanberg M, Loesche W J. The salivary concentration of Streptococci mutans and Streptococci sanguisand their colonization of artificial tooth fissures in man. Arch Oral Biol. 1977;22:441–447. doi: 10.1016/0003-9969(77)90125-x. [DOI] [PubMed] [Google Scholar]
- 33.Tanner A, Maiden M F, Macuch P J, Murray L L, Kent R L., Jr Microbiota of health, gingivitis, and initial periodontitis. J Clin Periodontol. 1998;25:85–98. doi: 10.1111/j.1600-051x.1998.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomas E L. Products of lactoperoxidase-catalyzed oxidation of thiocyanate and halides. In: Pruitt K M, Tenovuo J O, editors. The lactoperoxidase system: chemistry and biological significance. New York, N.Y: Dekker; 1985. pp. 31–54. [Google Scholar]
- 35.van der Hoeven J S, Camp P J. Mixed continuous cultures of Streptococcus mutans with Streptococcus sanguis or with Streptococcus oralisas a model to study the ecological effects of the lactoperoxidase system. Caries Res. 1993;27:26–30. doi: 10.1159/000261511. [DOI] [PubMed] [Google Scholar]
- 36.van Loveren C, van de Plassche-Simons Y M, de Soet J J, de Graaff J, Ten Cate J M. Acidogenesis in relation to fluoride resistance of Streptococcus mutans. Oral Microbiol Immunol. 1991;6:288–291. doi: 10.1111/j.1399-302x.1991.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 37.Whelton H. The anatomy and physiology of the salivary glands. In: Edgar W M, O'Mullane D M, editors. Saliva and oral health. London, England: British Dental Association; 1996. pp. 1–8. [Google Scholar]
- 38.Wolinsky L E. Caries and cariology. In: Newman M G, Nisengard R, editors. Oral microbiology and immunology. W. B. Philadelphia, Pa: Saunders Co.; 1988. pp. 389–409. [Google Scholar]
- 39.Zitzelsberger W F, Gotz F, Schleifer K H. Distribution of superoxide dismutases, oxidases, and NADH peroxidase in various streptococci. FEMS Microbiol Lett. 1984;21:243–246. [Google Scholar]