Abstract
Aims
The aim of this study was to derive a weighted score model predicting success/failure of antegrade wire crossing in chronic total occlusion (CTO) percutaneous coronary intervention (PCI).
Methods and results
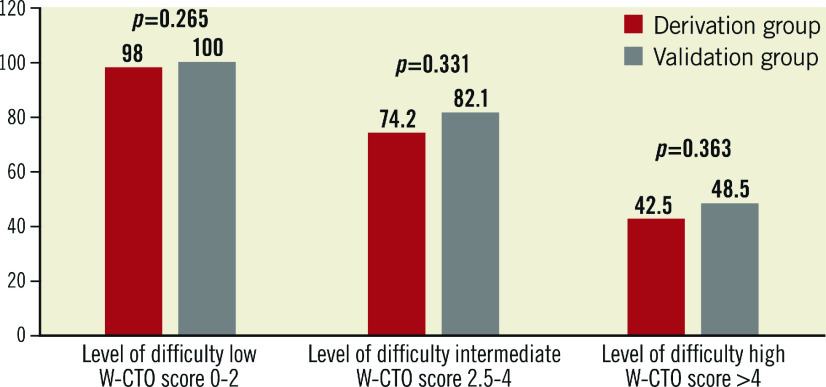
Four hundred and four consecutive CTO cases (408 lesions) undergoing CTO-PCI between January 2009 and March 2015 were included. Data were divided into two sets, namely “derivation” and “validation”, in a 70:30 ratio. The score was derived using multivariate analysis to identify independent predictors of wire crossing failure from the derivation set (n=285 lesions) and validated on the remaining 123 lesions (validation set). The overall procedural success rate was 83.6%. Independent predictors of CTO-PCI failure and their contribution to the weighted score were a blunt stump (beta coefficient 2.12), length of occlusion >20 mm (beta coefficient 1.71), presence of calcification (beta coefficient 0.72), presence of tortuosity (beta coefficient 1.06) and collateral with Rentrop grade <2 (beta coefficient 1.06). The respective scores allotted were +2.0, +1.5, +1, +1, +1 (total 6.5), rounding the coefficient to the nearest 0.5. Score values of 0-2, >2-4 and >4 were classified as low, intermediate and high levels of difficulty for CTO-PCI success and were associated with 98%, 74.2%, and 42.5% (p<0.0001), respectively, of antegrade wire crossing success in the derivation set. This was also validated on the validation set with CTO success in the three derived difficulty levels being 100%, 82.4% and 48.4%, respectively.
Conclusions
Our weighted angiographic CTO score is a strong predictor of final antegrade wire crossing success and could be used in day-to-day clinical practice of CTO interventions.
Introduction
With improving techniques and hardware for percutaneous coronary intervention (PCI) and the increasing role of PCI in the management of obstructive coronary artery disease (CAD), chronic total occlusions (CTO), being the most challenging subset, remain the last or unconquered frontier1,2,3,4. Success in CTO-PCI, in addition to operator expertise, also depends on detailed angiographic analysis of the CTO lesion5. The Japanese chronic total occlusion score (J-CTO score) identifies five independent parameters which could predict antegrade wire crossing success/failure with an equal contribution for each predictor6.
We hypothesised that all independent angiographic predictors may not have an equal contribution in predicting antegrade wire crossing success/failure and therefore a weighted score might be more accurate and useful for case selection in day-to-day practice. We therefore aimed to study the lesion characteristics of our cohort of about 400 CTO patients over the last six years and to establish a weighted score model predicting the level of difficulty of antegrade wire crossing.
Methods
Consecutive CTO-PCI cases between January 2009 and March 2015 were included in this study. The study protocol was approved by the local ethics committee, and all procedures were performed according to current international guidelines7. Baseline clinical and angiographic parameters were recorded. Lesions were defined as chronic total occlusion if the duration of occlusion was estimated to be three months or more with TIMI 0 antegrade flow. The duration of occlusion was determined from the time of onset of clinical symptoms, including timing of myocardial infarction or worsening of chest symptoms. When the duration of occlusion was uncertain and there was angiographic evidence of chronic occlusion it was labelled as of indeterminate duration.
DEFINITIONS OF ANGIOGRAPHIC PREDICTORS
Detailed specific angiographic features of the CTO lesions are described below.
1. Proximal stump/entry point: angiographic morphology of the entry point was described as “tapered” in its favourable format if the occluded segment started as a funnel-shaped ending of the leading vessel or “blunt” in its adverse format.
2. Calcification: the presence of fluoroscopic calcification on either still or moving images within the CTO segment was assigned positive for calcification.
3. Bending/tortuosity: tortuosity was defined as bending of at least >45° as assessed by angiography within the occluded segment or the vessel in close proximity.
4. Occlusion length: occlusion length was measured from the proximal end of the occlusion site to the most proximal point of retrograde filling from the ipsilateral/contralateral collateral. A simultaneous contralateral injection technique was used if distal filling was not clear on a single antegrade shot. Occlusion length was categorised into either <20 mm (favourable) or >20 mm (negative predictor) as per the consensus of the EuroCTO Club8.
5. Presence of side branch at occlusion site: a side branch was defined as being present if a branch originated within 3 mm from the proximal endpoint of the occlusion (conventionally a negative predictor for CTO wire crossing).
6. Ostial location: an ostial location was identified if the occlusion was within 5 mm of the origin of the vessel (conventionally a negative predictor for CTO wire crossing).
7. Collateral filling of the distal segment: the grade of collateral filling of the distal vessel was based on a collateral grading system of 0 to 3, according to the Rentrop and Cohen classification9. For the purpose of analysis, collateral filling grades 0-1 were combined into one category while grades 2 and 3 were combined into another category using filling of the distal vessel as the primary criterion irrespective of the source of the collateral. Finally, a good opacification of the distal vessel (grades 2 and 3) vs. poor collateral filling (grade 0, 1) was compared.
8. Antegrade trickle was defined as a faint delayed trickle beyond the proximal stump in a frame-by-frame analysis, which often equates to the presence of microchannels in the absence of bridging collaterals.
Technical success was defined as the ability to cross the occluded segment successfully with a CTO wire antegradely, and <20% residual stenosis with TIMI 3 flow. The primary endpoint was final antegrade guidewire crossing success irrespective of the time required in a single procedure. In cases of antegrade failure, and the procedure was completed by the retrograde technique either in the same or in a second sitting, the case was still considered as an antegrade failure. Cases which previously failed, but succeeded in the second antegrade attempt were on the contrary categorised as CTO success.
STATISTICAL ANALYSIS
For each angiographic adverse predictor, considering a baseline probability of success of 0.25 with its presence in a negative format and an alternative probability of success of 0.5 in its absence at 95% power, and 5% level of significance, the minimum sample size was estimated to be 190. Adding a design effect of 1.5, a total of 285 cases were required for the study.
As failure of antegrade wire crossing was the primary event, predictors of failure were identified using binary logistic regression analysis. The variables were not pre-specified and were identified using univariate analysis considering antegrade wire crossing failure as the final outcome. Such identified univariate variables were then used for multivariable logistic regression.
At the outset, the data were divided into two sets in a ratio of 70:30, the first set being the “derivation set” and the second set being the “validation set”, based on simple random sampling. A weighted score was then derived for each identified multivariate predictor in proportion to the respective beta coefficient obtained for the same, rounded off to the nearest 0.5. A level of difficulty score for a CTO procedure was then determined for each case in the derivation set by assigning points for each of these predictors present and then summing them all into a weighted CTO score (W-CTO). As a next step, the derived scores were then applied to each individual patient in the derivation data set and a hypothetical level of difficultly determined using cut-offs for probability of success of more than 90%, 50-89% and <50% and assigning them difficulty levels of low, intermediate and high, respectively. The score model thus derived was then validated using a computation of the W-CTO score for each patient in the validation set and matched for consistency using a Z-test. A p-value <0.05 was considered significant. A goodness of fit was derived using the Hosmer-Lemeshow test.
Having validated the W-CTO score, as a second step, our derived W-CTO score was additionally applied to the entire data set again and a level of difficulty for individual cases worked out using this score model. The J-CTO score was also simultaneously computed for each patient in the entire data set using the methodology described by Morino et al6.
The real-time predictability of the two scores was then compared using receiver operating characteristic (ROC) curve analysis10. All computations were carried out using SPSS, Version 22 (IBM Corp., Armonk, NY, USA).
Results
Between January 2009 and March 2015, a total of 404 consecutive patients with 408 CTO lesions underwent PCI at our centre and formed the study cohort.
BASELINE DEMOGRAPHICS AND CTO ANGIOGRAPHIC DETAILS
Table 1 shows the baseline demographics of the entire patient subset including the age/sex distribution, risk factor profile, clinical parameters and the CTO angiographic details and the success and failure in the entire patient cohort. The mean age of subjects was 57.1±9.4 years, with the majority (88.5%) male. About one third of patients were diabetic, half were hypertensive, and two fifths were smokers. About half of our patients had stable angina alone. History of previous myocardial infarction was present in 14.5%. The majority (80.9%) of cases had a relatively preserved left ventricle ejection fraction (LVEF >50%). Single-vessel disease was present in 57%, double-vessel disease in 36%, and only 7% had triple-vessel disease. One hundred and eighty-two (44.6%) of our patients had a total occlusion duration of less than one year, while 192 (47.1%) had occlusion of more than a year, and 34 (8.3%) had an indeterminate duration of occlusion.
Table 1. Clinical and angiographic lesion characteristics in the study patients.
| Variables | Total population, N=408 (%) | Derivation set, N=285 (%) | Validation set, N=123 (%) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 57.1±9.4 | 57.7±9.5 | 56.1±9.3 | 0.18* | |
| Male sex | 361 (88.5%) | 251 (88.1%) | 110 (89.4%) | 0.69 | |
| Diabetes | 131 (32.1%) | 84 (29.5%) | 47 (38.2%) | 0.08 | |
| Hypertension | 204 (50%) | 141 (49.5%) | 63 (51.2%) | 0.75 | |
| Smoking | 173 (42.1%) | 119 (41.8%) | 54 (43.9%) | 0.69 | |
| LVEF | >50% | 330 (80.9%) | 18 (6.3%) | 11 (8.9%) | 0.45 |
| 30-50% | 49 (12%) | 37 (13%) | 12 (9.8%) | ||
| <30% | 29 (7.1%) | 230 (80.7%) | 100 (81.3%) | ||
| Clinical symptom | CSA | 233 (57.1%) | 155 (54.4%) | 78 (63.4%) | 0.08 |
| ACS | 116 (28.4%) | 82 (28.8%) | 34 (27.6%) | ||
| MI | 59 (14.5%) | 48 (16.8%) | 11 (9.0%) | ||
| Lesion site | LAD | 174 (42.6%) | 114 (40%) | 60 (48.8%) | 0.26 |
| LCx | 71 (17.4%) | 52 (18.2%) | 19 (15.4%) | ||
| RCA | 163 (40%) | 119 (41.8%) | 44 (35.8%) | ||
| Duration of occlusion | 3 months to 1 year | 182 (44.6%) | 132 (46.3%) | 50 (40.7%) | 0.33 |
| >1 year | 192 (47.1%) | 133 (45.9%) | 61 (49.5%) | ||
| Indeterminate | 34 (8.3%) | 22 (7.7%) | 12 (9.7%) | ||
| Proximal cap blunt | 150 (36.8%) | 97 (34%) | 53 (43.1%) | 0.08 | |
| Side branch present | 108 (26.5%) | 71 (24.9%) | 37 (30.1%) | 0.28 | |
| Bridging collaterals present | 53 (13%) | 37 (13%) | 16 (13%) | 0.99 | |
| Length of occlusion >20 mm | 191 (46.8%) | 131 (46%) | 60 (48.8%) | 0.60 | |
| Calcification present | 131 (32.1%) | 92 (32.3%) | 39 (31.7%) | 0.91 | |
| Tortuosity present | 127 (31.1%) | 90 (31.6%) | 37 (30.1%) | 0.76 | |
| Ostial | 53 (13%) | 34 (11.9%) | 19 (15.4%) | 0.33 | |
| Antegrade trickle present (microchannels) | 148 (36.3%) | 107 (37.5%) | 41 (33.3%) | 0.42 | |
| Rentrop grade <2 | 355 (87%) | 243 (85.3%) | 112 (91.1%) | 0.11 | |
| Ipsilateral collateral present | 189 (46.3%) | 134 (47%) | 55 (44.7%) | 0.67 | |
| Contralateral collateral present | 184 (45.1%) | 124 (43.5%) | 60 (48.8%) | 0.33 | |
| In-stent lesion | 14 (3.4%) | 9 (3.2%) | 5 (4.1%) | 0.77 | |
| Previous failed attempt | 22 (5.4%) | 17 (6%) | 5 (4.1%) | 0.44 | |
| J-CTO score | 1.52±1.1 | 1.5±1.1 | 1.6±1.2 | 0.52* | |
| Total AG success | 341 (83.6%) | 235 (82.5%) | 106 (86.2%) | 0.35 | |
| *Mann-Whitney test. ACS: acute coronary syndrome; AG: antegrade; CSA: chronic stable angina; LAD: left anterior descending artery; LCx: left circumflex artery; MI: myocardial infarction; RCA: right coronary artery | |||||
LESION CHARACTERISTICS
Table 1 also presents a summary of the different angiographic characteristics of the CTO lesions in the entire patient subset and the related procedural success. The left anterior descending (LAD) artery was the most common CTO vessel (42.6%), followed by the right coronary artery (RCA: 40%) and left circumflex (LCx: 17.4%). An occlusion length of >20 mm was present in 46.8%, 36.8% had a blunt stump, 31.1% had a bend of >45° and 32.1% of lesions had calcification. Bridging collaterals were seen in 13% and the presence of a side branch at the proximal stump was present in 26.5% of lesions. Previously attempted lesions were only 5.4%.
Classifying the lesions in accordance with the J-CTO score, 22.3% of lesions were classified as easy, 27.5% as intermediate, 30.4% as difficult, and 19.9% as very difficult with their J-CTO scores being 0, 1, 2 or ≥3, respectively, as depicted in Table 2. Antegrade wire crossing success at the first attempt was obtained in a total of 334 (81.9%) CTO lesions. Amongst lesions where an antegrade attempt failed (T4), a retrograde attempt was made in 30 cases of which 24 cases (80%) were successful and a second antegrade attempt was made in twenty-two (22) lesions, of which 17 (77.2%) were successful. Primarily 22 lesions were kept on medical follow-up alone.
Table 2. The J-CTO score distribution and related success in the entire patient data set.
| J-CTO score | Number of patients (%) N=408 | 100% | |
|---|---|---|---|
| 1 | 112 (27.5%) | 95.5% | |
| 2 | 124 (30.4%) | 81.5% | |
| ≥3 | CTO success (%) | ||
| 0 | 91 (22.3%) | 81 (19.9%) | 51.9% |
PROCEDURAL CHARACTERISTICS
The procedural characteristics are presented in Table 3. The femoral approach was used in 13% of cases, the radial approach in 43%, a combined radial and femoral approach in 17% of cases, and a bilateral femoral approach in 26%. Contralateral injection to visualise the occluded segment filling through contralateral collaterals was carried out in 285 (69.8%). In the remaining 31.2% of cases, the distal vessel was visualised either through ipsilateral collaterals or antegrade trickle through a recanalised segment or bridging collaterals. In all cases, the antegrade approach was tried initially with a wire escalation strategy followed by parallel wiring if the wire went subintimal. If both techniques failed, then it was perceived as CTO antegrade failure and a retrograde approach was carried out depending upon the operator decision. Microcatheter support plus a Fielder XT (Asahi Intecc, Aichi, Japan) wire technique was used in 244 (59.6%) with success achieved in 164 (40.2%) cases. A parallel wire technique was attempted in 72 (17.6%) cases and a penetration technique to cross the CTO lesion was attempted in 99 (24.3%) cases. Gaia wires (Asahi Intecc) were available for use only after August 2013 (the last one and a half years) so the experience to a large extent is from the pre-Gaia era. In total, 24 (5.8%) cases had a Gaia wire used, with success achieved in 22 (91.6%). Crusade catheter-based parallel wiring (Kaneka, Osaka, Japan) was also available only after August 2013. The antegrade dissection re-entry technique using the CrossBoss™️ and Stingray™️ coronary CTO crossing and re-entry devices (Boston Scientific, Marlborough, MA, USA) were not available during this study.
Table 3. Procedural characteristics in the study patients.
| N=408 (%) | ||
|---|---|---|
| Approach | Bilateral femoral | 157 (39%) |
| Radial | 123 (30%) | |
| Femoral+radial | 128 (31%) | |
| Contralateral injection needed | 285 (69.8%) | |
| CTO wiring technique (microcatheter) | 408 (100%) | |
| Microcatheter | +Fielder XT (loose tissue tracking) | 244 (59.6%) |
| +Penetration technique | 99 (24.3%) | |
| +Parallel wire technique | 72 (17.6%) | |
| +Gaia wire | 24 (5.8%) | |
| Balloon crossing difficulty | 36 (8.8%) | |
| Anchoring balloon technique | 10 (2.5%) | |
| Rotablation | 2 (0.5%) | |
| GuideLiner | 5 (1.2%) | |
| Tornus | 6 (1.4%) | |
| Newer devices (after August 2013) | Gaia wire | 24 (5.8%) |
| Crusade | 17 (4.2%) | |
| Mean contrast volume (ml) | 295 (150-800) | |
| Mean number of stents per patient | 1.68 | |
DERIVATION AND VALIDATION SET ANALYSIS AND DEVELOPMENT OF WEIGHTED SCORES
The baseline clinical characteristics of cases stratified into two data sets in a proportion of 70:30 with n=285 in the “derivation set” and n=123 in the “validation set” are shown in Table 1. The two sets were matched for all independent variables.
UNIVARIATE/MULTIVARIATE PREDICTORS
Table 4 shows the univariate predictors of antegrade wire crossing failure in the derivation set. Factors with a significant p-value on univariate analysis were then assessed by multivariate analysis. The multivariate predictors including their beta coefficients (β) and odds ratio (OR) predicting failure are shown in Table 5. Blunt stump (β=2.07; OR 7.9, 95% CI: 3.66-17.08; p<0.001), length of occlusion >20 mm (β=1.54; OR 4.68, 95% CI: 2.09-10.47; p<0.001), presence of calcification (β=0.81; OR 2.25, 95% CI: 1.06-4.77; p=0.036), presence of tortuosity (β=1.11; OR 3.02, 95% CI: 1.43-6.39; p=0.004), and collateral with Rentrop grade <2 (β=1.09; OR 2.97, 95% CI: 1.22-7.21; p=0.016) emerged as independent predictors of CTO-PCI failure.
Table 4. Univariate predictors amongst clinical and lesion-related variables in the “derivation” data set.
| Variables | OR | 95% CI | β coefficient | p-value | |
|---|---|---|---|---|---|
| Age (years) | 1.03 | 0.99-1.06 | 0.03 | 0.07 | |
| Male sex | 2.38 | 0.69-8.12 | 0.87 | 0.17 | |
| Diabetes | 0.92 | 0.47-1.81 | –0.09 | 0.80 | |
| Hypertension | 1.25 | 0.68-2.29 | 0.22 | 0.48 | |
| Smoking | 1.23 | 0.67-2.28 | 0.21 | 0.50 | |
| Presenting problem (ref: CSA) | ACS | 0.53 | 0.25-1.11 | –0.63 | 0.09 |
| MI | 0.31 | 0.11-0.93 | –1.17 | 0.036 | |
| Duration (ref: <1 year) ≥1 year | 1.68 | 0.89-3.15 | 0.52 | 0.109 | |
| Lesion (ref: LAD) | RCA | 1.35 | 0.69-2.64 | 0.29 | 0.386 |
| LCx | 0.97 | 0.39-2.4 | –0.03 | 0.947 | |
| LVEF fraction (ref: >50%) | 30-50% | 1.31 | 0.56-3.08 | 0.27 | 0.535 |
| <30% | 0.59 | 0.13-2.68 | –0.52 | 0.498 | |
| Proximal cap blunt | 8.30 | 4.14-16.66 | 2.12 | <0.001 | |
| Side branch present | 2.68 | 1.41-5.09 | 0.99 | 0.003 | |
| Bridging collaterals present | 1.62 | 0.71-3.69 | 0.48 | 0.249 | |
| Length of occlusion >20 mm | 5.51 | 2.69-11.31 | 1.71 | <0.001 | |
| Calcification present | 2.05 | 1.10-3.82 | 0.72 | 0.024 | |
| Tortuosity present | 2.89 | 1.55-5.41 | 1.06 | <0.001 | |
| Ostial present | 2.60 | 1.17-5.76 | 0.96 | 0.019 | |
| Antegrade trickle present | 4.55 | 1.96-10.54 | –1.5 | <0.001 | |
| Rentrop grade <2 | 4.55 | 1.38-5.98 | 1.06 | 0.005 | |
| In-stent | 0.58 | 0.07-4.74 | –0.55 | 0.61 | |
| Previous attempt | 1.48 | 0.46-4.76 | 0.39 | 0.506 | |
| ACS: acute coronary syndrome; CI: confidence interval; CSA: chronic stable angina; LCx: left circumflex artery; LVEF: left ventricular ejection fraction; MI: myocardial infarction; OR: odds ratio; RCA: right coronary artery | |||||
Table 5. Independent predictors for CTO-PCI failure on multivariate analysis and their weighted contribution to W-CTO score.
| Variables | β coefficient | p-value | OR (95% CI) | Score |
|---|---|---|---|---|
| Proximal cap blunt | 2.07 | <0.0001 | 7.90 (3.65-17.08) | +2 |
| Length of occlusion >20 mm | 1.54 | <0.0001 | 4.68 (2.09-10.47) | +1.5 |
| Tortuosity present | 1.11 | 0.004 | 3.02 (1.43-6.39) | +1 |
| Rentrop grade <2 | 1.09 | 0.016 | 2.97 (1.22-7.21) | +1 |
| Calcification present | 0.81 | 0.036 | 2.25 (1.06-4.77) | +1 |
| Total score (W-CTO) | +6.5 | |||
| CI: confidence interval; OR: odds ratio | ||||
The score assigned to each independent variable was derived in proportion to its beta coefficient (Table 5). For each CTO lesion, all applicable score values were then summed to obtain a total difficulty level of that lesion as per our new score model (W-CTO). The total weighted score thus derived with a maximum of 6.5 (W-CTO score) was then segregated into three levels of difficulty as per predefined criteria in methodology. The difficulty levels thus derived ascribed a low level of difficulty to 0-2, intermediate to 2.5-4 and high >4. The success rate of CTO-PCI was 98%, 74.2% and 42.5%, in low, intermediate and high levels of difficulty W-CTO scores, respectively.
The levels of difficulty cut-offs as derived from the new score model in the derivation set were then applied to the validation set and a predictive value for CTO-PCI success was worked out on the validation set. Figure 1 shows the matching of the difficulty level computation in the derivation and validation sets using the Z-test. The goodness of fit for our model using the Hosmer-Lemeshow test was significant at a level of 0.86 with an x2 of 2.594.
Figure 1. Matching of success rate in different lesions in the “derivation” and “validation” sets stratified into three levels of difficulty, namely low, intermediate and high, using the derived W-CTO scores.
COMPARISON OF THE WEIGHTED W-CTO SCORE WITH THE J-CTO SCORE
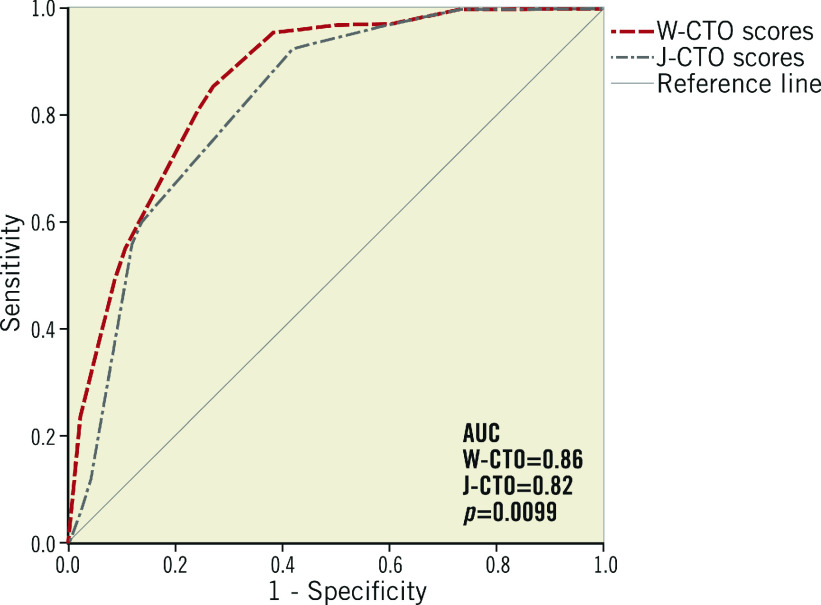
Figure 2 shows the predictive accuracy of our weighted score model (W-CTO) and the existing J-CTO score by applying both the scores on the entire data set (Figure 2). The predictability of antegrade wire crossing success was demonstrated to be better with the W-CTO score as compared to the J-CTO score (ROC AUC of 0.82 [J-CTO] vs. 0.86 [W-CTO], p=0.009).
Figure 2. ROC curves comparing the predictive value of guidewire crossing success/failure using the W-CTO and J-CTO scores from the entire data set.
PROCEDURE-RELATED COMPLICATIONS
In-hospital death occurred in a total of four cases (1%). Coronary perforation leading to pericardial tamponade occurred in four cases (1%). No patient required temporary or permanent haemodialysis and no cases of radiation-induced cutaneous injury occurred in our study population. No patient required emergency CABG.
Discussion
This is a single-centre analysis of 404 consecutive patients who underwent antegrade CTO-PCI. An angiographic scoring system to predict CTO-PCI success/failure has been attempted before5,6,11,12,13,14,15. A large proportion of this data is from a Japanese or European population and none from Indian patients, who have smaller calibre vessels, more diffuse disease and a younger age at presentation16,17. The J-CTO score is a commonly used scoring model and was derived from a study on 479 CTO cases. Our data included an almost equally large number of CTO lesions (n=408), if not more. However, the time duration during which the cases were collected was about two years in the J-CTO registry and six years in our study, ours being a single-centre study.
Our total success rates of 100%, 95.5%, 81.5% and 51.9% for lesions with J-CTO levels of difficulty of 0, 1, 2, and >3, respectively, were in line with the success rates observed in the J-CTO registry of 97.8%, 92.3%, 88.4%, and 73.3% with levels of difficulty 0, 1, 2 & ≥3, respectively. The distribution pattern of the level of difficulty of different lesions in our data set using the already published J-CTO scores conformed to the spectrum of CTO cases in the J-CTO registry, suggesting no major difference of lesion types in terms of the level of difficulty between the two data sets.
WHY A WEIGHTED SCORE
Predictors of antegrade wire crossing may not contribute equally to the outcome of the CTO success. The weighted contribution of each variable was also evident through the differing beta coefficients and odds ratios observed in the multivariate regression analysis of the J-CTO registry data, a point which was brought out by the authors in discussion but was somehow discarded in working out the score model considering the different coefficients to be in close proximity. However, on in-depth analysis of the J-CTO results, one finds that a few of the coefficients were at least twice the minimum, giving a relative importance of at least two times. In the light of the same thought, our weighted score model (W-CTO score), with a total score of 6.5 and individual values as shown in Table 3, could be more accurate when used on a wider patient base by different operators in different patient subsets in day-to-day clinical practice.
OTHER SCORE MODELS
In a recent study, Alessandrino et al proposed a clinical and lesion-related (CL) score which included two clinical variables (history of CABG and history of MI) and four lesion-related variables (blunt stump, lesion calcification, non-LAD CTO, and lesion length >20 mm) as independent predictors of unsuccessful CTO-PCI with a weighted score given to each parameter11. In our study, since there were only two cases of history of CABG, this did not affect the outcome analysis.
Recently, Galassi et al developed a weighted predictive score model for technical failure in CTO-PCI. The ORA score included three predictors of CTO failure: ostial location, Rentrop collateral grade <2, and age >75, assigning 1, 2, and 1 points, respectively18. However, this study included cases carried out via both the antegrade and retrograde techniques, which was different from our study which evaluated only antegrade wiring. As such, the requirement for retrograde success could be somewhat different from antegrade success.
PREDICTORS AS OBSERVED IN OUR STUDY VS. PREDICTORS OBSERVED IN THE J-CTO REGISTRY
In this study, multivariate predictors of failure included a proximal blunt cap, lesion length >20 mm, presence of calcification and tortuosity or bending within the lesion, similar to those noted in the J-CTO registry. A “previous failed attempt”, which was a predictor in the J-CTO score, did not however stand out in our data set, which might have been because of a difference in the types of repeat cases or numbers of those being accepted for repeat attempts by us vs. those in the J-CTO registry. Also, Syrseloudis et al did not find a correlation between a previously failed attempt and final CTO success12.
In addition to the J-CTO score predictors, one important predictor of failure in our study was a poor distal target vessel and/or its poor opacification with collaterals, i.e., collaterals with Rentrop grade <2. Louvard et al had also observed a mean Rentrop collateral grade to be significantly higher in cases with CTO success compared to failure (1.5+1 vs. 1.3+0.7, p=0.002), making it a predictor of CTO antegrade wire crossing failure. We summed Rentrop collaterals grade 2 and 3 together as a group with better collateral filling and better distal vessel visualisation: this turned out to be a favourable predictor for CTO success as compared to poor distal vessel filling (Rentrop collateral grade 0-1 only) which proved to be negative.
There was no significant difference in the CTO-PCI success rate with respect to the artery involved. This is different from one of the earlier studies by Hasegawa et al, which showed lower procedural success rates in RCA (71.8%) followed by LAD (74.8%) and LCx (79.0%)13. Maeremans et al showed a similar success rate of CTO-PCI in all three vessels, as in our study19. The periprocedural complication rates were also comparable to the earlier studies20,21,22.
Limitations
Firstly, this study included only those cases which, as per the operator, qualified to be managed interventionally as a whole. It is likely that cases with very heavy calcification, very long occlusions, or occlusion as part of diffuse TVD may have been left out, leading to selection bias. Secondly, being a single-centre and a single primary operator study there could be a case selection bias and results could differ because of the different complexity of cases selected for analysis. However, as mentioned earlier in the discussion, our data set and the J-CTO registry data set were not dissimilar with respect to distribution of level of difficulty of the lesions and total success rates, both features helping to override the above limitation to a large extent, if not completely.
Conclusions
We conclude that the weighted angiographic CTO score model (W-CTO) derived by us is highly predictive of final antegrade wire crossing success/failure and could be used as a predictive tool in day-to-day practice in elective CTO-PCI patients.
Impact on daily practice
We believe that our weighted CTO score model, with a predictive capacity as good as or even better than the preva-lent J-CTO score, could prove useful in prospective selec-tion of cases for CTO intervention. It should, however, be kept in mind that, with rapidly advancing technology, new scoring models will need to include the newer approaches and hardware.
Acknowledgments
Acknowledgements
We acknowledge the contribution of Mr Mritunjay Mishra, research assistant, for data collection and documentation for the study.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Abbreviations
- β
beta coefficient
- CAD
coronary artery disease
- CTO
chronic total occlusion
- J-CTO score
Japanese chronic total occlusion score
- OR
odds ratio
- PCI
percutaneous coronary intervention
- TIMI
Thrombolysis In Myocardial Infarction
- W-CTO score
weighted chronic total occlusion score
Contributor Information
Roopali Khanna, Department of Cardiology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
Chandra M. Pandey, Department of Biostatistics, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
Sonam Bedi, Department of Biostatistics, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
Fauzia Ashfaq, Department of Cardiology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
Pravin Kumar Goel, Department of Cardiology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
References
- Fefer P, Knudtson ML, Cheema AN, Galbraith D, Osherov AB, Yalonetsky S, Gannot S, Samuel M, Weisbrod M, Bierstone D, Sparkes JD, Wright GA, Strauss BH. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59:991–7. doi: 10.1016/j.jacc.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta-analysis. Am Heart J. 2010;160:179–87. doi: 10.1016/j.ahj.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Shah PB. Management of coronary chronic total occlusion. Circulation. 2011;123:1780–4. doi: 10.1161/CIRCULATIONAHA.110.972802. [DOI] [PubMed] [Google Scholar]
- Stone GW, Kandzari DE, Mehran R, Colombo A, Schwartz RS, Bailey S, Moussa I, Teirstein PS, Dangas G, Baim DS, Selmon M, Strauss BH, Tamai H, Suzuki T, Mitsudo K, Katoh O, Cox DA, Hoye A, Mintz GS, Grube E, Cannon LA, Reifart NJ, Reisman M, Abizaid A, Moses JW, Leon MB, Serruys PW. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part I. Circulation. 2005;112:2364–72. doi: 10.1161/CIRCULATIONAHA.104.481283. [DOI] [PubMed] [Google Scholar]
- Rathore S, Matsuo H, Terashima M, Kinoshita Y, Kimura M, Tsuchikane E, Nasu K, Ehara M, Asakura Y, Katoh Y, Suzuki T. Procedural and in-hospital outcomes after percutaneous coronary intervention for chronic total occlusions of coronary arteries 2002 to 2008: impact of novel guidewire techniques. JACC Cardiovasc Interv. 2009;2:489–97. doi: 10.1016/j.jcin.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, Hiasa Y, Doi O, Yamashita T, Hinohara T, Tanaka H, Mitsudo K J-CTO Registry Investigators. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213–21. doi: 10.1016/j.jcin.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Sianos G, Werner GS, Galassi AR, Papafaklis M, Escaned J, Hildick-Smith D, Christiansen EH, Gershlick A, Carlino M, Karlas A, Konstantinidis NV, Tomasello SD, Di Mario, Reifart N EuroCTO Club. Recanalisation of chronic total occlusions: 2012 consensus document from the EuroCTO club. EuroIntervention. 2012;8:139–45. doi: 10.4244/EIJV8I1A21. [DOI] [PubMed] [Google Scholar]
- Cohen M, Rentrop KP. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation. 1986;74:469–76. doi: 10.1161/01.cir.74.3.469. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- Alessandrino G, Chevalier B, Lefèvre T, Sanguineti F, Garot P, Unterseeh T, Hovasse T, Morice MC, Louvard Y. A Clinical and Angiographic Scoring System to Predict the Probability of Successful First-Attempt Percutaneous Coronary Intervention in Patients With Total Chronic Coronary Occlusion. JACC Cardiovasc Interv. 2015;8:1540–8. doi: 10.1016/j.jcin.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Syrseloudis D, Secco GG, Barrero EA, Lindsay AC, Ghione M, Kilickesmez K, Foin N, Martos R, Di Mario. Increase in J-CTO lesion complexity score explains the disparity between recanalisation success and evolution of chronic total occlusion strategies: insights from a single-centre 10-year experience. Heart. 2013;99:474–9. doi: 10.1136/heartjnl-2012-303205. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Godino C, Basavarajaiah S, Takagi K, Rezq A, Latib A, Alaide C, Montorfano M, Carlino M, Colombo A. Differences in the clinical and angiographic characteristics of chronic total occlusion lesions in the three major coronary arteries. J Interv Cardiol. 2014;27:44–9. doi: 10.1111/joic.12085. [DOI] [PubMed] [Google Scholar]
- Tan KH, Sulke N, Taub NA, Watts E, Karani S, Sowton E. Determinants of success of coronary angioplasty in patients with a chronic total occlusion: a multiple logistic regression model to improve selection of patients. Br Heart J. 1993;70:126–31. doi: 10.1136/hrt.70.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Miyazaki MD, Morii I, Daikoku S, Goto Y, Nonogi H. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Determinants of primary success and long-term clinical outcome. Catheter Cardiovasc Interv. 2000;49:258–64. doi: 10.1002/(sici)1522-726x(200003)49:3<258::aid-ccd7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Kaul U, Bhatia V. Perspective on coronary interventions and cardiac surgeries in India. Indian J Med Res. 2010;132:543–8. [PMC free article] [PubMed] [Google Scholar]
- Varghese PJ, Arumugum SB, Cherian KM, Wailey V, Farb A, Virmani R. Atheromatous plaque reflects serum total cholesterol levels: a comparative morphologic study of endarterectomy coronary atherosclerotic plaques removed from patients from the southern part of India and Caucasians from Ottawa, Canada. Clin Cardiol. 1998;21:335–40. doi: 10.1002/clc.4960210507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galassi AR, Boukhris M, Azzarelli S, Castaing M, Marzà F, Tomasello SD. Percutaneous Coronary Revascularization for Chronic Total Occlusions: A Novel Predictive Score of Technical Failure Using Advanced Technologies. JACC Cardiovasc Interv. 2016;9:911–22. doi: 10.1016/j.jcin.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Maeremans J, Selleslagh P, Di Serafino, Barbato E, Dens J. Impact of negative lesion characteristics of chronic total occlusions on procedural outcome and strategy. Acta Cardiol. 2013;68:455–61. doi: 10.1080/ac.68.5.2994467. [DOI] [PubMed] [Google Scholar]
- Lansky AJ, Stone GW. Periprocedural myocardial infarction: prevalence, prognosis and prevention. Circ Cardiovasc Interv. 2010;3:602–10. doi: 10.1161/CIRCINTERVENTIONS.110.959080. [DOI] [PubMed] [Google Scholar]
- Klein LW. Coronary artery perforation during interventional procedures. Catheter Cardiovasc Interv. 2006;68:713–7. doi: 10.1002/ccd.20855. [DOI] [PubMed] [Google Scholar]
- Tepel M, Aspelin P, Lameire N. Contrast induced nephropathy: a clinical and evidence based approach. Circulation. 2006;113:1799–806. doi: 10.1161/CIRCULATIONAHA.105.595090. [DOI] [PubMed] [Google Scholar]