Abstract
Takayasu’s arteritis (TA) is a chronic non-specific vasculitis with variable presentation in different ethnicities and countries. Treatment options vary and are dependent on the stage and presentation of the disease. We aimed to review current literature related to TA, focusing on the role of endovascular treatment in revascularisation. The temporal course of the disease and stage at presentation influence the management of TA. Treatment options include medical therapy, endovascular intervention or surgical vascular reconstruction. The decision to intervene is individualised according to vascular anatomy and the presence of haemodynamically significant lesions. There are currently no clear guidelines regarding the choice between the endovascular and open surgical approaches, but studies have shown that endovascular procedures are associated with slightly higher rates of restenosis while surgical procedures have higher rates of thrombosis. Periprocedural immunosuppression is suggested if the disease is active at the point of intervention. This improves outcomes but at the cost of immunosuppression-related side effects. Careful long-term follow-up is essential due to the risk of disease activation or flare-up, requiring appropriate evaluation of the diseased vessels.
Introduction
Takayasu’s arteritis (TA) is a chronic non-specific granulomatous large-vessel vasculitis, which affects the aorta and its main branches. It may also affect the coronary and pulmonary arteries1,2. TA has historically been described as having a strong association with female patients, though the degree of association with the female gender may vary for different populations3. In the Japanese population where TA was originally described, the majority (80-90%) of the TA patient population consists of females. By comparison, Indian, Thai and Israeli populations demonstrate greater gender heterogeneity, with a larger (31-38%) proportion of male patients3,4. The disease commonly presents in the second or third decade of life regardless of ethnicity, although a small percentage may present in childhood2,4,5. The incidence of TA has been described worldwide as up to 3.3 per million, and is generally considered to be most common in Asia6.
TA has been described as the infiltration of inflammatory cells into the adventitia and media resulting in a cell-mediated immune response, involving NK T cells and CD4 T cells which form characteristic granulomas and giant cells. This is different from atherosclerotic lesions which consist of the accumulation of lipid-laden foam cells involving the intima layer. These features make atherosclerotic lesions respond better to percutaneous transluminal angioplasty (PTA) as compared with TA lesions, with 15.5% residual stenosis after subclavian artery PTA in TA compared with only 8.3% in atherosclerotic lesions7.
CLINICAL MANIFESTATIONS
In a series of 60 patients described by Kerr et al, only 33% had systemic symptoms on presentation5. Hypertension is most often associated with renal artery stenosis. Sixty-eight percent (68%) of patients had extensive vascular disease; stenotic lesions were 3.6-fold more common than were aneurysms (98% compared with 27%)5. Most of the aneurysms were located in the thoracic aorta (83.3%), with a smaller proportion (19%) in the abdominal aorta8. It has been noted that the site of affected lesions differs amongst various countries, therefore causing different sets of symptoms. Occlusive disease seems to be more prevalent in Japan, the USA, and Europe, whereas aneurysmal disease is more common in India, Thailand, Mexico, and Africa9.
The clinical course of TA can be subdivided into two phases - the early or active phase, and the late, chronic, or inactive phase10. During the early or active phase, the initial intimal inflammation process is followed by oedema and subsequent infiltration of lipids and blood cells. The resultant calcification and intimal thickening that follows the inflammatory phase represents the chronic phase of TA, which is similar to atherosclerosis. Clinically, constitutional symptoms are predominant during the acute phase, whereas the chronic phase is characterised by symptoms related to arterial compromise11. Though TA patients have often been described as having high rates of relapse despite being on medical treatment12, a small cohort study (n=26) demonstrated that a significant proportion of patients (76%) had no angiographically significant relapse over more than three years13.
Geographically, TA demonstrated significant anatomical variability in terms of the site of the disease. This can potentially be explained by the heterogeneous genetic presentation of TA patients around the globe14. In Japan and South America, cervical and thoracic arterial lesions are more prevalent, but in Israel and other Asian countries abdominal lesions are more frequent15. In a study of 106 Japanese patients, the presentation was as follows: 41.5% had thoracic aorta lesions, 31.1% had abdominal aorta lesions, 22.6% had moderate to severe aortic regurgitation, 21.7% renal artery lesions, 8.5% had coronary lesions, 4.7% pulmonary artery lesions and 2.8% had loss of vision16. Japanese patients often present with “pulseless disease”, with the majority of the stenotic lesions occurring in the ascending aorta, the aortic arch, and/or its branches (58%) and occasionally extending into the thoracic and abdominal aorta4,17. On the other hand, in Korean patients, vasculitis generally occurs in the abdominal aorta (30%) involving renal arteries4,17,18. Indian patients tend to present with hypertension, with a number of studies having noted the characteristic involvement of the abdominal aorta and the renal arteries in the majority of cases (71-92%)19,20,21. In an Indian observational study, patients with early TA were observed to have isolated abdominal and renal artery lesions at presentation, which over the course of 10-20 years progressed to involve the entire aorta and its branches4. Approximately 20-26% of Indian patients with TA have aneurysmal lesions of the abdominal aorta22, with the only case series of 30 patients with the aneurysmal form of TA being published in India in 199023. In the UK, a study of 97 patients revealed that the majority of patients (95%) had arterial stenosis or occlusions, as compared to the other 5% who had a discrete aneurysm24. The supra-aortic vessels were involved more frequently, comprising 45.9% of affected segments, with the next most common site being the aorta (25.3%)24. Examples of anatomic involvement on imaging scans are illustrated in Figure 1-Figure 4.
Figure 1. A 7-year-old female with stenosis of the descending thoracic aorta demonstrated on a CT aortogram.

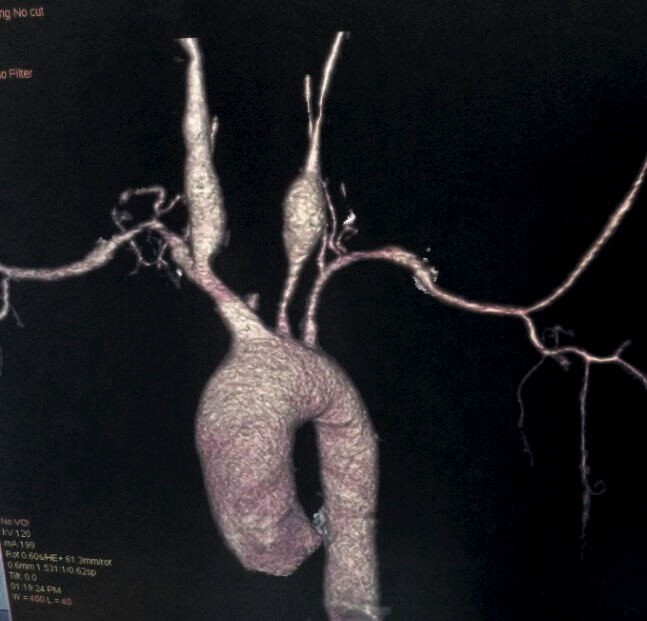
Figure 4. A CT aortogram showing bilateral common carotid artery aneurysms.
PRESENTATION
In Japanese patients, more common presentations include aortic regurgitation, ischaemic heart disease and visual disturbances, while amongst Korean and Indian patients the more frequent presentations were hypertension, headache, exertional dyspnoea, dizziness and malaise4,17,18. In a study of 106 patients in India, hypertension was the most common mode of presentation (51.3%) and was detected in 82 patients (77.4%) at the time of presentation. Other presentations include vascular bruits which were heard in 72 patients (67.9%), while 13 (12.3%) patients were found to be in congestive heart failure20. In Beijing, 530 patients were studied, of whom 60% had hypertension, 57.5% had vascular bruits in the upper abdomen, 47.4% had carotid bruits, 37.2% had pulse deficit at the extremities and 24.7% had intermittent claudication25. In essence, the affected lesions vary in different countries and therefore present differently.
The differences in clinical presentation based on geographical distribution are summarised in Table 1.
Table 1. Common presentation of Takayasu’s arteritis.
| Country | Symptoms | Site of affected lesions |
|---|---|---|
| Korea17 | – Headache (60%) – Exertional dyspnoea (42%) – Dizziness (36%) – Malaise or weakness (34%) – Hypertension | – Abdominal aorta (46%) – Descending thoracic aorta (37%) – Ascending aorta (1%) – Aortic arch (2%) |
| Japan16,23 | – Aortic regurgitation (22.6%) – Ischaemic heart disease (8.5%) – Visual disturbances/loss (2.8%) | – Thoracic aorta (41.5%) – Abdominal aorta (31.1%) – Moderate to severe aortic regurgitation (22.6%) – Renal artery lesions (21.7%) – Coronary lesions (8.5%) – Pulmonary artery lesions (4.7%) |
| India19 | – Hypertension (51.3%) – Vascular bruit (67.9%) – Congestive heart failure (12.3%) | – Type I (branches of aortic arch) (6.6%) – Type II (aortic arch, its branches and descending thoracic aorta) (6.6%) – Type III (descending thoracic aorta and abdominal aorta) (3.8%) – Type IV (abdominal aorta only) (27.3%) – Type V (aortic arch, descending thoracic aorta and abdominal aorta) (55.7%) |
| China24 | – Hypertension (60%) – Vascular bruits upper abdomen (57.5%) – Carotid bruits (47.4%) – Pulse deficit (37.2%) – Intermittent claudication (24.7%) | – Type I (20.8%) – Type II (37.1%) – Type III (42.1%) – Type IV (52.3%) |
DIAGNOSIS
The first set of diagnostic criteria for TA was initially established by Ishikawa in 1988. This was then replaced by a new set of criteria by the American College of Rheumatology (ACR) in 199026. The current ACR classification consists of six criteria: (1) onset at age less than or equal to 40 years, (2) claudication of an extremity, (3) decreased brachial artery pulse, (4) greater than 10 mmHg difference in systolic blood pressure between arms, (5) a bruit over the subclavian arteries or the aorta, and (6) arteriographic evidence of narrowing or occlusion of the entire aorta, its primary branches, or large arteries in the proximal upper or lower extremities. The presence of at least three of the six criteria was found to have demonstrated a sensitivity of 90.5% and a specificity of 97.8%26.
A modification to the 1988 Ishikawa criteria by Sharma et al was validated and compared to the ACR 1990 criteria in a small Indian population (n=106) with a reported higher sensitivity (96% vs. 77.4%) and similar specificity27. However, this has not been validated in other populations/studies; the ACR criteria remain the most widely used in studies and clinical practice14,28,29,30,31.
The current “gold standard” investigation is digital subtraction angiography (DSA); however, it is invasive and only identifies late, structural changes in the vasculature32. Angiographic findings can be classified into six types, according to the vessels involved (Figure 5)33,34. Additionally, involvement of the coronary and pulmonary arteries is indicated as C (+) or P (+), respectively. Type V has been documented as the most common type35.
Figure 5. Angiographic types of Takayasu’s arteritis.
Recent advances in non-invasive vascular imaging, on the other hand, have provided new insights into TA26. Contrast-enhanced computed tomography angiography and magnetic resonance angiography are useful in demonstrating vascular anatomy, wall enhancement, oedema, as well as thickening, which might enable early disease detection while the luminal diameter is still preserved. 18F-fluorodeoxyglucose positron emission tomography has also been found to provide important additional information by highlighting areas of increased metabolic activity and is therefore useful to detect inflammation with a reported high sensitivity and specificity in TA, allowing diagnosis of early pre-stenotic disease36,37. However, there are limitations with this technique, which include a lack of standardised technique for quantification of uptake, limited availability, and lack of reliable evidence for evaluation of disease activity38. Histological diagnosis of TA includes granulomatous arteritis with infiltration of Langhans giant cells in the media with smooth muscle cell necrosis and destruction of the internal elastic membrane39.
TREATMENT OPTIONS
Current treatment options include medical therapy, endovascular intervention and surgical vascular reconstruction. Briefly, the modality of treatment is largely dependent on the individual patient’s clinical course and extent of disease activity. Patients who present during the active stage of the disease require cortico-steroids and immunosuppressive agents to curb the systemic and vascular inflammatory response, whereas patients who have either progressed into or present during the chronic phase of TA may require revascularisation, either by an endovascular or by an open approach, for haemodynamically significant arterial lesions40.
MEDICAL TREATMENT
The administration of corticosteroids and other immunosuppressive agents has demonstrated positive anti-inflammatory effects in a majority of patients with TA1. Although glucocorticoids have long been considered the mainstay of treatment for TA due to excellent initial response rates of 40-93%, sustained remission is maintained in only 28% of cases and up to 80% of patients experience steroid-related adverse effects1,5,28,41,42,43. Immunosuppressive drugs such as cyclophosphamide, methotrexate and azathioprine are usually added due to glucocorticoid resistance, relapse upon reduction of glucocorticoid dose or serious side effects from steroid therapy1. About 40% of all steroid-resistant patients respond to the addition of cytotoxic agents28. Although remission can be achieved in the majority of patients with immunosuppression, over 90% have some form of relapse12.
Biologic agents such as anti-TNF-α agents, anti-IL-6R, anti-CD20, anti-IL-12/23 p40, and the soluble CTLA4 receptor fusion protein have recently been used in patients with resistant TA, including those patients who fail to achieve remission despite glucocorticoid steroids and other immunosuppressive therapy. A recent meta-analysis in 2014, based on three small randomised controlled trials, showed that anti-TNF agents (infliximab, etanercept and adalimumab) are not effective in the ability to induce remission or reduce the amount of corticosteroids required44. However, the same meta-analysis also observed that data from numerous case series suggested that tocilizumab and infliximab may be effective in the management of large vessel vasculitis and refractory TA44, with 37-67% of patients achieving complete remission and 53% having a partial response45,46,47. Adverse events such as infections and hypersensitivity in approximately 20% of patients result in discontinuation of anti-TNF-α therapy45.
INTERVENTION
TIMING
Intervention in TA is often challenging due to the complexity of the lesions and the high rates of restenosis. Approximately 20% of patients are resistant to any kind of medical treatment28,46. This in turn leads to the need for endovascular or surgical interventions, which are usually recommended at a time of quiescent TA10,28,29. This can be achieved by opting to revascularise patients during the chronic inactive phase or by administering preprocedural immunosuppression, which has been found to lower restenosis rates24,28,48,49. Studies have demonstrated that both endovascular and open surgery during active TA were associated with a higher incidence of arterial complications as well as the need for repeat revascularisation10,28,29,50. Therefore, assessment of disease activity plays an important role in determining the optimal timing for revascularisation. Clinical symptoms coupled with haemoglobin levels, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), as well as radiological imaging (e.g., CT angiography, magnetic resonance angiography, 18F-FDG positron emission tomography), can be used for assessment of disease activity28,36,51.
INDICATIONS AND PATIENT SELECTION
Broadly speaking, the decision to intervene in TA has to be individualised based on the anatomic location of disease of each patient. For each lesion, i.e., renal artery stenosis, subclavian artery stenosis or aortic regurgitation, the decision to intervene is based on standard indications (Table 2)2. Indications for intervention are generally the presence of haemodynamically significant lesions, such as hypertension caused by severe renal artery stenosis, severe limb claudication, progressive aneurysm enlargement, cerebrovascular ischaemia or critical stenosis of three or more cerebral vessels, coronary artery ischaemia, moderate to severe aortic regurgitation and severe aortic coarctation5,16,52. For abdominal aneurysms, the indications are similar to those due to atherosclerosis and connective tissue disease. There are no other special situations that will alter the indication for surgery2. Patients with two or more major complications of TA, defined as Ishikawa’s prognostic criteria stage III, were noted to derive the most benefit from revascularisation as compared to those with less extensive disease52,53.
Table 2. Intervention of Takayasu's arteritis based on organ involvement5,15,51.
| Anatomy | Intervention | Indication for intervention |
|---|---|---|
| Aorta | 1) Open surgery 2) Percutaneous endovascular repair | – Progressive aneurysm enlargement – Moderate to severe aortic regurgitation – Severe aortic coarctation |
| Renal artery stenosis | 1) Percutaneous angioplasty 2) Open surgery/ reconstruction | – Hypertension |
| Coronary artery stenosis | 1) Percutaneous intervention with drug-eluting stent and immunosuppressive steroids 2) CABG | – Coronary artery ischaemia |
| Carotid and subclavian artery occlusion | 1) Open surgery 2) Percutaneous angioplasty | – Severe limb claudication – Cerebrovascular ischaemia – Critical stenosis of 3 or more cerebral vessels |
ENDOVASCULAR VERSUS OPEN APPROACH
Currently there are no clear guidelines regarding the selection of endovascular versus open surgical intervention in TA patients with chronic inactive disease. The decision hinges on many factors including the anatomy of the lesion, local practices and expertise, and perioperative risk.
In general, a number of case control and cohort studies have reported lower restenosis rates for patients who underwent open surgery as compared to an endovascular approach24,28,31,41,54,55. A review of recent comparative studies published from 2007 to 2015 showed that 15-50% of patients who underwent surgery had recurrent disease requiring revision surgery after 5-20 years. For endovascular interventions, the restenosis rates ranged from 17-70% at 5-10 years. Conversely, a small US cohort study in 2009 found that treatment of late inactive stage TA lesions with either just angioplasty or angioplasty with stenting resulted in excellent to good clinical improvement at follow-up at 46.8 months56. Symptom recurrence occurred in 31.4%, which was then successfully treated with repeat angioplasty and stenting56. The contrasting, limited and retrospective evidence suggests that there may not be a “one size fits all” approach to revascularisation. Instead, each patient and lesion must be evaluated individually to determine the best mode of revascularisation.
In the modern era, survival rates for patients undergoing either open or endovascular revascularisation are generally good: 94.3% at 6.5 years and 73.5% at 20 years10,24,29,50,52. According to Saadoun, the rates of early complications (<30 days) of surgical and endovascular procedures were similar in terms of restenosis, thrombosis and stroke28. However, in terms of late complications (>30 days), endovascular procedures had slightly higher rates of restenosis at 64.5% compared to 46.1%, while surgical procedures had higher rates of thrombosis (15.4% versus 3.2%)28. For surgically treated patients, it is important to identify a disease-free area of artery for the bypass graft anastomosis, to prevent the development of anastomotic strictures and/or the formation of pseudo-aneursyms postoperatively56. The high restenosis rates published are similar to those quoted in another recent 25-year retrospective cohort study (open surgery 44%, endovascular approach 66%, p=0.33)54. This implies that, regardless of the approach, restenosis remains a frequent late complication and may necessitate repeat intervention.
RENAL ARTERY STENOSIS
The optimal approach for the treatment of Takayasu’s arteritis-induced renal artery stenosis (TARAS) remains a topic for discussion, with evidence to support both endovascular and open approaches. Aortorenal bypass was shown in two earlier studies (2003-2004) to have five-year patency rates of 79% with a postoperative morbidity of 19% and 17%, respectively57,58. More recently, primary angioplasty of TARAS has demonstrated procedural success of more than 90%, five-year patency of 67-91% and lower complication rates (5.7% in one study and 0% in another). Stenting is occasionally performed for ostial lesions, long-segment lesions, or incomplete dilatation of stenosis and dissection10,59,60,61. Given the similar long-term outcomes of both approaches coupled with the reduced complication rate of endovascular techniques, angioplasty is currently favoured by most centres50.
CORONARY ARTERY STENOSIS
Coronary stenosis in patients with TA tends to be ostial, involving the left main artery. A recent small retrospective analysis suggests that percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) have similar long-term outcomes in patients with TA and stable disease. Patients with active TA who went for CABG had a lower incidence of major adverse cardiac events (MACE) as compared to PCI62. Use of periprocedural immunosuppression for three months prior to PCI has been found to maintain coronary stent patency for up to 10 years24.
CAROTID AND SUBCLAVIAN ARTERY OCCLUSION
Common carotid and subclavian artery lesions in TA are often long, irregular and fibrotic. Surgical bypass has superior patency compared to endovascular intervention but with higher rates of major complications24,63. Endovascular intervention is usually reserved for stenoses less than 5 cm, with initial success rates of up to 81% when combined with periprocedural immunosuppression63,64,65.
THORACIC AND ABDOMINAL AORTA
Historically, aneurysmal disease of the aorta in TA was treated with open surgery to varied outcomes66,67,68. In contemporary practice, it is now commonly treated with standard techniques and devices such as aortic stent grafts69,70.
PROGNOSIS
A literature review revealed the overall survival at 15 years after diagnosis of TA to be 82.9%53. Aortic regurgitation, retinopathy, aneurysm and secondary hypertension are associated with poorer prognosis, the presence of two or more of which led to higher five-year (69.9%) and 10-year (36.7%) mortality rates71,72. Common causes of death include congestive cardiac failure, renal failure, cerebrovascular accident, and pulmonary infections.
Conclusion
Takayasu’s arteritis is a chronic non-specific granulomatous vasculitis affecting the aorta and its main branches. Its presentation is different in different ethnicities and countries. Management includes medical therapy with immunosuppressive therapy during the acute phase. Surgical bypass/reconstruction or endovascular therapy may be required for haemodynamically significant or symptomatic disease during the late phase. Careful long-term follow-up is essential due to the risk of disease activation or flare-up, requiring appropriate evaluation of the diseased vessels56.
Impact on daily practice
Endovascular interventions are recommended at a time of quiescent TA, which can be achieved by timing them during the chronic inactive phase or by administering preprocedural immunosuppression. Limited data exist but suggest that surgery results in lower restenosis rates than endovascular intervention. Primary angioplasty for TARAS is currently favoured by most centres, due to procedural success rates of more than 90% and lower complications as compared to the open approach.
Figure 2. CT aortogram of a 12-year-old male showing stenosis of the left subclavian artery and left renal artery.

Figure 3. A 23-year-old female with stenosis of the descending abdominal aorta with collaterals from celiac to inferior mesenteric artery.

Acknowledgments
Acknowledgements
Permission for the use of the image labelled Figure 5 was obtained from RadioGraphics.
Conflict of interest statement
K.K. Yeo has received research support from Medtronic, and honoraria from Abbott Vascular and St. Jude Medical. The other authors have no conflicts of interest to declare.
Abbreviations
- CABG
coronary artery bypass graft
- CRP
C-reactive protein
- CTA
computed tomography angiography
- MACE
major adverse cardiac events
- MRA
magnetic resonance angiography
- PCI
percutaneous coronary intervention
- PET
positron emission tomography
- PTA
percutaneous transluminal angioplasty
- TA
Takayasu’s arteritis
- TARAS
Takayasu’s arteritis-induced renal artery stenosis
Contributor Information
Rachel Wenrui Lim, National Heart Centre Singapore, Singapore.
Yann Shan Keh, National Heart Centre Singapore, Singapore.
Khung Keong Yeo, National Heart Centre Singapore, Singapore; Duke-NUS Medical School, Singapore.
Narendra Nath Khanna, Indraprastha Apollo Hospitals, New Delhi, India.
References
- Wen D, Du X, Ma CS. Takayasu arteritis: diagnosis, treatment and prognosis. Int Rev Immunol. 2012;31:462–73. doi: 10.3109/08830185.2012.740105. [DOI] [PubMed] [Google Scholar]
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med. 2013;2013:618910. doi: 10.1155/2013/618910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numano F, Kobayashi Y. Takayasu arteritis--beyond pulselessness. Intern Med. 1999;38:226–32. doi: 10.2169/internalmedicine.38.226. [DOI] [PubMed] [Google Scholar]
- Moriwaki R, Noda M, Yajima M, Sharma BK, Numano F. Clinical manifestations of Takayasu arteritis in India and Japan--new classification of angiographic findings. Angiology. 1997;48:369–79. doi: 10.1177/000331979704800501. [DOI] [PubMed] [Google Scholar]
- Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, Hoffman GS. Takayasu arteritis. Ann Intern Med. 1994;120:919–29. doi: 10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- Watts R, Al-Taiar A, Mooney J, Scott D, Macgregor A. The epidemiology of Takayasu arteritis in the UK. Rheumatology (Oxford) 2009;48:1008–11. doi: 10.1093/rheumatology/kep153. [DOI] [PubMed] [Google Scholar]
- Tyagi S, Verma PK, Gambhir DS, Kaul UA, Saha R, Arora R. Early and long-term results of subclavian angioplasty in aortoarteritis (Takayasu disease): comparison with atherosclerosis. Cardiovasc Intervent Radiol. 1998;21:219–24. doi: 10.1007/s002709900248. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Hirano T, Takeda K, Matsuda A, Nakagawa T, Yamaguchi N, Yuasa H, Kusakawa M, Nakano T. Incidence of aneurysms in Takayasu’s arteritis. Angiology. 1991;42:308–15. doi: 10.1177/000331979104200408. [DOI] [PubMed] [Google Scholar]
- Desiron Q, Zeaiter R. Takayasu’s arteritis. Acta Chir Belg. 2000;100:1–6. [PubMed] [Google Scholar]
- Ogino H, Matsuda H, Minatoya K, Sasaki H, Tanaka H, Matsumura Y, Ishibashi-Ueda H, Kobayashi J, Yagihara T, Kitamura S. Overview of late outcome of medical and surgical treatment for Takayasu arteritis. Circulation. 2008;118:2738–47. doi: 10.1161/CIRCULATIONAHA.107.759589. [DOI] [PubMed] [Google Scholar]
- Parra JR, Perler BA. Takayasu’s disease. Semin Vasc Surg. 2003;16:200–8. doi: 10.1016/s0895-7967(03)00025-5. [DOI] [PubMed] [Google Scholar]
- Cong XL, Dai SM, Feng X, Wang ZW, Lu QS, Yuan LX, Zhao XX, Zhao DB, Jing ZP. Takayasu’s arteritis: clinical features and outcomes of 125 patients in China. Clin Rheumatol. 2010;29:973–81. doi: 10.1007/s10067-010-1496-1. [DOI] [PubMed] [Google Scholar]
- Mandalam KR, Subramanyan R, Joseph S, Rao VR, Gupta AK, Unni NM, Rao AS, Kumar S, Balakrishnan KG, Neelakandhan KS. Natural history of aortoarteritis: an angiographic study in 26 survivors. Clin Radiol. 1994;49:38–44. doi: 10.1016/s0009-9260(05)82912-5. [DOI] [PubMed] [Google Scholar]
- Terao C, Yoshifuji H, Mimori T. Recent advances in Takayasu arteritis. Int J Rheum Dis. 2014;17:238–47. doi: 10.1111/1756-185X.12309. [DOI] [PubMed] [Google Scholar]
- Isobe M. Takayasu arteritis revisited: current diagnosis and treatment. Int J Cardiol. 2013;168:3–10. doi: 10.1016/j.ijcard.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Ohigashi H, Haraguchi G, Konishi M, Tezuka D, Kamiishi T, Ishihara T, Isobe M. Improved prognosis of Takayasu arteritis over the past decade--comprehensive analysis of 106 patients. Circ J. 2012;76:1004–11. doi: 10.1253/circj.cj-11-1108. [DOI] [PubMed] [Google Scholar]
- Yajima M, Moriwaki R, Numano F, Park YB, Cho YD. Comparative studies between Japanese and Korean patients: comparison of the findings of angiography, HLA-Bw52, and clinical manifestations. Heart Vessels Suppl. 1992;7:102–5. doi: 10.1007/BF01744553. [DOI] [PubMed] [Google Scholar]
- Park YB, Hong SK, Choi KJ, Sohn DW, Oh BH, Lee MM, Choi YS, Seo JD, Lee YW, Park JH. Takayasu arteritis in Korea: clinical and angiographic features. Heart Vessels Suppl. 1992;7:55–9. doi: 10.1007/BF01744545. [DOI] [PubMed] [Google Scholar]
- Yajima M, Numano F, Park YB, Sagar S. Comparative studies of patients with Takayasu arteritis in Japan, Korea and India--comparison of clinical manifestations, angiography and HLA-B antigen. Jpn Circ J. 1994;58:9–14. doi: 10.1253/jcj.58.9. [DOI] [PubMed] [Google Scholar]
- Jain S, Sharma N, Singh S, Bali HK, Kumar L, Sharma BK. Takayasu arteritis in children and young indians. Int J Cardiol. 2000;75 Suppl 1:S153–7. doi: 10.1016/s0167-5273(00)00180-7. [DOI] [PubMed] [Google Scholar]
- Jain S, Kumari S, Ganguly NK, Sharma BK. Current status of Takayasu arteritis in India. Int J Cardiol. 1996;54 Suppl:S111–6. doi: 10.1016/s0167-5273(96)88780-8. [DOI] [PubMed] [Google Scholar]
- Sharma BK, Jain S, Radotra BD. An autopsy study of Takayasu arteritis in India. Int J Cardiol. 1998;66 Suppl 1:S85–90. doi: 10.1016/s0167-5273(98)00155-7. [DOI] [PubMed] [Google Scholar]
- Kumar S, Subramanyan R, Mandalam KR, Rao VR, Gupta AK, Joseph S, Unni NM, Rao AS. Aneurysmal form of aortoarteritis (Takayasu’s disease): analysis of thirty cases. Clin Radiol. 1990;42:342–7. doi: 10.1016/s0009-9260(05)82150-6. [DOI] [PubMed] [Google Scholar]
- Perera AH, Youngstein T, Gibbs RG, Jackson JE, Wolfe JH, Mason JC. Optimizing the outcome of vascular intervention for Takayasu arteritis. Br J Surg. 2014;101:43–50. doi: 10.1002/bjs.9372. [DOI] [PubMed] [Google Scholar]
- Zheng D, Fan D, Liu L. Takayasu arteritis in China: a report of 530 cases. Heart Vessels Suppl. 1992;7:32–6. doi: 10.1007/BF01744541. [DOI] [PubMed] [Google Scholar]
- Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, Lightfoot RW, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–34. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- Sharma BK, Jain S, Suri S, Numano F. Diagnostic criteria for Takayasu arteritis. Int J Cardiol. 1996;54 Suppl:S141–7. doi: 10.1016/s0167-5273(96)88783-3. [DOI] [PubMed] [Google Scholar]
- Saadoun D, Lambert M, Mirault T, Resche-Rigon M, Koskas F, Cluzel P, Mignot C, Schoindre Y, Chiche L, Hatron PY, Emmerich J, Cacoub P. Retrospective analysis of surgery versus endovascular intervention in Takayasu arteritis: a multicenter experience. Circulation. 2012;125:813–9. doi: 10.1161/CIRCULATIONAHA.111.058032. [DOI] [PubMed] [Google Scholar]
- Fields CE, Bower TC, Cooper LT, Hoskin T, Noel AA, Panneton JM, Sullivan TM, Gloviczki P, Cherry KJ. Takayasu’s arteritis: operative results and influence of disease activity. J Vasc Surg. 2006;43:64–71. doi: 10.1016/j.jvs.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Lee TH, Chen IM, Chen WY, Weng CF, Hsu CP, Shih CC. Early endovascular experience for treatments of Takayasu’s arteritis. J Chin Med Assoc. 2013;76:83–7. doi: 10.1016/j.jcma.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Lee GY, Jeon P, Do YS, Sung K, Kim DI, Kim YW, Kim DK. Comparison of outcomes between endovascular treatment and bypass surgery in Takayasu arteritis. Scand J Rheumatol. 2014;43:153–61. doi: 10.3109/03009742.2013.822096. [DOI] [PubMed] [Google Scholar]
- Andrews J, Al-Nahhas A, Pennell DJ, Hossain MS, Davies KA, Haskard DO, Mason JC. Non-invasive imaging in the diagnosis and management of Takayasu’s arteritis. Ann Rheum Dis. 2004;63:995–1000. doi: 10.1136/ard.2003.015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol. 1996;54 Suppl:S155–63. doi: 10.1016/s0167-5273(96)02813-6. [DOI] [PubMed] [Google Scholar]
- Nastri MV, Baptista LP, Baroni RH, Blasbalg R, de Avila, Leite CC, de Castro, Cerri GG. Gadolinium-enhanced three-dimensional MR angiography of Takayasu arteritis. Radiographics. 2004;24:773–86. doi: 10.1148/rg.243035096. [DOI] [PubMed] [Google Scholar]
- Zhu FP, Luo S, Wang ZJ, Jin ZY, Zhang LJ, Lu GM. Takayasu arteritis: imaging spectrum at multidetector CT angiography. Br J Radiol. 2012;85:e1282–92. doi: 10.1259/bjr/25536451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Goodman PC, Leder RA. FDG-PET finding in early-phase Takayasu arteritis. J Comput Assist Tomogr. 1999;23:16–8. doi: 10.1097/00004728-199901000-00004. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Ishii K, Oda K, Nariai T, Tanaka Y, Ishiwata K, Numano F. Aortic wall inflammation due to Takayasu arteritis imaged with 18F-FDG PET coregistered with enhanced CT. J Nucl Med. 2005;46:917–22. [PubMed] [Google Scholar]
- Arnaud L, Haroche J, Malek Z, Archambaud F, Gambotti L, Grimon G, Kas A, Costedoat-Chalumeau N, Cacoub P, Toledano D, Cluzel P, Piette JC, Amoura Z. Is (18)F-fluorodeoxyglucose positron emission tomography scanning a reliable way to assess disease activity in Takayasu arteritis? Arthritis Rheum. 2009;60:1193–200. doi: 10.1002/art.24416. [DOI] [PubMed] [Google Scholar]
- Gravanis MB. Giant cell arteritis and Takayasu aortitis: morphologic, pathogenetic and etiologic factors. Int J Cardiol. 2000;75 Suppl 1:S21–33. doi: 10.1016/s0167-5273(00)00184-4. [DOI] [PubMed] [Google Scholar]
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014;53:793–801. doi: 10.1093/rheumatology/ket320. [DOI] [PubMed] [Google Scholar]
- Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56:1000–9. doi: 10.1002/art.22404. [DOI] [PubMed] [Google Scholar]
- Hoffman GS, Leavitt RY, Kerr GS, Rottem M, Sneller MC, Fauci AS. Treatment of glucocorticoid-resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum. 1994;37:578–82. doi: 10.1002/art.1780370420. [DOI] [PubMed] [Google Scholar]
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703–8. doi: 10.1002/art.11388. [DOI] [PubMed] [Google Scholar]
- Osman M, Pagnoux C, Dryden DM, Storie D, Yacyshyn E. The role of biological agents in the management of large vessel vasculitis (LVV): a systematic review and meta-analysis. PloS One. 2014;9:e115026. doi: 10.1371/journal.pone.0115026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comarmond C, Plaisier E, Dahan K, Mirault T, Emmerich J, Amoura Z, Cacoub P, Saadoun D. Anti TNF-alpha in refractory Takayasu’s arteritis: cases series and review of the literature. Autoimmun Rev. 2012;11:678–84. doi: 10.1016/j.autrev.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Hoffman GS, Merkel PA, Brasington RD, Lenschow DJ, Liang P. Anti-tumor necrosis factor therapy in patients with difficult to treat Takayasu arteritis. Arthritis Rheum. 2004;50:2296–304. doi: 10.1002/art.20300. [DOI] [PubMed] [Google Scholar]
- Nunes G, Neves FS, Melo FM, de Castro, Zimmermann AF, Pereira IA. Takayasu arteritis: anti-TNF therapy in a Brazilian setting. [Article in English, Portuguese]. Rev Bras Reumatol. 2010;50:291–8. [PubMed] [Google Scholar]
- Park MC, Lee SW, Park YB, Lee SK, Choi D, Shim WH. Post-interventional immunosuppressive treatment and vascular restenosis in Takayasu’s arteritis. Rheumatology (Oxford) 2006;45:600–5. doi: 10.1093/rheumatology/kei245. [DOI] [PubMed] [Google Scholar]
- Min PK, Park S, Jung JH, Ko YG, Choi D, Jang Y, Shim WH. Endovascular therapy combined with immunosuppressive treatment for occlusive arterial disease in patients with Takayasu’s arteritis. J Endovasc Ther. 2005;12:28–34. doi: 10.1583/12-01-04-1329.1. [DOI] [PubMed] [Google Scholar]
- Mason JC. Takayasu arteritis: surgical interventions. Curr Opin Rheumatol. 2015;27:45–52. doi: 10.1097/BOR.0000000000000127. [DOI] [PubMed] [Google Scholar]
- Tezuka D, Haraguchi G, Ishihara T, Ohigashi H, Inagaki H, Suzuki J, Hirao K, Isobe M. Role of FDG PET-CT in Takayasu arteritis: sensitive detection of recurrences. JACC Cardiovasc Imaging. 2012;5:422–9. doi: 10.1016/j.jcmg.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Miyata T, Sato O, Koyama H, Shigematsu H, Tada Y. Long-term survival after surgical treatment of patients with Takayasu’s arteritis. Circulation. 2003;108:1474–80. doi: 10.1161/01.CIR.0000089089.42153.5E. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Maetani S. Long-term outcome for 120 Japanese patients with Takayasu’s disease. Clinical and statistical analyses of related prognostic factors. Circulation. 1994;90:1855–60. doi: 10.1161/01.cir.90.4.1855. [DOI] [PubMed] [Google Scholar]
- Labarca C, Makol A, Crowson CS, Kermani TA, Matteson EL, Warrington KJ. Retrospective Comparison of Open versus Endovascular Procedures for Takayasu Arteritis. J Rheumatol. 2016;43:427–32. doi: 10.3899/jrheum.150447. [DOI] [PubMed] [Google Scholar]
- Ham SW, Kumar SR, Wang BR, Rowe VL, Weaver FA. Late outcomes of endovascular and open revascularization for nonatherosclerotic renal artery disease. Arch Surg. 2010;145:832–9. doi: 10.1001/archsurg.2010.183. [DOI] [PubMed] [Google Scholar]
- Lee BB, Laredo J, Neville R, Villavicencio JL. Endovascular management of takayasu arteritis: is it a durable option? Vascular. 2009;17:138–46. doi: 10.2310/6670.2009.00012. [DOI] [PubMed] [Google Scholar]
- Feng R, Wei X, Zhao Z, Bao J, Feng X, Qu L, Lu Q, Jing Z. Aortorenal bypass with autologous saphenous vein in Takayasu arteritis-induced renal artery stenosis. Eur J Vasc Endovasc Surg. 2011;42:47–53. doi: 10.1016/j.ejvs.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Weaver FA, Kumar SR, Yellin AE, Anderson S, Hood DB, Rowe VL, Kitridou RC, Kohl RD, Alexander J. Renal revascularization in Takayasu arteritis-induced renal artery stenosis. J Vasc Surg. 2004;39:749–57. doi: 10.1016/j.jvs.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Sharma BK, Jain S, Bali HK, Jain A, Kumari S. A follow-up study of balloon angioplasty and de-novo stenting in Takayasu arteritis. Int J Cardiol. 2000;75 Suppl 1:S147–52. doi: 10.1016/s0167-5273(00)00192-3. [DOI] [PubMed] [Google Scholar]
- Sawada S, Tanigawa N, Kobayashi M, Morioka N, Kotani K, Senda T, Okuda Y, Ohta Y. Treatment of Takayasu’s aortitis with self-expanding metallic stents (Gianturco stents) in two patients. Cardiovasc Intervent Radiol. 1994;17:102–5. doi: 10.1007/BF00193926. [DOI] [PubMed] [Google Scholar]
- Bali HK, Bhargava M, Jain AK, Sharma BK. De novo stenting of descending thoracic aorta in Takayasu arteritis: intermediate-term follow-up results. J Invasive Cardiol. 2000;12:612–7. [PubMed] [Google Scholar]
- Wang X, Dang A, Lv N, Cheng N, Cheng X, Yang Y, Song Y. Long-term outcomes of coronary artery bypass grafting versus percutaneous coronary intervention for Takayasu arteritis patients with coronary artery involvement. Semin Arthritis Rheum. 2017;47:247–52. doi: 10.1016/j.semarthrit.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Kim YW, Kim DI, Park YJ, Yang SS, Lee GY, Kim DK, Kim K, Sung K. Surgical bypass vs endovascular treatment for patients with supra-aortic arterial occlusive disease due to Takayasu arteritis. J Vasc Surg. 2012;55:693–700. doi: 10.1016/j.jvs.2011.09.051. [DOI] [PubMed] [Google Scholar]
- Joseph S, Mandalam KR, Rao VR, Gupta AK, Unni NM, Rao AS, Neelakandhan KS, Unnikrishnan M, Sandhyamani S. Percutaneous transluminal angioplasty of the subclavian artery in nonspecific aortoarteritis: results of long-term follow-up. J Vasc Interv Radiol. 1994;5:573–80. doi: 10.1016/s1051-0443(94)71556-6. [DOI] [PubMed] [Google Scholar]
- Chen B, Yu HX, Zhang J, Li XX, Wu XG, Yang SJ, Qi YX, Yan C, Wang ZG. Endovascular revascularization for carotid artery occlusion in patients with Takayasu arteritis. Eur J Vasc Endovasc Surg. 2015;49:498–505. doi: 10.1016/j.ejvs.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Kieffer E, Chiche L, Bertal A, Koskas F, Bahnini A, Blã Try, Cacoub P, Piette JC, Thomas D. Descending thoracic and thoracoabdominal aortic aneurysm in patients with Takayasu’s disease. Ann Vasc Surg. 2004;18:505–13. doi: 10.1007/s10016-004-0073-y. [DOI] [PubMed] [Google Scholar]
- Robbs JV, Abdool-Carrim AT, Kadwa AM. Arterial reconstruction for non-specific arteritis (Takayasu’s disease): medium to long term results. Eur J Vasc Surg. 1994;8:401–7. doi: 10.1016/s0950-821x(05)80957-0. [DOI] [PubMed] [Google Scholar]
- Ueno A, Maruyama Y, Tada Y, Fukushima K. Surgical treatment of dissecting aortic aneurysm. Twenty years’ experience. J Cardiovasc Surg (Torino) 1976;17:408–12. [PubMed] [Google Scholar]
- Shimizu H, Ueda T, Koizumi J, Kobayashi S, Enoki C, Kawada S. Endoluminal stent-grafting for thoracoabdominal aortic aneurysm in Takayasu’s disease. Ann Thorac Cardiovasc Surg. 2001;7:59–61. [PubMed] [Google Scholar]
- Bonilla-Abadia F, Echeverri AF, Carbonell JP, Canas CA. Multiple endovascular stent-graft implantations in a patient with aortic thoracic and abdominal aneurysms due Takayasu arteritis. Rheumatol Int. 2014;34:723–5. doi: 10.1007/s00296-012-2598-7. [DOI] [PubMed] [Google Scholar]
- Park MC, Lee SW, Park YB, Chung NS, Lee SK. Clinical characteristics and outcomes of Takayasu’s arteritis: analysis of 108 patients using standardized criteria for diagnosis, activity assessment, and angiographic classification. Scand J Rheumatol. 2005;34:284–92. doi: 10.1080/03009740510026526. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu M, Li M, Zheng W, Zhao J, Tian X, Zeng X. Cause of death in Chinese Takayasu arteritis patients. Medicine (Baltimore) 2016;95:e4069. doi: 10.1097/MD.0000000000004069. [DOI] [PMC free article] [PubMed] [Google Scholar]



