Abstract
Objectives
This study aims to compare the efficacy of the eccentric exercise (EE) and extracorporeal shock wave therapy (ESWT) on chronic midportion Achilles tendinopathy and evaluate the efficacy of these treatment modalities on tendon thickness, vascularity, and elasticity.
Patients and methods
In this randomized controlled trial, a total of 63 patients (40 females, 23 males; mean age: 37.3±12.2; range, 18 to 55 years) with chronic midportion Achilles tendinopathy were enrolled between April 2017 and December 2019. The patients were allocated randomly to two groups: the first group was treated with EE every day for three months with the Alfredson protocol, and the second group received four sessions of ESWT at weekly intervals. The study was terminated at the end of three months. Visual Analog Scales (VAS), Victorian Institute of Sports Assessment-Achilles (VISA-A) questionnaires, and ultrasonography measurements were assessed before and after treatment. Patient pain was evaluated at the two-year follow-up.
Results
At the three-month follow up, VAS scores decreased, and VISA-A scores increased in both groups (p<0.001). At the two-year-follow-up, VAS scores significantly decreased in the EE group (p<0.001), but the difference was statistically insignificant in the ESWT group (p=0.095). Tendon thickness and stiffness increased in the EE group (p=0.003 and p=0.03, respectively) while the difference was statistically insignificant in the ESWT group after treatment (p=0.173 and p=0.702, respectively).
Conclusion
Eccentric exercise and ESWT are efficient in the short term, whereas EE is efficient on tendon pain in the long term. While EE increases tendon thickness and stiffness, ESWT has no effect on these measures.
Keywords: Achilles tendon, exercise, tendinopathy.
Introduction
Achilles tendinopathy (AT) is an overuse injury that is particularly common among those competing in athletic sports, but it may also affect sedentary people.[1] Achilles tendinopathy frequently presents with pain, swelling, and disability. The incidence of midportion AT is 2.35 per 1,000 individuals in the general adult population.[2] The incidence of running-related AT is 9.1 to 10.9%, and the lifetime risk is 52% for elite athletes.[3,4] The histopathology of tendinopathy manifests as increased ground substance and number of cells, disorganized neovascularization, and collagen bundles.[5] The exact cause of AT is unknown (neovascularization is cited as a possible cause); however, most researchers consider AT to be a primarily inflammatory condition.[6] In addition to the histopathological changes identified in the tendinopathy, there are also changes in the elasticity of affected tendons, defined as the measured amount of deformation in tissue by force, which can be examined by ultrasonography (USG).[7]
Although there are various treatment modalities in midportion AT, the most recommended and effective treatment modality is an exercise-based intervention.[8,9] Alfredson protocol, containing heavy calf training, is mostly used in clinical practice, although the mechanism of eccentric exercise (EE) in promoting tendon recovery is not clearly explained.[10] Chronic AT responds to a 12-week EE protocol, which increases the rate of collagen synthesis and decreases tendon neovascularization and tendon pain in chronic AT.[11,12]
Extracorporeal shock wave therapy (ESWT) is another treatment modality for Achilles tendon disorders. Shock waves provide cell-to-cell contact and cell interactions with the extracellular matrix. This leads to conformational changes in membrane proteins and, subsequently, in the intracellular signal generation that modifies gene expression and release of growth factors (also called mechanotransduction), allowing the tendon to heal.[13] Many studies have investigated the relationship between EE and tendon structure; however, the number of studies investigating tendon structure following ESWT is limited.[14] In preclinical studies, increased vascularization was reported in the bone-tendon junction with the use of ESWT; however, tendon neovascularization with ESWT has not been demonstrated in clinical studies.[15,16] To our knowledge, there is no clinical trial examining the efficacy of ESWT on tendon thickness and stiffness in midportion AT.
This study aimed to compare the efficacy of EE and ESWT in chronic midportion AT and evaluate the efficacy of these treatment modalities on tendon thickness, vascularity, and elasticity with the help of virtual touch quantification software.
Patients and Methods
This randomized controlled study was conducted at the outpatient clinic of the Sports Medicine of Dokuz Eylül University between April 2017 and December 2019. A total of 63 patients (40 female 23 males; mean age: 37.3±12.2; range, 18 to 55 years) with chronic midportion AT that persisted for more than six weeks were enrolled in the study. Exclusion criteria were as follows: peritendinous injections with local anesthetics or corticosteroids and physiotherapy within the last four to six weeks before the study, bilateral or insertional AT of more than six weeks, a neurological or muscle disease, peripheral vascular or systemic inflammatory disease, presence of knee and ankle deformities, previous ankle or Achilles surgery, previous Achilles tendon rupture, or previous ankle dislocation or fracture. Exclusion criteria for ESWT were as follows: presence of systemic conditions, such as pregnancy, thrombosis, or hemophilia, use of anticoagulant medications, and the presence of local disease, such as active infection or malignancy.
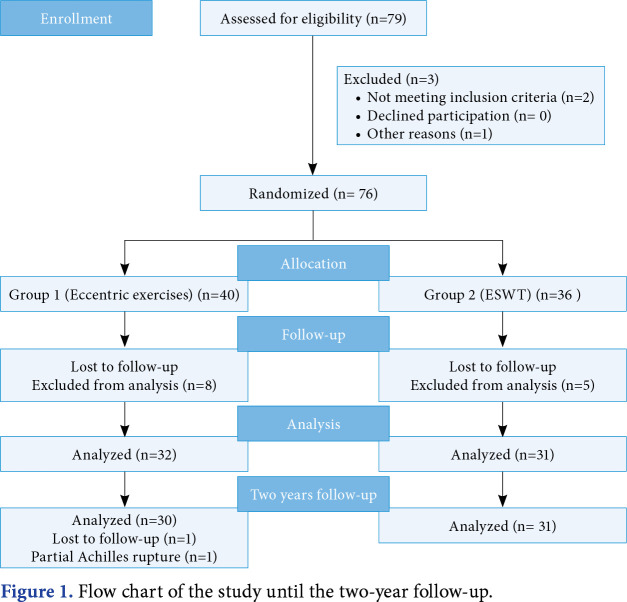
The patients were allocated to two groups by block randomization according to the treatment method, EE (Group 1) or ESWT (Group 2), by an individual not involved in any other part of the study. Patients were allocated to treatment groups by randomization in blocks of 20 in a 1:1 ratio. Ten blocks were also randomly selected from 1 and 20 in Microsoft Excel (Microsoft Corp., Redmond, WA, USA). In addition, patients were contacted by telephone two years after treatment to assess whether tendon pain had occurred (evaluated by the Visual Analog Scale [VAS]). One patient in Group 1 suffered a partial rupture according to the patient's note and a magnetic resonance imaging report taken from a different hospital. One patient did not respond to repeated telephone calls for the two-year follow-up (Figure 1).
Figure 1. Flow chart of the study until the two-year follow-up.
All patients were informed not to receive other medical treatments, such as analgesics, steroids, and injections, or other alternative treatments method for pain due to AT. During treatment with either modality, patients were not limited in their daily activity.
Procedure of eccentric exercises in Group 1
Patients were instructed to perform EE with the Alfredson protocol: 3 sets x 15 repetitions, twice per day for three months at home.[2] Patients in the EE group were shown how to perform and progress through the EE program, and the ankle of the injured leg was maximally dorsiflexed. These exercises were performed without a concentric phase during movement. The patients were advised to inform the researchers and to terminate the exercise if they experienced severe pain during the exercise. If the patients could tolerate the exercises with body weight, they were instructed to do these exercises with a 5 kg backpack and increase by 1 kg every two weeks.
Procedure of extracorporeal shock wave therapy in Group 2
For all patients in Group 2, ESWT was applied to the midportion Achilles tendon by the same physician without local anesthesia for four sessions at weekly intervals. Shock waves were applied to the midportion Achilles tendon using the R15 applicator with an energy of 2.0 bar, a pulse rate of 1,500 pulses, and a frequency of 12 Hz, and to gastrocnemius-soleus muscle groups using the D20 applicator with an energy of 1.8 bar, a pulse rate of 2,000 pulses, and a frequency of 15 Herz with the ESWT device (Masterpuls MP200; Storz Medical, Tagerwilen, Switzerland). Extracorporeal shockwave therapy application did not have any side effects on the patients.
Outcome measurement
All outcomes were assessed by a single observer who was unblinded to the patients’ problems and experienced in the diagnosis and treatment of AT. The radiologists and the statistician were blinded to the treatment groups. At the first visit, the patients were examined for local tenderness in the midportion of the tendon using a VAS that rated the severity of pain on a scale of 0 to 10, where 0 indicated no pain and 10 signified severe pain. The Achilles tendon function was evaluated with the Victorian Institute of Sports Assessment-Achilles questionnaire (VISA-A).[19] The Turkish version of VISA-A is also a reliable and valid scale.[20] Patients who had a VISA-A score of 70 points or more and patients with a VAS of 0 (no pain during daily activities or sports) were considered clinically recovered after three months.[19]
All USG examinations were performed with an Acuson S3000 ultrasound system (Siemens Medical Solution, Erlangen, Germany) equipped with a 9L4 linear transducer and virtual touch quantification software. The middle part of the tendon was measured 5 cm proximal to the tendon. Three consecutive measurements were performed and the average of these measurements was calculated. Tendon vascularization was assessed by color Doppler USG, and the presence or absence of vascularization was noted in the midportion of the tendon.
Elastography was performed by an experienced radiologist who held the probe longitudinally without compressing the tendon. The region of interest (ROI) was evaluated for shear-wave velocity values in the targeted area. The Achilles tendon strain (ROI 1) and Kager’s fat strain (ROI 2) were measured and calculated using built-in software and the strain ratio (SR), respectively (SR: ROI 1/ROI 2). Three ROI measurements were done and the average SR of the tendon was calculated. A grading system consisting of four grades was used to evaluate the color scale using the colors red, yellow, green, and blue. Relative tendon stiffness was estimated by color codes ranging from red (soft) to blue (stiff).
Reliability of USG measurements
The agreement and reproducibility of mean SR values and mean tendon thickness values were calculated by the intraobserver correlation coefficient (intra-CC), respectively. The intra-CC estimates the reliability between the three measurements taken by the same observer. Values below 0.50 represented poor reliability, values between 0.50-0.74 represented moderate reliability, values between 0.75-0.90 represented good reliability, and values above 0.90 represented excellent reliability.[21]
Statistical analysis
G*Power version 3.08 software (Heinrich-Heine Universität Düsseldorf, Düsseldorf, Germany) was used to calculate the sample size for this study. The sample size was calculated based on the primary outcome of VISA-A. For each group, a total of 27 patients were needed to achieve an effect size of 0.69, a power of 80%, and a significance level of 0.05. The effect size was calculated as 0.69 based on the VISA-A score found in the study by Rompe et al.[17] Anticipating dropouts, we planned to include a total of 30 patients in each group.
Statistical analyses were performed using the IBM SPSS version 22.0 software (IBM Corp, Armonk, NY, USA). A p value of <0.05 was considered statistically significant. Continuous variables were presented in mean ± SD or median (interquartile range), while categorical variables were presented in number and frequency. The normal distributions of the variables were investigated with the Shapiro-Wilk test. In the comparison of descriptive characteristics between the groups before treatment, age, height, and weight were assessed by the independent samples t-test, duration of symptoms and sporting time were determined by the Mann-Whitney U test, and other descriptive features were evaluated by the chi-square test. According to the suitability of continuous variables, analysis was made with the Student’s t-test or the Mann-Whitney U test. The continuity correction chi-square test or Fisher exact test were used for categorical data. While the McNemar test was used to compare the groups pre-and post-treatment in terms of neovascularization, the McNemar-Bowker test was used to compare the change with treatment in color classification. The paired samples t-test was used to compare pre-and post-treatment continuous measurements within each treatment group. Intraclass correlation estimates and their 95% confidence intervals were calculated based on a mean-rating (k=3), two-way, mixed-effects model with absolute agreement.
Results
Although all 63 patients completed the study, only 61 of these could be followed at the end of two years (Figure 1). The basic characteristics of the study participants show that the difference was statistically insignificant between the two treatment groups (Table 1). The types of exercises and sports were as follows: fitness (n=15), walking (n=12), pilates (n=5), soccer (n=4), volleyball (n=3), orienteering (n=2), basketball (n=1), taekwondo (n=1), and yoga (n=1).
Table 1. Basic characteristics of the patients.
| Characteristics | Group 1 | Group 2 | p | ||||||||
| n | % | Mean±SD | Median | IQR | n | % | Mean±SD | Median | IQR | ||
| Mean age (year) | 34.8 ±13.3 | 40.0±10.5 | 0.09 | ||||||||
| Height (cm) | 170.8±10.4 | 167.1±7.1 | 0.112 | ||||||||
| Weight (kg) | 75.1±13.5 | 76.0±11.3 | 0.766 | ||||||||
| Body mass index (kg/m2) | 25.8±4 | 25.7 ±4 | 0.064 | ||||||||
| Sporting time (month) | 24 | 9-93 | 48 | 4-10 | 0.107 | ||||||
| Frequency of sporting (hours/week) | 9 | 7-14 | 7 | 6-90 | 0.152 | ||||||
| Duration of symptoms (month) | 120 | 24-120 | 80 | 32-260 | 0.089 | ||||||
| Sex | 0.225 | ||||||||||
| Female | 18 | 56 | 22 | 71 | |||||||
| Male | 14 | 9 | |||||||||
| Smoking | 8 | 25 | 6 | 19 | 0.590 | ||||||
| Sports participation | 23 | 72 | 21 | 68 | 0.721 | ||||||
| Dominant extremity | 0.492 | ||||||||||
| Right | 32 | 31 | |||||||||
| Left | 0 | 1 | |||||||||
| Symptomatic extremity | 0.898 | ||||||||||
| Right | 16 | 16 | |||||||||
| Left | 16 | 15 | |||||||||
| SD: Standard deviation; IQR: Interquartile range; Group 1: Eccentric exercise; Group 2: Extracorporeal shock wave therapy. | |||||||||||
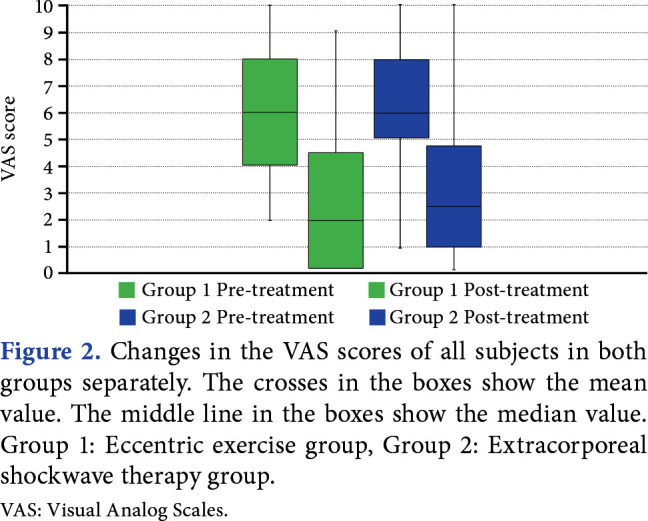
The difference in mean pre-treatment VAS scores was not statistically insignificant between groups (Group 1, 5.9±2.4; Group 2, 6.3±2.2; p=0.551). At the three-month follow-up, both groups showed improved post-treatment scores (Group 1, 2.6±2.5; Group 2, 2.6±2.3). Visual Analog Scale scores were decreased following treatment compared to the pre-treatment levels (Group 1, effect size: 1.193, p<0.001; Group 2, effect size: 1.276, p<0.001). The difference between Group 1 and Group 2 in the change of VAS scores after treatment was statistically insignificant (p=0.643; Figure 2). At the two-year follow-up, VAS scores decreased in Group 1, but the difference was statistically insignificant in Group 2 (Group 1, 1.2±1.3, p<0.001; Group 2, 5.4±3.5, p=0.095).
Figure 2. Changes in the VAS scores of all subjects in both groups separately. The crosses in the boxes show the mean value. The middle line in the boxes show the median value. Group 1: Eccentric exercise group, Group 2: Extracorporeal shockwave therapy group. VAS: Visual Analog Scales.
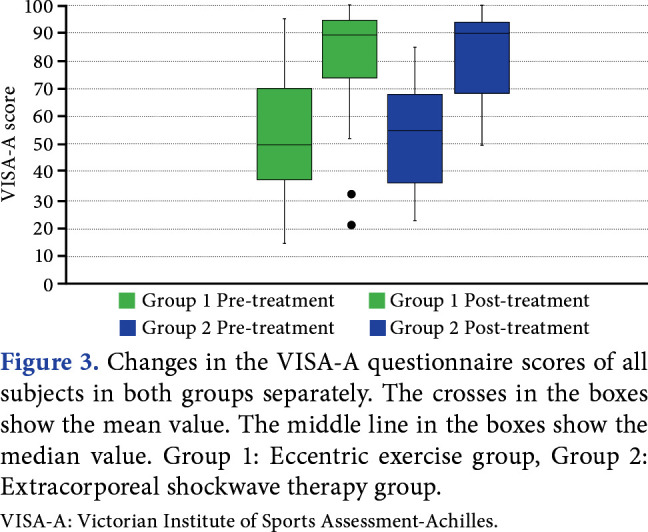
The mean pre-treatment VISA-A scores showed no significant difference between groups (Group 1, 52.6±20.5; Group 2, 53.3±18.4; p=0.882). In post-treatment, both groups showed significantly better results compared to pre-treatment scores (Group 1, 80.0±24.3; Group 2, 81.4±15.6). The VISA-A scores were increased following treatment compared to the pre-treatment levels (Group 1, effect size: 1.398, p<0.001; Group 2, effect size: 1.488, p<0.001). The difference between the increased VISA-A scores of Group 1 and Group 2 after treatment was statistically insignificant (p=0.883; Figure 3).
Figure 3. Changes in the VISA-A questionnaire scores of all subjects in both groups separately. The crosses in the boxes show the mean value. The middle line in the boxes show the median value. Group 1: Eccentric exercise group, Group 2: Extracorporeal shockwave therapy group. VISA-A: Victorian Institute of Sports Assessment-Achilles.
In this study, intra-CC values were moderate to excellent, ranging between 0.74 to 0.99 (Table 2). Tendon thickness in the Group 1 increased after treatment (p=0.002), while it decreased in the Group 2 (statistically insignificant; p=0.173). The change in tendon vascularization was not significant statistically (p=0.011). Tendon SRs of the Group 1 increased significantly after treatment (p=0.03), while the difference statistically insignificant in Group 2 (p=0.702), (Table 3).
Table 2. Intraobserver reliability of the USG measurement.
| Pre-treatment | Post-treatment | |||||
| ICC (r) | 95% CI | p | ICC (r) | 95% CI | p | |
| Tendon thickness | 0.990 | 0.984-0.994 | <0.001* | 0.993 | 0.990-0.996 | <0.001* |
| Strain ratio | 0.737 | 0.630-0.823 | <0.001* | 0.762 | 0.666-0.840 | <0.001* |
| USG: Ultrasonography; ICC: Intraclass correlation; CI: Confidence interval; * p value of <0.05 is significant. | ||||||
Table 3. Measurements of Achilles tendon structure by USG.
| Group 1 | Group 2 | |||||||||||||||
| Pre-treatment | Post-treatment | p | Effect size | Pre-treatment | Post-treatment | p | Effect size | |||||||||
| n | % | Mean±SD | n | % | Mean±SD | n | % | Mean±SD | n | % | Mean±SD | |||||
| Tendon thickness (cm) | 4.7±1.2 | 5.2±2.0 | 0.002* | 1.168 | 5.9±1.9 | 5.7±2.0 | 0.173 | 0.420 | ||||||||
| Neovascularization | 0.062 | N/A | 0.161 | N/A | ||||||||||||
| Yes | 2 | 6.3 | 1 | 3.1 | 5 | 16.1 | 1 | 3.2 | ||||||||
| No | 30 | 31 | 26 | 30 | ||||||||||||
| Strain ratio | 2.1±1.5 | 3.1±2.2 | 0.039* | 0.701 | 1.8±1.4 | 1.9±1.7 | 0.702 | 0.702 | ||||||||
| Color classification | ||||||||||||||||
| Red | 22 | 69 | 20 | 63 | N/A | 25 | 79 | 22 | 71 | N/A | ||||||
| Yellow | 8 | 25 | 7 | 22 | N/A | 2 | 7 | 2 | 7 | N/A | ||||||
| 0.423 | 0.306 | |||||||||||||||
| Green | 1 | 3 | 2 | 6 | N/A | 2 | 7 | 6 | 19 | N/A | ||||||
| Blue | 1 | 3 | 3 | 9 | N/A | 2 | 7 | 1 | 3 | N/A | ||||||
| USG: Ultrasonography; SD: Standard deviation; N/A: Not available; Group 1: Eccentric exercise; Group 2: Extracorporeal shock wave therapy; * p value of <0.05 is significant. | ||||||||||||||||
After treatment, 50 of the 63 (79%) patients recovered, and 13 (21%) patients did not. Twenty-six (81%) patients recovered in Group 1, while 24 (77%) patients recovered in Group 2. The mean duration of symptoms of the patients who did not recover was 50±34 months, while that of those who recovered was 20±13 months, and the duration of complaints of patients who recovered was significantly longer (p=0.008). Of the recovered patients, 84% were participating in sports, while 42% of the nonrecovered patients were not, and recovery was statistically significant in the group playing sports (p<0.001).
Discussion
This study found that EE and ESWT both have positive clinical effects in patients with chronic midportion AT regarding pain and function in the short term, while the effects of EE persist on tendon pain in the long term. In the short term, EE also cause structural changes in tendon thickness and stiffness, while ESWT does not cause any changes.
Eccentric exercise is the most efficient treatment modality for chronic midportion AT, and success rates vary between 49 and 90%, as confirmed by the present study.[17,22-25] In the study by Rompe et al.,[17] which is closest to our treatment protocol, 60% of the patients were recovered with EE, and only 32% of the patients in the intervention group were athletes. In this study, 70% of the patients performed at least one exercise activity. Eccentric exercises provide more benefits in active individuals than in sedentary. Therefore, the VISA-A score may have increased less in this study since it was found to be more active in the previous study.[17] There are three studies in the literature in which ESWT was applied only to patients with chronic midportion AT.[17,26,27] In these studies, success rates are between 53 and 87.5%, as confirmed by the present study.
The VAS score is evaluated by the amount of pain and is not specific for AT. Robinson et al.,[19] showed that it is a reliable and valid scale for determining the severity of AT. It has been validated specifically for AT, and evaluate tendon function. Victorian Institute of Sports Assessment-Achilles was determined to show an improvement above 70 points in the study by Robinson et al.;[19] therefore, we used this value as well. We believe that it would be more appropriate to evaluate the recovery in AT treatment to use both parameters in a common framework instead of only with pain or function of the tendon. However, there is no established recovery criteria for AT in the literature. Therefore, recovery criteria consisting of VAS and VISA-A were created to evaluate the efficacy of treatment in this study.
The Achilles tendon plays a substantial role in the storage and release of energy during movement. Greater tendon strain may occur with less tendon stiffness when the load is applied.[28] While tendon stiffness decreases in AT, chronic mechanical loading increases.[29] Siu et al.[30] reported that the stiffness in the Achilles tendons of people who exercise frequently was significantly higher than the stiffness in the tendons of people who exercised infrequently but not in the dominant ankle. The Achilles tendons of athletes are stiffer than those of sedentary people; therefore, altering the viscoelastic properties of the tendon can prevent and treat tendinopathies.[7,31]
In this study, we found that tendon stiffness increased with EE, whereas it was unchanged with ESWT. In a study by Mahieu et al.,[32] Achilles tendon stiffness remained unchanged with EE after six weeks; however, the changes were measured with a dynamometer. Leung et al.[33] reported that tendon stiffness, measured by shear-wave elastography, increased immediately after EE of 10 sets with 15 repetitions. Geremia et al.[34] found that tendon stiffness increased after 12 weeks with high-load plantar flexor training. Increased tendon stiffness is consistent with other studies in the literature.[33,34] The mechanism of change in tendon stiffness response is not clear; however, it was shown that the rate of Type 1 collagen synthesis is increased by EE, which may be due to the combination of new collagen fiber formation and increased density of cross-links.[12,35]
This is the first research to show the efficacy of ESWT on the structural properties of AT. Altivi et al.[14] reported that the thickness and stiffness of plantar fascia increased with three sessions of ESWT in patients with plantar fasciopathy. Additional studies are needed to investigate the relationship between ESWT and tendon stiffness. While both treatments have similar short-term improvement rates, EE is superior to ESWT in long-term results, possibly due to the increased tendon stiffness in the present study. This hypothesis requires additional studies with longer follow-up periods.
Five studies demonstrated decreased tendon thickness following 12 weeks of EE application in midportion AT, while two studies did not.[36-42] Interestingly, the tendon thickness increased in the present study after EE. We attribute this result to the strengthening mechanism of the exercise, and the premise that tendon thickness might increase with persistent exercise, regaining its original structure. This hypothesis requires additional studies with longer follow-up periods.
In our study, ESWT did not change tendon thickness as demonstrated in other recent studies.[17,43] More studies investigating the efficacy of ESWT need to be conducted on tendon thickness in midportion AT.
Tendon pain is associated with the presence of tendon neovascularization.[6] Some researchers observed that both EE and ESWT reduced tendon vascularization;[11,16,38] however, we did not find any change in tendon vascularization with these treatments. The studies of Beyer et al.[38] and Ohberg and Alfredson[11] demonstrated that Achilles tendon vascularization could be decreased after EE (Alfredson protocol). A study conducted by Stefansson et al.[42] showed no change in Achilles tendon vascularization with EE. In the present study, no change was observed in the neovascularization of the tendon with ESWT. Santamato et al.[16] reported that neovascularization did not disappear in 91.7% of the patients with midportion AT after five sessions of focused ESWT. Extracorporeal shock wave therapy caused angiogenesis in the rabbit tendon, contributing positively to tendon improvement.[15] However, these studies seem to contradict studies in which neovascularization causes tendon pain. Rompe et al.[17] calculated the power of the study based on the VISA-A, and no significant efficacy on vascularization was observed. There was no significant efficacy on tendon vascularization with either treatment modality in the current study.
This study has some limitations. Some of the data collection tools used were based on the patients’ notifications, and the reliability of the data was limited by the accuracy of the information provided by the participants. Futhermore, patients may have made unrealistic declarations, and adaptation to treatment may have decreased in time due to the home-based exercise design. Another potential limitation is that a control group could not be established in patients who applied to the outpatient clinic with Achilles tendon pain, as it was not ethically appropriate to deny these patients treatment. This study can be generalized to patients with chronic midportion AT but not be to the athlete or insertional ATs.
Eccentric exercises should be performed with supervision. More research is needed to determine a cut-off value of tendon stiffness in tendinopathy and tendon healing. Although changes due to EE in tendon structure are shown with elastography, any correlations should also be investigated with histopathology. Biochemical and histopathological studies are needed to better understand the structural effects and changes in tendons with these treatment modalities commonly used in the management of AT.
In conclusion, EE and ESWT are both efficient treatment modalities for chronic midportion AT. Eccentric exercises and ESWT are efficient in the short term, whereas EEs are efficient on tendon pain in the long term. While EE increases tendon thickness and stiffness, there is no change with ESWT in the short term.
Acknowledgments.
The authors would like to thank all the participants for graciously donating their time and also like to thank would Cenk Benli, MD. from Karşıyaka District Health Director, Izmir, Türkiye, for reviewing the results and the statistical analysis. Thank you Prof. Dr. Metin Ergün, MD. from Department of Sports Medicine of Ege University, Izmir, Türkiye for the correction and control at the writing stage.
Footnotes
Ethics Committee Approval: The study protocol was approved by the Dokuz Eylül University Clinical Research Ethics Committee (No: 366-SBKAEK) and Republic of Türkiye Medical Devices and Drug Institute (No: 62752). The study was conducted in accordance with the guidelines of the Declaration of Helsinki.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: This study design was created: M.D.B., H.T.; The patients were treated: M.D.B., A.B.T.K., M.A.T.; Images were scanned with ultrasonography: A.B., A.P., K.M.S.; Manuscript was drafted: M.D.B.; Critical revision of the manuscript for important intellectual content: M.D.B., H.T., O.Y.; All authors read and approved the final version of the manuscript.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Patient Consent for Publication: A written informed consent was obtained from the participants.<br><br><b>Data Sharing Statement:</b><br> The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Järvinen TA, Kannus P, Maffulli N, Khan KM. Achilles tendon disorders: Etiology and epidemiology. Foot Ankle Clin. 2005;10:255–266. doi: 10.1016/j.fcl.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.de Jonge S, van den Berg C, de Vos RJ, van der Heide HJ, Weir A, Verhaar JA, et al. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med. 2011;45:1026–1028. doi: 10.1136/bjsports-2011-090342. [DOI] [PubMed] [Google Scholar]
- 3.Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15:133–135. doi: 10.1097/01.jsm.0000165347.55638.23. [DOI] [PubMed] [Google Scholar]
- 4.Lopes AD, Hespanhol Júnior LC, Yeung SS, Costa LO. What are the main running-related musculoskeletal injuries. A Systematic Review. Sports Med. 2012;42:891–905. doi: 10.1007/BF03262301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfredson H. Conservative management of Achilles tendinopathy: New ideas. Foot Ankle Clin. 2005;10:321–329. doi: 10.1016/j.fcl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: An ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc. 2001;9:233–238. doi: 10.1007/s001670000189. [DOI] [PubMed] [Google Scholar]
- 7.Dirrichs T, Schrading S, Gatz M, Tingart M, Kuhl CK, Quack V. Shear Wave Elastography (SWE) of asymptomatic Achilles tendons: A comparison between semiprofessional athletes and the nonathletic general population. Acad Radiol. 2019;26:1345–1351. doi: 10.1016/j.acra.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: A systematic review on different protocols. Scand J Med Sci Sports. 2015;25:3–15. doi: 10.1111/sms.12208. [DOI] [PubMed] [Google Scholar]
- 9.Kingma JJ, de Knikker R, Wittink HM, Takken T. Eccentric overload training in patients with chronic Achilles tendinopathy: A systematic review. e3Br J Sports Med. 2007;41 doi: 10.1136/bjsm.2006.030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy MC, Travers MJ, Chivers P, Debenham JR, Docking SI, Rio EK, et al. Efficacy of heavy eccentric calf training for treating mid-portion Achilles tendinopathy: A systematic review and meta-analysis. Br J Sports Med. 2019;53:1070–1077. doi: 10.1136/bjsports-2018-099934. [DOI] [PubMed] [Google Scholar]
- 11.Ohberg L, Alfredson H. Effects on neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2004;12:465–470. doi: 10.1007/s00167-004-0494-8. [DOI] [PubMed] [Google Scholar]
- 12.Langberg H, Ellingsgaard H, Madsen T, Jansson J, Magnusson SP, Aagaard P, et al. Eccentric rehabilitation exercise increases peritendinous type I collagen synthesis in humans with Achilles tendinosis. Scand J Med Sci Sports. 2007;17:61–66. doi: 10.1111/j.1600-0838.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 13.d’Agostino MC, Craig K, Tibalt E, Respizzi S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int J Surg. 2015;24:147–153. doi: 10.1016/j.ijsu.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Alviti F, D'Ercole C, Schillizzi G, Mangone M, Bernetti A, Ioppolo F, et al. Elastosonographic evaluation after extracorporeal shockwave treatment in plantar fasciopathy. Med Ultrason. 2019;21:399–404. doi: 10.11152/mu-1976. [DOI] [PubMed] [Google Scholar]
- 15.Wang CJ, Wang FS, Yang KD, Weng LH, Hsu CC, Huang CS, et al. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J Orthop Res. 2003;21:984–989. doi: 10.1016/S0736-0266(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 16.Santamato A, Beatrice R, Micello MF, Fortunato F, Panza F, Bristogiannis C, et al. Power Doppler ultrasound findings before and after focused extracorporeal shock wave therapy for Achilles tendinopathy: A pilot study on pain reduction and neovascularization effect. Ultrasound Med Biol. 2019;45:1316–1323. doi: 10.1016/j.ultrasmedbio.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Rompe JD, Nafe B, Furia JP, Maffulli N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: A randomized controlled trial. Am J Sports Med. 2007;35:374–383. doi: 10.1177/0363546506295940. [DOI] [PubMed] [Google Scholar]
- 18.Alfredson H, Pietilä T, Jonsson P, Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360–366. doi: 10.1177/03635465980260030301. [DOI] [PubMed] [Google Scholar]
- 19.Robinson JM, Cook JL, Purdam C, Visentini PJ, Ross J, Maffulli N, et al. The VISA-A questionnaire: A valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35:335–341. doi: 10.1136/bjsm.35.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dogramaci Y, Kalaci A, Kücükkübas N, Inandi T, Esen E, Yanat AN. Validation of the VISA-A questionnaire for Turkish language: The VISA-A-Tr study. Br J Sports Med. 2011;45:453–455. doi: 10.1136/bjsm.2009.060236. [DOI] [PubMed] [Google Scholar]
- 21.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahlström M, Jonsson P, Lorentzon R, Alfredson H. Chronic Achilles tendon pain treated with eccentric calfmuscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327–333. doi: 10.1007/s00167-003-0418-z. [DOI] [PubMed] [Google Scholar]
- 23.de Vos RJ, Weir A, Visser RJ, de Winter T, Tol JL. The additional value of a night splint to eccentric exercises in chronic midportion Achilles tendinopathy: A randomised controlled trial. e5Br J Sports Med. 2007;41 doi: 10.1136/bjsm.2006.032532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yelland MJ, Sweeting KR, Lyftogt JA, Ng SK, Scuffham PA, Evans KA. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: A randomised trial. Br J Sports Med. 2011;45:421–428. doi: 10.1136/bjsm.2009.057968. [DOI] [PubMed] [Google Scholar]
- 25.Mafi N, Lorentzon R, Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2001;9:42–47. doi: 10.1007/s001670000148. [DOI] [PubMed] [Google Scholar]
- 26.Furia JP. High-energy extracorporeal shock wave therapy as a treatment for chronic noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:502–508. doi: 10.1177/0363546507309674. [DOI] [PubMed] [Google Scholar]
- 27.Lakshmanan P, O'Doherty DP. Chronic Achilles tendinopathy: Treatment with extracorporeal shock waves. Foot and Ankle Surgery. 2004;10:125–130. [Google Scholar]
- 28.Mafi N, Lorentzon R, Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Arthrosc. 2001;9:42–47. doi: 10.1007/s001670000148. [DOI] [PubMed] [Google Scholar]
- 29.Aubry S, Nueffer JP, Tanter M, Becce F, Vidal C, Michel F. Viscoelasticity in Achilles tendonopathy: Quantitative assessment by using real-time shear-wave elastography. Radiology. 2015;274:821–829. doi: 10.1148/radiol.14140434. [DOI] [PubMed] [Google Scholar]
- 30.Siu WL, Chan CH, Lam CH, Lee CM, Ying M. Sonographic evaluation of the effect of long-term exercise on Achilles tendon stiffness using shear wave elastography. J Sci Med Sport. 2016;19:883–887. doi: 10.1016/j.jsams.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Wiesinger HP, Kösters A, Müller E, Seynnes OR. Effects of increased loading on in vivo tendon properties: A systematic review. Med Sci Sports Exerc. 2015;47:1885–1895. doi: 10.1249/MSS.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahieu NN, McNair P, Cools A, D'Haen C, Vandermeulen K, Witvrouw E. Effect of eccentric training on the plantar flexor muscle-tendon tissue properties. Med Sci Sports Exerc. 2008;40:117–123. doi: 10.1249/mss.0b013e3181599254. [DOI] [PubMed] [Google Scholar]
- 33.Leung WKC, Chu KL, Lai C. Sonographic evaluation of the immediate effects of eccentric heel drop exercise on Achilles tendon and gastrocnemius muscle stiffness using shear wave elastography. e3592PeerJ. 2017;5 doi: 10.7717/peerj.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geremia JM, Baroni BM, Bobbert MF, Bini RR, Lanferdini FJ, Vaz MA. Effects of high loading by eccentric triceps surae training on Achilles tendon properties in humans. Eur J Appl Physiol. 2018;118:1725–1736. doi: 10.1007/s00421-018-3904-1. [DOI] [PubMed] [Google Scholar]
- 35.Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009;19:500–510. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 36.Ohberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: Normalised tendon structure and decreased thickness at follow up. Br J Sports Med. 2004;38:8–11. doi: 10.1136/bjsm.2001.000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Plas A, de Jonge S, de Vos RJ, van der Heide HJ, Verhaar JA, Weir A, et al. A 5-year follow-up study of Alfredson's heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214–218. doi: 10.1136/bjsports-2011-090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beyer R, Kongsgaard M, Hougs Kjær B, Øhlenschlæger T, Kjær M, Magnusson SP. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: A randomized controlled trial. Am J Sports Med. 2015;43:1704–1711. doi: 10.1177/0363546515584760. [DOI] [PubMed] [Google Scholar]
- 39.von Wehren L, Pokorny K, Blanke F, Sailer J, Majewski M. Injection with autologous conditioned serum has better clinical results than eccentric training for chronic Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2019;27:2744–2753. doi: 10.1007/s00167-019-05465-8. [DOI] [PubMed] [Google Scholar]
- 40.Grigg NL, Wearing SC, Smeathers JE. Eccentric calf muscle exercise produces a greater acute reduction in Achilles tendon thickness than concentric exercise. Br J Sports Med. 2009;43:280–283. doi: 10.1136/bjsm.2008.053165. [DOI] [PubMed] [Google Scholar]
- 41.Petersen W, Welp R, Rosenbaum D. Chronic Achilles tendinopathy: A prospective randomized study comparing the therapeutic effect of eccentric training, the AirHeel brace, and a combination of both. Am J Sports Med. 2007;35:1659–1667. doi: 10.1177/0363546507303558. [DOI] [PubMed] [Google Scholar]
- 42.Stefansson SH, Brandsson S, Langberg H, Arnason A. Using pressure massage for Achilles tendinopathy: A single-blind, randomized controlled trial comparing a novel treatment versus an eccentric exercise protocol. Orthop J Sports Med. 2019;7:2325967119834284–2325967119834284. doi: 10.1177/2325967119834284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng Y, Zhang J, Cai Y. Utility of ultrasonography in assessing the effectiveness of extracorporeal shock wave therapy in insertional Achilles tendinopathy. Biomed Res Int. 2016;2016:2580969–2580969. doi: 10.1155/2016/2580969. [DOI] [PMC free article] [PubMed] [Google Scholar]