Abstract
Objectives
This study aims to compare the short-term efficacy of mud-pack (MP) and hot-pack (HP) treatments with the same temperature and duration on sleep, function, depression, and quality of life for chronic non-specific neck pain (CNNP) patients.
Patients and methods
Between December 2018 and September 2019, a total of 70 patients with CNNP diagnosis (12 males, 58 females; mean age: 50.2±9.4 years; range, 24 to 65 years) were included. The patients were divided into two groups. The MP group (n=35) had a total of 15 sessions of MP for 20 min + transcutaneous electrical nerve stimulation (TENS) for 20 min + home exercise (HE) on five days per week for three weeks. The HP group (n=35) had 15 similar sessions of HP for 20 min + TENS for 20 min + HE. The patients were assessed with the Visual Analog Scale (VAS-pain), VAS physician’s and patient’s global assessments, modified Neck Disability Index (mNDI), Beck Depression Inventory (BDI), Pittsburgh Sleep Quality Index (PSQI), and Short Form-36 (SF-36) measures before treatment, at the end of post-treatment third week and one month later.
Results
In the MP group, there were statistically significant improvements in all parameters at the end of treatment three-week and one-month follow-up (p<0.05), apart from SF-36 Vitality/Energy (SF-36V/E) at the end of treatment and SF-36 General Health (SF-36GH) at one month. In the HP group, there were statistically significant improvements observed for all parameters (p<0.05), apart from the SF-36 Physical Role and SF-36GH at the end of treatment third week and SF-36V/E at the first-month assessment. The VAS-pain(p<0.001), mNDI (p=0.019), BDI (p=0.002), SF-36GH (p<0.001), SF-36V/E (p<0.001) and SF-36 mental health (p<0.001) showed statistically significantly superior improvements in the MP group (p<0.05).
Conclusion
In CNNP patients, both MP and HP treatments are effective. However, MP therapy has more positive effects on pain, function, depression, and quality of life parameters. The MP treatment may be used in addition to TENS treatment for CNNP patients.
Keywords: Chronic neck pain, home exercise, hot pack, mud pack, thermotherapy.
Introduction
Neck pain (NP) is one of the most common musculoskeletal system disorders and is the fourth leading cause of disability worldwide. A systemic review estimated the point, annual, and lifelong prevalence rates as 7.6% (range, 5.9 to 22.2%), 37.2% (range, 16.7 to 75.1%) and 48.5% (range 14.2 to 71%), respectively. Symptoms longer than three months may be referred to as chronic neck pain (CNP).[1,2] Neck pain is associated with risk factors like physiopathology, genetics, sleep problems, smoking, obesity, sedentary lifestyle, previous NP, and poor general health status.[1] The most common NP is chronic non-specific neck pain (CNNP) which cannot be attributed to a specific cause. Treatment includes pharmacological and conservative approaches. Pharmacological treatments may use paracetamol, steroids, non-steroidal anti-inflammatory drugs (NSAIDs), opiates, and topical analgesics.[3] Conservative approaches may be listed as patient education, cognitive-behavioral therapy, exercise, electrotherapy, diathermia, acupuncture, laser therapy, ultrasound, manual therapy, heat therapy, and balneotherapy.[3-8]
In the conservative approach, exercise reduces pain while increasing muscle strength, and improving motor functions and quality of life (QoL).[9] Administration of transcutaneous electrical nerve stimulation (TENS) in CNP has been shown to be more effective than placebo in reducing pain.[10] Thermotherapy is the application of heat to reduce pain. Thermotherapy applications are treatments with beneficial effects shown in clinical and physiological research. Nociception in soft tissue, local circulation, metabolism, and effect on functionality play roles in reducing NP. It is frequently applied with different treatment approaches like exercise.[4]
Mud-pack (MP) therapy is a balneological intervention defined as the external application of medicinal mud. Medicinal muds are usually applied when warm and are a perfect tool for heat transfer.[7,11,12] Many musculoskeletal discomforts are included among indications for hyperthermic medicinal mud application including back pain, fibromyalgia, lumbago, sprains/strains, and osteoarthritis (OA).[13]
In the present study, we aimed to compare the short-term efficacy of MP and hot-pack (HP) treatments applied for the same duration at the same temperature for patients with CNNP.
Patients and Methods
This single-center, single-blind, randomizedcontrolled study was conducted at University of Health Sciences, Konya Training and Research Hospital, Department of Physical Therapy and Rehabilitation between December 20th, 2018 and September 6th, 2019. A total of 70 patients with CNNP diagnosis (12 males, 58 females; mean age: 50.2±9.4 years; range, 24 to 65 years) with NP lasting at least three months and with degenerative disc disease on radiological assessment with no neurological complaints, having the pain of at least ≥3 on the 0-10 cm Visual Analog Scale (VAS-pain) who abided by the participation criteria for the study were included. Patients receiving physical therapy, Spa treatment, MP treatment or neck exercises within the last six months, with invasive treatment to the neck region in the last six months, with spinal stenosis, disc herniation, surgical history in the cervical region, trauma, infection, malignancy or inflammatory arthritis in the cervical vertebrae, pregnancy, using psychiatric medication, with pacemakers, neurological or vascular disease diagnosis or advanced degenerative findings on radiological images were excluded from the study.
Intervention
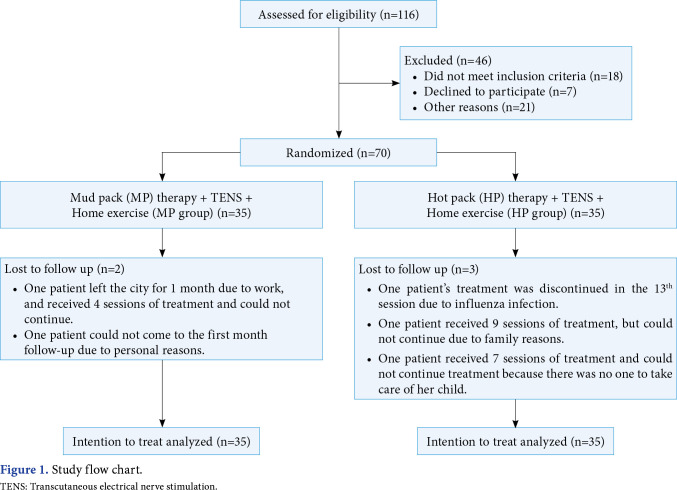
The patients were divided into two equal groups. Patients in Group 1 (MP group, n=35) had a total of 15 sessions of 20 min/day MP + 20 min/day TENS and daily home exercise (HE) program on five days per week for three weeks. Patients in Group 2 (HP group, n=35) had a total of 15 sessions of 20 min/day HP + 20 min/day TENS and HE treatment on five days per week for three weeks. Medical treatment used before treatment and during the study was recorded in both groups. The use of three or more analgesic pills per week was included in the assessment as using. The participants were requested not to take any analgesic medication in the 24 h before assessments (Figure 1).
Figure 1. Study flow chart. TENS: Transcutaneous electrical nerve stimulation.
Patients in the MP group had 1.5 to 2-cm thickness of MP applied at 45°C to the neck region for 20 min per session for a total of 15 sessions. The heat-conserving process and cleaning method for the applied medicinal mud are similar to previous studies. The medicinal mud used is rich in humic acid, humin, lignin, organic and inorganic substances.[14] In the HP group, Hydrocollator hot packs (Chattanooga Group, Hixson, TN, USA) with 25 to 50-cm dimensions were applied at 45°C for 20-min durations in a total of 15 sessions. Both groups had TENS treatment (ITO ES-320, Enraf Sonopuls 692, and Chattanooga Intelect) with 80 Hz frequency, 10 to 30 mA intensity through electrodes in the neck region for 20 min duration in a total of 15 sessions. The HE given to both groups was explained by an experienced physician and included cervical range of motion, cervical and back extensor stretches, cervical isometric, relaxation, and posture exercises. The patients were requested to perform the exercises once every day for three weeks and to repeat each exercise 10 times. Exercises were revised with small adjustments according to the patient’s pain status and functional limitations.
Evaluation parameters
VAS: The pain levels of patients were assessed using a 10-cm horizontal line. Pain level was assessed as 0= no pain and 10= most intense pain imaginable. Patient’s and physician’s global assessments VAS (VAS-PG and VAS PhG) were evaluated with a similar line.[15]
Modified Neck Disability Index (mNDI): This scale is used to assess disability. The mNDI comprises 10 sections assessing pain intensity in the neck, personal care, lifting weights, reading, headache, concentration, driving, sleep, and free-time activities. Each section is given points from 0 (no disability) to 5 (complete disability) with six possible responses. Total scale points are from 0 (no disability) to 50 (complete disability). The Turkish validity and reliability study was conducted by Kesiktas et al.[16]
Beck Depression Inventory (BDI): The BDI is a 21-item scale that evaluates the symptoms of depression. The maximum score is 63. High scores indicate an increased tendency toward depression. The Turkish validity and reliability study was investigated by Hisli.[17]
Short Form-36 (SF-36): The Turkish version of the SF-36 questionnaire, including the Turkish validity and reliability study, was used for the assessment of patients' QoL.[18] The SF-36 consists of eight subscales: physical function, physical role functioning (PR), emotional status, social function, general health (GH), mental health (MH), energy/vitality (E/V), and bodily pain. On a 0-100 scale, 0 indicates the worst QoL, whereas 100 indicates the best QoL.
Pittsburgh Sleep Quality Index (PSQI): The PSQI is a survey comprising 18 questions assessing sleep quality and disturbances over one month. It comprises seven components of subjective sleep quality, sleep latency (delay), sleep duration, habitual sleep efficiency, sleep disturbances, sleep medication use, and daytime function disorder. Each component is given points from 0 to 3. The total points for the seven components are the scale points. Total points above 5 show poor sleep quality. The validity and reliability of PSQI in the Turkish population was conducted by Ağargün et al.[19]
Sample size
Power analysis accepted effect size (d=0.68), type 1 error (alpha) 0.05, and test power 0.80 and calculated the sample size as 70 patients.
Randomization
The separation of participants into groups was completed by an independent researcher using a simple equal (1:1) randomization table created by a computer (SPSS for Windows version 15.0 software). Treatment groups were announced by other researchers. Pre-treatment, post-treatment, and one-month later evaluations were performed by a physician who was blinded to the study.
Statistical analysis
Statistical analysis was performed with the intention-to-treat (ITT) principle. Calculations accepted the type 1 error rate as alpha 0.05. Analyses were performed using the R Core Team, 2020 software.[20] Continuous variables were expressed in mean ± standard deviation (SD) and median (interquartile range [IQR]) values, while categorical variables were expressed in number and frequency. Normality was tested with the Shapiro-Wilk test. For multiple comparisons, all parameters were separately collected and longitudinal variations in the three periods were assessed with the mixed linear model. We created six different models. We included VAS-pain, VAS physician’s and patient’s global assessments, mNDI, BDI, PSQI, and SF-36 as the dependent variable and added fixed effects of treatment. We included participants as a random effect. Significance was calculated using the lmerTest package, which applies Satterthwaite’s method to estimate degrees of freedom and generate p-values for mixed models.[21] Univariate analyses used the chisquare and Fisher exact test for categorical variables and the Student t-test and Mann-Whitney U test for continuous variables. Suitable tests were selected according to whether assumptions were met or not. Additionally, for comparisons of measures at the end of treatment third-week and first-month follow-up with initial measures, the paired-sample t-test, and Wilcoxon test were used according to suitability.
Results
Figure 1 shows the flow diagram of the MP group (n=35) and HP group (n=35). The majority of the sample were females in MP group and HP group (n=26; 74.29% and n=32; 91.43%, respectively) and 84.29% of the participants were married. The mean age of the MP group was 49.4±10.9 years, and the other group was 51.2±8.1 years. In addition, 82.86% of the participants had attained primary school graduate or high school graduate, diploma, or the equivalent. The employment status of 91.45% of the participants was housewives or retired or unemployed. The rate of no smokers was 84.29%. There was no statistically significant difference between baseline demographic and disease characteristics in the groups (p>0.05) (Table 1).
Table 1. Demographic and disease characteristics of the patients and medical treatment use of patients.
| MP group (n=35) | HP group (n=35) | |||||||
| n | % | Mean±SD | n | % | Mean±SD | p | ||
| Demographic characteristics | ||||||||
| Age (year) | 49.4±10.9 | 51.2±8.1 | 0.621 | |||||
| Sex | 0.110 | |||||||
| Female | 26 | 74.29 | 32 | 91.43 | ||||
| Male | 9 | 25.71 | 3 | 8.57 | ||||
| BMI (kg/m2) | 29.8±5.5 | 31.8±6.4 | 0.240 | |||||
| Disease characteristics | ||||||||
| Disease duration (month) | 69.2±51.6 | 83.4±58.8 | 0.288 | |||||
| Evaluation time | ||||||||
| Baseline | Medical treatment | |||||||
| Count (%) | 35 | 50.00 | 35 | 50.00 | ||||
| 0 | 12 | 34.29 | 13 | 37.14 | ||||
| 1 | 6 | 17.14 | 5 | 14.29 | 0.224 | |||
| 2 | 7 | 20.00 | 13 | 37.14 | ||||
| 3 | 10 | 28.57 | 4 | 11.43 | ||||
| End of treatment | Medical treatment | |||||||
| Count (%) | 35 | 50.00 | 35 | 50.00 | ||||
| 0 | 28 | 80.00 | 14 | 40.00 | ||||
| 1 | 3 | 8.57 | 5 | 14.29 | 0.003** | |||
| 2 | 2 | 5.71 | 12 | 34.29 | ||||
| 3 | 2 | 5.71 | 4 | 11.43 | ||||
| 1st month | Medical treatment | |||||||
| Count (%) | 35 | 50.00 | 35 | 50.00 | ||||
| 0 | 26 | 74.29 | 17 | 48.57 | ||||
| 1 | 1 | 2.86 | 2 | 5.71 | 0.051 | |||
| 2 | 4 | 11.43 | 13 | 37.14 | ||||
| 3 | 4 | 11.43 | 3 | 8.57 | ||||
| MP: Mud pack, HP: Hot pack; SD: Standard deviation; BMI: Body mass index; Medical treatment= 0: No treatment; 1: Paracetamol; 2: NSAID (non-steroidal anti-inflammatory drug); 3: Myorelaxant drug; * p<0.05; ** p<0.01. | ||||||||
When the clinical assessment scales are compared with baseline assessments in the groups, apart from the SF-36 MH parameter (p=0.046), no parameters had statistically significant differences compared to initial assessments (p>0.05) (Tables 2, 3).
Table 2. Clinical evaluation parameters .
| Variables | MP group (n=35) | HP group (n=35) | Overall | MP group | HP group | |||||||
| Mean±SD | Mean | 95% CI | Effect size(d) |
Mean±SD | Mean | 95% CI | Effect size(d) |
p | p | p | p | |
| VAS-pain | ||||||||||||
| Baseline | 7.5±1.1 | 7.0±1.1 | 0.066 | |||||||||
| End of treatment | 4.2±2.2 | 3.3 | 2.383 to 4.016 | 1.365 | 4.6±1.9 | 2.42 | 1.14 to 4.85 | 0.564 | 0.937 | <0.001*** | <0.001*** | 0.002** |
| 1st month | 3.7±2.1 | 3.77 | 2.857 to 4.313 | 1.716 | 4.3±2.2 | 2.72 | 1.83 to 6.11 | 0.646 | 0.324 | <0.001*** | <0.001*** | |
| VAS-PG | ||||||||||||
| Baseline | 7.5±1.2 | 7.8±1.4 | 0.299 | |||||||||
| End of treatment | 4.7±1.7 | 2.8 | 2.128 to 3.214 | 1.714 | 5.0±2.1 | 2.79 | 1.50 to 2.77 | 1.175 | 0.489 | 0.284 | <0.001*** | <0.001*** |
| 1st month | 4.6±2.0 | 2.93 | 2.067 to 3.332 | 1.488 | 5.2±2.2 | 2.64 | 1.65 to 3.16 | 1.114 | 0.253 | <0.001*** | <0.001*** | |
| VAS-PhG | ||||||||||||
| Baseline | 7.7±0.9 | 7.7±1.1 | 0.819 | |||||||||
| End of treatment | 4.1±1.5 | 3.58 | 2.913 to 3.972 | 2.264 | 4.5±1.7 | 3.28 | 1.74 to 3.16 | 1.208 | 0.344 | 0.873 | <0.001*** | <0.001*** |
| 1st month | 3.8±1.9 | 3.9 | 3.013 to 4.244 | 2.054 | 4.6±1.9 | 3.18 | 1.60 to 3.02 | 1.134 | 0.097 | <0.001*** | <0.001*** | |
| NDI | ||||||||||||
| Baseline | 20.8±7.2 | 22.2±6.6 | 0.408 | |||||||||
| End of treatment | 14.4±7.8 | 6.39 | 4.310 to 8.432 | 1.077 | 15.9±5.9 | 6.29 | 3.41 to 7.55 | 0.922 | 0.392 | 0.019* | <0.001*** | <0.001*** |
| 1st month | 13.6±8.3 | 7.25 | 4.655 to 9.458 | 1.024 | 14.8±6.8 | 7.39 | 4.25 to 8.71 | 1.012 | 0.515 | <0.001*** | <0.001*** | |
| BDI | ||||||||||||
| Baseline | 11.8±7.1 | 15.9±8.8 | 0.034 | |||||||||
| End of treatment | 8.5±7.9 | 3.27 | 1.162 to 5.352 | 0.541 | 12.4±7.8 | 3.53 | 0.21 to 2.41 | 0.417 | 0.050 | 0.002** | 0.003** | 0.02* |
| 1st month | 7±5.0 | 4.77 | 2.033 to 6.252 | 0.684 | 11.3±7.6 | 4.6 | 1.72 to 4.27 | 0.820 | 0.01* | <0.001*** | <0.001*** | |
| PSQI | ||||||||||||
| Baseline | 8.2±3.7 | 9.9±4.1 | 0.074 | |||||||||
| End of treatment | 6.2±4.0 | 2.08 | 0.628 to 3.428 | 0.504 | 7.9±3.7 | 1.95 | -32. to -5.6 | -0.49 | 0.061 | 0.105 | 0.005** | 0.006** |
| 1st month | 6.1±4.1 | 2.11 | 0.237 to 3.419 | 0.400 | 6.1±4.1 | 3.8 | -48 to -16 | -0.70 | 0.947 | 0.025* | <0.001*** | |
| MP: Mud pack; HP: Hot pack; SD: Standard deviation; CI: Confidence interval; VAS-pain: Visual Analog Scale pain assessment; VAS-PG: Visual Analog Scale patient’s global assessment; VAS PhG: Visual Analog Scale physician’s global assessment; NDI: Neck disability index; BDI: Beck depression inventory; PSQI: Pittsburgh sleep quality index; * p<0.05; *** p<0.001; ** p<0.01. | ||||||||||||
Table 3. Clinical evaluation parameters for quality of life.
| Variables | MP group (n=35) | HP group (n=35) | Overall | MP group | HP group | |||||||
| Mean±SD | Mean | 95% CI | Effect size(d) |
Mean±SD | Mean | 95% CI | Effect size(d) |
p | p | p | p | |
| SF-36PF | ||||||||||||
| Baseline | 49.6±22.1 | 40.4±20.3 | 0.067 | |||||||||
| End of treatment | 63.4±23.5 | -13.81 | -16.9 to -7.08 | -0.85 | 52.3±21.5 | -11.91 | -16. to -5.1 | -0.66 | 0.072 | 0.144 | <0.001*** | <0.001*** |
| 1st month | 69.7±21.7 | -20.13 | -23.5 to -11.3 | -0.99 | 55±23.8 | -14.57 | to -7.7 | -0.84 | 0.011* | <0.001*** | <0.001*** | |
| SF-36PR | ||||||||||||
| Baseline | 22.1±32.5 | 18.6±31.1 | 0.640 | |||||||||
| End of treatment | 44.9±41.2 | -22.71 | -35.1 to -7.74 | -0.54 | 28.1±43.0 | -9.55 | to 4.92 | -0.20 | 0.112 | 0.581 | 0.003** | 0.2374 |
| 1st month | 51.5±42.8 | -29.38 | -42.9 to -9.90 | -0.55 | 34.4±42.0 | -15.81 | -28. to 2.64 | -0.28 | 0.108 | 0.002** | 0.101 | |
| SF-36BP | ||||||||||||
| Baseline | 38.6±14.9 | 34.9±16.4 | 0.295 | |||||||||
| End of treatment | 60.6±20.6 | -21.99 | -27.1 to -14.6 | -1.17 | 54.1±17.0 | -19.23 | -21. to -11.0 | -1.14 | 0.130 | 0.138 | <0.001*** | <0.001*** |
| 1st month | 63.3±20.6 | -24.7 | -30.1 to -15.9 | -1.13 | 54.1±16.5 | -19.17 | -21. to -11.0 | -1.05 | 0.088 | <0.001*** | <0.001*** | |
| SF-36GH | ||||||||||||
| Baseline | 46.1±21.0 | 40.7±16.4 | 0.233 | |||||||||
| End of treatment | 52.8±20.5 | -6.65 | -10.6 to -0.23 | -0.36 | 45.9±21.5 | -5.23 | -7.0 to 2.16 | -0.18 | 0.190 | <0.001*** | 0.04* | 0.289 |
| 1st month | 52.3±21.9 | -6.13 | -10.0 to 1.218 | -0.27 | 41.7±20.5 | -1.01 | -19. to 4.92 | -0.20 | 0.049* | 0.237 | 0.120 | |
| SF-36VE | ||||||||||||
| Baseline | 49.3±23.5 | 41.3±18.5 | 0.105 | |||||||||
| End of treatment | 56.0±25.9 | -6.74 | -11.9 to 0.824 | -0.30 | 49.4±20.6 | -8.09 | -9.3 to -0.3 | -0.37 | 0.207 | <0.001*** | 0.085 | 0.032* |
| 1st month | 60.5±22.6 | -11.16 | -15.6 to -0.94 | -0.39 | 50±21.1 | -8.71 | -6.4 to 4.47 | -0.06 | 0.045* | 0.028* | 0.7128 | |
| SF-36SF | ||||||||||||
| Baseline | 53.9±23.6 | 51.7±24.2 | 0.701 | |||||||||
| End of treatment | 69.3±22.5 | -15.4 | -20.4 to -7.86 | -0.78 | 65.1±21.4 | -13.43 | -10. to -2.1 | -0.52 | 0.442 | 0.821 | <0.001*** | 0.004** |
| 1st month | 74.0±21.5 | -20.17 | -20.4 to -7.86 | -0.78 | 70.9±19.1 | -19.25 | -13. to -0.9 | -0.40 | 0.538 | <0.001*** | 0.024* | |
| SF-36ER | ||||||||||||
| Baseline | 26.7±37.8 | 20.9±33.5 | 0.554 | |||||||||
| End of treatment | 46.1±43.5 | -19.4 | -9.79 to -1.62 | -0.48 | 43.8±44.4 | -22.81 | -18. to -4.3 | -0.55 | 0.791 | 0.702 | 0.007** | 0.002** |
| 1st month | 53.6±44.1 | -26.89 | -10.7 to -1.12 | -0.42 | 58.3±44.8 | -37.4 | -18. to -4.3 | -0.55 | 0.645 | 0.017* | 0.002** | |
| SF-36MH | ||||||||||||
| Baseline | 61.8±19.2 | 52.3±19.9 | 0.046* | |||||||||
| End of treatment | 68.5±19.4 | -6.64 | -30.9 to -5.25 | -0.49 | 59.6±21.9 | -7.28 | -9.7 to -0.9 | -0.42 | 0.088 | <0.001*** | 0.007** | 0.018* |
| 1st month | 70.4±19.1 | -8.59 | -38.4 to -9.23 | -0.56 | 62.5±18.5 | -10.16 | -13. to -2.6 | -0.51 | 0.095 | 0.002** | 0.004** | |
| MP: Mud pack; HP: Hot pack; SD: Standard deviation; CI: Confidence interval; SF-36PF: Short form-36 physical functioning; SF-36PR: Short form-36 physical role; SP-36BP: Short form-36 bodily pain; SF-36GH: Short form 36 general health; SF-36VE: Short form 36 Vitality/Energy; SF-36SF: Short form 36 social functioning; SF-36ER: Short form 36 emotional role; SF-36MH: Short form 36 mental health; * p<0.05; ** p<0.01; *** p<0.001. | ||||||||||||
Comparisons within groups observed that in the MP group, all parameters had statistically significant improvements (p<0.05) at the end of treatment and one-month follow-up, apart from the SF-36V/E parameter at the end of treatment and the SF-GH at the one-month follow-up. In the HP group, all parameters showed statistically significant improvements (p<0.05) at the end of treatment and one-month follow-up, apart from SF-36PR and SF-36GH at the end of treatment and one-month follow-up and SF-36V/E at one-month assessment (Tables 2, 3).
Comparisons of differences between the groups observed that improvements in the MP group were statistically significantly better for VAS-pain (p<0.001***), mNDI (p=0.019*), BDI (p=0.002**), SF-36GH (p<0.001***), SF-36V/E (p<0.001***) and SF-36MH (p<0.001***) (p<0.05) (Tables 2, 3).
Discussion
In this randomized-controlled study, we compared the short-term efficacy of MP with HP applied at the same temperature to CNNP patients. Our study results showed reduced pain, increased functionality and sleep quality, and improvements in depression and QoL assessments both at the end of treatment and 1 month later (p<0.05). Of these improvements, the improvements in pain reduction, functioning, depression, and QoL assessments appeared to be more effective in the MP treatment group (p<0.05). The reduction in analgesic consumption in the assessment at the end of treatment was statistically significant and more pronounced in the MP group (p=0.003).
Exercise is an important part of treatment in CNNP. The combination of strengthening, stretching and aerobic exercises were stated to reduce excessive fatigue and disability and increase general well-being in reviews and clinical guidelines.[9,22] A single-blind, randomized study including 27 women from 18-50 years with chronic cervical pain separated patients into manipulative treatment (n=13) and HE (n=14) groups. Assessments before and after treatment showed significant reduction in the NDI and VAS-pain scores in both groups.[23] In our study, it is thought that HE treatment may have positively contributed to reduced pain, increased functionality, and improved QoL and depressive mood in both groups. The results of our study are consistent with similar studies.
A review assessing the efficacy of TENS treatment in CNNP showed positive effects in only two out of 11 studies. These studies showed reduced pain and improvements in functional limitations were provided until the sixth month.[24] A randomized-controlled study by Chiu et al.[25] compared the efficacy of TENS and neck exercise treatment with a control group and showed that pain reduced, and functional improvement was provided in a statistically significant way (p<0.05) during the sixth-week and sixth-month assessments. A randomized-controlled study administering exercise + placebo TENS (n=20), exercise + high-frequency TENS (n=20), and exercise + low-frequency TENS (n=20) for CNP reported that TENS treatment in addition to exercise may have contributed to clinical improvements and might be beneficial.[26] In our study, TENS administration in the treatment of both groups may have contributed to reduced pain and improved functionality, consistent with previous studies.
There are many studies in the literature about the efficacy of thermotherapeutic treatments for CNP.[7,27,28] A randomized-controlled study assessing the efficacy of naturopathic administrations in addition to local thermotherapy and acupuncture for CNP observed that complementary thermotherapy and acupuncture administered to patients supported reductions in pain and improvements in functionality.[27] A randomized-controlled study by Cramer et al.[7] compared the efficacy of heated MP with a treatmentfree control group for chronic mechanical neck pain (CMNP). Heated MP treatment was given for 14 days with 20 min duration. Assessments before and after treatments showed a reduction in pain and improvements in somatosensorial functional status. A study comparing Spa therapy + HE with HE alone for CMNP randomized 70 patients into two equal groups. The Spa therapy group was administered a total of 15 sessions, on five days in three weeks, of full-body immersion in thermal water + neck region mud therapy + neck region massage + HE. The exercise group was only given the same HE programs as the Spa group. The patients were assessed with clinical assessment scales one week after the end of the third-week treatment and three months later. Assessments one week after the end of treatment showed statistically significant improvements for all parameters in both groups (VAS-pain, VAS-PG, VAS-PhG, Nottingham Health Profile (NHP), Neck Pain Disability Scale (NPDS) (p<0.05). The VAS assessments and NPDS assessments were statistically significantly superior in the Spa therapy group. At the three-month assessment, there was no significance compared to baseline in both groups. In conclusion, Spa therapy + HE were shown to be more effective in reducing pain and increasing functionality in the short term compared to HE.[28] In our study, in accordance with these studies, thermotherapy administration with heated MP and HP treatments was shown to reduce pain, increase functionality and improve QoL in the short term in patients with CNP.
The efficacy of heated MP treatments for musculoskeletal system diseases was shown in many studies. Sarsan et al.[29] compared the efficacy of MP and HP administration for knee OA patients and found statistical superiority in the MP group for up to three to six months on pain, functional status, and QoL assessments. Another double-blind, randomized study comparing the efficacy of MP and HP administration for knee OA administered a total of 10 sessions for 30-min durations on five days in two weeks to both groups. The patients were assessed before treatment, in the second week, sixth week, and 12th week after treatment. A statistically significant decrease was observed in analgesic consumption in the MP treatment group until the 12th week. Pain, functional status, and QoL assessments observed statistically significant improvements in both groups, though improvements were better with MP treatment.[30] The MP treatments have also been shown to have positive effects on chronic low back pain, fibromyalgia syndrome, and hand OA.[31-33] The results of our study suggested that MP treatment reduced pain and analgesic consumption, provided improvements in functionality and QoL. This is compatible with similar studies. The MP treatment is an inexpensive, easily applicable, and effective non-pharmacological treatment method and may be recommended for CNNP patients.
Increased blood flow, reduced tissue injury, reduced muscle spasm, and increased connective tissue elasticity are proposed to play roles in the efficacy of topical thermotherapy applications.[7,34] With analgesic effect due to the increase in β-endorphin levels obtained with thermotherapy, the anti-inflammatory effect is associated with increases in serum catecholamine and cortisol levels.[35] Mud treatment suppresses inflammatory mediators such as interleukin-1-beta, tumor necrosis factor-alpha, prostaglandin E2, and leukotriene B4 and has positive effects on antioxidant parameters like myeloperoxidase and nitric oxide.[30] A few studies have shown chemical efficacy of MP administration. Beer et al.[36] showed that water-soluble mud components like fulminic acid, ulmic acid and humic acid passed through the skin and had a stimulating effect on smooth muscle contractility mediated by alpha-2 adrenergic and dopamine D2 receptors. In vitro studies showed absorption of many chemical elements and inorganic elements from peloids by the skin.[37] Assessments in the MP group after treatment and one month later had higher reduction in pain, functional improvement and QoL improvement, and significantly higher reduction in analgesic consumption at the end of treatment which may be explained by the chemical efficacy of MP treatment.
Nonetheless, this study has some limitations. First, the study only included a patient and control group attending a single center. To obtain stronger results, there is a need for further large-scale, multi-center studies. Another limitation is that the effect of TENS + HE treatment is unknown. The lack of a placebo control group and short duration of follow-up can be deemed as the other limitations.
In conclusion, our study results showed that, in both groups, HE + TENS and thermotherapy administration reduced pain, provided functional improvements, improved QoL, and provided improvements in sleep quality and depression levels of CNNP patients. These improvements were observed to be statistically significantly better in the MP treatment group based on the assessment of pain with VAS, function with mNDI, depression with BDI, and subscales of SF-36V/E, SF-36GH, and SF-36MH for QoL with SF-36 after treatment and one month later. The reduction in analgesic use was more pronounced in the MP group. Based on these findings, the MP treatment is more effective than HP treatment for CNNP patients. However, additional randomized-controlled studies with longer followup periods are needed in which the mechanisms of action can be explained by biochemical parameters.
Footnotes
Ethics Committee Approval: The study protocol was approved by the Selçuk University, Faculty of Medicine Clinical Research Ethics Committee (No: 2018/394). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Consept, design and supervision: F.K., H.Y.; Data collection and/or processing: F.K., F.M.K., H.E.A.; Analysis and/or interpretation: F.K., E.Ş.Y.; Literature search: F.K., F.M.K., E.Ş.Y.; Writing manuscript: F.K., H.Y., H.E.A., E.Ş.Y.; Critical review: F.K., H.Y.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
Patient Consent for Publication: A written informed consent was obtained from each patient.<br><br><b>Data Sharing Statement:</b><br> The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Cohen SP, Hooten WM. Advances in the diagnosis and management of neck pain. j3221BMJ. 2017;358 doi: 10.1136/bmj.j3221. [DOI] [PubMed] [Google Scholar]
- 2.Popescu A, Lee H. Neck pain and lower back pain. Med Clin North Am. 2020;104:279–292. doi: 10.1016/j.mcna.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Pillastrini P, Castellini G, Chiarotto A, Fasciani F, Marzioni F, Vanti C, et al. Comparative effectiveness of conservative and pharmacological interventions for chronic non-specific neck pain: Protocol of a systematic review and network meta-analysis. e16762Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000016762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pangarkar S, Lee PC. Conservative treatment for neck pain: Medications, physical therapy, and exercise. Pangarkar S, Lee PC. Conservative treatment for neck pain: Medications, physical therapy, and exercise. Phys Med Rehabil Clin N Am 2011;22:503-20, ix. doi: 10.1016/j.pmr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Suvarnnato T, Puntumetakul R, Uthaikhup S, Boucaut R. Effect of specific deep cervical muscle exercises on functional disability, pain intensity, craniovertebral angle, and neck-muscle strength in chronic mechanical neck pain: A randomized controlled trial. J Pain Res. 2019;12:915–925. doi: 10.2147/JPR.S190125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price J, Rushton A, Tyros I, Tyros V, Heneghan NR. Effectiveness and optimal dosage of exercise training for chronic non-specific neck pain: A systematic review with a narrative synthesis. e0234511PLoS One. 2020;15 doi: 10.1371/journal.pone.0234511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer H, Baumgarten C, Choi KE, Lauche R, Saha FJ, Musial F, et al. Thermotherapy self-treatment for neck pain relief - A randomized controlled trial. e371-e378European Journal of Integrative Medicine. 2012;4 [Google Scholar]
- 8.Koyuncu E, Ökmen BM, Özkuk K, Taşoğlu Ö, Özgirgin N. The effectiveness of balneotherapy in chronic neck pain. Clin Rheumatol. 2016;35:2549–2555. doi: 10.1007/s10067-016-3199-8. [DOI] [PubMed] [Google Scholar]
- 9.O'Riordan C, Clifford A, Van De Ven P, Nelson J. Chronic neck pain and exercise interventions: Frequency, intensity, time, and type principle. Arch Phys Med Rehabil. 2014;95:770–783. doi: 10.1016/j.apmr.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Kroeling P, Gross A, Graham N, Burnie SJ, Szeto G, Goldsmith CH, et al. Electrotherapy for neck pain. CD004251Cochrane Database Syst Rev. 2013;(8) doi: 10.1002/14651858.CD004251.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutenbrunner C, Bender T, Cantista P, Karagülle Z. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int J Biometeorol. 2010;54:495–507. doi: 10.1007/s00484-010-0321-5. [DOI] [PubMed] [Google Scholar]
- 12.Gálvez I, Torres-Piles S, Ortega-Rincón E. Balneotherapy, immune system, and stress response: A hormetic strategy. Int J Mol Sci. 2018;19:1687–1687. doi: 10.3390/ijms19061687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groven MD. In: Textbook of Natural Medicine. Chapter 45. 4th ed. Pizzorno JE, Murray MT, editors. London: Churchill Livingstone; 2013. Peat therapeutics and balneotherapy; pp. 385–394. [Google Scholar]
- 14.Karaarslan F, Yılmaz H, Akkurt HE, Gül S, Kardeş S. Effectiveness of peloid therapy in patients with chronic low back pain: A single-blind controlled study. Int J Biometeorol. 2021;65:1799–1809. doi: 10.1007/s00484-021-02137-6. [DOI] [PubMed] [Google Scholar]
- 15.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 16.Kesiktas N, Ozcan E, Vernon H. Clinimetric properties of the Turkish translation of a modified neck disability index. BMC Musculoskelet Disord. 2012;13:25–25. doi: 10.1186/1471-2474-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hisli N. A study on validity and reliability test of the Beck Depression Scale. Turkish J Psychology. 1988;6:118–122. [Google Scholar]
- 18.Koçyiğit H, Aydemir Ö, Fişek G, Ölmez N, Memiş A. Kısa Form-36 (KF-36)'nın Türkçe versiyonunun güvenilirliği ve geçerliliği: Romatizmal hastalığı olan bir grup hasta ile çalışma. İlaç ve Tedavi Dergisi. 1999;12:102–106. [Google Scholar]
- 19.Ağargün MY, Kara H, Anlar Ö. Pittsburgh uyku kalitesi indeksinin geçerliği ve güvenirliği. Türk Psikiyatri Dergisi. 1996;7:107–115. [Google Scholar]
- 20.The R Project for Statistical Computing. Vienna, Austria. Available at: https://www.R-project.org/ [Accessed: July 23, 2019] [Google Scholar]
- 21.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software. 2017;82:1–26. [Google Scholar]
- 22.Childs JD, Cleland JA, Elliott JM, Teyhen DS, Wainner RS, Whitman JM, et al. Neck pain: Clinical practice guidelines linked to the International Classification of Functioning, Disability, and Health from the Orthopedic Section of the American Physical Therapy Association. A1-A34J Orthop Sports Phys Ther. 2008;38 doi: 10.2519/jospt.2008.0303. [DOI] [PubMed] [Google Scholar]
- 23.Galindez-Ibarbengoetxea X, Setuain I, Ramírez-Velez R, Andersen LL, González-Izal M, Jauregi A, et al. Short-term effects of manipulative treatment versus a therapeutic home exercise protocol for chronic cervical pain: A randomized clinical trial. J Back Musculoskelet Rehabil. 2018;31:133–145. doi: 10.3233/BMR-169723. [DOI] [PubMed] [Google Scholar]
- 24.Damgaard P, Bartels EM, Ris I, Christensen R, JuulKristensen B. Evidence of physiotherapy interventions for patients with chronic neck pain: A systematic review of randomised controlled trials. ISRN Pain. 2013;2013:567175–567175. doi: 10.1155/2013/567175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu TT, Hui-Chan CW, Chein G. A randomized clinical trial of TENS and exercise for patients with chronic neck pain. Clin Rehabil. 2005;19:850–860. doi: 10.1191/0269215505cr920oa. [DOI] [PubMed] [Google Scholar]
- 26.Martins-de-Sousa PH, Guimarães Almeida MQ, da Silva Junior JM, Santos AS, Costa Araújo GG, de Oliveira Pires F, et al. Program of therapeutic exercises associated with electrotherapy in patients with chronic neck pain: Protocol for a randomized controlled trial. J Bodyw Mov Ther. 2020;24:25–30. doi: 10.1016/j.jbmt.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Pullan JE, Sujatha KJ, Shetty P, Shetty GB. Comparative study on effect of moist heat therapy and acupuncture as an adjuvant to a comprehensive naturopathy treatment in management of chronic neck pain-A randomized control trial. IOSR Journal of Dental and Medical Sciences. 2016;15:139–144. [Google Scholar]
- 28.Türel A, Solak Ö, Dündar Ü, Toktaş H, Demirdal ÜS, Subaşı V, et al. Evaluation of the efficacy of spa therapy on pain and quality of life in patients with chronic mechanical neck pain. Arch Rheumatol. 2015;30:298–306. [Google Scholar]
- 29.Sarsan A, Akkaya N, Ozgen M, Yildiz N, Atalay NS, Ardic F. Comparing the efficacy of mature mud pack and hot pack treatments for knee osteoarthritis. J Back Musculoskelet Rehabil. 2012;25:193–199. doi: 10.3233/BMR-2012-0327. [DOI] [PubMed] [Google Scholar]
- 30.Tefner IK, Gaál R, Koroknai A, Ráthonyi A, Gáti T, Monduk P, et al. The effect of Neydharting mud-pack therapy on knee osteoarthritis: A randomized, controlled, double-blind follow-up pilot study. Rheumatol Int. 2013;33:2569–2576. doi: 10.1007/s00296-013-2776-2. [DOI] [PubMed] [Google Scholar]
- 31.Yücesoy H, Geçmen İ, Adıgüzel T, Karagülle M, Karagülle MZ. Efficacy of balneological outpatient treatment (hydrotherapy and peloidotherapy) for the management of chronic low back pain: A retrospective study. Int J Biometeorol. 2019;63:351–357. doi: 10.1007/s00484-018-01668-9. [DOI] [PubMed] [Google Scholar]
- 32.Eröksüz R, Erol Forestier FB, Karaaslan F, Forestier R, İşsever H, Erdoğan N, et al. Comparison of intermittent and consecutive balneological outpatient treatment (hydrotherapy and peloidotherapy) in fibromyalgia syndrome: A randomized, single-blind, pilot study. Int J Biometeorol. 2020;64:513–520. doi: 10.1007/s00484-019-01838-3. [DOI] [PubMed] [Google Scholar]
- 33.Tenti S, Manica P, Cheleschi S, Fioravanti A. Sulfurousarsenical-ferruginous balneotherapy for osteoarthritis of the hand: Results from a retrospective observational study. Int J Biometeorol. 2020;64:1561–1569. doi: 10.1007/s00484-020-01937-6. [DOI] [PubMed] [Google Scholar]
- 34.Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127:57–65. doi: 10.1080/00325481.2015.992719. [DOI] [PubMed] [Google Scholar]
- 35.Odabasi E, Turan M, Erdem H, Tekbas F. Does mud pack treatment have any chemical effect. A randomized controlled clinical study. J Altern Complement Med. 2008;14:559–565. doi: 10.1089/acm.2008.0003. [DOI] [PubMed] [Google Scholar]
- 36.Beer AM, Junginger HE, Lukanov J, Sagorchev P. Evaluation of the permeation of peat substances through human skin in vitro. Int J Pharm. 2003;253:169–175. doi: 10.1016/s0378-5173(02)00706-8. [DOI] [PubMed] [Google Scholar]
- 37.Tateo F, Ravaglioli A, Andreoli CY, Bonina FP, Coiro V, Degetto S, et al. The in-vitro percutaneous migration of chemical elements from a thermal mud for healing use. Applied Clay Science. 2009;44:83–94. [Google Scholar]