Abstract
Variations in exposure and treatment may contribute to heterogeneity in immunity and granuloma-induced pathology in human schistosomiasis. To examine this hypothesis, olive baboons were either repeatedly infected with Schistosoma mansoni cercariae or received an equivalent dose in a single infection. They were then cured with praziquantel and reinfected with a single exposure. Serial liver biopsies were obtained throughout the course of the experiment, and cytokine responses by peripheral blood mononuclear cells were measured every 2 to 3 weeks. Reinfection after treatment resulted in a twofold-smaller granuloma size at 6 and 9 weeks after infection compared to the size for the same period after primary infection (P < 0.001) but had no effect at 16 or 19 weeks postinfection. The pattern of exposure did not influence granuloma size. During primary infection schistosome-soluble egg antigen (SEA)-induced cytokine production correlated with granulomatous inflammation. Cytokine levels peaked during the acute infection, declined with chronic infection, and became undetectable after treatment. Reinfection after treatment stimulated a two- to three-fold increase in SEA-specific interleukin-4 (IL-4), IL-5, IL-10, IL-2, and transforming growth factor β (TGF-β) production and a marked rise in SEA-specific immunoglobulin E (IgE) and IgG regardless of the type of exposure. Cytokine production was significantly greater in repeatedly exposed animals (P < 0.001). SEA-induced gamma interferon production, however, did not increase with reinfection after treatment. SEA-induced TGF-β was the only cytokine that remained elevated as the infection become chronic and correlated with diminished hepatic granuloma size, implying its participation in down-modulation. These studies demonstrate that baboons partially retain their ability to down-modulate the granulomatous response after treatment.
Schistosomiasis is a widespread chronic helminth infection that contributes to the death of over half a million people yearly (30). The major form of disease results from the chronic granulomatous response to parasite ova trapped in host tissues. Most infected individuals, however, tolerate chronic infection without debilitating illness. This is thought to occur because of down-modulation of the host's granulomatous response (30). Failure to modulate can ultimately lead to hepatic periportal fibrosis, portal hypertension, and death. The mechanisms associated with modulation of the granulomatous response have been the subject of intense study and have important implications for control of schistosome-induced liver disease and other diseases associated with granulomatous inflammation.
The precise role that cytokines and antibodies have in regulating the granulomatous response is not fully understood. Most of our knowledge about the mechanisms of granuloma induction and modulation derives from studies of the murine model of schistosomiasis. These reports show that granuloma formation correlates with increased production of egg antigen (Ag)-specific interleukin-4 (IL-4), IL-5, and IL-13 (6, 7, 23, 33, 47) and that its down-modulation is partially mediated by IL-10 and parasite Ag-specific antibodies (18, 26, 46).
It is unknown whether the mechanisms that regulate granulomatous responses and disease in humans parallel those observed in murine schistosomiasis. Human studies are limited because of the difficulty in obtaining tissue samples in the acute phase of disease, though observations of the immune response in chronically infected humans have been made. Peripheral lymphocytes (or spleen cells) from asymptomatic Schistosoma mansoni-infected patients proliferate poorly and make little gamma interferon (IFN-γ) in response to egg antigens compared with those from (i) acutely infected individuals, (ii) subjects after curative chemotherapy, and (iii) patients with clinically apparent disease (9, 11, 40, 41, 48). One of the mechanisms that contribute to the diminished Th-cell proliferation and IFN-γ production results from active suppression by IL-10 (17, 20). Increased tumor necrosis factor alpha TNF-α production (29) and a failure to adequately down-modulate lymphocyte proliferation and IFN-γ production correlate with development of clinically overt hepatosplenic disease or severe bladder pathology. Down-regulation of Ag-specific IL-4 and IL-5 appears variable (16, 24, 34).
Individual immune responses to schistosomiasis, however, are highly variable, and the reasons why only some infected individuals progress to clinically overt disease remains poorly understood. A number of factors may contribute to the heterogeneity of immune responses and disease observed with human schistosomiasis. Humans experience widely different patterns of exposure to and intensity and duration of infection, and undergo occasional treatment. Two factors in particular, exposure and treatment, may affect the immune response and development of disease in humans. Treatment can stimulate an acute rise in parasite-specific antibody production (14, 36) and an alteration in isotypes (22, 28), along with an increase in parasite Ag-specific IFN-γ production and lymphocyte proliferation (34, 48). The effect on Ag-specific IL-4 and IL-5 production appears variable depending on the schistosome species and the time after treatment that people were examined (15, 25, 34). The effect of treatment on pathology, however, was not examined in these studies. Other population-based studies suggest that reinfection after interrupted treatment programs may result in worse pathology (32). The pattern of exposure may also affect the immune response. Our own studies of S. mansoni-infected baboons illustrate this point. Repeatedly exposed baboons develop high levels of egg Ag-specific IL-4, IL-5, and IFN-γ production by peripheral blood mononuclear cells (PBMC) and elevated serum immunoglobulin E (IgE) levels compared to baboons exposed once to a comparable number of S. mansoni cercariae (31). However, a detailed study of exposure, treatment, and reinfection for the immune and granulomatous responses has not been previously reported.
This study examines the hypothesis that an enhanced Th2-type immune response induced by repeated exposure and treatment will produce worse hepatic pathology, as indicated by larger acute and chronic granulomas with reinfection. To examine this hypothesis, olive baboons (Papio cynocephalus anubis) were either repeatedly infected or received a single exposure to a comparable number of cercariae and were allowed to develop a chronic infection (>19 weeks) before treatment. Following reinfection animals underwent serial liver biopsies to evaluate the granulomatous responses during the acute and chronic phases of infection. The study describes the relationship between schistosome-soluble egg Ag (SEA)-specific cytokine and antibody responses in peripheral blood and the corresponding hepatic pathology throughout the course of infection.
MATERIALS AND METHODS
Animals and parasites.
Juvenile male baboons (6 to 8 kg), P. cynocephalus anubis, were captured from schistosome-free areas in Kenya and confirmed as infection free based on lack of anti-schistosome antibodies as previously described (13). Animals were maintained in open enclosures according to international standards for primates and screened for common bacterial infections and intestinal helminths. Any infected animals were treated at least 3 months prior to beginning the experiment (13).
Experimental protocol.
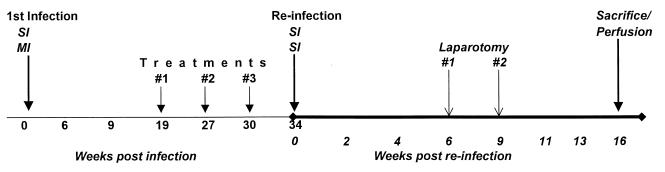
Two groups of seven juvenile baboons each were infected percutaneously by the pouch method (38) with either 100 cercariae weekly for a total of 10 weeks (termed multiple infection [MI]) or 1,000 cercariae once (termed single mass infection [SI]). After a chronic infection was established, animals were treated orally with praziquantel (PZQ; 60 mg/kg of body weight) on weeks 19, 27, and 30 postinfection (Fig. 1). All animals in both infection groups were then challenged percutaneously with a single dose of 1,000 S. mansoni cercariae at week 34 postinfection and perfused 16 weeks later to recover adult worms as described previously (13). Following perfusion, 10% (by weight) of the liver and small and large intestines was sampled separately and digested in 5% KOH to recover and count the ova (12). Peripheral venous blood was obtained every 2 to 3 weeks throughout the course of the experiment. Cumulative stool collections were obtained weekly to determine egg output by the Kato technique.
FIG. 1.
Experimental design with time indicated in weeks. The weeks listed indicate time points at which serum and PBMC were sampled. SI, single infection of baboons with 1,000 S. mansoni cercariae; MI, multiple infection of 100 cercariae per week for 10 consecutive weeks.
Two control groups of previously uninfected baboons were simultaneously infected once with 1,000 cercariae or multiply infected with 100 cercariae over 10 weeks. The separate control groups were studied to limit the number of survival surgeries required for liver biopsy.
Histopathology.
The details of preparation and measurement of granulomas and composition have been described elsewhere (13). Only nonconfluent granulomas containing ova at their centers were measured.
Cytokine assays.
PBMC were cultured for cytokine production at 2 × 106/ml in complete RPMI medium (RPMI 1640, 10% fetal calf serum, 4 mM l-glutamine, 25 mM HEPES, 80 μg of gentamicin/ml) in 48-well tissue culture plates (Falcon; Becton Dickinson Co., Franklin Lakes, N.J.). Media alone, SEA, prepared as previously described (2) at 5 μg/ml, or the mitogens phorbol myristate acetate (Sigma, St. Louis, Mo.) at 50 ng/ml with ionomycin at 1 μg/ml (Behring Corp., San Diego, Calif.) were added to cell cultures, and the cultures were incubated at 37°C in a humidified atmosphere with 5% CO2. Supernatants were harvested after 24 h for the measurement of IL-2 and IL-4 production and on day 5 for the measurement of IL-5, IL-10, transforming growth factor β (TGF-β), and IFN-γ production.
Cytokine measurements were performed by enzyme-linked immunosorbent assay (ELISA) (24) using different human reagents to detect baboon cytokines. The genes encoding the baboon cytokines examined and the cytokines themselves show 93 to 99% homology at the nucleic acid and protein levels, respectively, with the human equivalents, and therefore many human reagents detected baboon cytokine products (42). ELISA plates (Immulon 4; Dynatech, Sterling, Va.) were coated with the various antibodies in phosphatase buffer at pH 9.6. For IFN-γ, the coating antibody was monoclonal antibody MAb (BMS 107) (BioWhittaker, Walkersville, Md.) at 1 μg/ml, followed by the detecting biotinylated MAb 7-B6-1 (Diapharma Group Inc., Franklin, Ohio) at 0.5 μg/ml. The capture MAb used for IL-4 was 25D2 (2.5 μg/ml; Pharmingen, San Diego, Calif.), and the biotinylated MAb 8D2 (2.5 μg/ml; Pharmingen) was used for detection. The coating MAb for IL-5 was TRFK5 (1 μg/ml; Pharmingen), and the biotinylated MAb was 5A10 (2 μg/ml; Pharmingen). For IL-10 the coating antibody used was MAb AHC8102 (3 μg/ml; Biosource International, Camarillo, Calif.), followed by the detecting biotinylated MAb AHC7109 (0.8 μg/ml; Biosource International). TGF-β1 was assayed as follows: the coating antibody, MAb MAB240 (2 μg/ml; R&D Inc., Minneapolis, Minn.), was followed by the detecting biotinylated MAb BAF24 (0.1 μg/ml; R&D Inc.). Prior to assaying for TGF-β, samples were activated by a 10-min incubation with 10 μl of 1 N HCl per 50 μl of culture supernatants, followed by neutralization with 1.2 N NaOH–0.05 HEPES. The coating antibody for IL-2, MAb 55.111 (4 μg/ml; R&D Inc.), was followed by the detecting biotinylated MAb BAF202 (2.5 μg/ml; Pharmingen). Steptavidin-alkaline phosphatase at a 1:2,000 dilution (Jackson ImmunoResearch, West Grove, Pa.) was used as a conjugate for all the cytokine ELISAs, while phosphatase tablets (Sigma) were used as the substrate. Values were obtained from standard curves for human recombinant cytokines and were expressed in picograms per milliliter. Limits of detection were as follows: 10 pg/ml for IFN-γ, 20 pg/ml for IL-4, 22 pg/ml for IL-5, 50 pg/ml for IL-2, 40 pg/ml for IL-10, and 25 pg/ml for TGF-β.
Antibody assays.
Levels of SEA-specific IgG and IgE in serum were measured by ELISA. The methods of detection, antibody specificity and lack of cross-reaction to other Ig isotypes have been described previously (31).
Statistical analysis.
Data had a normal distribution after log transformation. Thus, all data in the experiment were analyzed for significant differences between experimental groups and control by Student's t test of log-transformed data. A paired t test was used to compare cytokine production by the same animals before and after treatment. Differences between the groups were considered significant at P < 0.05.
RESULTS
The effect of treatment on egg output and granuloma size after reinfection.
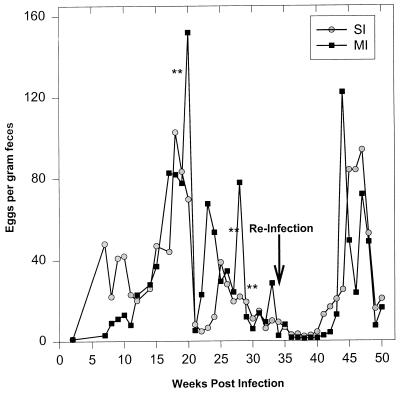
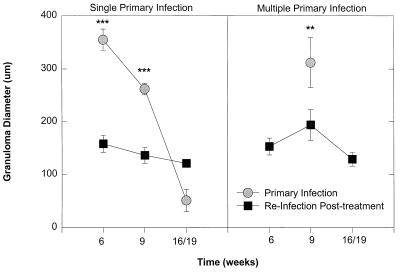
Treatment resulted in cure of all animals based on at least two consecutive egg-negative stools as determined by the Kato technique (Fig. 2). Hepatic granuloma size was serially examined at 6, 9, and 16 weeks postinfection in the same animals, corresponding to acute (6 and 9 weeks) and chronic phases (16 weeks) of infection following PZQ treatment (Fig. 1). To limit the number of survival surgeries, a separate group of animals served as pathological controls. These animals had not been previously infected or treated and had hepatic biopsies performed at similar time points during the primary infection. Prior to treatment peak granuloma size occurred at 6 weeks postinfection, and granulomas diminished in size as the infection became chronic in animals exposed to a single infection (Fig. 3, left panel). The mean worm burdens at perfusion were 901 ± 89 in this singly infected control group. In multiply infected animals, peak granuloma size occurred at 9 weeks after primary infection (Fig. 3, right panel). However, no granulomas that warranted measurement were observed at 16 weeks postinfection. The mean worm burdens in this multiply infected control group were lower than those in singly infected control animals (767 ± 65; P < 0.05). Infection following treatment resulted in a twofold reduction in acute granuloma size in both groups (P < 0.01). Few hepatic granulomas were found in most chronically infected animals both after the primary infection and with reinfection after treatment. There was no significant difference in granuloma size among chronically infected animals. Infection following treatment resulted in significantly fewer adult worms in the previously multiply exposed baboons (161 ± 89) than in baboons in the singly infected group (466 ± 182; P < 0.05).
FIG. 2.
Egg output per gram of feces as determined by the Kato method throughout the course of the experiment. The double asterisks represent the times of PZQ treatment. The arrow indicates the time of reinfection. No ova were detected in stools between weeks 36 and 40.
FIG. 3.
Mean granuloma size after treatment and reinfection, which resulted in reduced acute-phase granuloma size compared to that for untreated animals that received a primary infection. (Left panel) results for baboons that received single mass infections prior to treatment; (right panel) results for baboons that received multiple exposures. Both groups received a single reinfection dose of 1,000 cercariae after treatment. Each point represents the mean ± standard error of the mean for seven animals. Significant differences in granuloma size before treatment and after treatment are indicated by asterisks (∗∗, P < 0.01; ∗∗∗, P < 0.001).
The effect of treatment on cytokine and antibody production after reinfection.
To examine the immunological mechanisms responsible for the initiation and modulation of the granulomatous response before and after infection, peripheral venous blood was collected approximately every 3 weeks throughout the course of the experiment to examine parasite Ag and mitogen-induced cytokine production by PBMC. The mean net egg Ag-specific cytokine responses from the same baboons (two from the SI group and two from the MI group) are shown at all sampling points throughout the course of primary infection, treatment, and reinfection (Fig. 4). The mean net SEA-driven cytokine response for all seven animals, corresponding to the acute and chronic phases of the first and second infections, is shown in Table 1. The sampling points were grouped into acute- and chronic-phase points. Thus, the positive or peak cytokine response at either 6 or 9 weeks (acute phase) or at 13, 16, or 19 weeks (chronic phase) is included in the geometric mean indicated in Table 1. Mitogen-driven cytokine production was observed for a majority of animals throughout the course of the experiment, and the geometric mean for all animals in a group is shown for each cytokine (Fig. 5).
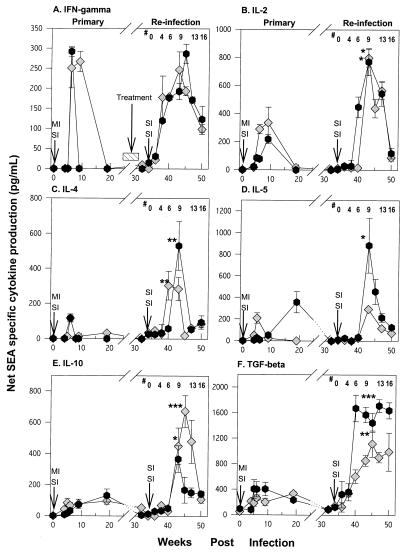
FIG. 4.
SEA-specific IFN-γ, IL-2, IL-4, IL-5, IL-10, and TGF-β cytokine levels during the course of primary infection and reinfection with S. mansoni in two representative baboons. PBMC were cultured at 2 × 106/ml in the presence of 5 μg of SEA/ml, and supernatants were harvested at 24 and 96 h. Cytokine secretion in the primary infection was compared to that at the corresponding time points after infection (secondary infection); significant differences are indicated by asterisks (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001). #, weeks after reinfection. Each time point represents the geometric mean of triplicate cultures ± the standard deviation. Arrows indicate the times of infection.
TABLE 1.
Geometric mean SEA-specific cytokine production from all animals (n = 7) during the acute and chronic phases of the primary infection and reinfection
| Cytokine | Infection | Geometric mean
production (pg/ml) ± SEM inb:
|
|||
|---|---|---|---|---|---|
| Acute phase
|
Chronic phase
|
||||
| Primary | Reinfection | Primary | Reinfection | ||
| IFN-γ | SI | 297 ± 32 | 203 ± 72 | 28 ± 8 | 120 ± 42 |
| MI | 291 ± 59 | 270 ± 61 | 31 ± 17 | 190 ± 93 | |
| IL-2 | SI | 85 ± 14 | 784 ± 173** | 119 ± 42 | 704 ± 316** |
| MI | 163 ± 51 | 1,483 ± 186** | 242 ± 83 | 727 ± 124** | |
| IL-4 | SI | 137 ± 28 | 251 ± 113* | 21 ± 14 | 173 ± 63* |
| MI | 62 ± 25 | 639 ± 217** | 20 ± 8 | 68 ± 31 | |
| IL-5 | SI | 285 ± 52 | 129 ± 64 | 17 ± 4 | 117 ± 46 |
| MI | 31 ± 14 | 616 ± 76* | 219 ± 58 | 160 ± 57 | |
| IL-10 | SI | 224 ± 38 | 496 ± 81** | 126 ± 61 | 138 ± 21 |
| MI | 71 ± 41 | 348 ± 136* | 130 ± 55 | 28 ± 3 | |
| TGF-β | SI | 1,141 ± 217 | 1,850 ± 261** | 767 ± 164 | 1,712 ± 312* |
| MI | 751 ± 192 | 2,852 ± 310*** | 862 ± 250 | 1,785 ± 351* | |
SI, single primary infection of 1,000 cercariae; MI, multiple primary infections of 100 cercariae per week for 10 weeks. All animals were reinfected singly with 1,000 cercariae after treatment.
Acute-phase results are peak cytokine responses at either 6 or 9 weeks in each animal after primary or secondary infection. Chronic-phase results are peak cytokine responses at 19 weeks in the primary infection and at either 13 or 16 weeks after reinfection. Asterisks indicate a significant difference compared to primary infection (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001).
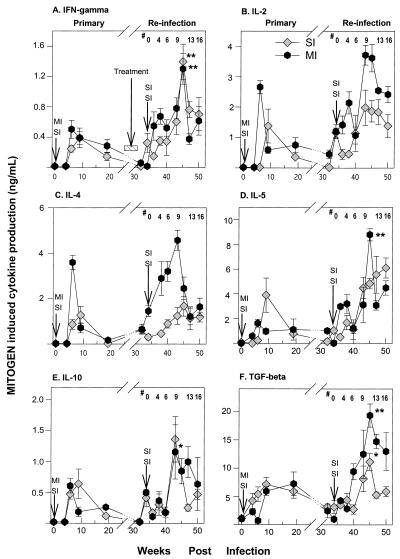
FIG. 5.
Serial mitogen-specific IFN-γ, IL-2, IL-4, IL-5, IL-10, and TGF-β cytokine levels during the course of primary infection and reinfection with S. mansoni. PBMC were cultured as described in the legend for Fig. 4. Each time point represents the geometric mean ± standard error of the mean for seven animals. Mitogen levels that are significantly different between corresponding time points of primary infection and reinfection phases are indicated by a single asterisk (P < 0.05) or a double asterisk (P < 0.01). Arrows show the times of infections. #, weeks after reinfection.
IFN-γ production peaked during the acute phase and then became undetectable during the chronic phase of the initial infection in representative animals (Fig. 4A). Reinfection produced a similar rise in IFN-γ release during the acute infection, while the levels tended to drop with chronic infection. The pattern of exposure, either SI or MI, did not alter the pattern of SEA-induced IFN-γ production. Geometric mean SEA-driven IFN-γ production levels for all the animals had similar profiles: they were elevated during the acute phase and declined in the chronic phase of the primary infection and with reinfection (Table 1). Mitogen-driven IFN-γ production was generally higher with reinfection than with primary infection but was equivalent between the MI and SI groups (Fig. 5A).
Egg Ag-driven IL-2 release (Fig. 4B) peaked during the acute phase of the primary infection and with reinfection and declined as the infection become chronic. Reinfection resulted in two- to eightfold more IL-2 production in both the SI and MI groups than the primary phase (Fig. 4B and Table 1; P < 0.001). Mitogen-driven IL-2 production was higher overall with reinfection than at primary infection, particularly in multiply infected animals (Fig. 5B).
Geometric mean SEA-driven IL-4 production showed a pattern similar to that observed for IL-2 (Fig. 4C and Table 1). SEA-driven IL-4 production was higher in the MI group than in the SI group during the acute phase after treatment (P < 0.05). Like IL-2 and IFN-γ, SEA-driven IL-4 declined dramatically with chronic infection. Mitogen-driven IL-4 production levels in the primary and reinfection phases were higher in the MI group (Fig. 5C).
Peak SEA-driven IL-5 production was more variable than production of the other cytokines. In singly infected animals it peaked at 6 weeks (Fig. 4D) after the primary infection and 9 weeks after reinfection (P < 0.05). In multiply infected animals peak SEA-driven IL-5 production occurred during the chronic phase of the first infection and 9 weeks after reinfection. These same patterns of cytokine responses were observed in all animals (Table 1). SEA-driven IL-5 production was significantly higher in the MI than in the SI group of animals during the acute phase but not in the chronic phase after treatment (P < 0.05) (Fig. 5D).
Egg Ag-driven IL-10 production increased two- to fivefold with reinfection after treatment (Fig. 4E). Cytokine production peaked during the acute phase of the infection and declined with chronic infection after treatment in representative animals. It is notable that egg Ag-induced IL-10 remained elevated during chronic infection after the first infection but not after reinfection. SEA-driven IL-10 production levels were equivalent for MI and SI groups throughout the course of the experiment.
Egg Ag-driven TGF-β production was also higher with reinfection after treatment than it was during infection prior to treatment (Fig. 4F). Unlike the other cytokines, however, SEA-driven TGF-β remained elevated during the chronic phase of infection both before and after treatment. Treatment eliminated the detection of SEA-driven TGF-β production in almost all animals. Overall, TGF-β production levels in SI and MI experimental groups were similar. Mitogen-driven TGF-β production was elevated with reinfection in the both groups (Fig. 5E).
Levels of SEA-specific IgE and IgG in serum.
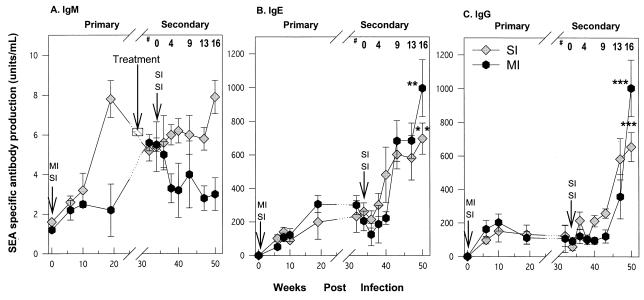
To examine the humoral correlates of modulation, levels of SEA-specific IgE, IgG, and IgM in serum were examined throughout the course of infection (Fig. 6). Egg Ag-specific IgM levels increased rapidly with the initial infection and remained at roughly the same levels throughout the course of the experiment. In contrast egg Ag-specific IgE and IgG increased significantly with reinfection after treatment and continued to rise with ongoing infection. It is notable that the rise in egg Ag-specific IgG with reinfection occurred later than that observed for egg Ag-specific IgE. Chronically infected animals with multiple infections had significantly higher levels of SEA-specific IgG and IgE in serum after treatment than singly infected animals (P < 0.05).
FIG. 6.
Levels of parasite-specific IgM (A), IgE (B), and IgG (C) in serum after S. mansoni infection. Each time point represents the geometric mean ± standard error of the mean for seven animals. Serum antibody titers that are significantly different between corresponding time points of primary and reinfection phases are indicated by single asterisks (P < 0.05), double asterisks (P < 0.01)., and triple asterisks (P < 0.001). #, weeks after reinfection. Arrows show times of infection.
DISCUSSION
These studies show that reinfection following treatment resulted in acute-phase granulomas significantly smaller than those produced during the primary infection. This demonstrates the persistence of partially modulated granulomas. These results imply that treatment followed by reinfection in areas where schistosomiasis is endemic is unlikely to enhance morbidity, a finding supported by studies of both mice (8, 10) and humans (39). Since most human infections will be reinfections, the modulated state is more typical of infection in populations.
The enhanced Th2-type immune response following reinfection contradicts our initial hypothesis that this should result in a more vigorous granulomatous reaction, as predicted from murine studies. We further observed that the patterns of Ag-specific cytokine production in baboons, like those observed in infected humans (45), do not always show a clear separation of Th1- and Th2-type immune responses, nor do they show consistent evidence of cytokine cross-regulation (e.g., a reciprocal decrease in IFN-γ with a rise in IL-4 and IL-5). It is possible that a Th2-type response may facilitate induction of smaller granulomas after treatment when other immunomodulatory mechanisms have been engaged. This study indicates that SEA-driven TGF-β may contribute to down-modulation of the granulomatous response. This was the only cytokine whose increased production consistently correlated with diminished granuloma size. The present observations also show that the frequency of exposure to the parasite affects the host's immune response, consistent with our earlier findings that repeated natural infection of baboons with S. mansoni generates higher levels of immunity than does a single exposure (31).
It is possible that egg Ag-specific cytokine production by PBMC may not accurately reflect cytokine responses in granuloma or draining lymphoid tissues. This possibility is unlikely since PCR analysis of hepatic tissue containing granulomas tended to show a pattern of cytokine response similar to that observed in PBMC (unpublished observations). We have also observed that Ag-specific cytokine production by draining LN cells and splenocytes shows the same pattern of cytokine responses as that by PBMC. In addition, recent reports suggest that lymphocytes from the peripheral circulation migrate into and populate granulomas, where they undergo cell death by interleukin deprivation and/or apoptosis, and that egg Ag-specific lymphocytes also fail to proliferate within granulomas (35). Therefore, granulomas must contain some egg-specific lymphocytes that arise from other sites that would be represented by PBMC.
The increase in IL-2, IL-4, IL-5, IL-10, and/or TGF-β with reinfection after treatment may participate in the early suppression of the granulomatous response. However, IL-2, IL-4, IL-5, and IL-10 are unlikely to play a pivotal role in ongoing suppression of the granulomatous response during chronic infection, since production of these cytokines wanes as granulomas become smaller. The exception was SEA-specific TGF-β release. TGF-β synthesis continued during the chronic phase of infection relative to that of other cytokines. It may suppress the granulomatous response because of its potent ability to inhibit lymphocyte proliferation and cytokine expression and to enhance the phagocytic activity of monocytes/macrophages within the granuloma (21). We postulate that continued production of TGF-β during the chronic phase of infection results from expansion of egg Ag-specific lymphocytes in gut-associated granulomas. The majority of eggs are deposited, and the majority of granulomas are formed, in the guts of baboons (13) and humans (4). The gut-associated lymphoid system favors the differentiation of lymphocytes that produce TGF-β that contributes to oral tolerance (44). Gut-derived egg Ag-specific T cells that produce TGF-β may migrate and populate hepatic granulomas and thus participate in their down-modulation.
We failed to detect egg Ag-induced cytokine production by PBMC within 2 weeks after completion of PZQ treatment, which suggests a rapid loss of parasite-specific memory lymphocytes. Therefore, ongoing egg deposition may be necessary to maintain the cytokine and/or antibody levels required for complete modulation of the granulomatous response. The large number of ova released as a consequence of the heavy infection rapidly expands Ag-specific lymphocytes. In order to maintain lymphocyte homeostasis after generation of specific T and B cells in response to persisting infection, Ag-specific lymphocytes, including many memory cells, probably undergo accelerated cell death (37). Treatment quickly kills adult worms and abruptly stops release of ova and further stimulation of specific lymphocytes. A similar phenomenon has been observed in virus-specific CD8+ T-cell memory (27). In humans, however, schistosome Ag-specific immunity usually persists after treatment (25, 34). This may occur because of the failure to completely eradicate infection or the possibility that individuals may be rapidly reexposed after treatment. It is also possible that the generally lighter and more persistent infections observed in humans maintain detectable levels of Ag-specific memory lymphocytes without widespread cell death.
Reinfection after treatment significantly boosted parasite-specific antibody responses, demonstrating the persistence of parasite-specific B-cell memory. It is also possible that the persisting antibody responses may contribute to modulating the acute-phase granulomatous response with reinfection. Antibodies and/or B cells have been shown to be important in regulating the granulomatous response in mice (5, 18, 19). This modulation was shown to be, in part, Fc receptor (FcR) dependent. Thus, antibodies may regulate the granulomatous responses by the formation of immune complexes or anti-idiotypic antibodies (26). This may occur at the time of egg release after reinfection when levels of egg-Ag-specific antibodies in serum are elevated. Immune complex binding to FcR (and complement) receptors on macrophages, B cells, and dendritic cells can impair their ability to present antigen (1, 13) and increase their phagocytic activity (37); both of these functions can participate in modulating the granuloma. TGF-β can participate in this regulatory network by its ability to up-regulate expression of cell surface FcγRIII receptors on monocytes (21).
The present study shows that repeated primary infection followed by treatment and reinfection produced a significant reduction in worm burden, compared to that in animals receiving a single exposure during their primary infection. Thus, repeatedly infected animals had approximately 65% fewer adult worms and a comparable reduction in total egg burden. This reduced worm burden and thus egg Ag load may affect the pattern of cytokine response and thereby the ability to modulate the granuloma. The relationship between the intensity of experimental infection and granuloma size has been examined in detail in different animal models of schistosomiasis (3). No consistent relationship between infection intensity and granuloma size has been found from experimental infection of rabbits or monkeys with any schistosome species or with S. mansoni infection in most murine strains. Thus, the observed differences in worm burdens between primary infection and reinfection are unlikely to account for the differences in cytokine production, antibody levels, or granuloma size. We also observed few hepatic granulomas in chronically infected animals after the primary or secondary infection. This was not related to intensity of infection, decreased egg output, or worm burdens at perfusion among the different groups. Although it is not well understood why fewer hepatic granulomas were observed in chronically infected animals, the finding may represent further migration of adults to the distal mesenteric vasculature such that fewer eggs embolize in the liver.
The present study suggests that selective-population chemotherapy for schistosomiasis is unlikely to exacerbate host-induced acute granulomatous responses with subsequent infection. However, the long-term effects on fibrosis and chronic modulation remain to be fully determined. The study also concludes that granuloma modulation was the result of a generalized suppression of cytokine synthesis, probably by the immunomodulatory function of TGF-β. In addition, these studies point to the value of the baboon as a model to study the immunopathology of human schistosomiasis and provide unique insights into its pathophysiology that may not be apparent from studies in mice.
ACKNOWLEDGMENTS
Support was provided by NIH grants AI-35935 and AI-01202.
We are indebted to J. Nyaundi, M. Njenga, and M. Suleman for surgical expertise and to Simon Kiarie, Fred Nyundo, and Sammy Kisara for excellent technical assistance. Francois Villinger kindly helped to develop and verify the baboon cytokine assays. Lynn Elson provided key help with initial infection and immunological assays. We thank Abram Stavitsky, Eric Pearlman, and Diana Martin for critical review of the manuscript.
REFERENCES
- 1.Barcy S, Wettendorff M, Leo O, Urbain J, Druger M, Ceuppens J, de Boer M. FcR cross-linking on monocytes results in impaired T cell stimulatory capacity. Int Immunol. 1995;7:179. doi: 10.1093/intimm/7.2.179. [DOI] [PubMed] [Google Scholar]
- 2.Boros D L, Lukacs N W. The role of egg antigens, cytokines in granuloma formation in murine schistosomiasis mansoni. Mem Inst Oswaldo Cruz. 1992;87:75–79. doi: 10.1590/s0074-02761992000800010. [DOI] [PubMed] [Google Scholar]
- 3.Cheever A W. The intensity of experimental schistosome infections modulates hepatic pathology. Am J Trop Med Hyg. 1986;35:124–133. doi: 10.4269/ajtmh.1986.35.124. [DOI] [PubMed] [Google Scholar]
- 4.Cheever A W, Andrade Z A. Pathological lesions associated with Schistosoma mansoniinfection in man. Trans R Soc Trop Med Hyg. 1967;61:626–639. doi: 10.1016/0035-9203(67)90125-3. [DOI] [PubMed] [Google Scholar]
- 5.Cheever A W, Byram J E, Hieny S, von Lichtenberg F, Lunde M N, Sher A. Immunopathology of Schistosoma japonicum and Schistosoma mansoniinfection in B cell depleted mice. Parasite Immunol. 1985;7:399–413. doi: 10.1111/j.1365-3024.1985.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 6.Chensue S W, Warmington K S, Ruth J, Lincoln P M, Kunkel S L. Cross-regulatory role of IFN-γ, IL-4 and IL-10 in schistosome egg granuloma formation: in vivo regulation of Th activity and inflammation. Clin Exp Immunol. 1994;98:395–400. doi: 10.1111/j.1365-2249.1994.tb05503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiaramonte M G, Schopf L R, Neben T Y, Cheever A W, Donaldson D D, Wynn T A. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansonieggs. J Immunol. 1999;162:920–930. [PubMed] [Google Scholar]
- 8.Coelho P M, Toppa N H, Feldmann J S, Goncalves R, Mello R T. Schistosoma mansoni: permanence of modulation of the granulomatous inflammatory response in mice cured in the chronic phase. Int J Parasitol. 1996;26:1393–1395. doi: 10.1016/s0020-7519(96)00090-2. [DOI] [PubMed] [Google Scholar]
- 9.Colley D G, Garcia A A, Lambertucci J R, Parra J C, Katz N, Rocha R S, Gazzinelli G. Immune responses during human schistosomiasis. XII. Differential responsiveness in patients with hepatosplenic disease. Am J Trop Med Hyg. 1986;35:793. [PubMed] [Google Scholar]
- 10.Domingo E O, Warren K S. Endogenous desensitization: changing host granulomatous response to schistosome eggs at different stages of infection with schistosoma mansoni. Am J Pathol. 1968;52:369–379. [PMC free article] [PubMed] [Google Scholar]
- 11.Ellner J J, Olds G R, Osman E S, El Kholy A, Mahmoud A A F. Dichotomies in the reactivity to worm Ag in human schistosomiasis mansoni. J Immunol. 1981;126:309. [PubMed] [Google Scholar]
- 12.Farah I O, Nyindo M. Schistosoma mansoniinduces in the Kenyan baboon a novel intestinal pathology that is manifestly modulated by an irradiated cercarial vaccine. J Parasitol. 1996;82:601–607. [PubMed] [Google Scholar]
- 13.Farah I O, Nyindo M, Suleman M A, Nyaundi J, Kariuki T M, Blanton R E, Elson L H, King C L. Schistosoma mansoni: development and modulation of the granuloma after single or multiple exposures in the baboon (Papio cynocephalus anubis) Exp Parasitol. 1997;86:93–101. doi: 10.1006/expr.1997.4152. [DOI] [PubMed] [Google Scholar]
- 14.Grogan J L, Kremsner P G, van Dam J G, Metzger W, Mordmuller B, Deelder A, Yazdanbakhsh M. Antischistosome IgG4 and IgE responses are affected differentially by chemotherapy in children versus adults. J Infect Dis. 1996;173:1242–1247. doi: 10.1093/infdis/173.5.1242. [DOI] [PubMed] [Google Scholar]
- 15.Grogan J L, Kremsner P G, Deelder A M, Yazdanbakhsh M. Elevated proliferation and interleukin-4 release from CD4+ cells after chemotherapy in human Schistosoma haematobiuminfection. Eur J Immunol. 1996;26:1365–1370. doi: 10.1002/eji.1830260628. [DOI] [PubMed] [Google Scholar]
- 16.Grogan J L, Kremsner P G, Deelder A M, Yazdanbakhsh M. Antigen-specific proliferation and interferon-gamma and interleukin-5 production are down-regulated during Schistosoma haematobiuminfection. J Infect Dis. 1998;177:1433–1437. doi: 10.1086/517832. [DOI] [PubMed] [Google Scholar]
- 17.Grogan J L, Kremsner P G, Deelder A M, Yazdanbakhsh M. The effect of anti-IL-10 on proliferation and cytokine production in human schistosomiasis: fresh versus cryopreserved cells. Parasite Immunol. 1998;20:345–349. doi: 10.1046/j.1365-3024.1998.00157.x. [DOI] [PubMed] [Google Scholar]
- 18.Jankovic D, Cheever A W, Kullberg M C, Wynn T A, Yap G, Caspar P, Lewis F A, Clynes R, Ravetch J V, Sher A. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J Exp Med. 1998;187:619–629. doi: 10.1084/jem.187.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankovic D, Kullberg M C, Dombrowicz D, Barbieri S, Caspar P, Wynn T A, Paul W E, Cheever A W, Kinet J P, Sher A. Fc epsilonRI-deficient mice infected with Schistosoma mansonimount normal Th2-type responses while displaying enhanced liver pathology. J Immunol. 1997;159:1868–1875. [PubMed] [Google Scholar]
- 20.King C L, Medhat A, Malhotra I, Nafeh M, Helmy A, Khaudary J, Ibrahim S, El-Sherbiny M, Zaky S, Stupi R J, Brustoski K, Shehata M, Shata M T. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J Immunol. 1996;156:4715–4721. [PubMed] [Google Scholar]
- 21.Letterio J J, Roberts A B. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, King C L, Ogundipe J O, Licate L S, Blanton R E. Preferential recognition by human IgE and IgG4 of a species-specific Schistosoma haematobiumserine protease inhibitor. J Infect Dis. 1995;171:416–422. doi: 10.1093/infdis/171.2.416. [DOI] [PubMed] [Google Scholar]
- 23.Lukacs N W, Boros D L. Lymphokine regulation of granuloma formation in murine schistosomiasis mansoni. Clin Immunol Immunopathol. 1993;68:57–63. doi: 10.1006/clin.1993.1095. [DOI] [PubMed] [Google Scholar]
- 24.Mahanty S, King C L, Kumaraswami V, Regunathan J, Maya A, Jayaraman K, Abrams J S, Ottesen E A, Nutman T B. IL-4- and IL-5-secreting lymphocyte populations are preferentially stimulated by parasite-derived antigens in human tissue invasive nematode infections. J Immunol. 1993;151:3704–3711. [PubMed] [Google Scholar]
- 25.Medhat A, Shehata M, Bucci K, Mohamed S, Diab A, Badary S, Galal H, Nafeh M, King C L. Increased interleukin-4 and interleukin-5 production in response to Schistosoma haematobiumadult worm antigens correlates with lack of re-infection after treatment. J Infect Dis. 1998;178:512–519. doi: 10.1086/515630. [DOI] [PubMed] [Google Scholar]
- 26.Montesano M A, Colley D G, Eloi-Santos S, Freeman G L, Secor W E. Neonatal idiotypic exposure alters subsequent cytokine, pathology, and survival patterns in experimental Schistosoma mansoniinfections. J Exp Med. 1999;189:637–645. doi: 10.1084/jem.189.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 28.Mutapi F, Ndhlovu P D, Hagan P, Spicer J T, Mduluza T, Turner C M, Chandiwana S K, Woolhouse M E. Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobiuminfection. J Infect Dis. 1998;178:289–293. doi: 10.1086/517456. [DOI] [PubMed] [Google Scholar]
- 29.Mwatha J K, Kimani G, Kamau T, Mbugua G G, Ouma J H, Mumo J, Fulford A J, Jones F M, Butterworth A E, Roberts M B, Dunne D W. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-gamma, but low levels of IL-5, are associated with hepatosplenic disease in human schistosomiasis mansoni. J Immunol. 1998;160:1992–1999. [PubMed] [Google Scholar]
- 30.Newport G R, Colley D G. Schistosomiasis. In: Warren K S, editor. Immunology and molecular biology of parasitic infections. Oxford, United Kingdom: Blackwell Scientific Publications; 1993. p. 387. [Google Scholar]
- 31.Nyindo M, Kariuki T, Mola P, Farah I, Elson L, Blanton R E, King C L. Role of adult worm antigen-specific immunoglobulin E in acquired immunity to Schistosoma mansoniinfection in baboons. Infect Immun. 1999;67:636–642. doi: 10.1128/iai.67.2.636-642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olveda R M, Daniel B L, Ramirez B D, Aligui G D, Acosta L P, Fevidal P, Tiu E, de Veyra F, Peters P A, Romulo R, Domingo E, Wiest P M, Olds G R. Schistosomiasis japonica in the Philippines: the long-term impact of population-based chemotherapy on infection, transmission, and morbidity. J Infect Dis. 1996;174:163–172. doi: 10.1093/infdis/174.1.163. [DOI] [PubMed] [Google Scholar]
- 33.Pearce E J, Caspar P, Grzych J M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts M, Butterworth A E, Kimani G, Kamau T, Fulford A J, Dunne D W, Ouma J H, Sturrock R F. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun. 1993;61:4984–4993. doi: 10.1128/iai.61.12.4984-4993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rumbley C A, Zekavat S A, Sugaya H, Perrin P J, Ramadan M A, Phillips S M. The schistosome granuloma: characterization of lymphocyte migration, activation, and cytokine production. J Immunol. 1998;161:4129–4137. [PubMed] [Google Scholar]
- 36.Shaker Z, Hassanein H, Kamel M, El-Bahairy N, El-Kalouby A, El-Raziky E. Effect of praziquantel on certain immune responses of schistosomal Egyptian patients. I. Changes of specific immunoglobulins. Parasitol Res. 1987;73:328–333. doi: 10.1007/BF00531087. [DOI] [PubMed] [Google Scholar]
- 37.Starzl T E, Zinkernagel R M. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sturrock R F, Butterworth A E, Houba V. Schistosoma mansoni in the baboon (Papio anubis): parasitological responses of Kenyan baboons to different exposures of a local parasite strain. Parasitology. 1976;73:239–252. doi: 10.1017/s003118200004693x. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian A, Mungai P, Ouma J, Magak P, King C H, Mahmoud A F, King C L. Long-term suppression of adult bladder morbidity and severe hydronephrosis following selective population chemotherapy for Schistosoma haematobium. Am J Trop Med Hyg. 1999;61:476–481. doi: 10.4269/ajtmh.1999.61.476. [DOI] [PubMed] [Google Scholar]
- 40.Todd C W, Goodgame R W, Colley D G. Immune responses during human schistosomiasis mansoni. V. Suppression of schistosome antigen-specific lymphocyte blastogenesis by adherent/phagocytic cells. J Immunol. 1979;122:1440–1446. [PubMed] [Google Scholar]
- 41.Viana I R, Sher A, Carvalho O S, Massara C L, Eloi-Santos S M, Pearce E J, Colley D G, Gazzinelli G, Correa-Oliveira R. Interferon-gamma production by peripheral blood mononuclear cells from residents of an area endemic for Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1994;88:466–470. doi: 10.1016/0035-9203(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 42.Villinger F, Brar S S, Mayne A, Chikkala N, Ansari A A. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J Immunol. 1995;155:3946–3954. [PubMed] [Google Scholar]
- 43.Virgin H W T, Wittenberg G F, Unanue E R. Immune complex effects on murine macrophages. I. Immune complexes suppress interferon-gamma induction of Ia expression. J Immunol. 1985;135:3735–3743. [PubMed] [Google Scholar]
- 44.Weiner H L, Friedman A, Miller A, Khoury S J, al-Sabbagh A, Santos L, Sayegh M, Nussenblatt R B, Trentham D E, Hafler D A. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 45.Williams M E, Montenegro S, Domingues A L, Wynn T A, Teixeira K, Mahanty S, Coutinho A, Sher A. Leukocytes of patients with Schistosoma mansoni respond with a Th2 pattern of cytokine production to mitogen or egg antigens but with a Th0 pattern to worm antigens. J Infect Dis. 1994;170:946–954. doi: 10.1093/infdis/170.4.946. [DOI] [PubMed] [Google Scholar]
- 46.Wynn T A, Cheever A W, Williams M E, Hieny S, Caspar P, Kuhn R, Muller W, Sher A. IL-10 regulates liver pathology in acute murine Schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J Immunol. 1998;160:4473–4480. [PubMed] [Google Scholar]
- 47.Yamashita T, Boros D L. IL-4 influences IL-2 production and granulomatous inflammation in murine schistosomiasis mansoni. J Immunol. 1992;149:3659–3664. [PubMed] [Google Scholar]
- 48.Zwingenberger K, Irschick E, Vergetti J G S, Decal A R C, Janssen-Rosseck R, Bienzle U, Huber C, Feldmeier H. Release of interleukin-2 and gamma interferon by peripheral mononuclear cells in human Schistosomiasis mansoni infection normalizes after chemotherapy. Scand J Immunol. 1989;30:463–471. doi: 10.1111/j.1365-3083.1989.tb02451.x. [DOI] [PubMed] [Google Scholar]