Abstract
Background:
Hepatitis C virus (HCV) infection can be cured, and the United States has joined the World Health Organization in calling for HCV elimination by 2030. However, historically low uptake of HCV treatment among people who inject drugs (PWID) threatens HCV elimination and exacerbates social and racial health disparities.
Objective:
To assess whether all-oral HCV treatments were accessed by PWID and reduced liver disease burden and mortality.
Design:
Community-based, longitudinal cohort study of persons with a history of injection drug use.
Setting:
Baltimore, Maryland.
Participants:
1323 participants enrolled in the ALIVE (AIDS Linked to the IntraVenous Experience) study from 2006 to 2019 and chronically infected with HCV.
Measurements:
Liver stiffness measures (LSMs) by transient elastography, HCV RNA, and mortality from the National Death Index.
Results:
Among 1323 persons with evidence of chronic HCV infection at baseline, the median age was 49 years. Most were Black (82%), male (71%), and HIV-negative (66%). The proportion in whom HCV RNA was detected decreased from 100% in 2006 to 48% in 2019. Across 10 350 valid LSMs, cirrhosis was detected in 15% of participants in 2006, 19% in 2015, and 8% in 2019. Undetectable HCV RNA was significantly associated with reduced odds of cirrhosis (adjusted odds ratio, 0.28 [95% CI, 0.17 to 0.45]) and reduced all-cause mortality risk (adjusted hazard ratio, 0.54 [CI, 0.38 to 0.77]).
Limitation:
Noninvasive markers of liver fibrosis have not been validated in persons with sustained virologic response.
Conclusion:
Many community-based PWID in Baltimore are receiving HCV treatment, which is associated with sharp decreases in liver disease and mortality. Additional efforts will be needed to reduce residual barriers to treatment and to eliminate HCV as a public health threat for PWID.
Primary Funding Source:
National Institutes of Health.
Advances in curative hepatitis C virus (HCV) treatments prompted the World Health Organization and the U.S. Department of Health and Human Services to call for the elimination of hepatitis C as a public health threat by 2030 (1-3). Because more than 95% of persons who are treated are cured, achieving the elimination target of a 65% reduction in HCV-related mortality requires identification and cure of infected persons (4). One model estimated that identifying at least 90% of HCV-infected persons and treating 80% of them is necessary to reduce mortality by 65%, but whether those projections will be realized is unclear (5, 6). However, several observational studies have reported a strong protective effect of HCV cure and all-cause mortality in patients chronically infected with HCV with varying liver disease burden (7-9).
In the United States and many high-income regions, most persons chronically infected with HCV are people who currently or formerly injected drugs (PWID). Long-standing structural barriers impede access to HCV testing and treatment among PWID (10). Because they are disproportionately uninsured or covered under Medicaid, restrictions on HCV treatment reimbursement based on liver disease severity have limited HCV treatment opportunities for PWID in many states (11). They also have a disproportionate burden of comorbidities, such as HIV and alcohol use disorder, that may substantially alter the net effect of HCV treatment on mortality (12). Thus, because injection drug use is the primary method of HCV transmission in the United States (13), plans for HCV elimination must incorporate HCV treatment uptake and its link with mortality.
Since 1988, we have been recruiting and following a community-based cohort of PWID in Baltimore, Maryland. Within that cohort, we have shown the high burden of HCV infection, the natural history of disease, and the effect of alcohol use and HIV infection on HCV-related cirrhosis and mortality (12, 14-16). In 2006, we added semiannual structured assessments of liver fibrosis by the most valid method available: liver stiffness measures (LSMs) via transient elastography (12). Because the cohort is community-based and no treatments are provided, it is the ideal setting to investigate whether the treatment uptake target for HCV elimination is being realized among PWID and to reassess the links among treatment uptake, liver disease burden, and mortality.
Methods
Setting and Participants
Data originated from participants enrolled in the ALIVE (AIDS Linked to the IntraVenous Experience) study, a community-recruited cohort of former and current PWID living in or near Baltimore (17). Eligibility criteria included being aged at least 18 years and having a history of injecting drugs. Enrollment began in 1988, with additional recruitment in 1994 to 1995, 1998, 2000, 2005 to 2008, and 2015 to 2018. Participant characteristics stratified by recruitment cohort are shown in Supplement Table 1 (available at Annals.org), and data on the number of visits over time are reported in Supplement Table 2 (available at Annals.org). Participants visited the clinic biannually; completed an interview on substance use behaviors, comorbidities, and health care use; and provided blood samples for laboratory testing. We limited this analysis to data collected from 2006 to 2019 among participants who had HCV RNA detected at least once and at least 1 valid LSM measured by transient elastography.
Out of 2058 participants who were eligible for inclusion, 355 were excluded because they did not have at least 1 valid LSM. Differences between participants with and without at least 1 valid LSM are shown in Supplement Table 3 (available at Annals.org). Another 362 participants were excluded because they were not positive for HCV RNA during the study interval. Observation time began at the first study visit for which there was both a valid LSM and detectable HCV RNA; this was at the same visit for 97% of participants, whereas HCV RNA was detected after an initial negative assessment in 34 cases. The Johns Hopkins University Institutional Review Board approved the study, and all participants provided written informed consent.
Measurements
Laboratory Measurements
We conducted HIV serologic testing for participants not known to have HIV and viral load testing for those with confirmed HIV. Detection of HIV-1 antibodies was assessed by an enzyme-linked immunosorbent assay and confirmed by Western blot. Plasma HIV-1 RNA levels were determined using reverse transcriptase polymerase chain reaction (Roche Amplicor HIV-1 Monitor Test; limit of detection, 50 copies/mL). Detection of HCV antibodies was assessed at the first available visit. We conducted HCV RNA testing on plasma collected at or near the time of the initial LSM, followed by biennial testing from 2006 to 2012 and yearly testing from 2014 to 2019 (Abbott Molecular or by a commercial laboratory). Platelet count, alanine aminotransferase level, and aspartate aminotransferase level were collected yearly.
Liver Fibrosis
Our primary outcome was LSM, which was collected semiannually by transient elastography using a FibroScan machine (Echosens). Measurement procedures, including quality control procedures and criteria for valid measurements, have been described previously (12, 18, 19). The median LSM was used. We considered LSM as both a continuous variable with natural log transformation and a dichotomous variable using a previously validated cutoff for severe fibrosis or cirrhosis (≥12.3 kPa) (12, 19, 20).
Mortality
Date of death occurring from 2006 to 2019 was obtained through linkage to the National Death Index Plus database. Each year, we provide names, dates of birth, and Social Security numbers to the National Death Index to determine whether any participants died in the previous year. A probabilistic score is generated to identify a potential match (21, 22).
Exposure
The primary exposure was undetectable HCV RNA (a proxy for sustained virologic response [SVR]), where the lower limit of quantification was 500 IU/mL. However, more recently conducted assays were more sensitive (15 to 200 IU/mL). Because HCV RNA data in the period before direct-acting antiviral (DAA) therapy were limited to biennial testing (2006, 2008, 2010, 2012), we carried forward the HCV RNA value until it was updated with a more recent test. Interferon-based treatment was minimal in the pre-DAA period, so carrying forward the HCV RNA value was reasonable, as it was unlikely that a person would have been cured or would have had spontaneous resolution of infection during this period (10).
Comorbidities and Other Covariates
To assess the independent association of HCV viremia with LSM, we considered comorbidities and covariates previously identified to be associated with the exposure and outcome (12). Comorbidities were based on self-report or laboratory measurement indicating hypertension (blood pressure >150/90 mm Hg for persons aged ≥60 years or >140/90 mm Hg for those aged <60 years), renal disease (estimated glomerular filtration rate <60 mL/min/1.73 m2), or diabetes (hemoglobin A1c level ≥6.5%). We grouped the cumulative number of comorbidities at each visit into 3 categories (0, 1, or ≥2). Other covariates included baseline age, race, sex, HIV viral load (detectable and undetectable), alcohol use (based on the Alcohol Use Disorders Identification Test [23]), body mass index (BMI), injection drug use in the previous 6 months (12), and methadone use in the previous 6 months.
Missing Data
Overall, out of 10 350 valid LSMs, approximately 1% were missing data on BMI (n = 106) and comorbidities (n = 102). Missingness for alcohol and drug use and HIV viral load was less than 0.5%. We performed multiple imputation with fully conditional specification to impute missing data (24). All of the aforementioned covariates were included as predictors. We imputed the data 10 times, assessed diagnostics, and confirmed low relative increases in variance and high relative efficiency.
Statistical Analysis
χ2 and Mann–Whitney tests were used to detect differences in frequency or median values of characteristics, respectively. To evaluate the effect of HCV cure on LSM, we used mixed-effects linear regression (continuous natural log LSM) and logistic regression (cirrhosis [LSM ≥12.3 kPa]). For the longitudinal models, we also included the LSM at the previous visit (lagged) in the model to adjust for potential time-varying confounding that could affect treatment status (see Supplement Figure 1, available at Annals.org). Other covariates included those listed earlier, as well as HCV RNA value at the previous visit (lagged) and calendar time based on when the visit occurred (from 2006 to 2019). We specified random intercepts and slopes, with an unstructured covariance matrix. To allow for individual-level curvature and direction, we included a quadratic time term. To account for the multiply imputed data sets, results were pooled across the data sets.
To evaluate the effect of HCV cure on mortality risk, we conducted proportional hazards modeling. The time scale for the analysis was age in years, which accounted for the possibility of left truncation. Participants entered the analysis at the age of their first LSM and were followed until death or were administratively censored at 31 December 2019. HCV RNA, LSM, HIV viral load, comorbidities, and drug use were treated as time-varying covariates. We estimated inverse probability treatment weights (IPTWs) to reduce bias when time-dependent covariates (such as cirrhosis) could be risk factors for the outcome (see the Supplement, available at Annals.org, for additional details). We also estimated differences in survival probability between participants with and without HCV cure at different age ranges. Ninety-five percent CIs were obtained using the 2.5th and 97.5th percentiles of the risk differences from 1000 bootstrap resamples.
To evaluate whether the effect of HCV cure differed by key factors that could influence both treatment access and mortality (cirrhosis before treatment and injection drug use in the prior 6 months), we assessed interaction terms in multivariable mixed models; however, these were not statistically significant. We also evaluated whether the association between HCV viral load and outcomes varied by recruitment wave by testing for an interaction, and these were also not statistically significant. In addition, we calculated E-values for the lower 95% confidence bound to determine the minimum strength an unmeasured confounder would need to explain the observed relative measure of association (25, 26). For example, an E-value of 5 indicates that an unmeasured confounder would need to be associated with both the exposure and the outcome by a relative risk of 5 each to explain away the effect. All statistical procedures were conducted in SAS, version 9.4 (SAS Institute), and statistical significance was set at a 2-tailed P value less than 0.05. The JM macro was used for joint modeling (27).
Sensitivity Analyses
To provide additional validation of post-SVR findings, we assessed the effect of reduced prevalence of chronic HCV on Fibrosis-4 (FIB-4) scores, a noninvasive measurement based on serum markers (platelet count, alanine aminotransferase, and aspartate aminotransferase) and age with a high specificity and positive predictive value for severe fibrosis or cirrhosis (>3.25) in patients with chronic HCV infection (28). Second, because informative dropout due to mortality could bias the association between longitudinal HCV RNA and LSM, we conducted joint longitudinal and time-to-event modeling (27). This family of models is appropriate when the longitudinal outcome of interest needs to be jointly modeled with time to study discontinuation to adjust for informative dropout (27). We used locally estimated scatterplot smoothing to plot the adjusted natural log LSM values generated from the linear predictor function of the longitudinal linear regression model, stratified by HCV treatment status. Participants were classified on the basis of their final HCV RNA status at the end of the observation period. Further details are provided in the Supplement. Third, we assessed self-reported treatment data rather than HCV RNA data on liver disease outcomes. Finally, because we have previously observed important sociodemographic and behavioral characteristics between the most recently recruited cohort (2015 to 2018) and older recruitment cohorts (29), we conducted analyses by excluding participants from the newer cohort who were on average younger, more likely to be non-Black, more likely to engage in more frequent injection-related risk behaviors, and more likely to live outside Baltimore City.
Role of the Funding Source
The National Institutes of Health had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Results
Sample Description
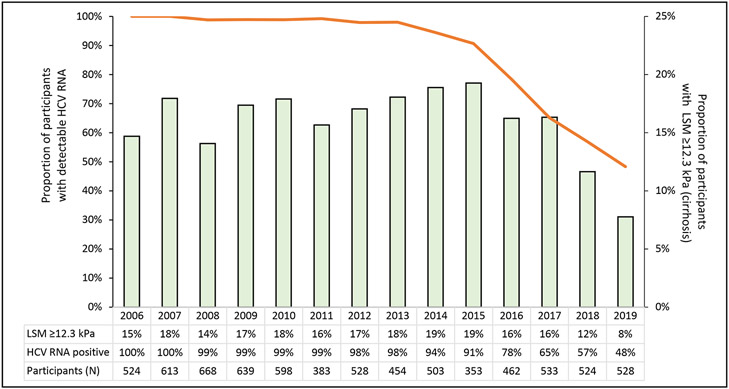
Among the 1323 participants who were positive for HCV RNA at their first LSM visit, the median age was 49 years (IQR, 44 to 54 years). Most were Black (82%), male (71%), and HIV-negative (66%) (Table 1). An LSM indicating cirrhosis (≥12.3 kPa) was observed in 15% of participants. Between 2006 and 2019, there were annual decreases in HCV viremia from 100% (by definition) in 2006 to 91% in 2015 and 48% by 2019 (Figure 1; Supplement Figure 2, available at Annals.org). Similarly, self-reported treatment increased from 3% in 2014 to 39% in 2019. From 2006 to 2019, 10 350 LSMs were conducted over a total of 7941 person-years and a median follow-up per person of 5.0 years (IQR, 1.5 to 11.2 years). The median number of LSMs per participant was 6 (IQR, 3 to 12), and the median number of RNA tests per participant was 4 (IQR, 2 to 8) (Supplement Figures 3 and 4, available at Annals.org). As shown in Figure 1, the proportion of visits with cirrhosis on LSM increased from 15% in 2006 to 19% in 2015 and then decreased to 8% by 2019. From 2006 to 2019, 430 deaths were recorded over 11 232 person-years (38 deaths per 1000 person-years). Twenty-nine percent (n = 126) of the deaths were drug- or trauma-related, and 41% (n = 176) were related to chronic disease. Six percent of all deaths were liver- or cirrhosis-related. Among persons with undetectable HCV RNA, 36 deaths occurred over 3981 person-years (9 deaths per 1000 person-years). However, among those who remained chronically infected, 394 deaths occurred over 7252 person-years (54 deaths per 1000 person-years).
Table 1.
Overview of Study Participants With Chronic HCV Infection at the First LSM Visit (n = 1323)*
| Characteristic | Value |
|---|---|
| Median age (IQR), y | 49 (43–54) |
| Sex | |
| Male | 936 (71) |
| Female | 387 (29) |
| Race | |
| Black | 1085 (82) |
| Non-Black | 238 (18) |
| AUDIT category † | |
| None | 587 (44) |
| Harmful/hazardous alcohol use | 295 (23) |
| Severe alcohol use/dependence | 439 (33) |
| Injection drug use in previous 6 mo | |
| No | 606 (46) |
| Yes | 714 (54) |
| Methadone use in previous 6 mo | |
| No | 958 (73) |
| Yes | 362 (27) |
| BMI ‡ | |
| Underweight/normal | 660 (51) |
| Overweight | 401 (31) |
| Obese | 238 (18) |
| Number of comorbidities § | |
| 0 | 626 (47) |
| 1 | 554 (42) |
| ≥2 | 135 (10) |
| HIV status | |
| Negative | 867 (66) |
| Positive, undetectable viral load | 235 (18) |
| Positive, detectable viral load | 217 (16) |
| Median log LSM (IQR), kPa | 1.95 (1.72–2.29) |
| LSM ≥12.3 kPa (cirrhosis) | |
| Yes | 194 (15) |
| No | 1129 (85) |
AUDIT = Alcohol Use Disorders Identification Test; BMI = body mass index; HCV = hepatitis C virus; LSM = liver stiffness measure.
Data are numbers (percentages) unless otherwise indicated. Some frequencies might not sum to the total because of missing values.
AUDIT scores between 8 and 14 were classified as harmful/hazardous alcohol use, and scores ≥15 were classified as severe alcohol use/dependence.
BMI <25 kg/m2 was classified as underweight/normal, 25–29.9 kg/m2 was classified as overweight, and ≥30 kg/m2 was classified as obese. Because there was a small number of underweight participants, they were grouped with those with normal weight.
Comorbidities were based on either 1) participants self-reporting that they had ever been told by a physician that they had kidney disease, diabetes, or hypertension, or 2) diagnostic criteria–based clinical/laboratory measurements (chronic kidney disease: estimated glomerular filtration rate <60 mL/min/1.73 m2; diabetes: hemoglobin A1c level >6.5%; hypertension: blood pressure >150/90 mm Hg for persons aged ≥60 y or >140/90 mm Hg for those aged <60 y).
Figure 1.
Temporal coincidence of HCV DAA availability in 2015 with declining prevalence of viremia (line, left Y-axis) and cirrhosis (bar, right Y-axis) in a cohort of Baltimore PWID, 2006-2019. HCV-hepatitis C virus; PWID – people who inject drugs; LSM – liver stiffness measures.
HCV Cure and Liver Stiffness
Undetectable HCV RNA was significantly associated with reduced liver disease burden (Table 2). In the univariable model, undetectable HCV RNA was associated with reduced average log LSM (β, −0.171 kPa [95% CI, −0.197 to −0.146 kPa]; P < 0.001) compared with detectable HCV RNA (Supplement Table 4, available at Annals.org). This association remained relatively unchanged in the multivariable model (adjusted β, −0.148 kPa [CI, −0.181 to −0.115 kPa]; P < 0.001; E-value, 1.55). Similarly, in the univariable model, we found undetectable HCV RNA to be associated with a 90% reduction in the odds of cirrhosis (odds ratio [OR], 0.10 [CI, 0.06 to 0.16]; P < 0.001). After other covariates were controlled for, undetectable HCV RNA was associated with a 72% reduction in the odds of cirrhosis (adjusted OR [aOR], 0.28 [CI, 0.17 to 0.45]; P < 0.001; E-value, 6.87). In multivariable analysis, other factors significantly associated with higher odds of cirrhosis were baseline age, BMI, number of comorbidities, and HIV infection. Predicted probability of cirrhosis over the entire observation period was lower among those who were cured (0.090 [CI, 0.071 to 0.108]) than among those with detectable HCV RNA (0.154 [CI, 0.147 to 0.162]).
Table 2.
Association of HCV RNA and Selected Covariates With Log LSM and Cirrhosis (LSM ≥12.3 kPa)
| Covariate | Log LSM (Continuous): Adjusted Mean (β) (95% CI) |
Cirrhosis (LSM ≥12.3 kPa): Adjusted Odds Ratio (95% CI) |
|---|---|---|
| HCV RNA status | ||
| Undetectable | −0.148 (−0.181 to −0.115) | 0.28 (0.17 to 0.45) |
| Detectable | Reference | Reference |
| Lagged HCV RNA status (previous visit) | ||
| Undetectable | −0.078 (−0.111 to −0.044) | 1.13 (0.67 to 1.90) |
| Detectable | Reference | Reference |
| Lagged LSM ≥12.3 kPa (previous visit) | ||
| Yes | 0.400 (0.370 to 0.429) | 37.3 (27.1 to 51.2) |
| No | Reference | Reference |
| Baseline age (1-y increase) | 0.007 (0.004 to 0.010) | 1.02 (0.99 to 1.05) |
| Sex | ||
| Female | −0.016 (−0.066 to 0.034) | 1.08 (0.82 to 1.41) |
| Male | Reference | Reference |
| Race | ||
| Black | −0.071 (−0.145 to 0.005) | 0.52 (0.33 to 0.81) |
| Non-Black | Reference | Reference |
| AUDIT category * | ||
| Severe alcohol use/dependence | 0.026 (0.004 to 0.048) | 1.36 (1.07 to 1.75) |
| Harmful/hazardous alcohol use | 0.011 (−0.008 to 0.031) | 1.22 (0.98 to 1.61) |
| None | Reference | Reference |
| Injection drug use in previous 6 mo | ||
| Yes | −0.019 (−0.036 to 0.001) | 0.74 (0.58 to 0.93) |
| No | Reference | Reference |
| Methadone use in previous 6 mo | ||
| Yes | 0.048 (0.027 to 0.068) | 1.31 (1.04 to 1.65) |
| No | Reference | Reference |
| BMI † | ||
| Obese | 0.119 (0.091 to 0.148) | 2.11 (1.56 to 2.86) |
| Overweight | 0.036 (0.016 to 0.055) | 1.48 (1.17 to 1.87) |
| Underweight/normal | Reference | Reference |
| Number of comorbidities ‡ | ||
| ≥2 | 0.052 (0.022 to 0.082) | 1.98 (1.44 to 2.72) |
| 1 | 0.036 (0.018 to 0.055) | 1.59 (1.26 to 2.01) |
| 0 | Reference | Reference |
| HIV status | ||
| Positive, detectable viral load | 0.133 (0.009 to 0.181) | 2.08 (1.48 to 2.94) |
| Positive, undetectable viral load | 0.075 (0.028 to 0.122) | 1.44 (1.05 to 1.97) |
| Negative | Reference | Reference |
| Recruitment cohort | ||
| 2015–2018 | −0.22 (−0.302 to −0.130) | 0.39 (0.20 to 0.75) |
| 2005–2008 | −0.058 (−0.114 to 0.002) | 0.72 (0.53 to 0.98) |
| 1998–2000 | −0.051 (−0.144 to 0.043) | 0.97 (0.47 to 1.33) |
| 1994 | 0.042 (−0.037 to −0.121) | 0.86 (0.58 to 1.29) |
| 1988–1989 | Reference | Reference |
AUDIT = Alcohol Use Disorders Identification Test; BMI = body mass index; HCV = hepatitis C virus; LSM = liver stiffness measure.
AUDIT scores between 8 and 14 were classified as harmful/hazardous alcohol use, and scores ≥15 were classified as severe alcohol use/dependence.
BMI <25 kg/m2 was classified as underweight/normal, 25–29.9 kg/m2 was classified as overweight, and ≥30 kg/m2 was classified as obese. Because there was a small number of underweight participants, they were grouped with those with normal weight.
Comorbidities were based on either 1) participants self-reporting that they had ever been told by a physician that they had kidney disease, diabetes, or hypertension, or 2) diagnostic criteria–based clinical/laboratory measurements (chronic kidney disease: estimated glomerular filtration rate <60 mL/min/1.73 m2; diabetes: hemoglobin A1c level >6.5%; hypertension: blood pressure >150/90 mm Hg for persons aged ≥60 y or >140/90 mm Hg for those aged <60 y).
HCV Cure and Mortality
In the unadjusted analysis, undetectable HCV RNA was associated with significantly lower risk for death (hazard ratio [HR], 0.42 [CI, 0.30 to 0.59; P < 0.001) (Table 3). In the IPTW models, all-cause mortality risk was significantly lower among participants who had undetectable HCV RNA than among those who did not (adjusted HR, 0.54 [CI, 0.38 to 0.77]; P < 0.001). Unadjusted and adjusted effect estimates strengthened when only deaths not related to drugs or trauma were included. Differences in survival probabilities between participants who were cured and those who were not increased with age (Supplement Figure 5, available at Annals.org).
Table 3.
Effect Estimates of HCV Cure on All-Cause Mortality and Non–Drug-Related or Non–Trauma-Related Mortality
| Effect Estimate | Undetectable HCV RNA | Detectable HCV RNA |
|---|---|---|
| All-cause mortality | ||
| Unadjusted hazard ratio (95% CI) | 0.42 (0.30–0.59) | Reference |
| IPTW-adjusted hazard ratio (95% CI)* | 0.54 (0.38–0.77) | Reference |
| Non–drug-related or non–trauma-related mortality | ||
| Unadjusted hazard ratio (95% CI) | 0.32 (0.21–0.50) | Reference |
| IPTW-adjusted hazard ratio (95% CI)* | 0.41 (0.26–0.64) | Reference |
AUDIT = Alcohol Use Disorders Identification Test; BMI = body mass index; HCV = hepatitis C virus; IPTW = inverse probability treatment weight; LSM = liver stiffness measure.
IPTWs were calculated using race, sex, recruitment cohort, age, AUDIT category, injection drug use in the previous 6 months, methadone use in the previous 6 months, BMI, number of comorbidities, HIV status, lagged HCV RNA status (previous visit), lagged LSM ≥12.3 kPa, lagged AUDIT category, lagged injection drug use in the previous 6 months, lagged methadone use in the previous 6 months, and lagged BMI.
Sensitivity Analyses
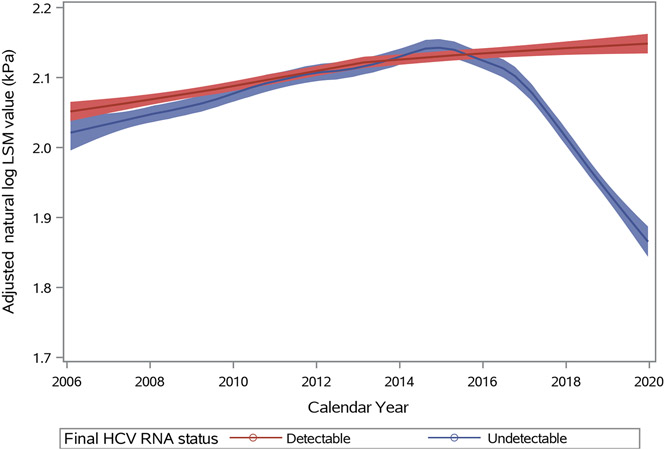
In the validation model with FIB-4 as the outcome, after adjustment for the same covariates as in the LSM model, undetectable HCV RNA was associated with 83% lower odds of having a FIB-4 value above 3.25 (aOR, 0.17 [CI, 0.08 to 0.37]; P < 0.001) (Supplement Table 5, available at Annals.org). Based on self-reported data rather than HCV RNA, participants who reported receiving HCV treatment had 39% lower odds of cirrhosis (aOR, 0.61 [CI, 0.43 to 0.89]). Furthermore, in the joint modeling analysis, we did not find substantial differences in the adjusted odds ratio or hazard ratios for HCV RNA with regard to the longitudinal and survival processes, respectively (Supplement Table 6, available at Annals.org). Adjusted natural log LSM trajectories of participants whose final HCV RNA status was undetectable diverged in 2015 from those who remained with detectable HCV RNA, with a sustained decrease as more participants received treatment over time (Figure 2). This was consistent with a sharp decrease in the adjusted natural log LSM occurring immediately after HCV clearance (Supplement Figure 6, available at Annals.org). After more recently recruited participants were removed, the associations between HCV RNA and liver disease outcomes were similar to those in the full cohort (Supplement Table 7, available at Annals.org).
Figure 2.
Adjusted liver stiffness measurements (LSM) in participants over calendar year by HCV RNA status at final observation. LSM are natural log transformed and adjusted for age, HIV, alcohol use and other factors then smoothed and depicted as blue if HCV RNA became negative by the final study visit through end of 2019 (as indication of cure) or as red (if HCV RNA remained detectable).
Discussion
In this study, we found that HCV treatment has expanded into the PWID community and has bent the rising arc of HCV-related liver disease. Conversely, given that 48% of participants in this sample remain chronically infected, the findings also underscore the heterogeneity of treatment uptake among PWID and the imperative to overcome these residual barriers to eliminate HCV infection in the United States.
These data strongly substantiate the link between increasing cure and decreasing HCV-related liver disease. By studying liver disease longitudinally using the most valid instruments, we had already characterized the increasing incidence of cirrhosis before the approval of sofosbuvir and ledipasvir in October 2014 (18). Continuing these structured measures on the same persons in the same setting provides rigorous evidence while also maintaining community representation. Moreover, SVR was confirmed by longitudinal testing of HCV RNA clearance, which is important because PWID can be reinfected (30). Our findings, which are supported by self-reported HCV treatment uptake and alternative measures of liver disease, indicate the benefit of treatment on liver disease and mortality. The mortality findings are especially important given the ultimate public health intent of HCV elimination.
Although other studies have shown that provision of HCV treatment results in high cure rates and reduced mortality (4, 31), we are unaware of other studies that have demonstrated in a community setting that HCV treatment uptake is sufficient among PWID to reduce the burden of cirrhosis and mortality at the population level. Thus, the critical question was not whether HCV treatment will work but whether it will be used in a community setting of PWID and the extent to which SVR will be sustained and will reduce cirrhosis and mortality despite HIV co-infection and ongoing alcohol use in some PWID. This is particularly salient given that more than 90% of persons with chronic hepatitis C in the United States are current or former PWID, and it is timely given the commitment by the U.S. Department of Health and Human Services to eliminate HCV infection by 2030 (1).
Transient elastography is the most valid method of ascertaining cirrhosis in persons with HCV infection (32-37). In addition, emerging data point to the value of a post-SVR LSM (or FIB-4) as a determinant of progression to liver failure and hepatocellular carcinoma (38-40). Just as SVR reduces but does not eliminate these risks, lower fibrosis scores in those noninvasive markers are associated with substantially reduced risk for clinical outcomes. Nonetheless, the absence of any validated marker of liver fibrosis after SVR is a challenge for monitorial global elimination of HCV infection.
Because the goal of a 65% reduction in mortality by 2030 is relative to 2015, progress toward achieving this target must include HCV epidemiologic data collected before 2015. We capitalized on a unique research ecosystem that for decades has rigorously and repeatedly collected HCV serologic and viremic data, LSMs, and self-reported treatment data from community-recruited PWID in and out of clinical care (12, 15, 41, 42). These empirical findings complement mathematical modeling indicating that with substantial testing and access to treatment, elimination targets can be achieved (5). For instance, after Australia removed restrictions on DAA access in 2016, treatment rates increased nearly 6-fold (43), and severe complications resulting from chronic HCV infection, such as decompensated cirrhosis and hepatocellular carcinoma, have either plateaued or decreased (44).
There are likely direct and indirect effects explaining the association between undetectable HCV RNA and mortality. Because cure is conditional on access to care, the direct effect of HCV cure was on reducing liver disease. However, indirect effects could also be related to management of chronic comorbidities and reduction of injection drug use after HCV cure, thereby reducing mortality risk. Thus, we recognize that lower mortality in persons who are cured might reflect not simply the effect of HCV cure but a constellation of social, environmental, and medical differences compared with those who are not cured.
Although the ALIVE cohort is replenished every few years to adapt to the changing profile of injection drug use, generalizability to populations outside Baltimore might be limited because injection drug use, HIV, and HCV infection have been endemic in Baltimore for decades. As discussed earlier, bias in HCV treatment uptake might affect the mortality estimates, potentially due to more motivated participants managing their own health. However, the more scientifically rigorous approach of randomly assigning participants to treatment or no treatment would be unethical. Moreover, the consistency in the findings and use of LSM as an intermediate step that was measured uniformly without bias is reassuring. Likewise, we could not estimate access to testing and awareness of HCV infection because, for ethical reasons, our study personnel counseled participants at each visit on the importance of HCV testing and treatment as well as delivering other harm reduction messages. Thus, it is possible that the effect of HCV testing and treatment is smaller in PWID not participating in this community-based cohort. Finally, the study was insufficiently powered to detect whether liver-related mortality decreased significantly in the post-DAA period, and death certificates likely undercount HCV infection as a contributing cause of liver death (45).
In conclusion, we found high uptake of curative HCV treatment in a community-based cohort of former and current PWID. In addition, concurrent decreases in HCV viremia and liver stiffness indicate that treatment is associated with substantial reductions in liver disease progression and mortality. With continued testing, treatment, and interventions to strengthen linkage to care and prevent HCV transmission, elimination of HCV infection could be achieved within the next decade.
Supplementary Material
Acknowledgment:
The authors thank all participants in the ALIVE study for their contribution.
Financial Support:
This work was supported by grants from the National Institutes of Health (NIH) (U01DA036297, R01DA048063, T32AI102623, and K01DA043421). This publication resulted in part from research supported by the Johns Hopkins University Center for AIDS Research, an NIH-funded program (1P30AI094189), which is supported by the following NIH co-funding and participating institutes and centers: the National Institute of Allergy and Infectious Diseases; the National Cancer Institute; the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; the National Institute on Drug Abuse; the National Institute on Aging; the National Institute of General Medical Sciences; the National Institute of Diabetes and Digestive and Kidney Diseases; and the National Institute on Minority Health and Health Disparities.
Footnotes
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M21-3846.
Contributor Information
Javier A. Cepeda, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
David L. Thomas, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, and Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland.
Jacqueline Astemborski, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Jacqueline Rudolph, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Rachel Gicquelais, School of Nursing, University of Wisconsin, Madison, Wisconsin.
Gregory D. Kirk, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, and Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland.
Shruti H. Mehta, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
References
- 1.U.S. Department of Health and Human Services. Viral Hepatitis National Strategic Plan for the United States: A Roadmap to Elimination (2021–2025). 2020. Accessed at www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strategic-Plan-2021-2025.pdf on 2 May 2021.
- 2.Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041–50. doi: 10.1056/NEJMra1810477 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Global hepatitis report, 2017. 2017:67.
- 4.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, et al. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166:637–48. doi: 10.7326/M16-2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heffernan A, Cooke GS, Nayagam S, et al. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet. 2019;393:1319–29. doi: 10.1016/S0140-6736(18)32277-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Interim guidance for country validation of viral hepatitis elimination. 8 June 2021. [DOI] [PubMed]
- 7.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878 [DOI] [PubMed] [Google Scholar]
- 8.Tada T, Kumada T, Toyoda H, et al. Viral eradication reduces all-cause mortality, including non-liver-related disease, in patients with progressive hepatitis C virus–related fibrosis. J Gastroenterol Hepatol. 2017;32:687–94. doi: 10.1111/jgh.13589 [DOI] [PubMed] [Google Scholar]
- 9.Janjua NZ, Wong S, Abdia Y, et al. Impact of direct-acting antivirals for HCV on mortality in a large population-based cohort study. J Hepatol. 2021;75:1049–57. doi: 10.1016/j.jhep.2021.05.028 [DOI] [PubMed] [Google Scholar]
- 10.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–33. doi: 10.1007/s10900-007-9083-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barua S, Greenwald R, Grebely J, et al. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163:215–23. doi: 10.7326/M15-0406 [DOI] [PubMed] [Google Scholar]
- 12.Kirk GD, Mehta SH, Astemborski J, et al. HIV, age, and the severity of hepatitis C virus–related liver disease: a cohort study. Ann Intern Med. 2013;158:658–66. doi: 10.7326/0003-4819-158-9-201305070-00604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trickey A, Fraser H, Lim AG, et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol. 2019;4:435–44. doi: 10.1016/S2468-1253(19)30085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas DL, Vlahov D, Solomon L, et al. Correlates of hepatitis C virus infections among injection drug users. Medicine (Baltimore). 1995;74:212–20. [DOI] [PubMed] [Google Scholar]
- 15.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–6. [DOI] [PubMed] [Google Scholar]
- 16.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–83. [DOI] [PubMed] [Google Scholar]
- 17.Vlahov D, Anthony JC, Munoz A, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 18.Cepeda JA, Thomas DL, Astemborski J, et al. Increased mortality among persons with chronic hepatitis C with moderate or severe liver disease: a cohort study. Clin Infect Dis. 2017;65:235–43. doi: 10.1093/cid/cix207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta SH, Kirk GD, Astemborski J, et al. Stability of liver fibrosis among HCV-infected injection drug users. Antivir Ther. 2012;17:813–21. doi: 10.3851/IMP2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirk GD, Astemborski J, Mehta SH, et al. Assessment of liver fibrosis by transient elastography in persons with hepatitis C virus infection or HIV–hepatitis C virus coinfection. Clin Infect Dis. 2009;48:963–72. doi: 10.1086/597350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics. National Death Index User’s Guide. 2013.
- 22.Rogot E, Sorlie P, Johnson NJ. Probabilistic methods in matching census samples to the National Death Index. J Chronic Dis. 1986;39:719–34. [DOI] [PubMed] [Google Scholar]
- 23.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res. 2015;4:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 26.Mathur MB, Ding P, Riddell CA, et al. Web site and R package for computing E-values. Epidemiology. 2018;29:e45–e47. doi: 10.1097/EDE.0000000000000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizopoulos D Joint Modeling of Longitudinal and Time-to-Event Data with Applications in R. Chapman and Hall/CRC; 2012. [Google Scholar]
- 28.Sterling RK, Lissen E, Clumeck N, et al. ; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 29.Cepeda JA, Astemborski J, Kirk GD, et al. Rising role of prescription drugs as a portal to injection drug use and associated mortality in Baltimore, Maryland. PLoS One. 2019;14:e0213357. doi: 10.1371/journal.pone.0213357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons B, Saleem J, Hill A, et al. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis. 2016;62:683–94. doi: 10.1093/cid/civ948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grebely J, Dalgard O, Conway B, et al. ; SIMPLIFY Study Group. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:153–61. doi: 10.1016/S2468-1253(17)30404-1 [DOI] [PubMed] [Google Scholar]
- 32.Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–50. [DOI] [PubMed] [Google Scholar]
- 33.Afdhal NH, Bacon BR, Patel K, et al. Accuracy of Fibroscan, compared with histology, in analysis of liver fibrosis in patients with hepatitis B or C: a United States multicenter study. Clin Gastroenterol Hepatol. 2015;13:772–9.e1–3. doi: 10.1016/j.cgh.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Li J, Zhou Q, et al. Liver stiffness measurement predicted liver-related events and all-cause mortality: a systematic review and nonlinear dose-response meta-analysis. Hepatol Commun. 2018;2:467–76. doi: 10.1002/hep4.1154 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Singh S, Muir AJ, Dieterich DT, et al. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152:1544–77. doi: 10.1053/j.gastro.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 36.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: final update of the series? J Hepatol. 2020;73:1170–218. doi: 10.1016/j.jhep.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 37.American Association for the Study of Liver Diseases; Infectious Diseases Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. 2020. Accessed at www.hcvguidelines.org on 3 February 2022. [DOI] [PMC free article] [PubMed]
- 38.Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151:457–471.e5. doi: 10.1053/j.gastro.2016.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyoda H, Tada T, Yasuda S, et al. Dynamic evaluation of liver fibrosis to assess the risk of hepatocellular carcinoma in patients with chronic hepatitis C who achieved sustained virologic response. Clin Infect Dis. 2020;70:1208–14. doi: 10.1093/cid/ciz359 [DOI] [PubMed] [Google Scholar]
- 40.Corma-Gómez A, Macías J, Téllez F, et al. Liver stiffness at the time of sustained virological response predicts the clinical outcome in people living with human immunodeficiency virus and hepatitis C virus with advanced fibrosis treated with direct-acting antivirals. Clin Infect Dis. 2020;71:2354–62. doi: 10.1093/cid/ciz1140 [DOI] [PubMed] [Google Scholar]
- 41.Wilson LE, Torbenson M, Astemborski J, et al. Progression of liver fibrosis among injection drug users with chronic hepatitis C. Hepatology. 2006;43:788–95. [DOI] [PubMed] [Google Scholar]
- 42.Rai R, Wilson LE, Astemborski J, et al. Severity and correlates of liver disease in hepatitis C virus–infected injection drug users. Hepatology. 2002;35:1247–55. [DOI] [PubMed] [Google Scholar]
- 43.Doyle JS, Scott N, Sacks-Davis R, et al. ; Eliminate Hepatitis C Partnership. Treatment access is only the first step to hepatitis C elimination: experience of universal anti-viral treatment access in Australia. Aliment Pharmacol Ther. 2019;49:1223–9. doi: 10.1111/apt.15210 [DOI] [PubMed] [Google Scholar]
- 44.Alavi M, Law MG, Valerio H, et al. Declining hepatitis C virus–related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia. J Hepatol. 2019;71:281–8. doi: 10.1016/j.jhep.2019.04.014 [DOI] [PubMed] [Google Scholar]
- 45.Mahajan R, Xing J, Liu SJ, et al. ; Chronic Hepatitis Cohort Study (CHeCS) Investigators. Mortality among persons in care with hepatitis C virus infection: the Chronic Hepatitis Cohort Study (CHeCS), 2006–2010. Clin Infect Dis. 2014;58:1055–61. doi: 10.1093/cid/ciu077 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.