Abstract
Background
Total body irradiation (TBI)-based-conditioning before allogeneic hematopoietic stem cell transplantation (allo-HSCT) is standard of care in patients with acute myeloid leukemia (AML) but can cause long-term morbidity. Data on the impact of chronic Graft-versus-host disease (cGvHD) on cognitive function (CF) and quality of life (QoL) of long-term transplant survivors are sparse.
Methods
We analyzed patient-reported outcomes focusing on progression-free AML patients and 1st allo-HSCT applying a standardized TBI-technique with an average dose rate of 4 cGy/min to the total body and lung shielding in case of doses > 8 Gy. Instruments included the Functional Assessment of Cancer Therapy-Bone marrow transplant (FACT-BMT, version 4), the FACT-Cognition Function (FACT-Cog, version 3) and the Patient Health Questionaire-4 (PHQ-4). We put focus on the impact of cGvHD and compared the results to normative data derived from the general population.
Results
Out of 41 eligible patients contacted, 32 (78.0%) patients with a medium follow-up of 154 months (Interquartile range 113, 191 months) participated in the study. Eleven patients (34.4%) had active cGvHD, 11 (34.4%) resolved cGvHD and 10 (31.3%) never had cGvHD. Patients with active cGvHD had poorer FACT-BMT, FACT-Cog and higher PHQ-4 scores compared to patients with resolved cGvHD or who never had cGvHD. Outcomes were similar in patients with resolved cGvHD and those who never had cGvHD. Patients with active cGvHD had similar FACT-Cog, but lower FACT-BMT in comparison to normative data. However, the overall patient sample had similar FACT-BMT and FACT-Cog in comparison to normative data.
Conclusion
Our data indicate that CF of long-term survivors upon TBI-based allo-HSCT is not impaired, even in the presence of active cGvHD. However, active cGvHD has a negative impact on QoL.
Trial registration The local Ethics Board of the University of Regensburg approved this study (Number 20-1810_1-101).
Keywords: Total body irradiation, Acute myeloid leukemia, Chronic graft-versus-host disease, Allogeneic hematopoietic cell transplantation, Quality of life, Cognitive function
Background
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative treatment modality for selected patients with acute myeloid leukemia (AML). Despite increasing survival rates, allo-HSCT can be associated with long-term morbidity and mortality [1]. The influence of total body irradiation (TBI) as part of the conditioning regimen and chronic graft-versus-host disease (cGvHD) on cognitive function (CF) and quality of life (QoL) of very long-term survivors is still unclear. In this study, we analyzed patient-reported CF and QoL focusing on long-term transplant survivors after 1st allo-HSCT applying a standardized TBI-technique as conditioning regimen. Since cGvHD can have a significant impact on QoL and CF [2] we analysed its impact as additional relevant covariable.
Patients and methods
Data collection
We analyzed patient-reported QoL, CF, and symptoms of depression and anxiety in patients with primary or secondary AML who received their 1st allo-HSCT with TBI-based protocols at the Department of Hematology of the University Hospital Regensburg between 1999 and 2017. All patients had a follow-up time of at least 2 years and were relapse-free for at least 2 years. The 2 year follow up was chosen since the primary aim was long term outcome and reports have indicated a protracted recovery of neurocognitive function [3]. Donors included matched sibling donors (MSD), matched unrelated donors (MUD), mismatched unrelated donors (MMUD) and haploidentical/mismatched related donors (MMRD). Source of stem cells were peripheral blood, bone marrow or cord blood. Patients completed the Functional Assessment of Cancer Therapy-Bone marrow transplant (FACT-BMT, version 4), the FACT-Cognition Function (FACT-Cog, version 3), the Patient Health Questionaire-4 (PHQ-4) and a questionnaire about sociodemographic data. All patients visited the Department of Hematology of the University Hospital Regensburg for routine follow-up visits. Clinical data including cGvHD status were abstracted from the medical charts of the Departments of Hematology and Radiation Oncology of the University Hospital Regensburg. Transplantation variables included gender, diagnosis, patient age, Karnofsky performance score (KPS), hematopoietic cell transplantation-comorbidity index (HCT-CI), as described by Sorror et al. [4], 2017 European LeukemiaNet (ELN) genetic risk stratification, as described by Döhner et al. [5], disease status, stem cell source, intensity of conditioning regimen, chemotherapeutic regimen, recipient and donor characteristics (donor type, donor age, HLA-compatibility, gender match, cytomegalovirus serostatus), GvHD prophylaxis and the use of rabbit anti-thymocyte globulin (ATG). Data closing was April 2021. The local Ethics Board of the University of Regensburg approved this study (Number 20-1810_1-101).
Treatment plan
The choice of conditioning regimen was based on the oncologists´ discretion and dependent on patient age, disease risk and comorbidities. All patients included in the analysis received TBI as part of a complex conditioning regimen. TBI was performed in a consistent manner with an average dose rate of 4 cGy/min to the total body and lung shielding in case of doses > 8 Gy. Over the years, four treatment protocols were used (8 Gy TBI/Cyclophosphamide/Fludarabine, FLAMSA-RIC/Cyclophosphamide/4Gy TBI, 12 Gy TBI/Cyclophosphamide and 8 Gy TBI/Fludarabine). From 2000 to 2013, two Siemens Primus linear accelerators (Siemens Medical Systems, Inc., Concord, CA) were used for TBI, and from 2013 to 2017 two linear accelerators of type Elekta Synergy ™ with an Agility ™ head (Elekta Ltd, Crawley, UK) were applied. We proved clinically good dose distributions and similar parameters with both linear accelerators [6]. All patients received 6 megavoltage (MV) photon beams. Patients were treated with a twice-daily fractionation and a minimum of 6 h between fractions. Patients were lying down on a couch at the floor level in supine and prone positions to extend the source-to-skin distance. A plate of Makrolon® polycarbonate of 1 cm thickness was placed on a stand above of the patient to neutralize the skin sparing by the buildup effect. The low diameter in the neck region was compensated by using a bolus of plastic modeling mass. Eight rotational arcs were used per patient position. The average time to deliver each fraction was 50–60 min per side (supine and prone). Additional fixed beams were used in cranial and caudal direction to compensate for the effects of inverse square variation with increasing distance. Two individual lung shields of MCP96 of calculated thickness were designed in case of doses > 8 Gy to reduce the total dose to the center of the lung to 3.5 Gy in supine and prone positions (total dose of 7 Gy). Radio-oncologists contoured two individual lung blocks for each patient on a CT scan with a 1–2 cm margin between the edge of the lung on the CT film and the edge of the block. Lung blocks were tailored to avoid shielding of the vertebrae. MV-imaging verified the shielding positions. Areas of the chest wall that were shielded by the blocks were supplemented once a day with electron beams to achieve the full dose to the thoracic walls. The electron fields delivered a supplemented dose of 5 Gy for 12 Gy regimens. In vivo dosimetry was used to verify the dose delivery on several points on the patient´s body, demonstrating the uniformity of the dose distribution [6].
QoL measures and other data sources
The FACT-BMT (Version 4.0) is a self-report questionnaire. The FACT-BMT combines the 27-item FACT-G total score (score range 0–108), an assessment of physical well-being (PWB, score range 0–28), social/family well-being (SWB, score range 0–28), emotional well-being (EWB, score range 0–24) and functional well-being (FWB, score range 0–28) with a 10 item Bone Marrow Transplant subscale (BMTS, score range 0–40) to evaluate self-reported concerns after transplantation. Patients rate on five-point Likert scale the frequency with which each concern was recognized in the past 7 days. The FACT-BMT-Trial Outcome Index (FACT-BMT-TOI, score range 0–96) is the sum of PWB, FWB and the BMTS-score. The FACT-BMT total score (score range 0–148) is the sum of the BMTS score and of the FACT-G total score. Higher scores indicate better QoL. The FACT-Cog (Version 3.0) is a validated measurement to analyze self-reported cognitive complaints in cancer patients. It includes perceived cognitive impairments (FACT-CogPCI, score range 0–72), impact of perceived cognitive impairments on quality of life (FACT-CogQoL, score range 0–16), comments from others (FACT-CogOth, score range 0–16) and perceived cognitive abilities (FACT-CogPCA, score range 0–28). Patients rate on five-point Likert scale the frequency of each complaint in the past 7 days. Higher scores indicate better QoL. All data are analyzed and expressed as mean according to the FACIT recommendations. The Patient Health Questionnaire-4 (PHQ-4) analyzes symptoms of depression and anxiety over the last 2 weeks on a 4 point Likert-type scale. Patients indicate if they feel nervous, anxious or on edge (item 1), if they are not able to stop and control worrying (item 2), if they have little interest or pleasure in doing things (item 3) and if they feel down, depressed or hopeless (item 4). The anxiety subscale (GAD-2) is the sum of the items 1 und 2 and the depression subscale (PHQ-2) is the sum of the items 3 and 4. In summary, there are four categories of psychological distress (None = 0–2, mild = 3–5, moderate = 6–8 and severe = 9–12). Patients with a GAD2 or PHQ2 of ≥ 3 are categorized as present for anxiety or depression. The presence and absence of cGvHD was extracted from the database of the Department of Hematology. Acute GvHD and cGvHD were defined according to described standard criteria [7–9]. Acute GvHD is classified as clinically significant at grade II-IV aGvHD. Patients have clinically active cGvHD (Group 1: Currently active inflammatory manifestations of cGvHD independently of the use of immunosuppression), resolved cGvHD (Group 2: All signs of clinically activity of cGvHD have disappeared, past history of cGvHD, no use of immunosuppression) or never had signs of cGvHD (Group 3: Never having cGvHD). The overall rate of completion was 100% for the FACT-Cog and the PHQ-4 as well as 98.1% for the FACT-BMT (Missing answers referred to satisfaction with sexual functioning).
Statistical analysis
Transplant-related characteristics were presented as absolute and relative frequencies for categorical variables and as median and interquartile range (IQR) for continuous variables. The Mann–Whitney U-test was used for comparisons of continuous variables and the chi-square test of independence for categorical variables. Spearman's rank correlation coefficients were calculated to analyze the association of the FACT-BMT, FACT-Cog and PHQ-4. One-way analyses of variance (ANOVA) were performed to explore the effect of cGvHD on QoL outcomes. Post hoc group comparisons were done using the Tukey-test. Median follow-up time was estimated by using the reverse Kaplan-Meier method. Normative data (unadjusted means and standard deviations) of a general U.S. adult population of Brucker et al. [10] were used for comparisons with the FACT-G and normative data (means and standard deviations) of a French healthy population of Lange et al. [11] for comparisons with the FACT-Cog. We excluded AML patients < 30 years of age (n = 4) for comparisons with the normative data of Lange et al. [11] because of missing reference values for patients < 30 years of age. Comparisons between the normative data and the patient population were made using the one-sample t-test. A minimum clinically important difference (MCID) in QoL scores was defined as half of standard deviation (0.5 SD), as reported by Norman et al. [12]. Missing data were treated according to the manual scoring guidelines. We used the cutpoints of van Dyk et al. [13] to discriminate cancer-related cognitive impairment (Perceived cognitive impairment-score, FACT-CogPCI < 54) from the healthy population (FACT-CogPCI ≥ 54). All p-values were two-sided and p-values < 0.05 were considered as significant. Statistical analysis was performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA) and graphics were performed with Excel (2013, Microsoft Office).
Results
Patient and transplantation characteristics
95 patients were identified who received TBI-based-conditioning before 1st allo-HSCT between 1999 and 2017. 54 patients (56.8%) died after allo-HSCT and were not available for the evaluation of the patient-reported outcomes. The causes of death were relapse (n = 37, 68.5%), GvHD (n = 8, 14.8%), infections (n = 6, 11.1%) and other causes (n = 3, 5.6%). The median survival time of the overall population sample (n = 95) was 152 months (IQR 188, 120 months). All 41 long-term transplant survivors were contacted via post between December 2021 and April 2022. 32 patients (78.0%) provided written informed consent and completed the questionnaires. Nine non-responders (22.0%) were excluded from the analysis after two unsuccessful contact attempts (response rate: 78.0%). At the time of the evaluation, all participants were in complete remission of their initially diagnosed AML. Three patients relapsed after 1st allo-HSCT but were successfully treated with azacitidine (n = 1) and donor lymphocyte infusions (DLI; n = 2) and remained in remission with a follow-up of at least 24 months after successful completion of treatment (min 24, max 154). Table 1 shows the baseline transplantation characteristics of the participants (n = 32). The median follow-up time of all participants was 153 months (IQR 113, 191 months).
Table 1.
Baseline transplantation characteristics
| All (n = 32) | Group 1: Never cGvHD (n = 10) | Group 2: Resolved cGvHD (n = 11) | Group 3: Active cGvHD (n = 11) | p-value | |
|---|---|---|---|---|---|
| Patient age at the time of transplantation, years | |||||
| Median (IQR) | 39 (26, 47) | 42 (34, 49) | 28 (21, 49) | 40 (31, 48) | .199 |
| Patient age at the time of survey, years | |||||
| Median (IQR) | 53 (37, 62) | 59 (45, 63) | 41 (29, 60) | 54 (36, 62) | .264 |
| Follow-up time, months | |||||
| Median (IQR) | 153 (113, 191) | 174 (103, 231) | 152 (113, 199) | 151 (120, 182) | .623 |
| Gender, n (%) | |||||
| Women | 13 (41.0%) | 3 (30.0%) | 5 (45.5%) | 5 (45.5%) | .712 |
| Men | 19 (59.0%) | 7 (70.0%) | 6 (54.5%) | 6 (54.5%) | |
| Diagnosis, n (%) | |||||
| Primary AML | 22 (68.8%) | 7 (70.0%) | 7 (63.6%) | 8 (72.7%) | .895 |
| Secondary AML | 10 (31.3%) | 3 (30.0%) | 4 (36.4%) | 3 (27.3%) | |
| 2017 European LeukemiaNet genetic risk stratification, n (%) | |||||
| Favorable | 6 (18.8%) | 2 (20.0%) | 3 (27.3%) | 1 (9.1%) | .829 |
| Intermediate | 18 (56.3%) | 6 (60.0%) | 5 (45.5%) | 7 (63.6%) | |
| Adverse | 8 (25.0%) | 2 (20.0%) | 3 (27.3%) | 3 (27.3%) | |
| Remission status at the time of transplantation, n (%) | |||||
| First complete remission (CR1) | 15 (46.9%) | 5 (50.0%) | 4 (36.4%) | 6 (54.5%) | .700 |
| First partial remission (PR1), CR2 | 12 (37.5%) | 3 (30.0%) | 6 (54.5%) | 3 (27.3%) | |
| > CR2, primary refractory AML | 5 (15.6%) | 2 (20.0%) | 1 (9.1%) | 2 (18.2%) | |
| Hematopoietic stem cell transplantation comorbidity index (HCT-CI), n (%) | |||||
| 0 | 16 (50.0%) | 6 (60.0%) | 4 (36.4%) | 6 (54.5%) | .712 |
| 1–2 | 11 (34.4%) | 2 (20.0%) | 5 (45.5%) | 4 (36.4%) | |
| ≥ 3 | 5 (15.6%) | 2 (20.0%) | 2 (18.2%) | 1 (9.1%) | |
| Karnofsky performance score, n (%) | |||||
| < 80 | 4 (12.5%) | 1 (10.0%) | 1 (9.1%) | 2 (18.2%) | .779 |
| ≥ 80 | 28 (87.5%) | 9 (90.0%) | 10 (90.9%) | 9 (81.8%) | |
| Donor type, n (%) | |||||
| Matched sibling donor | 10 (31.3%) | 2 (20.0%) | 3 (27.3%) | 5 (45.5%) | .637 |
| Matched unrelated donor | 17 (53.1%) | 7 (70.0%) | 5 (45.5%) | 5 (45.5%) | |
| Mismatched unrelated donor | 4 (12.5%) | 1 (10.0%) | 2 (18.2%) | 1 (9.1%) | |
| Haploidentical, mismatched related donor | 1 (3.1%) | 0 (-) | 1 (9.1%) | 0 (–) | |
| Stem cell source, n (%) | |||||
| Peripheral blood | 30 (93.8%) | 8 (80.0%) | 11 (100%) | 11 (100%) | .320 |
| Bone marrow | 1 (3.1%) | 1 (10.0%) | 0 (–) | 0 (–) | |
| Cord blood | 1 (3.1%) | 1 (10.0%) | 0 (–) | 0 (–) | |
| Conditioning regimen, n (%) | |||||
| Myeloablative (MAC) | 23 (71.9%) | 8 (80.0%) | 10 (90.9%) | 5 (45.5%) | .047 |
| Reduced intensity (RIC) | 9 (28.1%) | 2 (20.0%) | 1 (9.1%) | 6 (54.5%) | |
| Chemotherapeutic regimen, n (%) | |||||
| 8 Gy TBI/CY2/Fludarabine | 15 (46.9%) | 7 (70.0%) | 5 (45.5%) | 3 (27.3%) | .090 |
| FLAMSA-RIC/CY2/4 Gy TBI | 9 (28.1%) | 2 (20.0%) | 1 (9.1%) | 6 (54.5%) | |
| 12 Gy TBI/CY2 | 5 (15.6%) | 0 (–) | 3 (27.3%) | 2 (18.2%) | |
| 8 Gy TBI/Fludarabine | 3 (9.4%) | 1 (10.0%) | 2 (18.2%) | 0 (–) | |
| GvHD-prophylaxis, n (%) | |||||
| Cyclosporine/MTX | 26 (81.2%) | 9 (90.0%) | 10 (90.9%) | 7 (63.6%) | .322 |
| Cyclosporine/Mycophenolate mofetil | 4 (12.5%) | 1 (10.0%) | 0 (–) | 3 (27.3%) | |
| Post-transplantation Cyclophosphamide/tacrolimus/Mycophenolate mofetil | 2 (6.3%) | 0 (–) | 1 (9.1%) | 1 (9.1%) | |
| Anti-Thymocyte Globulin (ATG), n (%) | |||||
| Yes | 23 (71.9%) | 9 (90.0%) | 9 (81.8%) | 5 (45.5%) | .051 |
| No | 9 (28.1%) | 1 (10.0%) | 2 (18.2%) | 6 (54.5%) | |
| Donor-recipient cytomegalic-virus-status, n (%) | |||||
| Negative/negative | 16 (50.0%) | 5 (50.0%) | 8 (72.7%) | 3 (27.3%) | .143 |
| Negative/positive | 6 (18.8%) | 1 (10.0%) | 0 (-) | 5 (45.5%) | |
| Positive/positive | 6 (18.8%) | 2 (20.0%) | 2 (18.2%) | 2 (18.2%) | |
| Positive/negative | 4 (12.5%) | 2 (20.0%) | 1 (9.1%) | 1 (9.1%) | |
| Female donor to male recipient, n (%) | |||||
| Yes | 5 (15.6%) | 1 (10.0%) | 2 (18.2%) | 2 (18.2%) | .840 |
| No | 27 (84.4%) | 9 (90.0%) | 9 (81.8%) | 9 (81.8%) | |
| Grade II–IV acute GvHD, n (%) | |||||
| Yes | 12 (37.5%) | 4 (40.0%) | 4 (36.4%) | 4 (36.4%) | .981 |
| No | 20 (62.5%) | 6 (60.0%) | 7 (63.6%) | 7 (63.6%) | |
| Acute GvHD grade (n = 12) | |||||
| Grade II | 9 (75.0%) | 3 (75.0%) | 3 (75.0%) | 3 (75.0%) | .558 |
| Grade III | 2 (16.7%) | 1 (25.0%) | 0 (–) | 1 (25.0%) | |
| Grade IV | 1 (8.3%) | 0 (–) | 1 (25.0%) | 0 (–) | |
| Survival in CR/relapse, n (%) | |||||
| Survival in CR | 29 (90.6%) | 10 (100%) | 9 (81.8%) | 10 (90.9%) | .361 |
| Relapse* | 3 (9.4%) | 0 (-) | 2 (18.2%)¶ | 1 (9.1%) § | |
Never cGvHD (chronic Graft-versus-Host Disease): never having cGvHD; Resolved cGvHD: all signs of clinically activity of cGvHD have disappeared, past history of cGvHD, no use of immunosuppression; Active cGvHD: physician reported inflammatory manifestations of cGvHD
TBI 8 Gy/Cy2/Fludarabine: 8 Gy TBI (four 2 Gy doses on two consecutive days), Cyclophosphamide 2 × 60 mg/kg on two consecutive days, Fludarabine 3 × 30 mg/m2 on three consecutive days
FLAMSA-RIC/TBI 4 Gy/Cy2: FLAMSA regimen (d -12 to d -9): Fludarabine 4 × 30 mg/m2, HD-Ara-C 4 × 2000 mg/m2, Amsacrine 4 × 100 mg/m2. Reduced intensity conditioning (RIC)-regimen after 3 days of rest: 4 Gy TBI on d-5 (two 2 Gy doses), Cyclophosphamide (2 × 40 mg/kg for MRD or 2 × 60 mg/kg for MUD, MMRD or MMUD) on d -4 to d -3 , Antithymocyte globulin 10 mg/kg for MRD or 20 mg/kg for MUD, MMRD, MMUD from d-4 to d-2, prophylactic donor lymphocyte infusions at day + 120 or 30 days after discontinuation of immunosuppression: 1–5 × 106 CD3+ cells/kg
TBI 12 Gy/Cy2: 12 Gy TBI (Six 2 Gy doses on three consecutive days, d -7 to d -5), Cyclophosphamide 2 × 60 mg/kg on 2 consecutive days (d -4 to d -3)
TBI 8 Gy/Fludarabine: 8 Gy TBI (four 2 Gy doses on 2 consecutive days, d-5 and d-4), Fludarabine 4 × 30 mg/m2 (d -5 to d -2)
*At time of the evaluation: All patients with relapse in history were in complete remission of the initial diagnosed AML after donor lymphocyte infusions (n = 2 ¶) and therapy with azacitidine (n = 1 §) for a median time of 43.8 months (IQR 34.0, 98.6 months)
The participants (n = 32) and non-responders (n = 9) showed no differences in patient age at the time of allo-HSCT (p = .374), sex (p = 1.0), diagnosis (p = .401), ELN-risk classification (p = .434), remission status (p = .372), HCT-CI (p = 893), KPS (p = .559), GvHD prophylaxis (p = .402), donor type (p = .646), stem cell source (p = .544), the use of a female donor to a male recipient (p =. 568), donor recipient CMV status (p = .527), use of ATG (p = 1.0), the current cGvHD status (p = .248), length of follow-up (p = .712) and the status of relapse (p = 1.0). Participants and non-responders had similar aGvHD ≥ grade II in history (31.3% vs. 0.0%, p = .083).
Chronic GvHD characteristics after transplantation
Table 2 shows the cGvHD characteristics and the NIH severity of cGvHD at maximum severity. Twenty-two patients (69%) developed cGvHD after allo-HSCT. The median time from allo-HSCT to the onset of cGvHD was 326 days (IQR 208, 601 days). At time of the evaluation, 11 patients (34.4%) had currently active cGvHD, 11 patients (34.4%) had resolved cGvHD and 10 patients (31.3%) never had signs of cGvHD.
Table 2.
Chronic Graft-versus-Host disease characteristics
| Chronic GvHD characteristics at time of onset of cGvHD (n = 22) | All | Resolved cGvHD (n = 11), n (%) | Active cGvHD (n = 11), n (%) | p-value |
|---|---|---|---|---|
| Median days from transplantation to onset of cGvHD (IQR) | 326 (208, 601) | 350 (224, 595) | 240 (151, 723) | 1.0 |
| Subcategories of cGvHD type at time of onset | ||||
| Classic cGvHD | 20 (90.9%) | 10 (90.9%) | 10 (90.9%) | 1.0 |
| Overlap cGvHD | 2 (9.1%) | 1 (9.1%) | 1 (9.1%) | |
| NIH severity of cGvHD at maximum severity | ||||
| Mild | 2 (9.1%) | 1 (9.1%) | 1 (9.1%) | .080 |
| Moderate | 11 (50.0%) | 8 (72.7%) | 3 (27.3%) | |
| Severe | 9 (40.9%) | 2 (18.2%) | 7 (63.6%) | |
| Organ manifestations of cGvHD at maximum severity | ||||
| Cutaneous | 16 (72.7%) | 8 (72.7%) | 8 (72.7%) | 1.0 |
| Oral | 13 (59.1%) | 5 (45.5%) | 8 (72.7%) | .387 |
| Eye | 9 (40.9%) | 4 (36.4%) | 5 (45.5%) | 1.0 |
| Lung | 5 (22.7%) | 3 (27.3%) | 2 (18.2%) | 1.0 |
| Gastrointestinal | 4 (18.2%) | 3 (27.3%) | 1 (9.1%) | .586 |
| Liver | 4 (18.2%) | – | 4 (36.4%) | .090 |
| Vaginal | 4 (18.2%) | 2 (18.2%) | 2 (18.2%) | 1.0 |
| Fascia | 2 (9.1%) | – | 2 (18.2%) | .476 |
| Joint | 2 (9.1%) | 1 (9.1%) | 1 (9.1%) | 1.0 |
| Platelet count at cGvHD onset, thrombocytes/µl | ||||
| Median (IQR) | 224 (90, 325) | 240 (90, 306) | 175 (77, 387) | 1.0 |
| Platelet count at cGvHD onset | ||||
| < 100/nL | 6 (27.3%) | 3 (27.3%) | 3 (27.3%) | 1.0 |
| ≥ 100/nL | 16 (72.7%) | 8 (72.7%) | 8 (72.7%) | |
Data presented are n (%) unless otherwise indicated
cGvHD: chronic Graft-versus-Host disease; Resolved cGvHD: all signs of clinically activity of cGvHD have disappeared, past history of cGvHD, no immunosuppression; Active cGvHD: physician reported inflammatory manifestations of cGvHD; IQR: interquartile range; NIH: National Institutes of Health
Socioeconomic characteristics
Table 3 shows the socioeconomic data at the time of the evaluation.
Table 3.
Socioeconomic aspects
| All (n = 32), n (%) | Group 1: Never cGvHD (n = 10), n (%) | Group 2: Resolved cGvHD (n = 11), n (%) | Group 3: Active cGvHD (n = 11), n (%) | p-value | |
|---|---|---|---|---|---|
| Relationship | |||||
| Single/never married | 8 (25.0%) | 1 (10.0%) | 4 (36.4%) | 3 (27.3%) | .246 |
| Married/living with a partner | 20 (62.5%) | 6 (60.0%) | 7 (63.6%) | 7 (63.6%) | |
| Divorced/Widowed | 4 (12.5%) | 3 (30.0%) | 0 (–) | 1 (9.1%) | |
| Employment | |||||
| Working all-day | 12 (37.5%) | 4 (40.0%) | 5 (45.5%) | 3 (27.3%) | .209 |
| Working part-time | 5 (15.6%) | 2 (20.0%) | 3 (27.3%) | 0 (-) | |
| Pensioners | 15 (46.9%) | 4 (40.0%) | 3 (27.3%) | 8 (72.7%) | |
| School-leaving qualifications | |||||
| Secondary modern school/intermediate school qualification | 21 (65.6%) | 6 (60.0%) | 7 (63.6%) | 8 (72.7%) | .816 |
| Advanced technical college entrance qualification/A-levels | 11 (34.4%) | 4 (40.0%) | 4 (36.4%) | 3 (27.3%) | |
| Care obligations to family | |||||
| Yes | 6 (18.8%) | 3 (30.0%) | 2 (18.2%) | 1 (9.1%) | .471 |
| No | 26 (81.3%) | 7 (70.0%) | 9 (81.8%) | 10 (90.9%) |
cGvHD: chronic Graft-versus-Host Disease; Never cGvHD: never having cGvHD; Resolved cGvHD: all signs of clinically activity of cGvHD have disappeared, past history of cGvHD, no immunosuppression; Active cGvHD: physician reported inflammatory manifestations of cGvHD
FACT-BMT, FACT-Cog and PHQ-4 according to the chronic GvHD-status
Table 4 shows the mean scores of the FACT-Cog, FACT-BMT and PHQ-4. Using the classification of Van Dyk et al. [13], 4 patients (12.5%) had present cognitive impairments (PCI-score < 54) (Mean 52.3, SD 0.5). Patients of group 1 (never cGvHD) and group 2 (resolved cGvHD) showed similar values of FACT-CogPCI, FACT-CogQoL and FACT-CogPCA. Patients of group 3 (currently active cGvHD) had the lowest FACT-Cog values of all of the three groups.
Table 4.
FACT-BMT, FACT-Cog and PHQ-4 according to cGvHD
| Mean (SD) | All (n = 32) | Group 1: Never cGvHD (n = 10) | Group 2: Resolved cGvHD (n = 11) | Group 3: active cGvHD (n = 11) | F-value | p-value |
|---|---|---|---|---|---|---|
| Functional Assessment of Cancer Therapy-Cognitive function (FACT-Cog) | ||||||
| Perceived cognitive impairment (PCI, score range 0–72) | 63.9(6.3) | 67.0(5.2)a | 66.1(4.6)a | 58.8(5.8)b | 7.8 | .002* |
| Comments from others (Oth, score range 0–16) | 15.6(.84) | 15.7(.9) | 15.7(.5) | 15.4(1.0) | .621 | .545 |
| Impact of perceived cognitive impairments on quality of life (QoL, score range 0–16) | 14.2(1.7) | 15.0(1.1)a | 15.0(1.0)a | 12.9(1.9)b | 7.8 | .002* |
| Perceived cognitive abilities (PCA, score range 0–28) | 22.5(4.1) | 24.8(3.2)a | 23.8(3.0)a | 19.2(3.9)b | 8.4 | .001** |
| Functional Assessment of Cancer Therapy-Bone marrow transplantation (FACT-BMT) | ||||||
| Physical wellbeing (PWB, score range 0–28) | 23.1 (4.8) | 26.3 (2.1)a | 25.8 (2.3)a | 17.4 (3.2)b | 39.9 | < .001*** |
| Emotional wellbeing (EWB, score range 0–24) | 19.3 (3.8) | 21.7 (2.5)a | 21.0 (2.2)a | 15.5 (3.1)b | 17.0 | < .001*** |
| Social/family wellbeing (SWB, score range 0–28) | 21.0 (3.8) | 23.7 (1.0)a | 22.5 (3.0)a | 17.0 (2.6)b | 23.6 | < .001*** |
| Functional wellbeing (FWB, score range 0–28) | 21.1 (3.6) | 22.8 (1.8)a | 23.8 (1.3)a | 16.8 (2.1)b | 46.7 | < .001*** |
| Bone marrow transplant subscale (BMTS, score range 0–40) | 30.8 (4.3) | 33.4 (2.5)a | 33.0 (2.7)a | 26.4 (3.3)b | 20.3 | < .001*** |
| FACT-G total score (score range 0–108) | 84.6 (14.0) | 94.4 (5.3)a | 92.8 (6.7)a | 67.5 (7.0)b | 59.1 | < .001*** |
| FACT-BMT-Trial Outcome Index (TOI, score range 0–96) | 75.1 (11.5) | 82.4 (4.6)a | 82.3 (5.0)a | 61.4 (7.1)b | 48.4 | < .001*** |
| FACT-BMT total score (score range 0–148) | 115.5 (17.8) | 127.8 (6.7)a | 125.8 (7.9)a | 93.9 (9.9)b | 56.5 | < .001*** |
| Patient Health Questionnaire-4 (PHQ-4, score range 0–12) | 1.94 (2.1) | 1.3 (1.4)a | .91 (.94)a | 3.6 (2.5)b | 7.0 | < .001*** |
| PHQ-2 (score range 0–6) | 1.09 (1.3) | .8 (.9)a | .45 (.82)a | 2.0 (1.4)b | 6.1 | < .01** |
| GAD-2 (score range 0–6) | .84 (1.0) | .5 (.7)a | .45 (.52)a | 1.5 (1.3) b | 5.0 | < .05* |
cGvHD: chronic graft-versus-host disease; Never cGvHD: never having cGvHD; Resolved cGvHD: patients with a past history of cGvHD, no immunosuppression; Active cGvHD: physician reported currently active inflammatory manifestations of cGvHD; FACT-BMT and FACT-Cog: higher scores indicate better quality of life; Patient Health Questionaire (PHQ-4): measurement of core symptoms of depression (PHQ-2) and anxiety (GAD-2)
Analysis of variance (ANOVA) with post-hoc Tukey-test: *p < .05, **p < .01, ***p < .001
Groups with different letters (a, b) differ significantly at 5% significance level by the Tukey-test. Equal letters do not differ by the Tukey-test
Patients of group 1 (never cGvHD) and group 2 (resolved cGvHD) showed similar values of all FACT-BMT scores, while patients of group 3 (currently active cGvHD) had the lowest values of all of the three groups.
15.6% (n = 5) of patients were categorized as present of depression (PHQ2 ≥ 3) and 9.4% (n = 3) as present of anxiety (GAD ≥ 3). Regarding the psychological aspect, patients of group 1 (never cGvHD) and group 2 (resolved cGvHD) showed similar values of PHQ-4 scores, while patients of group 3 (currently active cGvHD) had the highest values.
Correlations between PHQ-4 and FACT-Cog and FACT-BMT
Table 5 shows the Spearman-Rho correlations between the PHQ-2 and the GAD-2 with the FACT-Cog and the FACT-BMT.
Table 5.
Spearmen-Rho correlations between the PHQ-4, the FACT-Cog and the FACT-BMT
| PHQ-4 | ||
|---|---|---|
| PHQ-2 depression | GAD-2 anxiety | |
| Functional Assessment of Cancer Therapy-Cognitive function (FACT-Cog) | ||
| Perceived cognitive impairment (PCI) | − .503** | − .490** |
| Comments from others (Oth) | − .286 | − .438* |
| Impact of perceived cognitive impairments on quality of life (QoL) | − .550*** | − .703*** |
| Perceived cognitive abilities (PCA) | − .624*** | − .487** |
| Functional Assessment of Cancer Therapy-Bone marrow transplantation (FACT-BMT) | ||
| Physical wellbeing (PWB) | − .634*** | − .389* |
| Emotional wellbeing (EWB) | − .555*** | − .520** |
| Social/family wellbeing (SWB) | − .536** | − .183 |
| Functional wellbeing (FWB) | − .639*** | − .401* |
| Bone marrow transplant subscale (BMTS) | − .605*** | − .374* |
FACT-BMT and FACT-Cog: higher scores indicate better quality of life; Patient Health Questionnaire (PHQ-4): measurement of core symptoms of depression (PHQ-2) and anxiety (GAD-2)
*p < .05, **p < .01, ***p < .001
Correlation of FACT-BMT and FACT-Cog
Table 6 shows the Spearman-Rho correlations between the FACT-BMT and the FACT-Cog scores.
Table 6.
Spearmen-Rho correlations between the FACT-Cog and the FACT-BMT
| Functional Assessment of Cancer Therapy-Cognitive function (FACT-Cog) | ||||
|---|---|---|---|---|
| Perceived cognitive impairment (PCI) | Comments from others (Oth) | Impact of perceived cognitive impairments on quality of life (QoL) | Perceived cognitive abilities (PCA) | |
| Functional Assessment of Cancer Therapy-Bone marrow transplantation (FACT-BMT) | ||||
| Physical wellbeing (PWB) | .582*** | .260 | .594*** | .559*** |
| Emotional wellbeing (EWB) | .538** | .343 | .707*** | .536** |
| Social/family wellbeing (SWB) | .619*** | .120 | .311 | .600*** |
| Functional wellbeing (FWB) | .441* | .168 | .549** | .432* |
| Bone marrow transplant subscale (BMTS) | .562*** | .312 | .510** | .528** |
FACT-BMT and FACT-Cog: higher scores indicate better quality of life
*p < .05, **p < .01, ***p < .001
FACT-BMT and FACT-Cog in comparison to normative data derived from the general population
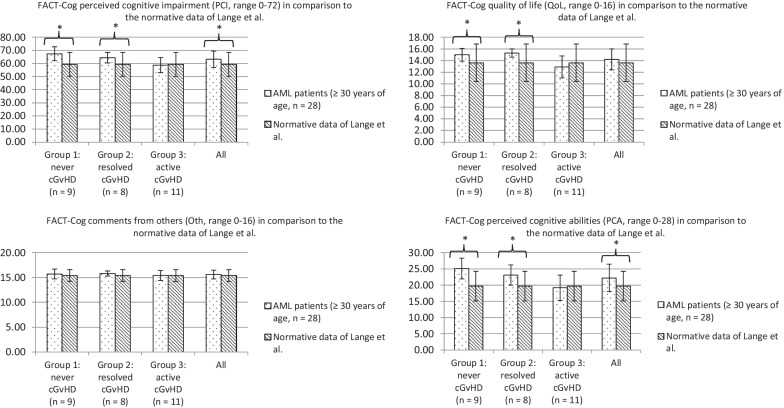
Figure 1 illustrates the FACT-Cog scores of the AML patient sample (≥ 30 years of age, n = 28) and of all cGvHD groups (Group 1: never cGvHD; Group 2: resolved cGvHD; Group 3: active cGvHD) compared to the normative data of a French healthy population of Lange et al. [11]. The overall patient cohort (n = 28) had similar FACT-CogQoL, FACT-CogOth as well as higher FACT-CogPCI and FACT-CogPCA (accompanied by MCID) compared to the normative data of Lange et al. [11]. Patients of group 1 (never cGvHD) and group 2 (resolved cGvHD) had better FACT-Cog scores (PCI, QoL and PCA) compared to the normative data, accompanied by MCID. Patients of group 3 (active cGvHD) had similar FACT-Cog scores (PCI, QoL, Oth and PCA) compared to the normative data of Lange et al. [11].
Fig. 1.
FACT-Cog according to cGvHD in comparison to the normative data of Lange et al. FACT-Cog: Functional Assessment of Cancer Therapy-Cognition; cGvHD: chronic Graft-versus-Host Disease; Never cGvHD: never having cGvHD; Resolved cGvHD: patients with a past history of cGvHD, no immunosuppression; Active cGvHD: physician reported currently active signs of inflammatory manifestations of cGvHD. Significance is indicated as p < .05 in brackets. Minimum clinically important differences (MCID) are indicated as *
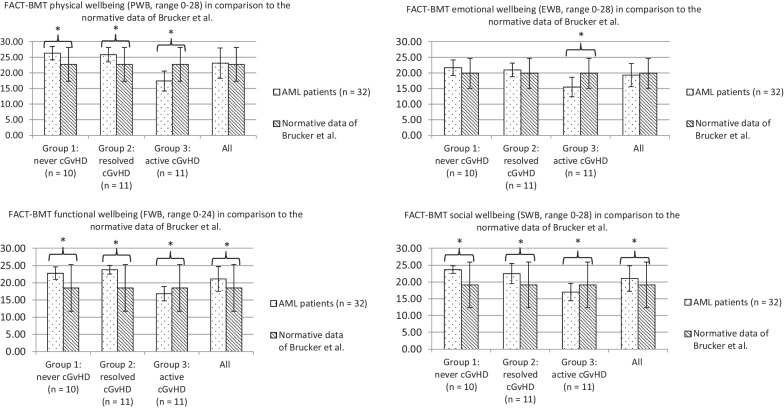
Figure 2 depicts the FACT-BMT/FACT-G scores of the overall patient sample (n = 32) in comparison to the normative data of Brucker et al. (FACT-G) [10]. The overall population had similar (PWB, EWB) or better FACT-BMT scores (FWB, SWB) compared to the normative data. Patients with active cGvHD had significantly lower FACT-BMT scores (PWB, EWB, FWB, SWB) compared to the normative data of Brucker et al. [10], accompanied by MCID.
Fig. 2.
FACT-BMT/(FACT-G) according to cGvHD in comparison to the normative data of Brucker et al. FACT-BMT: Functional Assessment of Cancer Therapy-Bone marrow transplantation; FACT-G: Functional Assessment of Cancer Therapy-General; cGvHD: chronic Graft-versus-Host Disease; Never cGvHD: never having cGvHD; Resolved cGvHD: patients with a past history of cGvHD, no immunosuppression; Active cGvHD: physician reported currently active signs of inflammatory manifestations of cGvHD. Significance is indicated as p < .05 in brackets. Minimum clinically important differences (MCID) are indicated as *
Discussion
In this study, we analyzed the patient-reported CF and QoL of long-term transplant survivors after TBI-based-conditioning, which delivers a homogenous dose to the whole body and examined the impact of cGvHD on outcome parameters. Impairment of CF [3], commonly named chemo-brain, and reduced QoL [14] are common concerns following chemotherapy. Our findings indicate that cognitive impairment is no significant problem of long-term survivors after TBI-based-conditioning. The overall patient sample reported similar CF compared to the normative data of Lange et al. [11]. Even patients with active cGvHD had no worse FACT-Cog scores compared to the normative data which is in line with the fact that neurological manifestations of cGvHD are rare [15, 16]. We used normative data from a healthy French population [11] for comparisons with the FACT-Cog because we did not find reference values from a healthy German population. The normative data of Lange et al. [11] didn’t include patients < 30 years of age. We therefore excluded four AML patients (12.5%) < 30 years of age from the comparisons with the general population. The excluded patients had overall good FACT-Cog PCI (mean 68.8, SD 4.3), PCA (mean 24.8, SD 2.5), Oth (mean 15.7, SD 0.5) and QoL (mean 14.5, SD 1.3). Patients with active cGvHD reported a consistent pattern of deficits in physical wellbeing, emotional wellbeing, functional wellbeing, and social/family wellbeing. Similar associations of cGvHD and worse QoL, e.g. physical wellbeing, social wellbeing and mental wellbeing have been reported independently of the conditioning regimen [17, 18]. A previous study found similar self-reported QoL between patients who never had cGvHD and those whose cGvHD had resolved [14]. Our results confirm this observation. Noteworthy, the overall AML population had no worse FACT-BMT scores compared to the normative data of Brucker et al. [10].
In summary, our data indicate that no relevant impairments persist after resolution of cGvHD. Patients with resolved cGvHD and those who were never diagnosed with cGvHD had comparable long-term QoL and CF [14, 18, 19]. A reason for the overall good CF and QoL of the AML patients may be the relatively young patient population with a median age of 53 years (IQR 37, 62 years) at the time of evaluation. Moreover, the median time interval between allo-HSCT and the evaluation was 154 months (IQR 113, 191 months). Literature reports moderate impairment of QoL after allo-HSCT that returns to baseline levels as time from transplantation increases and more than 60% of patients have good to excellent QoL 1 to 4 years after allo-HSCT [20, 21]. Additionally, our results indicate that patients may have personal benefits after allo-HSCT for AML and report better patient-reported results because of a better appreciation for life [22, 23].
Our data show a negative association of depression and anxiety with QoL and CF. The relatively high frequency of anxiety and depression in long-term survivors with active cGvHD support the need for psychological support [14, 24]. We didn’t analyse fatigue or the impact of fatigue on QoL or CF. Long-term survivors of allo-HSCT can suffer from persisting fatigue several years after allo-HSCT and mental fatigue may be associated with cognitive dysfunction and reduced QoL [25, 26].
The low number of patients limits this study, which is the consequence of the long follow-up. In addition, we did not compare the outcome with patients, who received a conditioning regimen not containing TBI precluding an analysis of the specific impact of the latter. Nevertheless, we analysed a homogenous group of AML patients who were treated with TBI as part of the conditioning regimen in a consistent manner in a 20-years-period and did not observe a major impact of the transplant procedure itself on QoL except the impact of cGvHD which occurs independent of the conditioning regimen. Additionally, cGvHD, the major variable of interest was based on physician-reported institutional databases and National Institutes of Health Criteria. Of note, the overall response rate was high (78%) and we found no differences between participants and non-participants in baseline characteristics.
Conclusion
The present study indicates that CF is not impaired after TBI-based conditioning, even in the presence of active cGvHD. However, active cGvHD has a significant impact on physical, emotional, functional and social wellbeing.
Acknowledgements
Not applicable.
Abbreviations
- TBI
Total body irradiation
- allo-HSCT
Allogeneic hematopoietic stem cell transplantation
- AML
Acute myeloid leukemia
- cGvHD
Chronic graft-versus-host disease
- CF
Cognitive function
- QoL
Quality of life
- FACT-BMT
Functional Assessment of Cancer Therapy-Bone marrow transplant
- FACT-Cog
Functional Assessment of Cancer Therapy-Cognition Function
- PHQ
Patient Health Questionnaire
- MSD
Matched sibling donors
- MUD
Matched unrelated donors
- MMUD
Mismatched unrelated donors
- MMRD
Mismatched related donors
- KPS
Karnofsky performance score
- HCT-CI
Hematopoietic cell transplantation-comorbidity index
- ELN
European LeukemiaNet
- ATG
Anti-thymocyte globulin
- MV
Megavoltage
- PWB
Physical wellbeing
- SWB
Social wellbeing
- EWB
Emotional wellbeing
- FWB
Functional wellbeing
- BMTS
Bone marrow transplant subscale
- PCI
Perceived cognitive impairments
- PCA
Perceived cognitive abilities
- IQR
Interquartile range
- ANOVA
Analysis of variance
- MCID
Minimum clinically important differences
- CR1
First complete remission
- PR1
First partial remission
- MAC
Myeloablative conditioning
- RIC
Reduced intensity conditioning
Author contributions
IG conducted the analysis and wrote the manuscript. DW directed the study and provided patient samples. DW, OK, WH, EH and ME reviewed the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors did not receive support from any organization for the submitted work.
Data availability
The data used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The local Ethics Board of the University of Regensburg approved this study (Ethics approval number 20-1810_1-101). The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
Written informed consent was obtained from all patients.
Competing interests
Daniel Wolff received honoraria from Neovii and Medac. The other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socié G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101(9):3373–85. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 3.Scherwath A, Schirmer L, Kruse M, et al. Cognitive functioning in allogeneic hematopoietic stem cell transplantation recipients and its medical correlates: a prospective multicenter study. Psychooncology. 2013;22(7):1509–16. doi: 10.1002/PON.3159. [DOI] [PubMed] [Google Scholar]
- 4.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Härtl PM, Treutwein M, Hautmann MG, et al. Total body irradiation-an attachment free sweeping beam technique. Radiat Oncol. 2016 doi: 10.1186/s13014-016-0658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for clinical trials in chronic graft-versus-host disease: I—The 2014 diagnosis and staging Working Group Report. Biol Blood Marrow Transpl. 2015;21(3):389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health Consensus Development Project on Criteria for clinical trials in chronic graft-versus-host disease: I—diagnosis and staging Working Group Report. Biol Blood Marrow Transpl. 2005;11(12):945–56. doi: 10.1016/J.BBMT.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology and guidance for graft-versus-host disease assessment. Bone Marrow Transpl. 2018;53:1401–15. doi: 10.1038/s41409-018-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the functional assessment of cancer therapy-general (FACT-G) Eval Heal Prof. 2005;28(2):192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 11.Lange M, Heutte N, Morel N, Eustache F, Joly F, Giffard B. Cognitive complaints in cancer: the french version of the Functional Assessment of Cancer Therapy-Cognitive function (FACT-Cog), normative data from a healthy population. Neuropsychol Rehabil. 2016;26(3):392–409. doi: 10.1080/09602011.2015.1036890. [DOI] [PubMed] [Google Scholar]
- 12.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in Health-related quality of life. Med Care. 2003;41(5):582–92. doi: 10.1097/01.mlr.0000062554.74615.4c. [DOI] [PubMed] [Google Scholar]
- 13.Van Dyk K, Crespi CM, Petersen L, Ganz PA. Identifying cancer-related cognitive impairment using the FACT-Cog perceived cognitive impairment. JNCI Cancer Spectr. 2020;4(1):2019–21. doi: 10.1093/jncics/pkz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SJ, Onstad L, Chow EJ, et al. Patient-reported outcomes and health status associated with chronic graft-versus-host disease. Haematologica. 2018;103(9):1535–41. doi: 10.3324/haematol.2018.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grauer O, Wolff D, Bertz H, et al. Neurological manifestations of chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation: report from the consensus conference on clinical practice in chronic graft-versus-host disease. AJ Neurol. 2010 doi: 10.1093/brain/awq245. [DOI] [PubMed] [Google Scholar]
- 16.Kraus PD, Wolff D, Grauer O, et al. Muscle cramps and neuropathies in patients with allogeneic hematopoietic stem cell transplantation and graft-versus-host disease. PLoS ONE. 2012;7(9):e44922. doi: 10.1371/journal.pone.0044922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Worel N, Biener D, Kalhs P, et al. Quality of life long-term outcome and quality of life of patients who are alive and in complete remission more than two years after allogeneic and syngeneic stem cell transplantation. Bone Marrow Transpl. 2002;30:619–26. doi: 10.1038/sj.bmt.1703677. [DOI] [PubMed] [Google Scholar]
- 18.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the bone marrow transplant Survivor Study. Published online 2006. 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed]
- 19.Kurosawa S, Yamaguchi T, Mori T, et al. Patient-reported quality of life after allogeneic hematopoietic cell transplantation or chemotherapy for acute leukemia. Bone Marrow Transpl. 2015;50(9):1241–9. doi: 10.1038/bmt.2015.137. [DOI] [PubMed] [Google Scholar]
- 20.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutherland HJ, Fyles GM, Adams G, et al. Quality of life following bone marrow transplantation: a comparison of patient reports with population norms. Bone Marrow Transpl. 1997;19(11):1129–36. doi: 10.1038/sj.bmt.1700806. [DOI] [PubMed] [Google Scholar]
- 22.Tierney DK, Facione N, Padilla G, Dodd M. Response shift: a theoretical exploration of quality of life following hematopoietic cell transplantation. Cancer Nurs. 2007;30(2):125–38. doi: 10.1097/01.NCC.0000265002.79687.AF. [DOI] [PubMed] [Google Scholar]
- 23.Andrykowski MA, Bishop MM, Hahn EA, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23(3):599–608. doi: 10.1200/JCO.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 24.Prieto JM, Atala J, Blanch J, et al. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(25):6063–71. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- 25.Boberg E, Kadri N, Winterling J, et al. Mental fatigue after allogeneic hematopoietic stem cell transplantation is associated with cognitive dysfunction, but not central nervous system inflammation. Haematologica. 2020;105(6):E310–4. doi: 10.3324/haematol.2019.225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esser P, Kuba K, Mehnert A, et al. Investigating the temporal course, relevance and risk factors of fatigue over 5 years: a prospective study among patients receiving allogeneic HSCT. Bone Marrow Transplant. 2017;52:753–8. doi: 10.1038/bmt.2016.344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and analysed during the current study are available from the corresponding author on reasonable request.