Abstract
Background
The spine is the most frequently affected part of the skeletal system to metastatic tumors. External radiotherapy is considered the first-line standard of care for these patients with spine metastases. Recurrent spinal metastases after radiotherapy cannot be treated with further radiotherapy within a short period of time, making treatment difficult. We aimed to evaluate the effectiveness and safety of MWA combined with cementoplasty in the treatment of spinal metastases after radiotherapy under real-time temperature monitoring.
Methods
In this retrospective study, 82 patients with 115 spinal metastatic lesions were treated with MWA and cementoplasty under real-time temperature monitoring. Changes in visual analog scale (VAS) scores, daily morphine consumption, and Oswestry Disability Index (ODI) scores were noted. A paired Student’s t-test was used to assess these parameters. Complications during the procedure were graded using the CTCAE version 5.0.
Results
Technical success was attained in all patients. The mean VAS score was 6.3 ± 2.0 (range, 4–10) before operation, and remarkable decline was noted in one month (1.7 ± 1.0 [P < .001]), three months (1.4 ± 0.8 [P < .001]), and six months (1.3 ± 0.8 [P < .001]) after the operation. Significant reductions in daily morphine consumption and ODI scores were also observed (P < .05). Cement leakage was found in 27.8% (32/115) of lesions, with no obvious associated symptoms.
Conclusion
MWA combined with cementoplasty under real-time temperature monitoring is an effective and safe method for recurrent spinal metastases after radiotherapy.
Keywords: Microwave ablation, Cementoplasty, Real-time temperature monitoring, Recurrent spinal metastases
Background
The bone is the third most common organ affected by metastases [1], Of which, the spine is the most frequently affected part [2]. Conventional, palliative, and short-course external radiotherapy is considered the first-line standard of care for these patients with spine metastases; however, the complete response rate to pain is low, usually between 10 and 20% [3]. Moreover, almost half of the remaining patients have recurrent pain at a median of 16 weeks following the treatment [4]. Recurrent spinal metastases after radiotherapy cannot be treated with further radiotherapy within a short period of time, making treatment difficult.
Percutaneous ablation techniques have recently gained acceptance; compared to other ablation methods, microwave ablation (MWA) provides several potential advantages [5]. Cementoplasty can enhance bone stability and effectively prevent and treat osteoporosis and pathological fractures [6, 7]. A small number of published studies have evaluated the treatment of spinal metastases treated by MWA combined with cementoplasty [8–11]. However, due to the rapid heating rate of MWA and the large ablation area, there is a potential for damage to the spinal cord and nerves. This makes real-time temperature monitoring during the ablation process very important. Therefore, we aimed to evaluate the effectiveness and safety of MWA combined with cementoplasty in the treatment of spinal metastases after radiotherapy under real-time temperature monitoring.
Methods
All the patients were not randomized. All the treatments were performed by the same team of operators, with two doctors having 10 years experience. The inclusion criteria were as follows: (1) pathologic evidence of primary cancer; (2) spinal metastasis confirmed by computed tomography (CT) and magnetic resonance imaging (MRI) scan; (3) recurrence of pain after radiotherapy, and (4) pain (visual analog scale [VAS] score ≥ 4) that severely affected the patient’s quality of life.
The exclusion criteria were as follows: (1) coagulation disorder and infections; (2) end-stage malignant tumors with heart and lung failure; or (3) metastases causing symptomatic spinal cord compression.
Microwave ablation
MWA was performed using the ECO-100A1 MW ablation system with a frequency of 2450 ± 10 MHz and an output level of 0 to 100 W (ECO Microwave Electronic Institute, Nanjing, China) under CT (SOMATOM Definition AS; Siemens Healthineers, Erlangen, Germany) guidance. The patients lay prone on the CT flatbed and were fixed with a vacuum negative pressure pad. The lateral position was an alternative if the patient could not lie prone. A positioning grid was placed on the skin surface, and a 5-mm slice CT was performed to choose the puncture path. The surgical area was disinfected using povidone-iodine followed by cleaning with a sterile surgical towel. Conscious sedation of patients was achieved with continuous intravenous infusion of fentanyl citrate (0.1 mg/2 mL), which was diluted 1:10 with saline solution; then, local anesthesia was administered with subcutaneous injection of 1% lidocaine hydrochloride and 0.25% ropivacaine hydrochloride. When 13-gauge bone needles were inserted into the pedicle step-by-step close to the anterior edges of the lesions under imaging guidance, continuous 3D reconstruction during needle insertion was performed to adjust the angle and direction of needle insertion and guide the bone puncture needle accurately into the target area. Subsequently, the cores were pulled out, the antenna was coaxially inserted into the lesion, the bone needle was retrograded to expose the anterior portion of the ablation antenna for 1.5–2.0 cm, and the thermocouple was coaxially inserted in the spinal canal or in the ipsilateral intervertebral foramen to monitor the temperature (Fig. 1). The maximum accepted temperature threshold was 42 ℃ [13, 14]. When the temperature exceeded 42℃, intrathecal carbon dioxide or 5% ice glucose was injected into the spinal canal to protect the spinal cord. According to ablation parameters provided by the manufacturer and the location of the lesion, appropriate power and time were selected empirically. The MWA power was 20–40 W (mean 31.7 ± 5.0 W). Short, repeated microwave cycles (30–90 s) were performed. The total ablation time was 3–5 min (mean, 3.6 ± 1.1 min). Unilateral ablation (20, 17.4%) was performed if the lesions were located on one side without crossing the midline; bilateral ablation (95, 82.6%) was performed if the lesions crossed the midline. Lower-limb clinical examinations (motor and sensory) were performed during the operation, and the patients were asked to inform us about the presence of lower-limb pain or paresthesia.
Fig. 1.
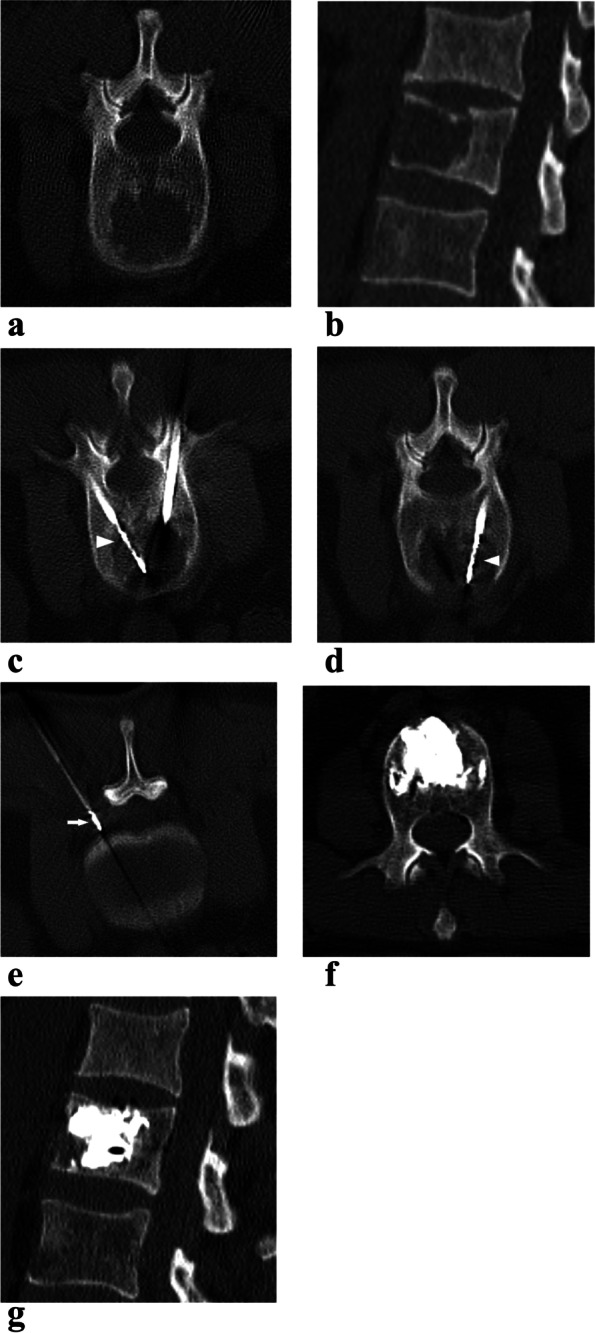
A 58-year-old man with lung adenocarcinoma with L2 osteolytic metastasis (recurrence after radiotherapy) treated with MWA in combination with cementoplasty. L2 osteolytic metastasis showed on axial and sagittal reconstructed CT (a, b). The microwave ablation antennas (arrowheads) were inserted into the lesion through bilateral access (c, d). The thermometric electrode (arrow) was inserted into the left intervertebral foramen (e). The cement deposited in the vertebral body without significant leakage on axial and sagittal CT (f, g)
Cementoplasty
Several 1-mL syringes filled with polymethylmethacrylate (PMMA) (Heraeus Medical GmbH, Wehrheim, Germany) were kept in ice saline to prolong the setting time. Ten minutes after MWA, the PMMA was slowly injected into the ablative lesion; CT was performed immediately to visualize the flow of PMMA after every 1 mL injection. When the PMMA was close to the posterior edge of the vertebral body, the dose of a single injection was reduced to 0.3–0.5 ml. A single vertebral body was scanned each time, and the scanning time was approximately 3 s. While filling the ablation area with PMMA as much as possible, if the PMMA leaked into the spinal canal and intervertebral foramen, the injection was stopped. The mean volume of bone cement per lesion was 5.7 ± 1.9 mL (range, 2–10 mL). CT was performed after the operation to evaluate adequate filling and leakage of cement.
Technical success was defined as precise placement of the antenna in the lesion, achievement of the target ablation power and time, and placement of an adequate amount of cement in the lesion.
Follow-up schedule
The follow-up time was at least 6 months. The Oswestry Disability Index (ODI), VAS scores, morphine dosage, side effects, and complications of all patients were recorded through telephonic enquiries or at the outpatient department. CT and MRI scans were performed 3 and 6 months after the operation.
Outcome assessment
The primary outcomes were VAS scores and morphine dosages, the secondary outcomes were complications. The VAS scores and morphine dosages were used to assess pain. The ODI score was used to assess the degree of disability [15]. Complications during the procedure were graded using the CTCAE version 5.0.
Statistical analyses
SPSS Statistics version 26 software (IBM Corp, Armonk, NY, USA) was used to perform the analysis. Mean and standard deviation or median and range were used to express the numerical date. A paired Student’s t-test was used to assess the VAS scores, morphine doses, and ODI scores. All tests were two-tailed and a P value < 0.05 was considered statistically significant.
Results
Clinical data
The local Institutional Review Board (Ethics number: 2020-Ethics Review-12) approved this study. Informed consent was obtained from all patients before treatment. From May 2015 to December 2021, 82 patients with 115 lesions were enrolled. Of these, 48 lesions were located in the thoracic vertebrae and 67 in the lumbar vertebrae. All primary lesions were histopathologically diagnosed, and the spinal metastases were confirmed by a combination of CT and MRI before the operation. All patients had received external irradiation with the scheme of 10 × 3 Gy prior to MWA combined with cementoplasty, of which 53 received concurrent or sequential systemic therapy.The median time after radiotherapy was 10.2 (rang, 5–21) months. According to the modified Shimony, the degree of epidural invasion and spinal cord compression is classified as type A, type B, and type C [12], of which type C was excluded from our study. An interdisciplinary team including interventional radiologists, surgeons, and oncologists evaluated all patients before the operation. The baseline characteristics are listed in Tables 1 and 2.
Table 1.
Baseline characteristics
| Number | Percent (%) | |
|---|---|---|
| Age (years) | ||
| Median (range) | 63 (18–88) | |
| Sex | ||
| Male | 49 | 59.8 |
| Female | 33 | 40.2 |
| Number of lesions | ||
| One | 57 | 69.5 |
| Two | 18 | 22 |
| Three | 6 | 7.3 |
| Four | 1 | 1.2 |
| Primary tumor | ||
| Lung | 29 | 35.4 |
| Bladder | 1 | 1.2 |
| Bile duct | 1 | 1.2 |
| Multiple myeloma | 1 | 1.2 |
| Liver | 5 | 6.1 |
| Femur | 1 | 1.2 |
| Colon | 2 | 2.4 |
| Choroidal malignant melanoma | 1 | 1.2 |
| Prostate | 3 | 3.7 |
| Breast | 11 | 13.4 |
| Kidney | 5 | 6.1 |
| Esophagus | 7 | 8.5 |
| Stomach | 7 | 8.5 |
| Lower jaw | 1 | 1.2 |
| Pancreas | 1 | 1.2 |
| Unidentified primary adenocarcinoma | 2 | 2.4 |
| Uterus | 2 | 2.4 |
| Mediastinum | 2 | 2.4 |
Table 2.
Baseline characteristics of lesions
| Number | Percent (%) | |
|---|---|---|
| Type of lesion | ||
| Lytic | 88 | 76.5 |
| Osteogenic | 6 | 5.2 |
| Mixed | 21 | 18.3 |
| Intact posterior edge | ||
| Yes | 67 | 58.3 |
| No | 48 | 41.7 |
| The degree of epidural invasion | 48 | 41.7 |
| Type A | 32 | 27.8 |
| Type B | 16 | 13.9 |
| Compression fracture | ||
| Yes | 46 | 40 |
| No | 69 | 60 |
MWA combined with cementoplasty under real-time temperature monitoring was successfully performed in all patients, and all patients were followed up for at least 6 months.
Pain relief
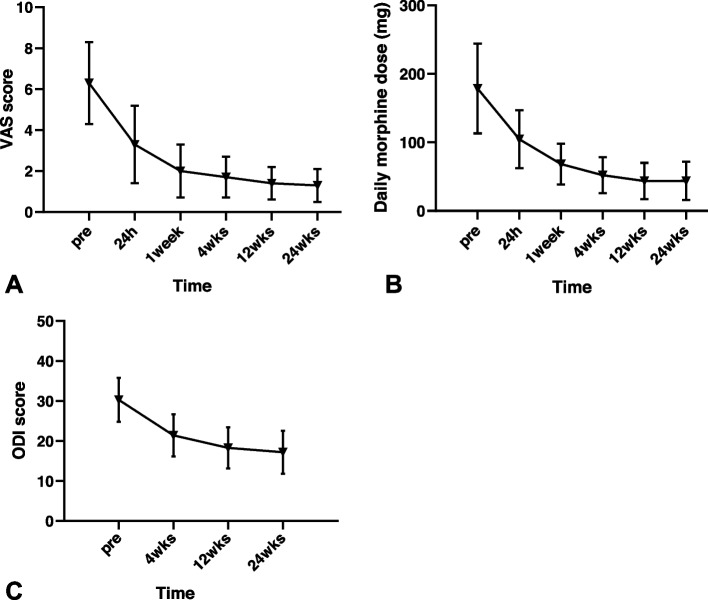
The preoperative VAS score was 6.3 ± 2.0 (range, 4–10); the postoperative VAS score decreased in 24 h (3.3 ± 1.9, P < 0.05), one week (2.0 ± 1.3, P < 0.001), four weeks (1.7 ± 1.0, P < 0.001), 12 weeks (1.4 ± 0.8, P < 0.001), and 24 weeks (1.3 ± 0.8, P < 0.001) (Fig. 2A). Compared to the preoperative morphine dose (178.5 ± 65.7 mg; range, 80–300 mg), the postoperative morphine doses decreased significantly in 24 h (104.5 ± 42.3 mg, P < 0.05), one week (68.4 ± 29.7 mg, P < 0.001), four weeks (52.0 ± 26.4 mg, P < 0.001), 12 weeks (43.5 ± 26.7 mg, P < 0.001) and 24 weeks (43.9 ± 28.1 mg, P < 0.001) (Fig. 2B). During the follow-up, eight patients experienced recurrence in pain, of which five underwent systemic treatment, three underwent pain management with morphine.
Fig. 2.
The changes of visual analog scale (VAS) scores (A), Daily morphine dose (mg) (B), and Oswestry Disability Index (ODI) (C) after operation
Degree of disability
Compared to preoperative values (30.3 ± 5.5; range, 20–45), the postoperative ODI score decreased in four weeks (21.4 ± 5.3, P < 0.005), 12 weeks (18.3 ± 5.2, P < 0.001), and 24 weeks (17.2 ± 5.4, P < 0.001) (Fig. 2C).
Complications
Asymptomatic cement leakage (one degree) was found in 32/115 (27.8%) lesions in our study. Forty sites of cement leakage were identified, of which nine (22.5%) were in the vertebral canal, five (12.5%) were in the posterior vertebral vein, 18 (45%) were in the intervertebral disc, and eight (20%) were in the paraspinal region. However, the cement leakages showed no obvious relationship with the presence of an intact posterior edge of the spine (Table 3). Other complications, such as hematoma, skin burns, bone cement embolism, infection and periprocedural death, were not observed in our study.
Table 3.
Relationship between spine posterior edge and bone cement leakage
| Intact posterior edge | Total (n) | Bone cement leakage | χ2 test | ||
|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | χ2 value | P value | ||
| Yes | 67 | 18 (26.9) | 49 (73.1) | 0.074 | 0.786 |
| No | 48 | 14 (29.2) | 34 (70.8) | ||
Discussion
Currently, painful spinal metastases are treated to relieve pain and improve the quality of life. Several effective mini-invasive treatments for the management of spinal metastases are currently available, including embolization, thermal ablation, electrochemotherapy, cementoplasty, and MRI-guided high-intensity focused ultrasound [16]. Of which, thermal ablation performed to relieve pain could cause coagulative necrosis of the tumor. Microwave and radiofrequency are commonly used for thermal ablation; compared with radiofrequency, microwave can provide faster heating and target larger ablation areas without grounding electrodes [17]. Microwave is more insensitive to the high impedance of bone desiccation than is radiofrequency, causing deeper thermal penetration, which efficiently heats the bone [15]. However, microwaves cannot improve the biomechanical stability of the bone. Cementoplasty can relieve pain and increase the biomechanical stability of the bone. Combining MWA and cementoplasty can lead to optimal cement distribution and bone stabilization [18, 19]. However, compared to MW-ablation alone, cementoplasty combined with MW-ablation increases the operation time, cost and the risk of complications.
Pusceddu et al. [19] reported that 35 patients with 37 osseous metastases treated by MWA achieved significant pain relief in one week, one month, and six months, without local tumor progression. Another retrospective study showed that the postoperative VAS scores of 69 patients with spinal metastases treated by MWA and cementoplasty decreased significantly at 2–4 weeks (2 ± 1.6) and 20–24 weeks (2 ± 2.1), compared to the preoperative VAS scores (7.0 ± 1.8). No local progression was found in 59/61 patients [9]. Wu L et al. [18] reported 33 high thoracic vertebral metastases treated by MWA and cementoplasty. The mean VAS score, morphine consumption doses, and ODI significantly decreased at 24 h and one, four, 12, and 24 weeks postoperatively, with no local tumor progression during the 24-week follow-up. The results are in accordance with previously reported studies.
Whether cementoplasty can be used in patients with an incomplete posterior wall is still controversial. Most authors consider that neurologic symptoms associated with nerve root- or spinal cord compression are contraindications to cementoplasty [20–22]. However, few studies have demonstrated the feasibility of cementoplasty. Researchers believe that epidural involvement with intractable pain, few other treatment options, and a short life expectancy should not be a contraindication to cementoplasty. Moreover, the patients must be properly screened, and cementoplasty should be performed with conscious sedation [12, 23, 24]. Halpin et al. [25] showed that thermal ablation before cementoplasty could decrease the risk of cement extravasation due to thrombosis of the venous plexus caused by ablation. We found no statistically significant difference in the incidence of cement leakage between groups with incomplete and intact posterior margins. We also found no spinal nerve injury in either group after surgery. Therefore, we believe that incomplete posterior margins should not be a contraindication for performing cementoplasty.
Thermal ablation has the intrinsic risk of nerve damage, which is a serious potential complication of the operation. Several aspects affect the extent and severity of nerve damage such as type of nerve fiber, distance from margins of the ablation zone, presence or absence of intact vertebral cortex, duration of thermal effect, and absolute temperature [26]. The threshold of irreversible nerve damage is 42.2 ℃ for 50–60 min or 70 ℃ for 5 min [27]. Studies have shown that the incidence of nerve injury was 1.4–17.4% [9, 28, 29]. Several methods are used to prevent thermal injury, including active warming/cooling with saline injection, passive insulation with CO2 insufflation [30, 31], and continuous temperature monitoring [30, 32, 33]. Additionally, repeated and short ablation cycles to control the diffusion of the ablation zone might be suitable for tumors close to vital structures [10]. In our study, thermocouples were positioned close to vital neural elements for continuous temperature monitoring, and repetitive, short ablation cycles were also used to ensure the safety of thermal ablation.
This study had some limitations. We did not include a control group. Additionally, the study was conducted at a single center, was retrospective in nature, and had a short follow-up duration. Thus, the findings outlining the benefits of MWA combined with cementoplasty need to be confirmed in further studies, and multicenter randomized controlled studies are needed to validate these results and obtain more conclusive findings.
MWA combined with cementoplasty results in reduced VAS and ODI scores, without serious complications, and is a safe and effective method for treating painful recurrent spinal metastases after radiotherapy.
Acknowledgements
None.
Authors’ contributions
B.W. K.Z. and X.Z. made substantial contribution in design and conception of the study, wrote the manuscript. S.Y. and M.H. performed acquisition of data and revisited the manuscript critically. P.L. and W.Y. participated in the design of the study and performed the statistical analysis. J.F. and C.X. performed acquisition of data and interpretation of data and revisited the manuscript critically. Q.Y. made substantial contribution in design the conception of the study. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Tengzhou Central People’s Hospital Review Board (Ethics number: 2020-Ethics Review-12) approved this study. Informed consent was obtained from all patients before treatment. All the methods were carried out in accordance with relevant guideline and regulation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hosono N, Yonenobu K, Fuji T, Ebara S, Yamashita K, Ono K. Orthopaedic management of spinal metastases. Clin Orthop Relat Res. 1995;312:148–159. [PubMed] [Google Scholar]
- 2.Bouras T, Zairi F, Arikat A, Vieillard M, Allaoui M, Assaker R. Decision Making for the Surgical Treatment of Vertebral Metastases Among Patients with Short Predicted Survival. World neurosurgery. 2018;111:e573–e580. doi: 10.1016/j.wneu.2017.12.107. [DOI] [PubMed] [Google Scholar]
- 3.Lutz S, Balboni T, Jones J, Lo S, Petit J, Rich S, Wong R, Hahn C. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7(1):4–12. doi: 10.1016/j.prro.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clinical Oncol (Royal College of Radiologists (Great Britain)) 2012;24(2):112–124. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Kurup AN, Callstrom MR. Ablation of skeletal metastases: current status. J Vasc Interv Radiol. 2010;21(8 Suppl):S242–250. doi: 10.1016/j.jvir.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Clark W, Bird P, Gonski P, Diamond T, Smerdely P, McNeil H, Schlaphoff G, Bryant C, Barnes E, Gebski V. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2016;388(10052):1408–1416. doi: 10.1016/S0140-6736(16)31341-1. [DOI] [PubMed] [Google Scholar]
- 7.Bae J, Gwak H, Kim S, Joo J, Shin S, Yoo H, Lee S. Percutaneous vertebroplasty for patients with metastatic compression fractures of the thoracolumbar spine: clinical and radiological factors affecting functional outcomes. Spine J. 2016;16(3):355–364. doi: 10.1016/j.spinee.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Jiao D, Yao Y, Li Z, Ren J, Han X. Simultaneous C-arm Computed Tomography-Guided Microwave Ablation and Cementoplasty in Patients with Painful Osteolytic Bone Metastases: A Single-center Experience. Acad Radiol. 2020;29(1):42–50. doi: 10.1016/j.acra.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Khan MA, Deib G, Deldar B, Patel AM, Barr JS. Efficacy and Safety of Percutaneous Microwave Ablation and Cementoplasty in the Treatment of Painful Spinal Metastases and Myeloma. AJNR Am J Neuroradiol. 2018;39(7):1376–1383. doi: 10.3174/ajnr.A5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kastler A, Alnassan H, Aubry S, Kastler B. Microwave thermal ablation of spinal metastatic bone tumors. J Vasc Interv Radiol. 2014;25(9):1470–1475. doi: 10.1016/j.jvir.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Pusceddu C, Sotgia B, Fele R, Melis L. Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol. 2013;24(2):229–233. doi: 10.1016/j.jvir.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Shimony J, Gilula L, Zeller A, Brown D. Percutaneous vertebroplasty for malignant compression fractures with epidural involvement. Radiology. 2004;232(3):846–853. doi: 10.1148/radiol.2323030353. [DOI] [PubMed] [Google Scholar]
- 13.Diehn F, Neeman Z, Hvizda J, Wood B. Remote thermometry to avoid complications in radiofrequency ablation. J Vasc Interv Radiol. 2003;14(12):1569–1576. doi: 10.1097/01.RVI.0000096769.74047.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupuy D, Liu D, Hartfeil D, Hanna L, Blume J, Ahrar K, Lopez R, Safran H, DiPetrillo T. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116(4):989–997. doi: 10.1002/cncr.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubner M, Brace C, Hinshaw J, Lee F. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papalexis N, Parmeggiani A, Peta G, Spinnato P, Miceli M, Facchini G. Minimally Invasive Interventional Procedures for Metastatic Bone Disease: A Comprehensive Review. Current Oncol (Toronto, Ont) 2022;29(6):4155–4177. doi: 10.3390/curroncol29060332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Ye X, Zhang K, Qiu Y, Fan W, Yuan Q, Fan J, Wu L, Yang S, Hu M, et al. Computed Tomography-Guided Microwave Ablation Combined with Osteoplasty for the Treatment of Bone Metastases: A Multicenter Clinical Study. J Vasc Interv Radiol. 2021;32(6):861–868. doi: 10.1016/j.jvir.2021.03.523. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Fan J, Yuan Q, Zhang X, Hu M, Zhang K. Computed tomography-guided microwave ablation combined with percutaneous vertebroplasty for treatment of painful high thoracic vertebral metastases. Int J Hyperthermia. 2021;38(1):1069–1076. doi: 10.1080/02656736.2021.1951364. [DOI] [PubMed] [Google Scholar]
- 19.Pusceddu C, Sotgia B, Fele R, Ballicu N, Melis L. Combined Microwave Ablation and Cementoplasty in Patients with Painful Bone Metastases at High Risk of Fracture. Cardiovasc Intervent Radiol. 2016;39(1):74–80. doi: 10.1007/s00270-015-1151-y. [DOI] [PubMed] [Google Scholar]
- 20.Mathis JM. Percutaneous Vertebroplasty. In: Johnson BA, Staats PS, Wetzel FT, Mathis JM, editors. Image-Guided Spine Interventions. New York: Springer New York; 2004. pp. 245–272. [Google Scholar]
- 21.Gangi A, Buy X, Irani F, Guth S, Guermazi A, Imbert J-P, Dietemann J-L. Percutaneous Cementoplasty. In: Gangi A, Guth S, Guermazi A, editors. Imaging in Percutaneous Musculoskeletal Interventions. Berlin: Springer Berlin Heidelberg; 2009. pp. 197–257. [Google Scholar]
- 22.Deramond H, Depriester C, Chiras J, Cotten A, Boutry N, Cortet B. Tumors. In: Mathis JM, Deramond H, Belkoff SM, editors. Percutaneous Vertebroplasty. New York: Springer New York; 2002. pp. 125–153. [Google Scholar]
- 23.Appel N, Gilula L. Percutaneous vertebroplasty in patients with spinal canal compromise. AJR Am J Roentgenol. 2004;182(4):947–951. doi: 10.2214/ajr.182.4.1820947. [DOI] [PubMed] [Google Scholar]
- 24.van der Linden E, Kroft L, Dijkstra P. Treatment of vertebral tumor with posterior wall defect using image-guided radiofrequency ablation combined with vertebroplasty: preliminary results in 12 patients. J Vasc Interv Radiol. 2007;18(6):741–747. doi: 10.1016/j.jvir.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Halpin R, Bendok B, Sato K, Liu J, Patel J, Rosen S. Combination treatment of vertebral metastases using image-guided percutaneous radiofrequency ablation and vertebroplasty: a case report. Surgical neurology. 2005;63(5):469–474; discussion 474–465. doi: 10.1016/j.surneu.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Tsoumakidou G, Garnon J, Ramamurthy N, Buy X, Gangi A. Interest of electrostimulation of peripheral motor nerves during percutaneous thermal ablation. Cardiovasc Intervent Radiol. 2013;36(6):1624–1628. doi: 10.1007/s00270-013-0641-z. [DOI] [PubMed] [Google Scholar]
- 27.Konno S, Olmarker K, Byröd G, Nordborg C, Strömqvist B, Rydevik B. The European Spine Society AcroMed Prize 1994. Acute thermal nerve root injury. Eur Spine J. 1994;3(6):299–302. doi: 10.1007/BF02200140. [DOI] [PubMed] [Google Scholar]
- 28.Nakatsuka A, Yamakado K, Maeda M, Yasuda M, Akeboshi M, Takaki H, Hamada A, Takeda K. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol. 2004;15(7):707–712. doi: 10.1097/01.RVI.0000133507.40193.E4. [DOI] [PubMed] [Google Scholar]
- 29.Clarençon F, Jean B, Pham H, Cormier E, Bensimon G, Rose M, Maksud P, Chiras J. Value of percutaneous radiofrequency ablation with or without percutaneous vertebroplasty for pain relief and functional recovery in painful bone metastases. Skeletal Radiol. 2013;42(1):25–36. doi: 10.1007/s00256-011-1294-0. [DOI] [PubMed] [Google Scholar]
- 30.Buy X, Tok C-H, Szwarc D, Bierry G, Gangi A. Thermal Protection During Percutaneous Thermal Ablation Procedures: Interest of Carbon Dioxide Dissection and Temperature Monitoring. Cardiovasc Intervent Radiol. 2009;32(3):529–534. doi: 10.1007/s00270-009-9524-8. [DOI] [PubMed] [Google Scholar]
- 31.Tsoumakidou G, Buy X, Garnon J, Enescu J, Gangi A. Percutaneous thermal ablation: how to protect the surrounding organs. Tech Vasc Interv Radiol. 2011;14(3):170–176. doi: 10.1053/j.tvir.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Diehn FE, Neeman Z, Hvizda JL, Wood BJ. Remote thermometry to avoid complications in radiofrequency ablation. J Vasc Interv Radiol. 2003;14(12):1569–1576. doi: 10.1097/01.RVI.0000096769.74047.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakatsuka A, Yamakado K, Takaki H, Uraki J, Makita M, Oshima F, Takeda K. Percutaneous radiofrequency ablation of painful spinal tumors adjacent to the spinal cord with real-time monitoring of spinal canal temperature: a prospective study. Cardiovasc Intervent Radiol. 2009;32(1):70–75. doi: 10.1007/s00270-008-9390-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.