Abstract
Background
The mobile integrated health-community paramedicine (MIH-CP) program affiliated with the University of Maryland Medical Center focuses on improving patient transitions from hospital to home by addressing both medical and social determinants of health. Until recently, only self-contained health systems could integrate inpatient and outpatient medication data. Without some means to track patients in transition, there is a significant risk of medication-related problems and errors.
Objective
To evaluate the impact of the MIH-CP program on medication adherence among patients with congestive heart failure (CHF) and/or chronic obstructive pulmonary disease (COPD).
Methods
This is a pilot observational study designed to compare adherence to drug regimens prescribed at hospital discharge (measured by the proportion of days covered [PDC]) between patients enrolled in the MIH-CP program and a propensity-matched control group. Propensity scores were calculated using 11 demographic, diagnostic, third-party payer, and patient care-associated variables. Discharge medication details were obtained from electronic medical records. PDC for each of the medications were calculated from pharmacy claims data.
Results
Eighty-three patients were included in the study; forty-three patients were placed in the intervention group and 40 were propensity-matched controls. After adjusting for age, sex, and third-party payer, findings indicated that medication adherence was higher among patients enrolled in the MIH-CP program compared with control during the first 30 days post-discharge, specifically among patients diagnosed with CHF (8% difference in PDC, 95% confidence interval [CI], ‐0.12-0.28%) and COPD (14% difference, 95% CI, ‐0.15-0.43%), although neither result achieved statistical significance. The differences in medication adherence between patients who were enrolled and those who were not enrolled in the MIH-CP program diminished after 30 days post-discharge.
Conclusion
This pilot study demonstrated a trend toward improved medication adherence among patients enrolled in the MIH-CP program. Future research involving a larger patient cohort will be required to confirm these preliminary findings.
Keywords: Medication adherence, Community paramedicine, Mobile integrated health, Transition of care, Telehealth, Data integration
Abbreviations: ALP, Advanced Licensed Provider; CHF, Congestive Heart Failure; CHW, Community Health Worker; CI, Confidence Interval; CMS, Centers for Medicare and Medicaid Services; COPD, Chronic Obstructive Pulmonary Disease; CRISP, Chesapeake Regional Information System for our Patients; EHR, Electronic Health Record; ICD, Institutional Classification of Diseases; IRB, Institutional Review Board; M-DRAW, Modified Drug Adherence Work-Up; MIH-CP, Mobile Integrated Health-Community Paramedicine; PDC, Proportion of Days Covered; PSM, Propensity Score Matching; SDoH, Social Determinants of Health; UMMC, University of Maryland Medical Center; UMMS, University of Maryland Medical System
1. Introduction
Unplanned readmissions represent an important challenge to hospitals across the United States, as payment models shift from the “fee for service” to the “value-based reimbursement” model through the use of financial incentives and penalties.1 Whilst hospital readmission is multifactorial in cause, several factors contributing to readmissions center around medication use. One national comparative study found significant variations in the patient-reported quality of communication of medicines (ComMed) across U.S. hospitals by region and by access to health information technology (HIT) infrastructure. The study called for hospital providers and policy makers to design pharmacy training programs for staff to improve hospital ComMed quality and manage performance outcomes like readmissions across care transitions.2 Such technology based interventions coupled with the inclusion of pharmacists on multidisciplinary transition of care teams may reduce patient readmissions to hospital.2, 3, 4, 5 In 2018, the University of Maryland Medical Center and the Baltimore City Fire Department partnered to implement a mobile integrated health-community paramedicine (MIH-CP) transitional health support program to address this problem. The program's objective was to assist patients in the transition from hospital to home by addressing medical needs and social determinants of health (SDoH) to decrease post-discharge adverse events, including 30-day unplanned hospital readmissions.6 MIH-CP is supported by evidence showing that in-home evaluation, multidisciplinary teams, and multifaceted interventions have been found to be effective in improving care transitions.7, 8, 9
MIH-CP programs use EMS providers to deliver in-home care with the multidisciplinary support of pharmacists, medical doctors, nurses, and community health workers (CHW) to assist patients with complex medical conditions and needs during the transition period. There is mixed evidence that this is effective at reducing healthcare utilization, and, more research is neededexamining patient-centered health outcomes.10,11
The leading causes of hospital readmission in patients with chronic diseases are pneumonia, congestive heart failure (CHF), cardiovascular disease, and chronic obstructive pulmonary disease (COPD).12,13 Approximately 1 in 4 patients with CHF are readmitted within 30 days.14 Similarly, 22.6% of Medicare beneficiaries admitted for acute exacerbation of COPD have a 30-day hospital readmission rate, while a concomitant diagnosis of CHF and COPD is the third leading cause of early readmission.15 In addition, chronic conditions such as congestive heart failure (CHF) and COPD are of particular concern because of their associated high healthcare costs as well as the financial penalties imposed by the Centers for Medicare and Medicaid Services (CMS) for 30-day unplanned readmissions. The U.S. is projected to incur $50 billion in direct costs and $100 billion in indirect costs, which can be attributed to COPD.16 Similarly, the total cost for CHF is projected to increase from $31 billion in 2021 to $70 billion by 2030.11 There is a growing body of evidence that shows that social adversities negatively impact health outcomes including mortality, disease prevalence, and health care utilization.13,14 A study by Wilder et al. (2021) showed that adverse social determinants of health negatively impact medication adherence in a Medicaid sample with good prescription medication coverage.17
Medication adherence is a known predictor of hospital readmissions.18, 19, 20 Randomized controlled studies have shown that pharmacists improve medication and lifestyle adherence as part of a multidisciplinary team. Some of these studies also show improvements in patients ‘clinical outcomes.21, 22, 23, 24 Pharmacists' role in multidisciplinary teams, MIH-CP programs is critical as they can identify and rectify medication-related problems and thus improve medication adherence.
Medication adherence is defined by the World Health Organization as “the degree to which use of medication by the patient corresponds with the prescribed regimen.”25 Medication adherence includes factors such as filling prescriptions as well as adherence to the appropriate dosing schedules and routes of administration. Medication adherence can be categorized into three distinct phases: initiation - the time at which a patient obtains and uses a new medication for the first time; implementation - the period after initiation during which a patient takes the prescribed medication at the appropriate dose and frequency until the final dose has been taken; and discontinuation - the time at which a patient stops taking prescribed medication on his or her own volition or in response to physician orders.26
Results from numerous studies have documented that medication non-adherence is associated with poor clinical outcomes, high healthcare costs, and lost productivity.27 Low medication adherence have been associated with 2.5-times greater odds of hospital readmission compared to high adherence.18 Results from a systematic review revealed that an estimated 50% of the medications prescribed to treat chronic diseases were not taken as intended and that 20% to 30% of these prescriptions were never filled.28 Medication non-adherence was projected to cost the United States healthcare system between 100 and 289 billion dollars per year, including costs associated with avoidable hospitalizations, nursing home admissions, and premature deaths.28
The objective of this pilot study was to evaluate the impact of the MIH-CP program on medication adherence, as measured by the proportion of days covered (PDC) by appropriate drug therapy among patients diagnosed with CHF and/or COPD. We hypothesize that patients enrolled in the program will demonstrate higher levels of medication adherence than eligible patients who are not enrolled in the program.
2. Methods
2.1. Study design and participants
2.1.1. Inclusion criteria
Patients enrolled in the program had to be (i) inpatients (observation or admitted status) at the University of Maryland Medical Center (UMMC, a large tertiary care academic medical center) or the UMMC Midtown Campus (a nearby community hospital closely affiliated with the UMMC) medicine and family medicine services; (ii) 18 years of age or older; and (iii) residing in West Baltimore ZIP codes 21201, 21216, 21217, 21223, 21229, or 21230. In addition, patients had to have stable housing so that the field team could conduct home visits.
2.1.2. Exclusion criteria
Patients who are pregnant and/or homeless were not enrolled in the program and therefore were excluded from this study.
2.1.3. Program enrollment
Patients were identified and referred to the program during interdisciplinary discharge rounds, during which inpatient physicians, administrators, social workers, case managers, and program CHWs identify eligible patients with complex medical and social needs who were likely to benefit from MIH-CP support. Participation is voluntary and patients must consent to enrollment (about 75% of the patients approached accepted enrollment). Not all eligible patients were enrolled due to patient preference, logistics, and MIH-CP program capacity. Program capacity (30–50 patients per month) may limit the number of eligible patients who can be offered MIH-CP enrollment. A patient priority ranking process was put in place. Many of the patients enrolled in the MIH-CP program experience multiple economic, social, environmental, and healthcare challenges and have high healthcare utilization including multiple hospital readmissions. A multidisciplinary team of physicians, nurse practitioners, paramedics, nurses, pharmacists, social workers, and CHWs provided tailored, patient-centered support to individuals in their homes for 30 days after hospital discharge, as previously described.29
2.1.4. Study cohort selection
This study is a retrospective cohort observational study. Within this patient population, the inclusion criteria for this study were a primary diagnosis or comorbidity that was documented by the healthcare provider during the index hospitalization as defined by International Classification of Diseases, version 10, Clinical Modification (ICD-10-CM) of CHF (I09.**, I26.** – I.28.**, I50.**, Z86.**) or COPD (J40.**, J43.**, J44.**, J45.**, J47.**).
Due to the pilot nature of the study, team chart review capacity, and data availability, we planned to enroll a convenience sample of approximately 50 patients in the MIH-CP program. The intervention group was enrolled in the MIH-CP program, while, the control group met the inclusion criteria but was not enrolled in the program during the study period.
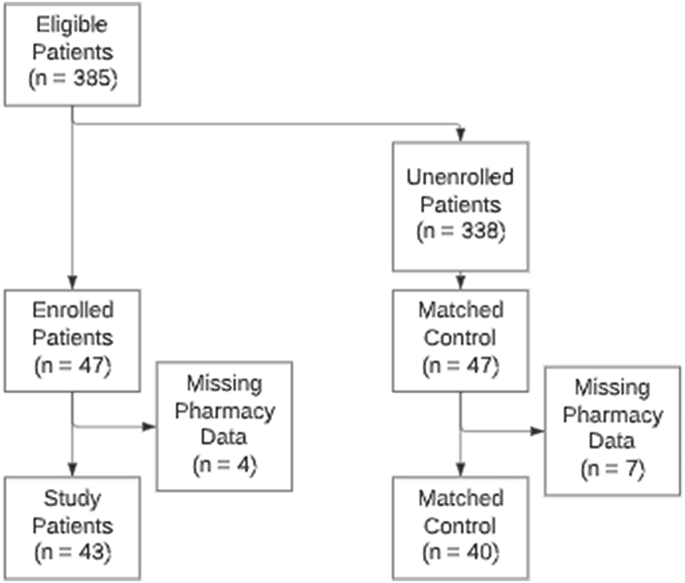
Between February 6, 2020, and May 11, 2020, 385 patients diagnosed with CHF or COPD discharged from UMMC or UMMC Midtown Campus were identified as eligible for enrollment in the MIH-CP program. Program enrollment records identified 47 of the eligible 385 patients who enrolled in MIH-CP (intervention group). A sample of 47 controls was selected from the remaining 338 patients who were eligible at discharge but did not enroll in the MIH-CP using 1-to-1 nearest neighbor propensity score matching (PSM) without replacement. Using this method, the means (or proportions for categorical variables) of the 11 pre-selected variables (age, CHF [Y/N], COPD [Y/N], sex, race, insurance provider, service, patient type [inpatient versus outpatient observation], zip code, discharge date, and pharmacy claim [Y/N]) did not differ significantly between the MIH-CP cases and the non-MIH-CP controls. Of the initial 94 discharges/subjects (47 cases versus 47 controls) 11 subjects were excluded because of missing pharmacy data. This resulted in a sample of 83 subjects (43 cases versus 40 controls) for the final analysis (See Fig. 1).
Fig. 1.
Study flow diagram for selection of patients in the intervention and control groups.
2.2. MIH-CP program intervention
Patients enrolled in the MIH-CP program were discharged from the UMMC or the UMMC Midtown Campus. The hospitals do not have a standard discharge care for medicine patients. Discharge care varies based on patient needs, which ranges from no coordinated follow-up to hospital-provided transitional services (such as the MIH-CP program), or other transitional services utilizing a nurse, CHW, or pharmacist to provide phone calls or in-clinic services. The hospitals have an outpatient pharmacy, however, they do not routinely provide a “meds-to-beds” medication delivery service to all patients before they leave the hospital.
2.2.1. MIH-CP team and training
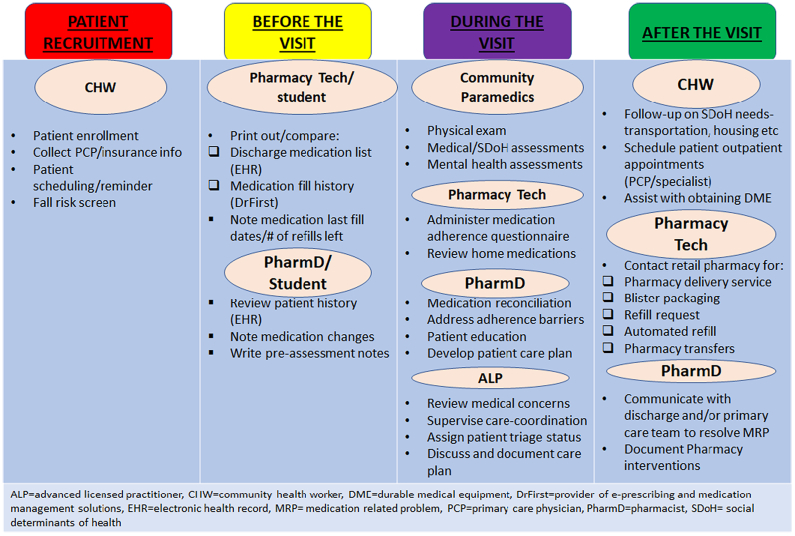
The MIH-CP field team included specially trained community paramedics (working under novel, state-approved, expanded practice protocols) and a pharmacy technician. The virtual team included a pharmacist, a CHW, and an advanced licensed provider (ALP) such as a nurse practitioner or a physician. The field team visited the patients in their home and linked up with the virtual team members during the home visit via a Health Insurance Portability and Accountability Act-compliant telehealth video (UMMS teleport) from the patient's home (See Fig. 2). All members of the care team underwent specific training to familiarize themselves with MIH protocols and guidelines. Initial training included EHR system training, process and operational review, tabletop exercises, patient scenario exercises, and a soft rollout when the program commenced.
Fig. 2.
The MIH-CP program intervention workflow.
Documentation: All members of the care team document the patient encounters in an hospital-based EHR that was linked with the Chesapeake Regional Information System for our Patients (CRISP).
2.2.2. Patient enrollment
(Patients enrolled in the MIH-CP programs were not enrolled in other transitional services. Patients were enrolled in the MIH-CP program prior to discharge by the CHW. CHWs provided patient outreach at the bedside, obtained patient informed consent, and collected some patient information (e.g., site of enrollment, primary care provider (PCP), health insurance coverage, employment status, assessments of activities of daily living and SDoH. CHWs also provided information to the patient about the home visit, scheduled home visits, and coordinated and executed the wide variety of activities needed to address or mitigate social and environmental needs. The CHW notified the MIH-team about the initial home visit appointment via the EHR.
2.2.3. Pharmacy pre-visit assessment
A pre-visit assessment was completed once the pharmacist received the notification (via EHR), the day before the home visit was scheduled. This assessment included a discharge medication reconciliation, which is completed by a pharmacy technician or pharmacy student and communicated to the pharmacist. This process involved comparing the discharge medication list in the EHR with the medication fill history obtained from DrFirst claims data. The pharmacy technician or student compiled a medication list, including the last fill dates for each medication, the number of refills left, and any medication discrepancies (i.e., dose discrepancies, refill gaps, medications that were filled according to DrFirst data but were not on the discharge list and vice versa) and communicated this to the pharmacist. The pharmacist, with access to the EHR, performed a medication therapy review, assessing medication therapies to identify medication-related problems, and developed a prioritized list of medication-related problems prior to the home visit.
2.2.4. Home visit
Patients enrolled in the MIH-CP program were visited in their homes 24–72 hours after hospital discharge by a field team of community paramedics and a pharmacy technician. During the home visit, the community paramedics collected vital signs and performed a physical exam, social and functional assessments, environmental and psychosocial assessments, and fall risk assessments. Meanwhile, the pharmacy technician gathered and reviewed all patient medications and completed the medication adherence assessment. The medication adherence assessment is a questionnaire developed to help with identifying barriers to medication use. This questionnaire was developed from the framework of the Modified Drug Adherence Work-Up (M-DRAW) tool, which is a validated 13-item checklist questionnaire30 but was slightly modified to include other pertinent questions relevant to our patient population, such as assessing for reading ability, dexterity with opening tablet bottles, problems with medication co-pays, and other medicine-taking behaviors (e.g., tendency to take other people's medications) (see Table S2). The home medication list obtained by the pharmacy technician, as well as the patient responses from the medication assessment questionnaire, were recorded directly in the EHR. The pharmacist reconciled the home medication list with the pre-visit assessment completed prior to the visit and then virtually joined the home visit via the UMMS teleport. Pharmacy students on Advanced Pharmacy Practice Experience (APPE) rotations, under the direct supervision of a pharmacist, were also involved in the medication reconciliation process. The field team connected with the virtual team via the UMMS teleport. The community paramedic presented the patient's case to the entire MIH-CP team, including findings from the various assessments. The pharmacist then provided patient education, addressed the identified adherence barriers, and created a plan of action to resolve any medication-related problems. See Table S2 and S3 for a list of pharmacist-driven interventions used to address medication adherence barriers. The ALP supervised the entire home visit, ensured that patients who do not have a PCP are connected with a practice, and created a comprehensive plan in collaboration with the patient, the MIH-CP team, and the patient's PCP to address health concerns and social determinants of health needs. Each patient was assigned a CHW/ALP pair who supervised the progress of the care plan throughout the 30 days.
2.2.5. Post-visit follow-up
The pharmacist intervened to address medication-related problems with the patient's PCP and documented these follow-up interventions and recommendations in the EHR. In addition, pharmacist communicated any medication changes to the patient's outpatient pharmacist and provided a warm handoff to the community pharmacist. A discharge visit at the end of the 30-day program was used to reassess outstanding needs and self-reported quality of life. The visit also provided an opportunity to extend enrollment if necessary or enact a warm handoff to the patient's primary care physician, case manager, or other care teams for ongoing care and support.
2.3. Data sources and collection
Data sources used for this study include: program data, including enrollment roster; hospital data, including EHRs from the UMMS; pharmacy data, including information on medication adherence via a review of pharmacy claims, electronic prescriptions, and medicine filling information from DrFirst (https://drfirst.com/).
EHR chart abstraction was conducted by pharmacists and pharmacy students to obtain information regarding medications prescribed to treat COPD and/or CHF at hospital discharge.
The discharge medication list for each patient obtained by EHR chart abstraction was used as a baseline regimen at the time of discharge. Patient adherence to medications was determined by comparing pharmacy claims data provided by DrFirst to each patient's discharge medication list. DrFirst provided access to each patient's pharmacy fill history over a period of 12 months (from November 8, 2019, through November 7, 2020). It is important to note that DrFirst data is only available for 12 months from any given day because Surescripts contractually only allows the downloading of 12 months' worth of data. Pharmacy claims provided by DrFirst were then used to track pre- and post-admission medication history by reviewing each patient's medication fill history for 3 months before and 6 months after the index discharge. Hospital EHRs were used to determine the medications initiated, implemented, or discontinued during the patient's hospital stay.
2.4. Outcomes
PDC calculates the ratio of the number of days that the patient is covered by the medication in a given period to the total number of days in the period. An adherence threshold of PDC ≥ 80% was used according to the CMS 2017 Quality Rating System, which accepts PDC ≥ 80% as the level above which the medication (for diabetes and cardiovascular disease) has a reasonable likelihood of achieving most of its potential clinical benefit.31,32 Clinical evidence provides support for a standard PDC threshold of 80%. However, the PDC: Antiretroviral Medications measure requires a 90% threshold.31 Average PDC were used to measure the impact of the 30-day MIH-CP program specifically on medication adherence. PDC for a given subject were scored zero if there was no match between the discharge medication from EHR review and prescriptions reported in DrFirst claims data; in all other cases, PDC were calculated for a given period after hospital discharge, excluding any time of overlap. The medication supply (in days) associated with the matching discharge medication (prescribed prior to admission to the hospital) was accounted for in the PDC calculation. The average PDC for all discharge medications included in a particular drug category (i.e., drugs used to treat CHF and drugs used to treat COPD; see Table S1) were then computed for each patient discharge. PDC were calculated for CHF medications prescribed for patients diagnosed with CHF and for COPD medications prescribed for patients diagnosed with COPD. PDC is a continuous variable, which is computed for each medication. The mean PDC for each drug category was calculated because a patient may have multiple medications in a particular drug category (see Table S1).
The final calculated PDC were assigned to one of six mutually exclusive 30-day intervals, including 0–30, 31–60, 61–90, 91–120, 121–150, and 151–180 days after hospital discharge.
2.5. Statistical analysis
Patient characteristics were summarized using descriptive statistics. Differences between the MIH-CP and the non-MIH-CP groups were evaluated using the Mann-Whitney U test for continuous variables and Chi-square test or Fisher's exact test for categorical variables, as appropriate. For both MIH-CP and non-MIH-CP patients diagnosed with CHF, the average PDC for CHF medications determined for each 30-day interval were compared using linear regression both with and without adjusting for age, sex, or payer. Similarly, for both MIH-CP and non-MIH-CP patients diagnosed with COPD, the average PDC for COPD medications determined at each 30-day interval were compared using the same approach. All data were originally managed in SAS (version 9.4) with statistical analyses performed in Stata/SE (version 17). All graphs were generated using Microsoft Excel. The project was reviewed and identified as exempt from review by the institutional review board (IRB) of the University of Maryland, Baltimore (HP-00085030).
3. Results
Table 1 includes patient demographics, clinical indicators, and other variables used to perform PSM between the intervention (MIH-CP) group and the controls (non-MIH-CP group). PSM balanced nearly all differences in observed variables of interest between the two groups except for the proportion of patients diagnosed with CHF, which remained different (p = 0.03) even after missing data were excluded. As shown, the outcomes of interest for patients diagnosed with CHF and those diagnosed with COPD were evaluated separately; sample sizes for drug-specific analyses are as indicated. (See Table 1)
Table 1.
Demographic characteristics of the study population.
| Total study population (N = 83) | MIH-CP group (n = 43) | Non-MIH-CP group (n = 40) | P-value | |
|---|---|---|---|---|
| Age, mean ± SD | 64.8 ± 13.1 | 64.1 ± 12.9 | 65.5 ± 13.3 | 0.601 |
| Male, n (%) | 26 (31.3) | 12 (27.9) | 14 (35.0) | 0.652 |
| Race, n (%) | 0.892 | |||
| African American | 70 (84.3) | 37 (86.1) | 33 (82.5) | |
| White | 13 (15.7) | 6 (13.9) | 7 (17.5) | |
| Payer, n (%) | 13 | |||
| Commercial | 3 (3.6) | 2 (4.7) | 1 (2.5) | |
| Medicaid | 31 (37.4) | 16 (37.2) | 15 (37.5) | |
| Medicare | 49 (59.0) | 25 (58.1) | 24 (60.0) | |
| Diagnosis/comorbidity, n (%) | ||||
| CHF | 58 (69.9) | 25 (58.1) | 33 (82.5) | 0.032 |
| COPD | 48 (57.83) | 28 (65.12) | 20 (50) | 0.242 |
| Diagnosis/comorbidity groups, n (%) | 0.052 | |||
| CHF only | 35 (42.17) | 15 (34.88) | 20 (50) | |
| COPD only | 25 (30.12) | 18 (41.86) | 7 (17.5) | |
| Both | 23 (27.71) | 10 (23.26) | 13 (32.5) | |
| Hospital services, n (%) | 13 | |||
| Hospitalist Service | 30 (36.1) | 16 (37.2) | 14 (35.0) | |
| General Internal Medicine | 20 (24.1) | 10 (23.3) | 10 (25) | |
| Cardiology, Medical Subspecialty, Medicine Transplant, Pulmonary |
7 (8.4) |
4 (9.3) |
3 (7.5) |
|
| Family Medicine | 9 (10.8) | 5 (11.6) | 4 (10.0) | |
| Infectious disease | 9 (10.8) | 4 (9.3) | 5 (12.5) | |
| Other | 8 (9.6) | 4 (9.3) | 4 (10.0) | |
| Hospital stay type, n (%) | 0.482 | |||
| Observation | 29 (34.94) | 13 (30.23) | 16 (40) | |
| Inpatient | 54 (65.06) | 30 (69.77) | 24 (60) | |
| Patient postal/ZIP code, n (%) | 0.943 | |||
| 21201 | 13 (15.7) | 5 (11.6) | 8 (2.0) | |
| 21217 | 10 (12.0) | 5 (11.6) | 5 (12.5) | |
| 21223 | 27 (32.5) | 15 (34.9) | 12 (30.0) | |
| 21229 | 24 (28.9) | 13 (30.2) | 11 (27.5) | |
| 21230 | 7 (8.4) | 4 (9.3) | 3 (7.5) | |
| 21201 | 2 (2.4) | 1 (2.3) | 1 (2.5) | |
| Days between discharge date and January 1, 2020; mean ± SD | 84.8 ± 29.8 | 81.4 ± 28.1 | 88.5 ± 31.5 | 0.311 |
| Pharmacy claim, n (%) | 54 (65.1) | 27 (62.8) | 27 (67.5) | 0.832 |
SD, standard deviation.
1Mann-Whitney U test.
2Chi-square test.
3Fisher's exact test.
Pharmacy claim = 1(if there was a pharmacy claim before or after the discharge date); = 0 (if there was no pharmacy claim).
Data provided by DrFirst revealed that 89% of newly prescribed medications had been retrieved by MIH-CP CHF patients within 30 days of discharge versus only 69.4% by non-MIH-CP CHF patients. Similarly, 75% of the newly prescribed medications were retrieved by MIH-CP COPD patients within 30 days of discharge versus only 50% by non-MIH-CP COPD patients.
Thus, 11% of the new CHF medications and 25% of the new COPD prescribed to patients enrolled in the MIH-CP program were not retrieved within 30 days after hospital discharge. Similarly, 30.6% and 50% of new medications prescribed to CHF and COPD patients, respectively, who were not enrolled in the MIH-CP program had not been retrieved at 30-days post-hospital discharge. Among the MIH-CP patients, the reasons provided for prescription abandonment included cost, inability to respond to pharmacy calls, and intentional non-adherence. Cost was the main reason for prescription abandonment among COPD patients enrolled in the MIH-CP program.
The findings presented in Table 2 document the time that new medications were retrieved after hospital discharge by patients diagnosed with CHF or COPD who were or were not enrolled in MIH-CP. (See Table 2.)
Table 2.
New prescriptions retrieved.
| CHF |
COPD |
|||
|---|---|---|---|---|
| Days after hospital discharge | MIH-CP patients (%) | Non-MIH-CP patients (%) | MIH-CP patients (%) | Non-MIH-CP patients (%) |
| 0–7 | 77.8 | 50 | 75 | 50 |
| 8–14 | 1.9 | 16.7 | 0 | 0 |
| 15–21 | 1.9 | 2.7 | 0 | 0 |
| 22–30 | 7.4 | 0 | 0 | 0 |
| >30 | 11 | 30.6 | 25 | 50 |
| Total | 100 | 100 | 100 | 100 |
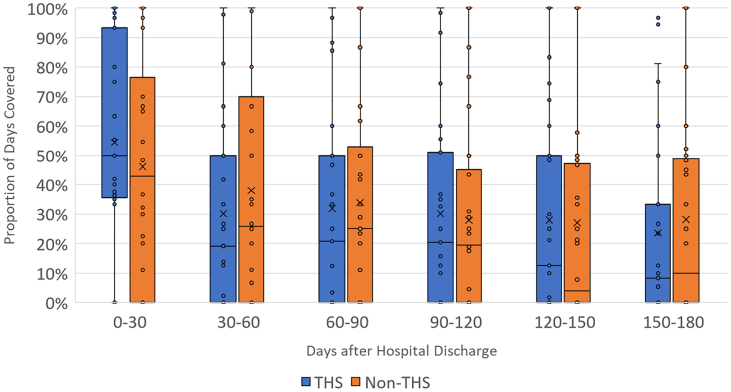
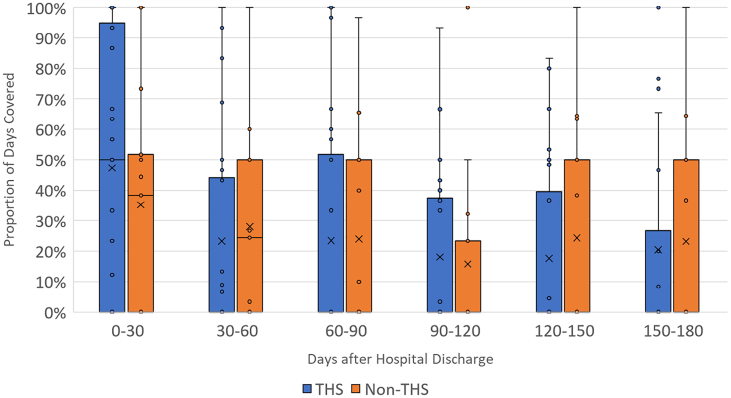
The findings shown in Fig. 3 compare the medians and distributions of PDC for CHF-related drugs between patients diagnosed with CHF in the intervention (enrolled in MIH-CP) and control (not enrolled in MIH-CP) within each 30-day period after hospital discharge. These findings reveal an increase in PDC (mean, median, and interquartile range [IQR]) during the first 30 days after hospital discharge in MIH-CP patients compared to controls, although this difference does not achieve statistical significance (median absolute difference, 0.08; 95% confidence interval [CI], −0.11-0.28). (See Table 3). No significant differences in PDC between intervention and control groups were observed during any of the subsequent periods. Similar results were obtained when comparing PDC for COPD-related medications among patients diagnosed with COPD (Fig. 4). In this latter group, an increase in overall PDC was observed during the first 30 days among patients enrolled in MIH-CP compared to controls who were not enrolled in this program, although this finding also did not achieve statistical significance (median absolute difference, 0.12; 95% CI, −0.16-0.41]. No substantial differences between the two groups were observed during any of the subsequent periods. Among the 48 patients with COPD, only 1 patient (2%) adhered to the PDC threshold of ≥80%; among the 58 patients with CHF, 10 patients (17.2%) met this adherence threshold (MIH-CP: 5/25 = 20% vs. non-MIH-CP: 5/33 = 15.2%).
Fig. 3.
Mean PDC by CHF medications among patients with CHF by MIH-CP enrollment status in 30-day increments after hospital discharge. The top and bottom whiskers represent the largest and smallest non-outlier values, respectively. The colored boxes denote the interquartile ranges. The horizontal bars within each box are the inclusive medians and “X” represents the mean. CHF = congestive heart failure, MIH-CP = mobile integrated health-community paramedicine.
Table 3.
Unadjusted and adjusted differences in Proportion Days Covered based on the underlying disease and enrollment status.
| Diagnosis | Days after hospital discharge | Unadjusted |
Adjusted⁎ |
||
|---|---|---|---|---|---|
| Difference [95% CI] | P-value | Difference [95% CI] | P-value | ||
| Congestive heart failure | 0–30 | 0.08 [−0.11–0.28] | 0.402 | 0.08 [−0.12–0.28] | 0.407 |
| 30–60 | −0.08 [−0.28–0.12] | 0.437 | −0.06 [−0.25–0.14] | 0.548 | |
| 60–90 | −0.02 [−0.21–0.17] | 0.838 | 0.00 [−0.18–0.18] | 0.988 | |
| 90–120 | 0.22 [−0.16–0.20] | 0.809 | 0.04 [−0.14–0.22] | 0.655 | |
| 120–150 | 0.01 [−0.18–0.20] | 0.927 | 0.03 [−0.16–0.22] | 0.733 | |
| 150–180 | −0.05 [−0.23–0.14] | 0.616 | −0.02 [−0.21–0.16] | 0.786 | |
| Chronic obstructive pulmonary disease | 0–30 | 0.12 [−0.16–0.41] | 0.397 | 0.14 [−0.15–0.43] | 0.341 |
| 30–60 | −0.05 [−0.28–0.18] | 0.677 | −0.04 [−0.28–0.20] | 0.757 | |
| 60–90 | −0.01 [−0.25–0.24] | 0.965 | −0.00 [−0.27–0.26] | 0.971 | |
| 90–120 | 0.02 [−0.18–0.22] | 0.822 | 0.02 [−0.19–0.24] | 0.836 | |
| 120–150 | −0.07 [−0.28–0.15] | 0.536 | −0.05 [−0.27–0.16] | 0.603 | |
| 150–180 | −0.03 [−0.27–0.21] | 0.820 | −0.01 [−0.26–0.24] | 0.931 | |
Adjusted for age, sex, and third-party payer.
Fig. 4.
Mean PDC by COPD medications among patients with COPD by MIH-CP enrollment status in 30-day increments after hospital discharge. The top and bottom whiskers represent the largest and smallest non-outlier values, respectively. The colored boxes denote the interquartile ranges. The horizontal bars within each box are the inclusive medians and “X” represents the mean. COPD = chronic obstructive pulmonary disease; MIH-CP = mobile integrated health-community paramedicine.
4. Discussion
The program interventions designed to address medication adherence were found to have an impact on medication adherence when comparing the results (mean PDC) from the intervention group to propensity-score matched controls within the 0–30-day interval after hospital discharge. The differences in medication adherence observed during the first 30 days did not persist beyond the 30-day duration of the MIH-CP program. These findings suggest that these high-risk patients with complex medical and social needs most likely require ongoing intervention, management, and follow-up that extends beyond the initial 30 days. An extension of these services may reveal a persistent impact on medication adherence.
Integration of inpatient and outpatient medication data was crucial toward the efforts to promote medication adherence. The inpatient medication data served as a baseline against which adherence was measured using outpatient prescription claims data. The home visit component of this program sets this care transition model apart from other models, which utilize a phone-call or other in-clinic services. This is because home visits provide pharmacists with a more realistic picture of a patient's medication-taking behavior, which results in a more thorough post-discharge medication reconciliation. This process helped to identify important changes and discrepancies to patient's medication regimen. During the process of in-home medication reconciliation conducted by the MIH-CP team, several discrepancies were identified including continuation of discontinued medications, therapeutic duplication, omitted medications, incorrect administration such as changes in drug strength, and/or changes to the frequency of their administration among others. Each enrolled patient was provided with an updated personal medication list; this list was then used to facilitate ongoing tracking of medication adherence using outpatient medication data. The integration of inpatient and outpatient medication data is critical for the accurate assessment of medication adherence across all phases of adherence, including initiation, implementation, and drug discontinuation, as well as the identification of any medication-related problems. The pharmacist identified cases of first fill failure, where a medication is started in the hospital, but the patient never filled the medication post-discharge. The process of integrating medication data involved manually abstracting medication data from the inpatient EHR which were then merged manually with outpatient medication data. This was a tedious and time-consuming process that would be challenging to scale upwards for a larger patient cohort. Some attention to computer-based automation might be considered in future studies.
The patients eligible to enroll in MIH-CP and included in this study were limited to those selected from a predetermined geographical area by postal ZIP code. This population includes a large proportion of individuals with a disproportionately high burden of chronic disease and health disparity issues that are largely fueled by poor SDoH, including low health literacy, low socioeconomic levels, lack of social support, and limited access to transportation. The impact of these factors on the lack of persistence beyond the program enrollment period is not yet known. The program might have a more sustained impact on populations with fewer health burdens and greater access to health care.
Pharmacists play a critical role in MIH-CP and similar types of programs by focusing on medication management and adherence. Crockett et al. (2017) described the role of a pharmacist in a community paramedicine team but could not assess the benefit of a pharmacist on the community paramedicine team due to small sample size.33 One way to ensure continuity and persistence of the impact of the MIH-CP might be a “pharmacist to pharmacist” intervention and referral model that would serve to link pharmacists working with MIH-CP to those based in the community. In this type of model, MIH-CP pharmacists coordinates patient care via an official “soft/warm handoff” to the community pharmacists at the end of the 30-day enrollment program. Community pharmacists then continue providing effective medication management services to these vulnerable patients in collaboration with their primary care providers.
This study has several important limitations. This was a pilot study that focused on a small number of patients diagnosed with CHF and COPD. The sample size was limited by the availability of pharmacy claims data, MIH-CP program capacity and the need for the manual abstraction of discharge medication lists. Some local dispensing pharmacies do not share their data with DrFirst due to the differing policies of local and independent pharmacies with regards to sharing prescription data. While some are “opt-out”, most are “opt-in”. As such, a few pharmacies are not able to share their data with a third-party data aggregator. Thus, a portion of patients may be missing specific pharmacy claims. The study was somewhat underpowered and may be responsible for the lack of statistical significance in medication adherence between the intervention and control groups. In addition, variation in factors such as the level of care provided (due to varying patient needs and preferences), team member performance, availability of medications, and caseload, could also decrease the impact of the intervention. Although PDC is considered to be a relatively strong measure of medication adherence, it is by definition a surrogate marker as it is not clear whether the patient ultimately administered the medication in question. Finally, the PDC adherence measure was based on discharge medications list and does not take into account any changes or adjustments made to the medication regimen after an outpatient or primary care visit or subsequent readmission.
Based on this pilot study, the adjusted difference in the PDC between the two groups was 8% for patients with CHF, which corresponds to an effect size (Cohen's d) of 0.23. The adjusted difference was 14% for patients with COPD, which corresponds to an effect size of 0.34.34 A future study with the same experimental design (i. e., a PSM observational study with a 1:1 sample size ratio between the intervention group and the control group), a sample size of 298 CHF patients per group (and/or 137 COPD patients per group) will achieve 80% power to detect an effect size of 0.23 (or 0.34) using a two-sided two-sample t-test with equal-variance assumption and a significance level of 5%.
5. Conclusions
Medication non-adherence continues to be a major contributor to preventable hospital readmissions and poor healthcare outcomes. Any efforts to improve medication adherence will require a clear understanding of each patient's medication regimen at hospital discharge. This will allow healthcare providers to track, assess, and evaluate medication non-adherence on a case-by-case basis. Results from this small pilot study revealed increased medication adherence among patients diagnosed with CHF and COPD who were enrolled in the MIH-CP program compared to propensity-matched controls. While these differences did not achieve statistical significance and did not persist beyond the 30-day enrollment period, they highlight several specific critical issues. Efforts to integrate inpatient and outpatient medication regimens remain critical for the prevention of medication non-adherence during transitions of care and help to identify medication non-adherence at timepoints. Transition of care programs such as MIH-CP, which incorporate pharmacists as part of the team, support the identification and resolution of critical medication-related problems and medication non-adherence. These types of programs can provide much-needed care and support for a largely underserved community.
Funding
The Baltimore City MIH-CP program is funded from a grant from the Maryland Health Services Cost Review Commission.
CRediT authorship contribution statement
Olufunke Sokan: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft, Writing – review & editing. Benoit Stryckman: Conceptualization, Methodology, Software, Formal analysis, Data curation, Writing – original draft, Visualization. Yuanyuan Liang: Formal analysis, Visualization. Sade Osotimehin: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Daniel B. Gingold: Methodology, Investigation, Visualization, Supervision. Weston W. Blakeslee: Conceptualization, Data curation. Colleen T. Landi: Methodology, Investigation. Magaly Rodriguez: Supervision.
Declaration of Competing Interest
None declared.
Acknowledgments
The authors thank the following students for their assistance with manual chart abstraction: Michael Obineme, Chukwudalu Umeh, Grace Victor, and Zachary Leppert.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rcsop.2022.100201.
Appendix A. Supplementary data
Supplementary material
References
- 1.Baechle C., Huang C.D., Agarwal A., Behara R.S., Goo J. Latent topic ensemble learning for hospital readmission cost optimization. Eur J Oper Res. 2020;281(3):517–531. [Google Scholar]
- 2.Mullings L., Sankaranarayanan J. Quality of communication about medicines in United States hospitals: a national retrospective study. Res Soc Adm Pharm RSAP. 2017;13(4):849–856. doi: 10.1016/j.sapharm.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Rottman-Sagebiel R., Cupples N., Wang C.P., et al. A pharmacist-led transitional care program to reduce hospital readmissions in older adults. Fed Pract. 2018;35(12):42–50. [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S. Impact of pharmacist-led medication management in care transitions. BMC Health Serv Res. 2017;17(1):722. doi: 10.1186/s12913-017-2684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McPhail E.J., Marshall V.D., Remington T.L., Vordenberg S.E. Readmission rates associated with pharmacist involvement in a geriatric transitional care management clinic. Innov Pharm. 2019;10(3) doi: 10.24926/iip.v10i3.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seidl K.L., Gingold D.B., Stryckman B., et al. Development of a logic model to guide implementation and evaluation of a mobile integrated health transitional care program. Popul Health Manag. 2021;24(2):275–281. doi: 10.1089/pop.2020.0038. [DOI] [PubMed] [Google Scholar]
- 7.Mistiaen P., Francke A.L., Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta-review. BMC Health Serv Res. 2007;7:47. doi: 10.1186/1472-6963-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kripalani S., Theobald C.N., Anctil B., Vasilevskis E.E. Reducing hospital readmission rates: current strategies and future directions. Annu Rev Med. 2014;65:471–485. doi: 10.1146/annurev-med-022613-090415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kansagara D., Englander H., Salanitro A., et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurman W.A., Moczygemba L.R., Tormey K., Hudzik A., Welton-Arndt L., Okoh C. A scoping review of community paramedicine: evidence and implications for interprofessional practice. J Interprof Care. 2021;35(2):229–239. doi: 10.1080/13561820.2020.1732312. [DOI] [PubMed] [Google Scholar]
- 11.Gregg A., Tutek J., Leatherwood M.D., et al. Systematic review of community paramedicine and EMS mobile integrated health care interventions in the United States. Popul Health Manag. 2019;22(3):213–222. doi: 10.1089/pop.2018.0114. [DOI] [PubMed] [Google Scholar]
- 12.Dharmarajan K., Hsieh A.F., Lin Z., et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jencks S.F., Williams M.V., Coleman E.A. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 14.30-Day Readmission Rates to U.S. Hospitals. Agency for Healthcare Research and Quality. Agency for Healthcare Research and Quality; Rockville, MD: 2021. https://www.ahrq.gov/data/infographics/readmission-rates.html Published August 2018. Accessed September 29. [Google Scholar]
- 15.Shah T., Press V.G., Huisingh-Scheetz M., White S.R. COPD readmissions. Chest. 2016;150(4):916–926. doi: 10.1016/j.chest.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Press V.G., Konetzka R.T., White S.R. Insights about the economic impact of chronic obstructive pulmonary disease readmissions post implementation of the hospital readmission reduction program. Curr Opin Pulm Med. 2018;24(2):138–146. doi: 10.1097/MCP.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilder M.E., Kulie P., Jensen C., et al. The impact of social determinants of health on medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2021;36(5):1359–1370. doi: 10.1007/s11606-020-06447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen O., Fridman R., Rosen B., Shane R., Pevnick J. Medication adherence as a predictor of 30-day hospital readmissions. Patient Prefer Adherence. 2017;11:801–810. doi: 10.2147/PPA.S125672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chisholm-Burns M.A., Kim Lee J., Spivey C.A., et al. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care. 2010;48(10):923–933. doi: 10.1097/MLR.0b013e3181e57962. [DOI] [PubMed] [Google Scholar]
- 20.Mueller S.K., Sponsler K.C., Kripalani S., Schnipper J.L. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14) doi: 10.1001/archinternmed.2012.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis E.M., Packard K.A., Jackevicius C.A. The pharmacist role in predicting and improving medication adherence in heart failure patients. J Manag Care Spec Pharm. 2014;20(7):741–755. doi: 10.18553/jmcp.2014.20.7.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadik A., Yousif M., McElnay J.C. Pharmaceutical care of patients with heart failure. Br J Clin Pharmacol. 2005;60(2):183–193. doi: 10.1111/j.1365-2125.2005.02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López Cabezas C., Falces Salvador C., Cubí Quadrada D., et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farm Hosp Organo Of Expresion Cient Soc Espanola Farm Hosp. 2006;30(6):328–342. doi: 10.1016/s1130-6343(06)74004-1. [DOI] [PubMed] [Google Scholar]
- 24.Murray M.D., Young J., Hoke S., et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146(10):714–725. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Sabaté E., World Health Organization . World Health Organization; 2003. Adherence to Long-Term Therapies: Evidence for Action. [Google Scholar]
- 26.Zullig L.L., Blalock D., Dougherty S., et al. The new landscape of medication adherence improvement: where population health science meets precision medicine. Patient Prefer Adherence. 2018;12:1225–1230. doi: 10.2147/PPA.S165404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson S.H., Eurich D.T., Majumdar S.R., et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viswanathan M., Golin C.E., Jones C.D., et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 29.Gingold D.B., Liang Y., Stryckman B., Marcozzi D. The effect of a mobile integrated health program on health care cost and utilization. Health Serv Res. 2021;56(6):1146–1155. doi: 10.1111/1475-6773.13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S., Bae Y.H., Worley M., Law A. Validating the modified drug adherence work-up (M-DRAW) tool to identify and address barriers to medication adherence. Pharm Basel Switz. 2017;5(3):E52. doi: 10.3390/pharmacy5030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.PQA_Measures_Overview.pdf https://www.pqaalliance.org/assets/Measures/PQA_Measures_Overview.pdf Accessed August 10, 2022.
- 32.Hamilton B.A. 2017. Quality Rating System Measure Technical Specifications. :228. [Google Scholar]
- 33.Crockett B.M., Jasiak K.D., Walroth T.A., Degenkolb K.E., Stevens A.C., Jung C.M. Pharmacist involvement in a community paramedicine team. J Pharm Pract. 2017;30(2):223–228. doi: 10.1177/0897190016631893. [DOI] [PubMed] [Google Scholar]
- 34.Linden A. ESIZEREG: Stata Module for Computing the Effect Size Based on a Linear Regression Coefficient. Stat Softw Compon. https://ideas.repec.org/c/boc/bocode/s458607.html Published online October 30, 2021. Accessed October 28, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material