To the editor
Growing evidence support that olfactory dysfunction (OD) is one of the major sequalae of long COVID-19.1 Long-term unrecovered OD may significantly reduce the quality of life of patients.2 However, previous studies were mostly based on self-reported symptoms and lacked control groups, making it difficult to evaluate the real association between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and persistent OD. Therefore, we read with great interest the recently published study in the Journal of Infection by M S Otte et al. on the topic of persistent OD in patients with COVID-19. Using psychophysical test, they found that 50% of patients still suffered from OD at 7 weeks after the start of COVID-19.3 Nevertheless, no study reported recovery rates of OD more than 2 years after infection. Long-term careful evaluation of OD recovery after SARS-CoV-2 infection is critical to understand the chronic symptoms of COVID-19 and to guide treatments.
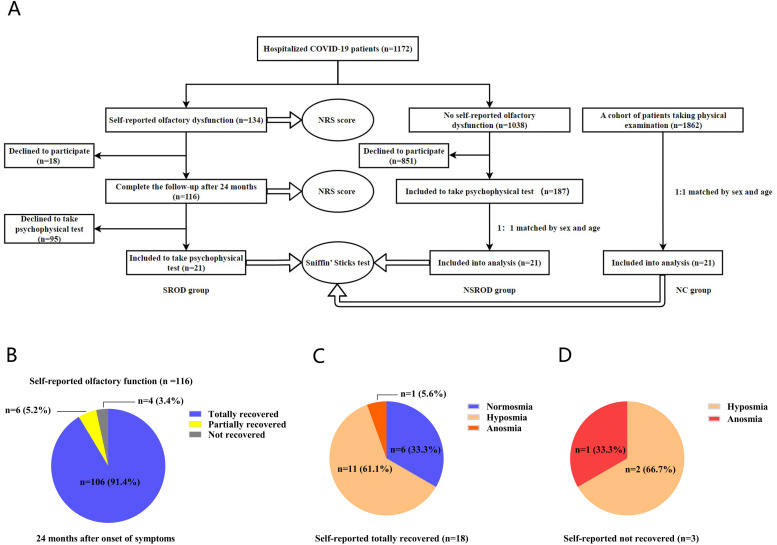
We followed up our previously reported cohort of 1,172 hospitalized COVID-19 patients, who were infected by alpha variant of SARS-CoV-2 during the first pandemic.4 The severity of OD was scored by patients on a numerical rating scale (NRS) of 0–10, with 0 being “sense of smell is completely normal” and 10 being “complete loss of sense of smell”. In this cohort, 134 patients reported OD during the onset of illness4, and 116 of them completed 2-year follow-up (Fig. 1 A). Patients whose NRS scores dropped to 0 were defined as fully recovered, while those with declined scores (>0) were defined as partially recovered, and the patients not having declined scores were defined as not recovered. Twenty-one of the 116 patients with self-reported OD (SROD group) finished the Sniffin’ Sticks test (Burghart Messtechnik, Holm, Germany). Among 1,038 patients without self-reported OD, 187 finished 2-year follow-up and completed the Sniffin’ Sticks test. As a disease control group, 21 of the 187 patients (NSROD group) were sex and age-matched to SROD group (Fig. 1A). In addition, 21 of 1,862 healthy subjects, who underwent Sniffin’ Sticks test and denied history of COVID-19, rhinitis, sinusitis and OD, were 1:1 sex and age-matched to SROD group as a normal control group (NC) (Fig. 1A). More information is provided in the Online Repository.
Fig. 1.
The flow chart and evaluation of OD.
A, The flow chart. B, Self-reported recovery rates of OD in SROD group. C, OD evaluated by Sniffin` Sticks test in patients with self-reported totally recovered. D, OD evaluated by Sniffin` Sticks test in patients self-reporting not recovered. Abbreviations: OD, olfaction dysfunction; NRS, numerical rating scale; SROD, self-reported olfactory dysfunction; NSROD, no self-reported olfactory dysfunction; NC, normal control.
The NRS scores of the patients in both NSROD and NC group were zero. At 24- month after symptom onset, we found that 91.4% (106 of 116) and 5.2% (6 of 116) patients in SROD group reported complete and partial recovery, respectively, while 3.4% (4 of 116) reported not recovered (Fig. 1B). No significant association was found between the self-reported OD 24 months after disease onset and the initial severity of COVID-19 and comorbidities (Table S1).
Both patients in SROD and NSROD group had significantly lower TDI scores compared to the subjects in NC group. Consistently, we found that only 28.6% (6/21) and 14.3% (3/21) patients were normosmia in SROD and NSROD groups, respectively, which were significantly lower than that in NC group (14/21; 66.7%) (Table 1 ). In contrast, the ratios of heterosmia were significantly higher in SROD and NSROD groups (71.4% and 85.7%, respectively) than that in NC group (33.3%) (Table 1). No difference was found between SROD and NSROD groups (Table 1).
Table 1.
The TDI score and age-adjusted olfactory function grading of subjects 24 months after onset symptoms evaluated by the Sniffin’ Sticks test.
| Characteristics | COVID-19 patients |
Normal subjects | |
|---|---|---|---|
| With self-reported olfactory dysfunction | Without self-reported olfactory dysfunction | ||
| Subject, N | 21 | 21 | 21 |
| Gender, male, N (%) | 11 (52.4%) | 14 (66.7%) | 10 (47.6%) |
| Age, years, median (IQR) | 63 (42, 66) | 51 (40, 59) | 54 (41, 58) |
| TDI score, median (IQR) | 23.5 (20.1, 29.9) * | 24 (21.5, 26.3) * | 29 (24.6, 30.5) |
| Threshold, median (IQR) | 4 (1, 5.8) | 1 (1, 4) * | 5.25 (2.5, 6.5) |
| Discrimination, median (IQR) | 9 (8, 13) | 11 (9, 12) | 11 (10, 13.5) |
| Identification, median (IQR) | 11 (9, 12) * | 11 (10, 12) | 12 (11, 13) |
| Normosmia, N (%) | 6 (28.6%) * | 3 (14.3%) ** | 14 (66.7%) |
| Heterosmia, N (%) | 15 (71.4%) * | 18 (85.7%) ** | 7 (33.3%) |
| Hyposmia, N (%) | 13 (61.9%) | 18 (85.7%) | 7 (33.3%) |
| Anosmia, N (%) | 2 (9.5%) | 0 | 0 |
Abbreviations: TDI, threshold, discrimination, and identification; COVID-19, coronavirus disease 2019; IQR, interquartile range; Data are presented as medians and interquartile ranges for continuous variables and numbers with percentage for categorical variables. P values were calculated from Mann-Whitney U 2-tailed test, or χ2 test or Fisher's exact test. *P<0.05 vs. Normal control, **P<0.01 vs. Normal control.
Among 21 patients finishing psychophysical test in SROD group, 18 of them reported complete recovery of OD and 3 reported not recovered. Surprisingly, the Sniffin’ Sticks test revealed that only 33.3% (6/18) of the self-reported completely recovered patients reached to normal sense of smell, while 61.1% (11/18) and 5.6% (1/18) of them were still hyposmia and anosmia, respectively (Fig. 1C). In addition, 2 of the 3 subjects who complained persistent OD were hyposmia (66.7%), and one (33.3%) was anosmia (Fig. 1D).
The persistence of OD in long COVID-19 over 2 years remains unclear. In this study, we found that although 96.6% of the patients reported that their sense of smell was recovered after 2 years, only one-third of them actually recovered according to the psychophysical test. This result is in line with another study, which reported that the prevalence of OD evaluated by psychophysical tests was higher than that estimated based on self-reported information at 6-month from diagnosis of SARS-CoV-2 infection.5 Collectively, it suggests that the self-reported evaluation is inadequate to evaluate OD in long COVID-19. Olfactory function varies with age and gender.6 To adjust these confounding factors, 21 individuals 1:1 matched to patients infected with SARS-CoV-2 were enrolled. The ratios of normosmia and heterosmia in NC group in this study were in line with those reported in general population.6 We found that the prevalence and severity of OD in SROD group were significantly higher than those in NC group. It indicates that SARS-CoV-2 infection is the cause of persistent OD which may last up to 24 months. High prevalence of persistent OD in long COVID-19 argues for more attentions with development of target therapies.7
A very recent study based on self-reported data showed that 88.2% of patients reporting COVID-19–related smell or taste dysfunction completely recovered within 2 years.8 However, no study, to the best of our knowledge, has investigated the olfactory function in COVID-19 patients after two years using psychophysical tests. Surprisingly, we found that 85.7% patients in NSROD groups also had heterosmia when evaluated by psychophysical test, further indicating that the prevalence of OD in long COVID-19 might be much higher than those self-reported, even for those without complain of OD at the onset of illness and 2-year follow up.
In conclusion, our current findings suggest that there is still a significant proportion of patients having persistent OD 2 years after SARS-CoV-2 infection. However, additional studies are required to clarify the long-term olfaction condition in COVID-19 patients infected by the other variants, since distinct SARS-CoV-2 variants may have different abilities to cause OD.9 Our study is limited to the small sample size of subjects completing the psychophysical test. Nevertheless, our study confirms the great discrepancy between psychophysical tests and self-reported function, which highlights the importance of psychophysical tests in the evaluation of OD in the long-term follow-up of COVID-19 patients. Last but not least, the high prevalence of OD in long COVID-19, even 2 years post-infection, argues for more attentions with the development of target therapies.
Author contribution
Yi-Ke Deng collected clinical data, performed data analysis and prepared manuscript; Ke-Tai Shi collected clinical data; Ming Zeng and Zheng Liu designed the study, interpreted data, and prepared manuscript.
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
Funding sources
This study was supported by the National Natural Science Foundation of China (NSFC) grants 82071025 (M.Z.), 82201260 (Y.K.D.), 82130030 (Z.L.) and 81920108011(Z.L.), and the Key Research and Development Program of Hubei Province 2021BCA119 (Z.L.).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.11.024.
Appendix. Supplementary materials
References
- 1.Groff D, Sun A, Ssentongo AE, et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otte MS, Klussmann JP, Luers JC. Persisting olfactory dysfunction in patients after recovering from COVID-19. J Infect. 2020;81:e58. doi: 10.1016/j.jinf.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song J, Deng YK, Wang Het. Self-reported Taste and Smell Disorders in Patients with COVID-19: Distinct Features in China. Curr Med Sci. 2021;41:14–23. doi: 10.1007/s11596-021-2312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boscolo-Rizzo P, Menegaldo A, Fabbris Cet. Six-Month Psychophysical Evaluation of Olfactory Dysfunction in Patients with COVID-19. Chem Senses. 2021;46 doi: 10.1093/chemse/bjab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the "Sniffin' Sticks" including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237–243. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- 7.Denis F, Septans AL. Periers Let al. Olfactory Training and Visual Stimulation Assisted by a Web Application for Patients With Persistent Olfactory Dysfunction After SARS-CoV-2 Infection: Observational Study. J Med Internet Res. 2021;23:e29583. doi: 10.2196/29583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boscolo-Rizzo P, Fabbris C, Polesel Jet. Two-Year Prevalence and Recovery Rate of Altered Sense of Smell or Taste in Patients With Mildly Symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. 2022 doi: 10.1001/jamaoto.2022.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menni C, Valdes AM, Polidori Let. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.