Abstract
Background
SARS-COV-2 infection has been associated to long-lasting neuropsychiatric sequelae, including cognitive deficits, that persist after one year. However, longitudinal monitoring has been scarcely performed. Here, in a sample of COVID-19 patients, we monitor cognitive, psychological and quality of life-related profiles up to 22 months from resolution of respiratory disease.
Methods
Out of 657 COVID-19 patients screened at Manzoni Hospital (Lecco, Italy), 22 underwent neuropsychological testing because of subjective cognitive disturbances at 6 months, 16 months, and 22 months. Tests of memory, attention, and executive functions were administered, along with questionnaires for depressive and Post-traumatic stress disorder (PTSD) symptoms, psychological well-being and quality of life. Cross-sectional descriptives, correlational, as well as longitudinal analyses considering COVID19-severity were carried out. A preliminary comparison with a sample of obstructive sleep apneas patients was also performed.
Results
Around 50% of COVID-19 patients presented with cognitive deficits at t0. The most affected domain was verbal memory. Pathological scores diminished over time, but a high rate of borderline scores was still observable. Longitudinal analyses highlighted improvements in verbal and non-verbal long term memory, as well as attention, and executive functioning. Depression and PTSD-related symptoms were present in 30% of patients. The latter decreased over time and were associated to attentional-executive performance.
Conclusions
Cognitive dysfunctions in COVID-19 patients may extend over 1 year, yet showing a significant recovery in several cases. Cognitive alterations are accompanied by a significant psychological distress. Many patients displaying borderline scores, especially those at higher risk of dementia, deserve clinical monitoring.
Keywords: COVID-19, Long-COVID, Cognitive functions, Neuropsychology, Longitudinal assessment
1. Introduction
Severe Acute Respiratory Syndrome due to Coronavirus 2 (SARS-COV-2) has been shown to impact multiple organs beyond the respiratory system, including the brain [1]. In fact, along the first studies highlighting neurological [[2], [3], [4]], as well as psychiatric symptoms that could persist up to six months after the respiratory syndrome [5,6], it became more and more evident that also fatigue and cognitive impairments (often described as “brain fog”) could persist well beyond the acute infection, leading to the so-called “Long COVID” [[7], [8]]. Such sequelae could pose psychological, occupational, and social problems, also affecting the caregivers [9]. Moreover, concerns were raised as COVID-19 patients who recovered from respiratory disease could be more likely to develop cognitive decline and Alzheimer's disease [10]. Thus, it is not surprising that a growing number of studies have been characterizing COVID-19-related cognitive alterations and their neural correlates, such as [11,12].
The pathophysiological mechanisms underlying de novo onset of cognitive decline, or the worsening of pre-existing cognitive impairments, in patients with COVID-19 are likely multiple, ranging from the noxious effects of neural cells hypo‑oxygenation to toxic effects by cytokines released in the context of widespread inflammation [13].
Overall, despite methodological differences - e.g., telephone vs. in-person assessment, screening vs. second-level assessment [14,15] – converging evidence shows that cognitive deficits in COVID-19 patients can be appreciated from the early sub-acute stage [16] and few days after hospital discharge [[17], [18], [19]], up to 5–7 months [14,[20], [21], [22]] and even 1 year after hospital discharge [23,24]. A recent review by Crivelli and colleagues [14] on 27 studies including 2049 individuals, highlights a broad spectrum of cognitive impairments mostly involving executive functions, attention and long-term memory (i.e., the ability to learn new information and/or recall it at a later time).
However, to date, longitudinal monitoring of cognitive performance has been scarcely performed [16,22,25,26], with the vast majority of studies presenting only cross-sectional evidence [14]. To our knowledge, only three studies explored longitudinal, long-term, neuropsychological changes up to one year follow-up [23,24,26]. For instance, Ferrucci and colleagues [23] assessed a sample of COVID-19 patients after five months and one year from hospital discharge, with an extended neuropsychological battery. The authors found that around 60% of patients had a deficit in at least one cognitive function at five months, mostly in speed of processing (41%), long-term verbal (around 20%) and visuospatial memory (18%). Interestingly, at one year follow up, around 50% of the sample still showed a pathological performance in at least one cognitive domain, mainly in the same cognitive functions of the first timepoint. Similar results were found by Miskowiak et al. [24] and Mendez et al. [26]. Nonetheless, Ferrucci and colleagues [23] observed that speed of processing, attention, and verbal memory significantly improved after one year. Moreover, worse oxygenation in the acute phase was associated to worse verbal memory performance five months from discharge.
In the present study, we aim to enrich longitudinal evidence of cognitive dysfunctions by investigating neuropsychological changes in a sample of COVID-19 patients tested about 6, 16, and up to 22 months after the recovery of acute respiratory symptoms. Importantly, we complement the characterization of impaired cognitive domains by also considering sub-clinical (i.e., borderline), cognitive performance, almost neglected so far. We further assessed the impact of disease severity in the acute phase (i.e., the need for oxygen therapy) on the evolution of neuropsychological symptoms, as previous works suggested a pivotal role of respiratory impairments in explaining the pattern and the severity of cognitive deficits [20,23]. Moreover, as suggested by [21], the association between cognitive performance and mood disorders was also tested, along with a characterization of psychological well-being, and quality of life.
Finally, to assess the role of hypoxia alone as opposed to other mechanisms active in neurocovid patients, we compare cognitive and emotional profiles of COVID-19 patients to those of a sample of patients suffering from a common respiratory disease, i.e., Obstructive Sleep Apnea Syndrome (OSAS).
2. Materials and methods
2.1. Participants
From April 2020 to March 2021, 657 patients infected by SARS-COV-2 who received care for acute infection at the Manzoni Hospital (Lecco, Italy) were screened by a team of infectious disease specialists, in order to monitor long-term symptoms of SARS-CoV2 infection, with a particular focus on persistent respiratory, neurological and psychological sequelae (see Fig. 1 ). Specifically, neurological and neuropsychiatric changes (i.e., memory impairments, mood changes, neurological symptoms) were evaluated by means a check-list provided by the Neurology Unit (for details, see the Supplementary Material). In light of new-onset neurological symptoms, 74 patients (29 F; mean age = 60 ± 15.87, range 19–94) were referred to neurological examination; of those, 34 reported subjective cognitive alterations following COVID-19 (i.e., 5.5% of the whole population study), and 21 of them agreed to undergo a neuropsychological assessment around 6 months (timepoint t0) after resolution of acute respiratory problems. A sub-group of patients was further evaluated at two supplementary timepoints i.e., on average, at 16 months (t1; N = 19) and 22 months (t2; N = 16). Demographic and clinical features of the recruited patients are reported in Table 1 .
Fig. 1.
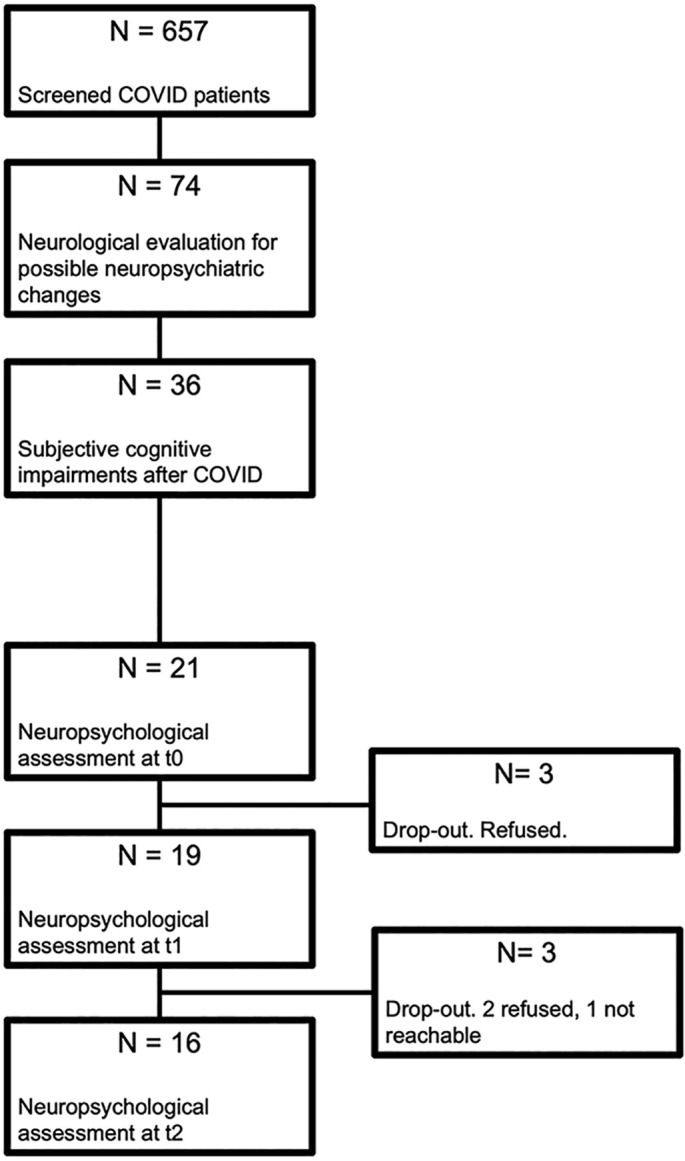
Flow chart.
Table 1.
Demographic and clinical variables of COVID-19 and OSAS patients.
| t0 | t1 | t2 | OSAS | |
|---|---|---|---|---|
| N | 21 | 19 | 16 | 8 |
| Sex | 6 F, 15 M | 5 F, 14 M | 4 F, 12 M | 2 F, 6 M |
| Age, years | 57 ± 15 [19–82] | 57 ± 15; [19–82] | 59 ± 15; [19–82] | 61.38 ± 6.78; [54–74] |
| Education, years | 11 ± 3; [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]] | 12 ± 3; [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]] | 11 ± 3; [[5], [6], [7], [8], [9], [10], [11], [12], [13]] | 9.5 ± 3; [[5], [6], [7], [8], [9], [10], [11], [12], [13]] |
| Oxygen therapy | 11/21 | 10/19 | 8/16 | |
| Hospital Stay, daysa | 23.19 ± 21; [0–86] | 24.11 ± 21.41; [0–86] | 21.5 ± 20.1; [0–86] | |
| Hyposmia/Hypogeusia | 6/21 | 5/19 | 4/16 |
Note: for age, education and hospital duration, mean, standard deviation, and range are reported. OSAS: obstructive sleep apnea syndrome.
Moreover, with the exploratory aim of comparing the cognitive-emotional profile of COVID-19 patients to that of control individuals suffering from respiratory problems, we recruited 8 additional individuals diagnosed with OSAS at the Respiratory Unit of the IRCCS INRCA (Italian National Research Centre On Aging), Mandich Hospital (Merate, Italy). None of them reported SARS-COV-2 infection. They were all tested before receiving night oxygen support by means continuous positive airway pressure (CPAP). They were all diagnosed with severe OSAS, i.e., Apnea Hypopnea Index (AHI) > 30 (range: 30–93). The average nocturnal saturation was 88% (range: 84%–94%); two patients did not present nocturnal desaturation. The average night time under 90% of saturation (i.e., CT90) was 31% (range: 5%–85%).
The study was approved by the local Ethics Committee (Protocol N°: 3477) and was conducted in accordance with the ethical standards of the Declaration of Helsinki. All participants provided their written informed consent.
2.2. Neuropsychological evaluation
The neuropsychological evaluation took place at around 6 months (t0; mean number of days: 185.29 ± 114.15 SD), 16 months (t1 mean number of days: 473.26 ± 97.67 SD) and 22 months (t2; mean number of days: 664 ± 100 SD) after resolution of respiratory disease.
2.2.1. Cognitive assessment
Cognitive assessment at t0 was carried out by two neuropsychologists and was tailored to the patients' age and education, at the discretion of the psychologist. It included several psychometric tests normed in the Italian population: the Mini Mental State Evaluation (MMSE) [27,28]; Attentional Matrices [29]; Trail Making Test (TMT) [30]; forward and backward digit span [31], Rey Verbal Learning Test (RVLT) [32]; Babcock Story Recall Test (BSRT) [29,33]; copy and recall of Rey-Osterrieth's complex fig. [34]; Frontal Assessment Battery (FAB) [[35], [36]]; verbal fluency by letter (i.e., phonemic fluency) and category (semantic fluency) [[37], [38]]; Weigl's Sorting Task [39]; Raven's Matrices [29,40].
At t1 and t2, in addition to the tests administered at t0, we sought to provide a more fine-grained characterization of the executive and attentional profile that may be part of long-COVID, by including: a computerized reaction time test (Open-source Open-access Reaction Time test, OORT) [41], both in its simple modality (i.e., reacting to a target with no distractors) and in a go-no-go modality; the oral version of the Symbol Digit Modalities Test (SDMT) [42]; the Clock Drawing Test (CDT) [43]; the Modified Five Point Test for non-verbal fluency [[44], [45]], and the alternate verbal fluency (Costa et al., 2013). In order to calculate the fluency-based Composite Shifting Index, Costa's version [38] of phonemic and semantic fluency tasks were administered. Moreover, at t2 the copy and recall of Taylor's fig. [46] replaced Rey-Osterrieth's to avoid learning effects. Likewise, parallel lists of words were used on the RVLT.
Raw scores were corrected for age and level of education, and whenever available, they were converted into equivalent scores (ES) [47,48], on an ordinal scale ranging from 0 to 4. ES from 2 to 4 are indicative of a non-pathological performance, whereas ES 1 and 0 can be regarded, respectively, as “borderline performance” (i.e., not pathological, but reflecting a significant decrease) and a pathological performance. For those tests which were not normed according to the ES method (i.e., the SDMT [42] and the CDT [43]), cut-off values were considered. For the statistical analyses (see below), corrected scores were used, whenever possible. ES were considered when different tests were used to test the same cognitive function in different patients. For example, logical reasoning was always tested with Raven's matrices, however, at the discretion of the neuropsychologist at t0, the more demanding Raven Standard Progressive Matrices [40] could be administered to some younger or more educated patients, whereas Raven Progressive Colored Matrices [29], relatively easier and shorter, to older participants. The use of standardized ES overcomes the problems of different score ranges.
2.2.2. Psycho-affective and quality of life questionnaires
Finally, at t1 and t2, we investigated participants' psychological state, as well as quality of life by means of self-administered questionnaires. The Beck Depression Inventory (BDI) [49] was administered to assess the presence and severity of depressive symptoms. BDI scores (range = 0–33) were classified as in: 0–9 = no depressive symptoms, 10–19 = mild symptoms, 20–29 = moderate symptoms; 30–33 = severe symptoms [50]. Moreover, participants completed the Impact of Event Scale-Revised (IES-R) [51] to assess the presence of post-traumatic stress disorders (PTSD) symptoms (range = 0–88); a score above 32 [52] indicated clinically relevant symptoms. We also administered the Psychological Well-Being Index (PGWBI) [53] considering both the total score (range = 0–110; higher scores, higher quality of life) and the six subscales Anxiety, Depression, Self-control, Health, Vitality, Positivity and Well-Being. Individual z-scores of the total score were calculated based on means and standard deviation of different age and sex groups [54]. Scores below −1.65 (i.e., below the theoretical 5th percentile) were regarded as a significant decrease of psychological well-being. Finally, all participants completed the short version of the World Health Organization Quality of Life Questionnaire (WHOQOL-BREF) [55]. We calculated both the total score (range = 26–130; higher score, higher quality of life) and the mean scores of the subscales (i.e., physical, psychological, social, and environmental) which were converted into a 0–100 scale.
OSAS patients were tested only once and were administered the same protocol of COVID-19 patients at t1, except for the IES-R.
The evaluation lasted about 105 min and was split into two daily sessions, whenever participant's fatigability made it necessary. Participants were allowed to take a short break among tests.
2.3. Analyses
All analyses were carried out with jamovi 1.6.23 [56]. Alpha was set 0.05.
For each timepoint, we calculated the percentage of patients showing pathological (i.e., ES score = 0 or scores below the cut-off) and borderline scores (i.e., ES = 1) at neuropsychological tests, as well as the psychological and quality-of-life-related profiles emerging from the questionnaires. Correlations between cognitive and psycho-affective measures at t1 and t2 were calculated by means of Spearman's rank correlation because several cognitive measures were not normally distributed.
Moreover, we tested longitudinal changes in cognitive and psycho-affective profiles, considering the need of oxygen support in the acute stage of the disease. In more detail, scores of neuropsychological tests (i.e., corrected scores or ES) and questionnaires were taken as dependent variables of Linear Mixed Models (LMMs), with timepoint (i.e., t0, t1, and t2) and oxygen therapy (i.e., O+, O-) as factors. The choice of models with no more than two predictors was made in light of the small size of our sample. Random intercepts were allowed for subjects, who were set as cluster variable. Degrees of freedom and p-values were calculated with Satterthwaite's method. Post-hoc comparisons were corrected with Bonferroni. In case the assumption of normality of residuals was violated (i.e., visual inspection of Q-Q plots and significant Kolmogorov-Smirnov test), longitudinal comparisons were carried out by means of simplified models, i.e., Friedman's Analysis of Variance (ANOVA) with Durbin-Conover post-hoc comparisons (for measures tested at three timepoints) or Wilcoxon's rank test (for measures tested at t1 and t2).
Finally, COVID-19 patients' cognitive and emotional/quality of life scores at each timepoint were compared to those of OSAS patients by means of non-parametric Mann-Whitney tests, as deemed more appropriate for small sample sizes.
3. Results
3.1. Cross-sectional analyses for each timepoint
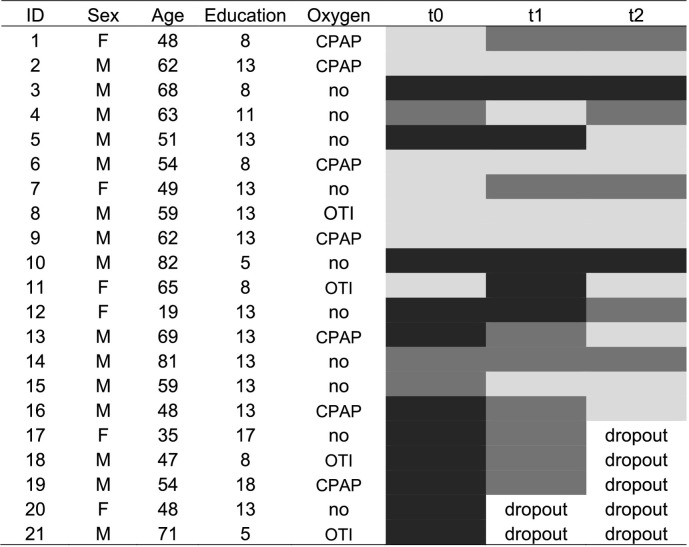
At t0, three patients reported possible cognitive decline even before COVID infection and all patients reported subjective memory complaints and reduced attentional span/impaired concentration, as compared to pre-COVID. Twelve out of 21 patients (52%) showed deficits (ES = 0 or score below the cut-off) in at least one cognitive domain: the most impaired domain was verbal memory. Specifically, long-term memory (i.e., RAVLT delayed recall) was compromised in 5 patients (24%), 4 (19%) patients showed deficits in visuo-constructive abilities (i.e., copy of the Rey-Osterrieth complex figure), followed by immediate recall – the learning phase of the RAVLT (3 patients, 14%), verbal short-term memory (3 patients), verbal working memory (2 patients, 9%), and recall of a short story (2 patients). Five patients showed cognitive impairments in multiple domains. When borderline performances were also considered (i.e., ES = 1), the number of patients showing cognitive alterations raised to 14/21 patients (67%). Individual trends are reported in Table 2 .
Table 2.
Demographics and individual cognitive performance at different timepoints.
Note: Black: impairment in at least one cognitive function, i.e., equivalent score (ES) = 0 or score below the cut-off; dark grey: ES = 1 in at least one cognitive measure; light grey: ES > 1 or score above the cut-off in all cognitive measures. CPAP: continuous positive airway pressure; OTI: orotracheal intubation.
At t1, in terms of subjective memory impairment, 6/19 (32%) patients reported a subjective recovery, whereas 6/19 experienced an improvement although not comparable to pre-COVID levels, and 7/19 (37%) referred no improvements. In terms of subjective attentional problems and cognitive fatigability, 6/19 reported no more problems, 3/19 referred some improvements, and 10/19 still suffered from reduced attentional abilities. By repeating the tests administered at t0, 3/19 patients (16%) showed pathological performance in at least one domain; however, when considering borderline performances, the number raised to 10/19 (53%). Notably, the majority of tests with borderline performance were memory tests, i.e., short-term memory (measured with the digit span) and verbal learning (measured with the RAVLT). Whereas some patients improved from pathological performance at t0, others maintained a borderline performance. It is important to note that two patients with cognitive impairments at t0 dropped out, thus leading to a possible underestimation of the actual rate. Moreover, at t1, supplementary tests were administered for a deeper characterization of attentional and executive functions. The addition of these tests highlighted a pathological slowdown of reaction times and speed of processing in two additional patients (now 5/19;26% of patients with impaired performance), as well as borderline performances in other patients, mostly in simple and go-no-go reaction times (now 13/19; 68% of patients with cognitive alterations). See also Table S1, for individual data.
Depressive symptoms (i.e., BDI scores >9) were present in 6/19 (32%) of COVID-19 patients, namely, 3/19 (16%) with mild symptoms, 2/19 (11%) with moderate symptoms, and 1/19 (5%) with severe symptoms. Moreover, 7/19 (37%) showed clinically relevant PTSD-related symptoms (IES-R > 32). The analyses of the PGWBI, revealed that all participants' psychological well-being was below the average (range: from −2.9 to −0.35), with 9/19 (47%) participants significantly below the cut-off.
Correlational analyses (see Table S2 in the Supplementary Material) showed that higher scores on the IES-R (i.e., stronger PTSD-related symptoms) were associated with a worse performance on the attentional matrices (rs = −0.555; p = 0.014) and the Weigl's test (rs = −0.627; p = 0.005). Moreover, higher psychological well-being was positively correlated to verbal learning (RAVLT immediate recall; rs = 0.493; p = 0.034), and a perceived better quality of life (WHOQOL-BREF) was correlated with better executive functions, such as logical reasoning (Raven's matrices; rs = 0.515; p = 0.024) and abstraction on the Weigl's test (rs = 0.647; p = 0.004).
At t2, all patients reported a stable pattern of subjective cognitive functioning, compared to t1. Test scores indicated that only 2/16 (12,5%) showed pathological performance in at least one cognitive domain (patient 1: delayed recall of the RAVLT; patient 2: TMT-A and Weigl sorting task). Importantly, these patients reported subjective cognitive decline even before COVID-19. However, when considering borderline performances, the number of patients with a reduced cognitive performance was 5/16 (31%), when considering baseline tests, and 7/16 (43%) when considering additional executive and attentional measures. Overall, compared to t0 and t1, all percentages decreased. The pattern of tests with borderline performance varied among patients.
As for the psycho-affective variables, depressive symptoms were present in 4/16 (25%) of COVID-19 patients - all with mild symptoms – and 3/16 (18%) showed clinically relevant PTSD-related symptoms. In terms of psychological well-being, 10/16 (62%) patients scored below the cut-off.
Correlations with cognitive tests showed a persisting negative association, although weaker than at t1, of IES-R with the Weigl's test (rs = 0.521; p = 0.039) and a positive association with execution times of the TMT-A (rs = −0.654; p = 0.008), i.e., those with higher levels of traumatic stress symptoms, were slower in the attentional task.
3.2. Longitudinal analyses
Linear Mixed Models showed different effects of timepoint and oxygen therapy for different attentional measures (see Table 3 and Table S4 in the Supplementary Material).
Table 3.
Longitudinal change in cognitive and psycho-affective measures.
| Measure | Timepoints | t0 | t1 | t2 | pa | Post-hoc |
|---|---|---|---|---|---|---|
| MMSE | t0 - t1 - t2 | 29.1 ± 1.78 | 28.6 ± 1.68 | 28.8 ± 1.74 | 0.175 | |
| Att Matr | t0 - t1 - t2 | 48.2 ± 7.84 | 51.5 ± 4.54 | 51.8 ± 5.81 | 0.120 | |
| TMT-A | t0 - t1 - t2 | 38.3 ± 22.9 | 26.8 ± 13.4 | 22.8 ± 22.0 | <0.001 | t2 < t1 < t0 |
| TMT-B | t0 - t1 - t2 | 59.9 ± 45.9 | 45.1 ± 37.1 | 41.6 ± 34.7 | 0.012 | t2 = t1 < t0 |
| TMT-BA | t0 - t1 - t2 | 28.4 ± 35.3 | 18.3 ± 29.4 | 23.9 ± 27.1 | 0.060 | |
| Span Forward | t0 - t1 - t2 | 5.48 ± 1.08 | 5.82 ± 0.92 | 5.85 ± 0.95 | 0.341 | |
| Span Backward | t0 - t1 - t2 | 4.13 ± 1.24 | 4.70 ± 1.02 | 4.40 ± 0.81 | 0.118 | |
| RAVLT Immediate | t0 - t1 - t2 | 39.2 ± 8.62 | 42.3 ± 6.99 | 47.7 ± 7.26 | 0.002 | t2 > t0 |
| RAVLT Delayed | t0 - t1 - t2 | 7.34 ± 3.51 | 8.56 ± 2.23 | 9.00 ± 2.92 | 0.144 | |
| BSRT (ES) | t0 - t1 - t2 | 2.44 ± 1.38 | 2.79 ± 1.23 | 2.94 ± 0.99 | 0.196 | |
| Figure Recall | t0 - t1 - t2 | 14.9 ± 6.55 | 20.2 ± 7.37 | 20.2 ± 5.11 | 0.005 | t2 = t1 > t0 |
| Figure Copy | t0 - t1 - t2 | 32.3 ± 3.55 | 32.2 ± 2.09 | 33.2 ± 1.84 | 0.157 | |
| Flu Phon (ES) | t0 - t1 - t2 | 3.14 ± 1.28 | 3.74 ± 0.45 | 3.56 ± 0.89 | 0.483b | |
| Flu Phon Costa (CS) | t1 - t2 | – | 41.9 ± 9.11 | 40.1 ± 10.1 | 0.429 | |
| Flu Sem (ES) | t0 - t1 - t2 | 3.33 ± 1.28 | 4.00 ± 0 | 4.00 ± 0 | 0.050b | |
| Flu Sem Costa (CS) | t1 - t2 | – | 52.8 ± 8.90 | 52.5 ± 5.74 | 0.865 | |
| Flu Alternate | t1 - t2 | – | 39.9 ± 11.2 | 43.6 ± 8.79 | 0.004 | t2 > t1 |
| Flu CSI | t1 - t2 | – | 0.82 ± 0.17 | 0.91 ± 0.17 | 0.004 | t2 > t1 |
| FAB | t0 - t1 - t2 | 16.1 ± 2.21 | 16.8 ± 1.78 | 16.6 ± 1.62 | 0.588 | |
| CDT | t1 - t2 | – | 9.42 ± 1.44 | 9.34 ± 1.57 | 0.665c | |
| Weigl Sorting Task | t1 - t2 | 12.9 ± 2.19 | 13.5 ± 2.61 | 13.5 ± 2.73 | 0.405 | |
| OORT Simple | t1 - t2 | – | 185 ± 78.3 | 168 ± 47.7 | 0.415 | |
| OORT Go-no-go | t1 - t2 | – | 364 ± 98.7 | 325 ± 74.2 | 0.015 | t2 < t1 |
| SDMT | t1 - t2 | – | 51.1 ± 11.8 | 51.9 ± 10.3 | 0.785 | |
| M5P - UDs | t1 - t2 | – | 34.9 ± 10.1 | 38.8 ± 11.6 | 0.022 | t2 > t1 |
| Raven Matrices (ES) | t0 - t1 - t2 | 3.24 ± 0.94 | 3.84 ± 0.38 | 3.75 ± 0.58 | 0.009 | t1 > t2 |
| BDI | t1 - t2 | – | 10.1 ± 8.03 | 6.56 ± 5.42 | 0.089 | |
| IES-R | t1 - t2 | – | 28.6 ± 25.9 | 15.9 ± 17.5 | 0.028c | t2 < t1 |
| PGWBI - tot - z | t1 - t2 | – | −1.62 ± 0.59 | −1.70 ± 0.55 | 0.636 | |
| PGWBI - Anxiety | t1 - t2 | – | 10.0 ± 3.71 | 8.63 ± 2.55 | 0.193 | |
| PGWBI - Depression | t1 - t2 | – | 5.32 ± 2.47 | 4.94 ± 0.93 | 0.751c | |
| PGWBI - PW | t1 - t2 | – | 9.53 ± 2.27 | 10.8 ± 3.00 | 0.186 | |
| PGWBI – Self-control | t1 - t2 | – | 9.21 ± 1.18 | 9.69 ± 0.79 | 0.174 | |
| PGWBI - Health | t1 - t2 | – | 7.63 ± 2.24 | 7.00 ± 1.93 | 0.282 | |
| PGWBI - Vitality | t1 - t2 | – | 11.6 ± 1.95 | 12.4 ± 1.55 | 0.065 | |
| QoL - tot | t1 - t2 | – | 92.5 ± 12.7 | 92.5 ± 13.8 | 0.939 | |
| QoL - Physical | t1 - t2 | – | 60.3 ± 16.8 | 69.2 ± 13.0 | 0.051 | |
| QoL - Social | t1 - t2 | – | 60.2 ± 16.3 | 63.5 ± 12.7 | 0.524 | |
| QoL - Psychological | t1 - t2 | – | 74.1 ± 12.4 | 71.9 ± 14.9 | 0.025 | t2 < t1 |
Note: athe p values refer to the main effect of “timepoint” in the Linear Mixed Models with timepoint and oxygenation as factors. For detailed results of the models, see Table S4 in the Supplementary Material. b Friedmann Analysis of Variance. c Wilcoxon Signed Rank Test.
MMSE: Mini-Mental State Examination; Att Mat: Attentional Matrices; TMT: Trail Making Test; RAVLT: Rey Auditory Verbal Learning Task; BSRT: Babcock Story Recall Test; Flu: fluency; Phon: phonological, by letter; Sem: semantic, by category; Costa: phonemic and semantic fluency according to Costa et al., 2013, administered at t1 and t2; ES: equivalent scores; CS: corrected stores; CSI: Composite Shifting Index; FAB: Frontal Assessment Battery; CDT: Clock Drawing Test; OORT: Open-access Open-source Reaction Times; SDMT: oral version of the Symbol Digit Modalities Test; M5P – UDs: unique designs of the Modified Five Point test; BDI: Beck Depression Inventory; IES-R: Impact of Event Scale Revised; PGWBI: Psychological General Well Being Index; PW: Positivity and Well-being; QoL: World Health Organization Quality of Life questionnaire.
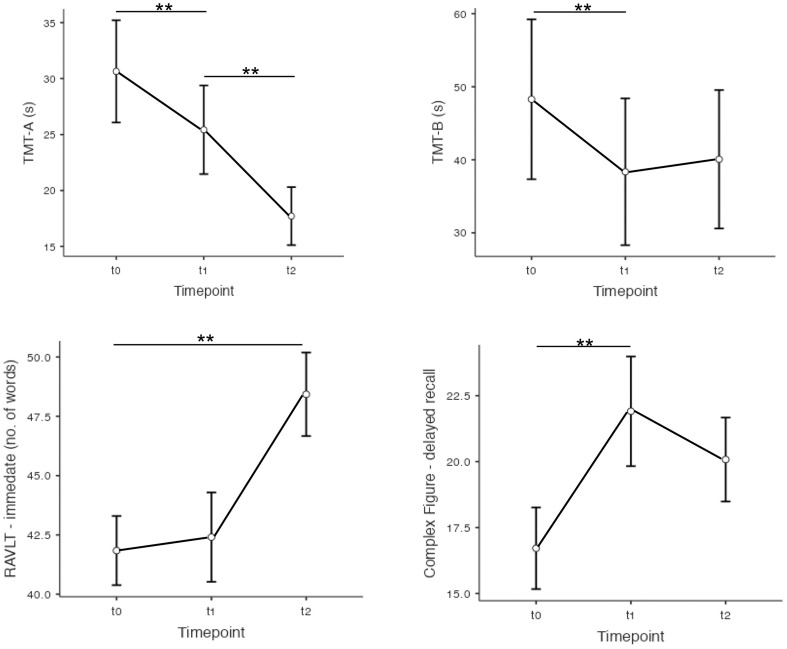
A significant reduction of execution times was observed for TMT-A (F 2,27.4 = 18.15; p < 0.001) and TMT-B (F 2,28.4 = 5.17; p = 0.012; see Fig. 2 ). Moreover, an effect of oxygen therapy was also observed (F 1,17 = 7.54; p = 0.014), with O+ performing faster (33.1 ± 10.5 s) than O- (75.1 ± 11.1 s). Accordingly, O+ showed better shifting abilities than O-, as indicated by the TMT B-A index (F 1,17.2 = 7.57; p = 0.014). An improvement of reaction times in a go-no-go context (OORT; F 1,25.7 = 7.61; p = 0.015) was observed from t1 to t2.
Fig. 2.
Longitudinal improvements in attentional and memory tasks. Relevant post-hoc comparisons are depicted. Error bars represent the standard error. TMT = Trail Making Test; s: seconds; RAVLT: immediate recall of the Rey Auditory Verbal Learning Task. **p < .008.
An improvement was also observed in verbal learning of the RAVLT (immediate recall; F 2,28.5 = 7.56) and non-verbal long-term memory, whereby the delayed recall of a complex figure improved over time (F 2,23.9 = 6.65; p = 0.005). See also Fig. 2.
As for the phonological fluency, the analyses highlighted no effect of timepoint, but an independent effect of oxygenation, namely, O+ scored better than O- (F 1,17.4 = 5.41; p = 0.032). The same pattern emerged for semantic fluency (F 1,15.8 = 8.08; p = 0.012). Interestingly, the analysis of alternate fluency, administered at t1 and t2 as supplementary test, showed an interaction of timepoint and oxygenation (F 1,12.7 = 4.96; p = 0.045), i.e., although O- patients performed overall worse (48.7 ± 1.86), than O+ (56.1 ± 1.80) they improved the most from t1 to t2 (p = 0.002).
Finally, longitudinal improvements were observed in the logical reasoning (Raven Matrices; F 2,25.7 = 5.63; p = 0.009), especially from t0 to t1(p = 0.009), and in graphic fluency of the M5P from t1 to t2 (F 1,13.1 = 12.31; p = 0.022).
Concerning, the psycho-affective scales, PTSD-related symptoms decreased from t1 to t2 (W = 77.5; p = 0.028). No changes in quality of life (WHOQOL-BREF total score) were found, with only a positive trend for the “Physical Health” (F 1;13.4 = 4.6; p = 0.051) and “Psychological” scales (F 1;14.3 = 6.31; p = 0.025), suggesting small improvements from t1 to t2.
In light of our findings showing that O+ patients performed better than O- in some cognitive tasks, we then looked for any difference in other demographic, clinical, or psychological variables. Of the 16 patients who completed all timepoints, 8 patients were O+ (6 CPAP +2 OTI) and 8 were O-. Mann-Whitney tests highlighted no significant differences with respect to age (p = 0.645), education (p = 1), days in hospital (p = 0.083), distance between hospital discharge and t0 (p = 0.636), and between t0 and t1 (p = 0.442), between t1 and t2 (p = 0.195). Furthermore, no differences were observed on the psychological and quality of life questionnaires (all ps > 0.431), besides a trend for PTSD-related symptoms (p = 0.081).
3.3. Exploratory analyses with OSAS patients
Only one OSAS patient reported attentional difficulties in everyday life, with sudden-onset sleep, and nobody showed pathological performance on the cognitive tests. Three patients obtained borderline scores on different cognitive domains (i.e., the copy of the Rey-Osterrieth's figure, non-verbal fluency, and go-no-go RTs). Two patients showed mild depressive symptoms, and three other patients showed a significant reduction of psychological well-being.
Mann-Whitney tests showed, at t0, that COVID-19 patients were significantly slower (38.3 ± 22.9 s) than OSAS (19.3 ± 6.82 s; U = 36; p = 0.035). At t1 and t2, no significant differences emerged in the cognitive tests (all ps > 0.106); the only difference was observed for the psychological domain of the WHOQOL-BREF, where, COVID-19 patients obtained higher scores at both t1 (74.1 ± 12.4; U = 22; p = 0.004) and t2 (71.9 ± 14.9; U = 28; p = 0.028). All results are reported in Table S5 of the Supplementary Material.
4. Discussion
In the present study, we provide longitudinal evidence of cognitive deficits in patients reporting subjective cognitive decline after COVID-19 infection. Overall, we observed a high prevalence of cognitive alterations around six months after the acute stage (i.e., t0) that progressively diminished more than 18 months after acute infection (i.e., t1 and t2). This pattern was also supported by longitudinal analyses showing improvements in attentional-executive and mnemonic functioning.
4.1. Cognitive functioning
Over 657 screened COVID-19 patients, around 5% of them reported subjective cognitive impairments after the infection. Another work (Hadad et al., 2022) reported slightly higher values (8%, i.e., 46 out of 523). Moreover, the prevalence of neuropsychological deficits at 6 months after acute recovery (52%) is slightly lower than previous works such as that by Ferrucci and colleagues [23] (63% at a 5-month follow-up), Miskowiak et al. [57] (59%–65% at a 4-month follow-up), and Poletti and colleagues (22) (75% at a 6-month follow-up). However, beyond methodological differences and different sample sizes, the actual estimates could be higher (see also below the reflections about borderline scores). The present study has focused the investigation on those patients reporting subjective cognitive impairments, thus possibly missing subtler cognitive alterations even in patients without subjective complaints. In this regard, it is worth mentioning that some patients may present anosognosia for their cognitive deficits [21] (however, see [24] for opposite findings). Moreover, only 21 out of 36 patients with subjective cognitive impairments decided to take part in the study.
Notably, the most impaired function resulted to be verbal learning, a finding in line with several neuropsychological works which found verbal memory as one of the most affected functions in the first six months following acute recovery [9,14,20,23].
Around 16 and 22 months after the acute disease (i.e., t1 and t2), by just repeating the tests administered at the first assessment, a much lower number of patients obtained pathological scores with an increased percentage of borderline ones, possibly reflecting a gradual improvement (see also the discussion of the longitudinal analyses). However, the addition of RTs test indicated a significant reduction in alertness in several patients. In our opinion, this highlights two crucial aspects: 1) the importance of computerized testing of attention as more sensitive measures than classic paper-and-pencil tests, and 2) the importance of considering both pathological and borderline scores (see also the work by Voruz and colleagues [21]). Although borderline scores could sometimes reflect situational variables (e.g., anxiety), we cannot fully exclude that they could represent red flags of persisting problems or even predictors of cognitive decline in the long run. Therefore, we believe they should not be underestimated.
Along the same line of reasoning, it is important to note that the only patients with persisting pathological scores more than 18 months after hospital discharge (i.e., at t2) were those who reported a subjective cognitive decline even before the pandemic. Although the lack of pre-COVID assessment does not allow us to appreciate the actual impact of the acute disease, we cannot exclude a link between SARS-COV-2 infection and cognitive decline [5]. Notably, studies on different viral infections, such as herpes viruses [58,59] and Spanish influenza [60], raised the possibility of increased risk of neurodegeneration following infections, likely mediated by hyperinflammation states. Along the same line, a systemic, pro-inflammatory cytokine storm has been hypothesized to underlie COVID-related neurological involvement [61]. It follows the need for future research to evaluate the trajectories of cognitive decline in COVID-19 survivors, in particular to longitudinally monitor individuals at risk of dementia. This line of research will benefit from the increasing availability of tele-neuropsychological tools [[62], [63], [64], [65]].
Moving beyond the discussion of pathological findings, longitudinal analyses demonstrated significant improvements from t0 in multiple cognitive domains. Ferrucci et al. [23], by testing their patients at 5 months and 1 year (roughly comparable to our t0 and t1), observed significant improvements in the verbal memory domain (i.e., in all measures of the Selective Reminding Test of the Brief Repeatable Battery of Neuropsychological tests). Interestingly, although we also found changes in the same cognitive domain, here, the greatest improvement emerged 20 months after acute recovery (i.e., at t2), especially in the immediate recall of the RAVLT - the encoding phase - with no effects on the delayed recall (i.e., the proper active retrieval from long-term storage). Furthermore, we observed an improvement in attentional-executive tests, as highlighted, for example, by the improvements on the TMT. Ferrucci et al. [23] came to similar conclusions by administering different tests, i.e., the SDMT and the complex Paced Auditory Serial Addition Test. Importantly, we showed that attentional-executive functions could keep improving after one year from the acute disease. Although methodological differences prevent direct comparison of the results, the observed trends point towards the same direction. Future works should ideally adopt a shared set of second-level cognitive tests to improve the comparability and favor aggregated meta-analysis, thus reducing the methodological gaps highlighted by recent reviews [14].
The present work also aimed at testing the effects of oxygen therapy. In light of recent works showing a negative impact of hypoxia in the acute stage on the following cognitive sequelae [20,23], we expected to find lower cognitive performances in patients requiring oxygen support. However, this was not the case for a number of attentional and executive tests. Other works have shown an unclear relationship between the incidence of cognitive deficits and the severity of acute respiratory deficits [[16], [66], [67]]. Manera and colleagues [68] found that patients admitted to intensive care because of respiratory distress symptoms performed better on the MMSE than those requiring non-invasive ventilation, suggesting that the former may have suffered less from cerebral hypoxia. However, the lack of objective oxygenation measure, relatively small sample sizes [21] and age effects [16] may have affected, at least in part, previous as well as the current findings.
Finally, to explore cognitive alterations due to low oxygenation levels, but in the absence of SARS-COV-2 infection, we compared COVID-19 and OSAS patients' cognitive-emotional profiles. Indeed, OSAS patients often report cognitive alterations in their everyday life (i.e., reduced attention, concentration and short-term memory) [69] that can be objectified through a neuropsychological assessment (see the meta-analysis by Stranks & Crowe [70]), often resulting in a reduced quality of life [71]. Here, we observed no cognitive impairments (besides few borderline scores) and a slower attentional performance (TMT-A execution times) of COVID-19 patients at t0 (i.e., the timepoint with the higher rate of cognitive alterations). We believe that longer-term effects of these pathologies (especially in older adults) are worth further investigation, considering the increased risk of cognitive decline related to impaired respiratory functions, common to both COVID-19 and OSAS.
4.2. Psycho-affective variables
Concerning the results on psycho-affective scales, we extend previous evidence on psychiatric sequelae six months after the acute stage [5]: more than 30% of our patients presented a certain degree of depressive symptoms and PTSD-related symptoms more than one year after acute recovery, with rates and severity decreasing over time. Notably, the correlations between psycho-affective and cognitive measures are in line with the recent findings of Voruz and colleagues [21], who recently confirmed the profound impact of psycho- affective variables on a wide spectrum of neuropsychological tests. Taken together, these results suggest that changes in cognitive functioning in COVID-19 patients cannot be fully understood without considering psychological aspects [22,57].
Finally, some limitations deserve consideration. The main limit of the present study is the small sample size that prevents from drawing definite conclusions about, e.g., the effects of COVID hypoxia on cognition, as well as differential cognitive profiles of COVID-19 and OSAS patients. Another limit regards the repeated administration of the same neuropsychological tests, potentially leading to practice effects and hampering the interpretation of repeated measurements. Here we used parallel versions whenever valid and normed forms were available (i.e., for the copy and recall of a complex figure and the RAVLT); however, we cannot fully exclude that test-retest effects may have partially influenced the previously discussed improvements.
In conclusion, our work confirms and extends previous literature, showing that cognitive alterations in COVID-19 patients may extend over one year from the resolution of acute respiratory problems but nonetheless face a significant recovery in several cases. Importantly: 1) such cognitive alterations are accompanied - and possibly affected - by significant and more prolonged psychological-emotional distress; 2) several patients may present with borderline scores in memory and executive tests that are worthy of attention; 3) neuropsychological monitoring is especially advised for patients with suspected or initial cognitive decline at higher risk of developing dementia.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgement
This work was funded by an unconditional donation from Novatex Italia S.p.A and partially supported by the Italian Ministry of Health to Nadia Bolognini.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jns.2022.120511.
Appendix A. Supplementary data
Supplementary Tables
References
- 1.Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P.…Smith S.M. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J.…Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8) doi: 10.1212/WNL.0000000000009937. e1060–e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L., Wang M., Chen S., He Q., Chang J., Hong C.…Hu B. 2020. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: A Retrospective Case Series Study. MedRxiv. [Google Scholar]
- 4.Moro E., Priori A., Beghi E., Helbok R., Campiglio L., Bassetti C.L.…EAN core COVID-19 Task Force The international European academy of neurology survey on neurological symptoms in patients with COVID-19 infection. Eur. J. Neurol. 2020;27(9):1727–1737. doi: 10.1111/ene.14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dini M., Poletti B., Tagini S., Reitano M.R., Allocco E., Mazzocco K.…Ferrucci R. Resilience, psychological well-being and daily functioning following hospitalization for respiratory distress due to sars-cov-2 infection. Healthcare. 2021, September;9(9):1161. doi: 10.3390/healthcare9091161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceban F., Ling S., Lui L.M., Lee Y., Gill H., Teopiz K.M.…McIntyre R.S. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crivelli L., Calandri I., Corvalán N., Carello M.A., Keller G., Martínez C.…Allegri R. Cognitive consequences of COVID-19: results of a cohort study from South America. Arq. Neuropsiquiatr. 2021;80:240–247. doi: 10.1590/0004-282X-ANP-2021-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambati L., Mattarozzi K., Mascia L., Tonetti T., Santoro R., Cretella L.…Guarino M. Cognitive and affective disorders in critical SARS-CoV-2 patients and caregivers. J. Neurol. Sci. 2021;429 [Google Scholar]
- 10.Heneka M.T., Golenbock D., Latz E., Morgan D., Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res. Ther. 2020;12(1):1–3. doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guedj E., Campion J.Y., Dudouet P., Kaphan E., Bregeon F., Tissot-Dupont H.…Eldin C. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging. 2021;48(9):2823–2833. doi: 10.1007/s00259-021-05215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellgren L., Thornberg U.B., Samuelsson K., Levi R., Divanoglou A., Blystad I. Brain MRI and neuropsychological findings at long-term follow-up after COVID-19 hospitalisation: an observational cohort study. BMJ Open. 2021;11(10) doi: 10.1136/bmjopen-2021-055164. e055164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y., Yuan K., Wang Z., Liu W.J., Lu Z.A., Liu L.…Lu L. Neuropsychiatric manifestations of COVID-19, potential neurotropic mechanisms, and therapeutic interventions. Transl. Psychiatry. 2021;11(1):1–20. doi: 10.1038/s41398-021-01629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crivelli L., Palmer K., Calandri I., Guekht A., Beghi E., Carroll W.…Kivipelto M. Alzheimer’s & Dementia; 2022. Changes in Cognitive Functioning after COVID-19: A Systematic Review and Meta-Analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daroische R., Hemminghyth M.S., Eilertsen T.H., Breitve M.H., Chwiszczuk L.J. Cognitive impairment after COVID-19—a review on objective test data. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.699582. 699582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alemanno F., Houdayer E., Parma A., Spina A., Del Forno A., Scatolini A.…Iannaccone S. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246590. e0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav. Immun. Health. 2020;9 doi: 10.1016/j.bbih.2020.100163. 100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaud V., Crottaz-Herbette S., Dunet V., Vaucher J., Bernard-Valnet R., Du Pasquier R.…Clarke S. Pattern of cognitive deficits in severe COVID-19. J. Neurol. Neurosurg. Psychiatry. 2021;92(5):567–568. doi: 10.1136/jnnp-2020-325173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Lorenzo R., Conte C., Lanzani C., Benedetti F., Roveri L., Mazza M.G.…Rovere-Querini P. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0239570. e0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrucci R., Dini M., Groppo E., Rosci C., Reitano M.R., Bai F.…Priori A. Long-lasting cognitive abnormalities after COVID-19. Brain Sci. 2021;11(2):235. doi: 10.3390/brainsci11020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voruz P., Allali G., Benzakour L., Nuber-Champier A., Thomasson M., Jacot de Alcântara I.…Péron J.A. Long COVID neuropsychological deficits after severe, moderate, or mild infection. Clin. Transl. Neurosci. 2022;6(2):9. [Google Scholar]
- 22.Poletti S., Palladini M., Mazza M.G., De Lorenzo R., Furlan R., Ciceri F.…Benedetti F. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: role of depression and impact on quality of life. Eur. Arch. Psychiatry Clin. Neurosci. 2022;272(5):773–782. doi: 10.1007/s00406-021-01346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrucci R., Dini M., Rosci C., Capozza A., Groppo E., Reitano M.R.…Priori A. One-year cognitive follow-up of COVID-19 hospitalized patients. Eur. J. Neurol. 2022;29(7):2006–2014. doi: 10.1111/ene.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miskowiak K.W., Fugledalen L., Jespersen A.E., Sattler S.M., Podlekareva D., Rungby J.…Johnsen S. Trajectory of cognitive impairments over 1 year after COVID-19 hospitalisation: pattern, severity, and functional implications. Eur. Neuropsychopharmacol. 2022;59:82–92. doi: 10.1016/j.euroneuro.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Brutto O.H., Wu S., Mera R.M., Costa A.F., Recalde B.Y., Issa N.P. Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: a longitudinal prospective study nested to a population cohort. Eur. J. Neurol. 2021;28(10):3245–3253. doi: 10.1111/ene.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Méndez R., Balanzá-Martínez V., Luperdi S.C., Estrada I., Latorre A., González-Jiménez P.…Menéndez R. Long-term neuropsychiatric outcomes in COVID-19 survivors: a 1-year longitudinal study. J. Intern. Med. 2022;291(2):247–251. doi: 10.1111/joim.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magni E., Binetti G., Bianchetti A., Rozzini R., Trabucchi M. Mini-mental state examination: a normative study in Italian elderly population. Eur. J. Neurol. 1996;3(3):198–202. doi: 10.1111/j.1468-1331.1996.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 28.Measso G., Cavarzeran F., Zappalà G., Lebowitz B.D., Crook T.H., Pirozzolo F.J.…Grigoletto F. The mini-mental state examination: normative study of an Italian random sample. Dev. Neuropsychol. 1993;9(2):77–85. [Google Scholar]
- 29.Spinnler H., Tognoni G. Standardizzazione e taratura italiana di test neuropsicologici. Ital. J. Neurol. Sci. 1987;8(Suppl):1–120. [PubMed] [Google Scholar]
- 30.Giovagnoli A.R., Del Pesce M., Mascheroni S., Simoncelli M., Laiacona M., Capitani E. Trail making test: normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 1996;17(4):305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- 31.Monaco M., Costa A., Caltagirone C., Carlesimo G.A. Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol. Sci. 2013;34(5):749–754. doi: 10.1007/s10072-012-1130-x. [DOI] [PubMed] [Google Scholar]
- 32.Carlesimo G.A., Caltagirone C., Gainotti G.U.I.D., Fadda L., Gallassi R., Lorusso S.…Parnetti L. The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur. Neurol. 1996;36(6):378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- 33.Capitani E., Della Sala S., Laiacona M., Marchetti C. Giunti Organizzazioni Speciali; 1994. Standardizzazione ed uso clinico di un test di memoria di prosa. [Google Scholar]
- 34.Caffarra P., Vezzadini G., Dieci F., Zonato F., Venneri A. Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol. Sci. 2002;22(6):443–447. doi: 10.1007/s100720200003. [DOI] [PubMed] [Google Scholar]
- 35.Aiello E.N., Esposito A., Gramegna C., Gazzaniga V., Zago S., Difonzo T.…Bolognini N. The frontal assessment battery (FAB) and its sub-scales: validation and updated normative data in an Italian population sample. Neurol. Sci. 2022;43(2):979–984. doi: 10.1007/s10072-021-05392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appollonio I., Leone M., Isella V., Piamarta F., Consoli T., Villa M.L.…Nichelli P. The frontal assessment battery (FAB): normative values in an Italian population sample. Neurol. Sci. 2005;26(2):108–116. doi: 10.1007/s10072-005-0443-4. [DOI] [PubMed] [Google Scholar]
- 37.Novelli G., Papagno C., Capitani E., Laiacona M. Archivio di Psicologia, Neurologia e Psichiatria. 1986. Tre test clinici di ricerca e produzione lessicale. Taratura su sogetti normali. [Google Scholar]
- 38.Costa A., Bagoj E., Monaco M., Zabberoni S., De Rosa S., Papantonio A.M.…Carlesimo G.A. Standardization and normative data obtained in the Italian population for a new verbal fluency instrument, the phonemic/semantic alternate fluency test. Neurol. Sci. 2014;35(3):365–372. doi: 10.1007/s10072-013-1520-8. [DOI] [PubMed] [Google Scholar]
- 39.Laiacona M., Inzaghi M.G., De Tanti A., Capitani E. Wisconsin card sorting test: a new global score, with Italian norms, and its relationship with the Weigl sorting test. Neurol. Sci. 2000;21(5):279–291. doi: 10.1007/s100720070065. [DOI] [PubMed] [Google Scholar]
- 40.Basso A., Capitani E., Laiacona M. Raven’s coloured progressive matrices: normative values on 305 adult normal controls. Funct. Neurol. 1987;2(2):189–194. [PubMed] [Google Scholar]
- 41.Rigoli M., Facchin A., Cardile D., Beschin N., Luzzatti C. Open-source open-access reaction time test (OORTT): an easy tool to assess reaction times. Neurol. Sci. 2021;42(6):2461–2469. doi: 10.1007/s10072-020-04839-y. [DOI] [PubMed] [Google Scholar]
- 42.Nocentini U., Giordano A., Vincenzo S.D., Panella M., Pasqualetti P. The Symbol Digit Modalities Test--Oral version: Italian normative data. Funct. Neurol. 2006;21(2):93–96. [PubMed] [Google Scholar]
- 43.Mondini S., Mapelli D., Vestri A., Bisiacchi P. 2003. ENB esame neuropsicologico breve. Raffaello Cortina, MI. [Google Scholar]
- 44.Battista P., Griseta C., Tortelli R., Guida P., Castellana F., Rivolta D., Logroscino G. The modified five-point test (MFPT): normative data for a sample of Italian elderly. Neurol. Sci. 2021;42(6):2431–2440. doi: 10.1007/s10072-020-04818-3. [DOI] [PubMed] [Google Scholar]
- 45.Cattelani R., Dal Sasso F., Corsini D., Posteraro L. The modified five-point test: normative data for a sample of Italian healthy adults aged 16–60. Neurol. Sci. 2011;32(4):595–601. doi: 10.1007/s10072-011-0489-4. [DOI] [PubMed] [Google Scholar]
- 46.Casarotti A., Papagno C., Zarino B. Modified Taylor complex figure: normative data from 290 adults. J. Neuropsychol. 2014;8(2):186–198. doi: 10.1111/jnp.12019. [DOI] [PubMed] [Google Scholar]
- 47.Capitani E., Laiacona M. Composite neuropsychological batteries and demographic correction: standardization based on equivalent scores, with a review of published data. J. Clin. Exp. Neuropsychol. 1997;19(6):795–809. doi: 10.1080/01688639708403761. [DOI] [PubMed] [Google Scholar]
- 48.Capitani E., Laiacona M. Outer and inner tolerance limits: their usefulness for the construction of norms and the standardization of neuropsychological tests. Clin. Neuropsychol. 2017;31(6–7):1219–1230. doi: 10.1080/13854046.2017.1334830. [DOI] [PubMed] [Google Scholar]
- 49.Beck A.T., Ward C., Mendelson M., Mock J., Erbaugh J.J.A.G.P. Beck depression inventory (BDI) Arch. Gen. Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 50.Beck A.T., Steer R.A., Carbin M.G. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8(1):77–100. [Google Scholar]
- 51.Weiss D., Marmar C. In: Assessing Psychological Trauma and PTSD: A Handbook for Practitioners. Wilson J., Keane T., editors. 1997. The impact of event scale—revised. W. [Google Scholar]
- 52.Creamer M., Bell R., Failla S. Psychometric properties of the impact of event scale—revised. Behav. Res. Ther. 2003;41(12):1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Dupuy H. 1984. The Psychological General Well-Being (PGWB) Index. (Assessment of quality of life in clinical trials of cardiovascular therapies) [Google Scholar]
- 54.Grossi E., Mosconi P., Groth N., Niero M., Apolone G. 2002. Questionario Psychological General Well-Being Index. Versione Italiana. [Google Scholar]
- 55.Whoqol Group Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol. Med. 1998;28(3):551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 56.The jamovi project jamovi. 2021. https://www.jamovi.org Retrieved from:
- 57.Miskowiak K.W., Johnsen S., Sattler S.M., Nielsen S., Kunalan K., Rungby J.…Porsberg C.M. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mancuso R., Sicurella M., Agostini S., Marconi P., Clerici M. Herpes simplex virus type 1 and Alzheimer’s disease: link and potential impact on treatment. Expert Rev. Anti-Infect. Ther. 2019;17(9):715–731. doi: 10.1080/14787210.2019.1656064. [DOI] [PubMed] [Google Scholar]
- 59.Warren-Gash C., Forbes H.J., Williamson E., Breuer J., Hayward A.C., Mavrodaris A.…Smeeth L. Human herpesvirus infections and dementia or mild cognitive impairment: a systematic review and meta-analysis. Sci. Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-41218-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCall S., Vilensky J.A., Gilman S., Taubenberger J.K. The relationship between encephalitis lethargica and influenza: a critical analysis. J. Neuro-Oncol. 2008;14(3):177–185. doi: 10.1080/13550280801995445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Virgiliis F., Di Giovanni S. Lung innervation in the eye of a cytokine storm: neuroimmune interactions and COVID-19. Nat. Rev. Neurol. 2020;16(11):645–652. doi: 10.1038/s41582-020-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aiello E.N., Esposito A., Giannone I., Diana L., Appollonio I., Bolognini N. Telephone interview for cognitive status (TICS): Italian adaptation, psychometrics and diagnostics. Neurol. Sci. 2022;43(5):3071–3077. doi: 10.1007/s10072-021-05729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aiello E.N., Esposito A., Giannone I., Diana L., Woolley S., Murphy J.…Appollonio I. ALS cognitive behavioral screen-phone version (ALS-CBSTM-PhV): norms, psychometrics, and diagnostics in an Italian population sample. Neurol. Sci. 2022;43(4):2571–2578. doi: 10.1007/s10072-021-05636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aiello E.N., Preti A.N., Pucci V., Diana L., Corvaglia A., di San Pietro C.B.…Bolognini N. The Italian telephone-based verbal fluency battery (t-VFB): standardization and preliminary clinical usability evidence. Front. Psychol. 2022;13 doi: 10.3389/fpsyg.2022.963164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aiello E.N., Pucci V., Diana L., Niang A., Preti A.N., Delli Ponti A.…Bolognini N. Telephone-based frontal assessment battery (t-FAB): standardization for the Italian population and clinical usability in neurological diseases. Aging Clin. Exp. Res. 2022;34(7):1635–1644. doi: 10.1007/s40520-022-02155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Priftis K., Velardo V., Vascello M.G.F., Villella S., Galeri S., Spada M.S., Algeri L. Limited evidence for neuropsychological dysfunction in patients initially affected by severe COVID-19. Neurol. Sci. 2022:1–3. doi: 10.1007/s10072-022-06373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadad R., Khoury J., Stanger C., Fisher T., Schneer S., Ben-Hayun R.…Adir Y. Cognitive dysfunction following COVID-19 infection. J. NeuroVirol. 2022:1–8. doi: 10.1007/s13365-022-01079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manera M.R., Fiabane E., Pain D., Aiello E.N., Radici A., Ottonello M.…Pistarini C. Clinical features and cognitive sequelae in COVID-19: a retrospective study on N= 152 patients. Neurol. Sci. 2022;43(1):45–50. doi: 10.1007/s10072-021-05744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kapur V.K., Auckley D.H., Chowdhuri S., Kuhlmann D.C., Mehra R., Ramar K., Harrod C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stranks E.K., Crowe S.F. The cognitive effects of obstructive sleep apnea: an updated meta-analysis. Arch. Clin. Neuropsychol. 2016;31(2):186–193. doi: 10.1093/arclin/acv087. [DOI] [PubMed] [Google Scholar]
- 71.Scarpina F., Bastoni I., Cappelli S., Priano L., Giacomotti E., Castelnuovo G.…Mauro A. Psychological well-being in obstructive sleep apnea syndrome associated with obesity: the relationship with personality, cognitive functioning, and subjective and objective sleep quality. Front. Psychol. 2021;12 doi: 10.3389/fpsyg.2021.588767. 588767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables