Abstract
Objectives
To explore mortality and causes of death among Norwegian patients with RA, PsA and axial spondyloarthritis (axSpA) compared with the general population by conducting a nationwide registry-based cohort study.
Methods
Patients with RA, PsA and axSpA were identified from the Norwegian Patient Registry based on ICD-10 codes between 2008 and 2017. Using age as the time variable, all-cause and cause-specific mortality were estimated between 2010 and 2017 with the Kaplan–Meier estimator and the cumulative incidence competing risk method, respectively. Sex-, education level-, health region- and age group-adjusted hazard ratios (HRs) for mortality were estimated using Cox regression models.
Results
We identified 36 095 RA, 18 700 PsA and 16 524 axSpA patients (70%, 53% and 45% women, respectively). RA and axSpA were associated with increased all-cause mortality (HR 1.45 [95% CI: 1.41, 1.48] and HR 1.38 [95% CI: 1.28, 1.38], respectively). Women but not men with PsA had a slightly increased mortality rate (HR 1.10 [95% CI: 1.00, 1.21] among women and 1.02 [95% CI: 0.93, 1.11] among men). For all patient groups as well as for the general population, the three leading causes of death were cardiovascular diseases, neoplasms and respiratory diseases. RA patients had increased mortality from all of these causes, while axSpA patients had increased mortality from cardiovascular and respiratory diseases.
Conclusion
Even in the era of modern treatments for IJDs, patients with RA and axSpA still have shortened life expectancy. Our findings warrant further attention to the prevention and management of comorbidities.
Keywords: inflammatory joint diseases, cardiovascular disease, mortality, epidemiology
Rheumatology key messages.
Mortality was increased among Norwegian RA and axSpA patients compared with the general population
RA and axSpA patients had excess risk of death from cardiovascular and respiratory diseases
Women but not men with PsA had slightly increased all-cause and cardiovascular mortality rates
Introduction
RA, PsA and axial spondyloarthritis (axSpA) affect ∼1.5% of the adult population [1], and may lead to joint destruction, disability, comorbidities such as cardiovascular disease (CVD), and even a reduced life span. The risk of mortality is increased in RA by a factor of 1.5 based on a meta-analysis of observational studies [2]. Previous reports examining the underlying causes of the excess mortality suggest increased risk from cardiovascular, infectious, gastrointestinal, renal and respiratory diseases [3]. Among PsA patients, mortality may be slightly higher than in the general population and be related to excess CVD, although the results are conflicting [4, 5]. Most reports on mortality in axSpA have evaluated patients with AS; these studies showed that the mortality and cardiovascular mortality of these patients is increased by a factor of ∼1.5 compared with the general population [6, 7]. Studies exploring mortality in axSpA populations that are not restricted to AS but include patients with non-radiographic axSpA are few, representing a gap in the current knowledge. Moreover, studies investigating mortality in RA, PsA and axSpA in the same population are lacking.
In RA, PsA and axSpA, excess mortality has been linked to high disease activity [4, 8, 9]. The early diagnostics and treatment of RA, PsA and axSpA have improved markedly during the past decades with improved imaging techniques, more sensitive classification criteria, and the implementation of early and active treatment strategies. The introduction and increasing use of biologic DMARDs (bDMARDs) and targeted synthetic DMARDs since the late 1990s and 2000s has improved outcomes of inflammatory joint diseases (IJDs) even further. However, it remains unclear whether these advances in anti-rheumatic treatment have translated into normalization of IJD patients’ life expectancy.
Our aim was to explore the mortality rates and causes of death among Norwegian RA, PsA and axSpA patients as well as the general population in the 2010s, when the use of bDMARDs has been more frequent, based on data from nationwide registries.
Methods
Registry data
The Norwegian Cardio-Rheuma Register is a nationwide register-linkage study comprising pseudonymized data on the total Norwegian population ≥18 years between 2008 and 2017 from multiple nationwide registries, including the Norwegian Patient Registry (NPR), the Norwegian Prescription Database (NorPD), the Norwegian Causes of Death Registry and the Norwegian National Population Register. Statistics Norway provided annual, individual-level data on highest education level.
Since 2008, the NPR contains individual-level data on episodes in specialized healthcare in Norway, including all public specialist healthcare services as well as private institutions and medical specialists contracted to the regional health authorities [10]. Data from primary healthcare is not included in NPR. For all inpatient, day and outpatient consultations, NPR includes up to 20 diagnostic codes per episode according to the ICD-10 classification.
The Norwegian Cause of Death Registry provided information on dates and underlying causes of death, reported by the EU Shortlist for Causes of death.
Study population
The study population included the total Norwegian adult population (≥18 years) between 1 January 2010 and 31 December 2017. The National Population Register provided information on date of birth, death and immigration/emigration as well as on sex and county and health region of residence (detailed information on health regions are provided in Supplementary Table S1, available at Rheumatology online). A person entered the study population if they immigrated to Norway (i.e. were newly registered in the National Population Register) or turned 18 years between 2010 and 2017.
Identification of patients with IJDs
Patients with RA, PsA and axSpA were identified from the NPR based on ICD-10 codes (M05–M06 for RA; M07.0–M07.3 and L40.5 for PsA; and M45, M46.0, M46.1, 46.8 and M46.9 for axSpA). To be classified as having RA, PsA or axSpA in this study, a patient had to have both of the following: (i) a first episode listing an RA, PsA or axSpA diagnostic code as either the main or a contributory diagnosis (index date); and (ii) a second, confirmatory episode listing an RA, PsA or axSpA diagnostic code as either the main or a contributory diagnosis within a 2-year period following the index date.
Follow-up and outcomes
The study period began on 1 January 2010. NPR data from the years 2008 and 2009 served to identify patients with an existing IJD at the start of the follow-up period. We identified and included both patients with an existing IJD at the start of follow-up period and patients who had their first record of IJD diagnosis during the follow-up period (between 1 January 2010 and 31 December 2017) (Supplementary Fig. S1, available at Rheumatology online). Patients who had their first episode listing a diagnostic code for IJD after 1 January 2010 and who fulfilled the case definition for RA, PsA or axSpA were included in the respective IJD cohort after that date. Follow-up ended at death, emigration or 31 December 2017.
The primary outcome of the study was all-cause mortality, and secondary outcomes included cause-specific mortality for the three most common causes of death (CVD, malignancies and respiratory diseases). Less common causes of death were avoided as including them would have introduced a larger survival bias for the risk group.
Other definitions
Seropositivity in RA was defined by ICD-10 codes: M05 for seropositive and M06 for seronegative RA. In cases where both M05 and M06 were present, RA cases were defined as seropositive if the number of episodes with a recorded M05 code was equal to or greater than the number with an M06 code.
Using the ICD-10 codes from the NPR, we identified comorbidities among the IJD patients (Supplementary Data S1, available at Rheumatology online). We were not able to obtain data on disease activity, smoking and/or obesity.
Patients’ medications between 2008 and 2017 were identified from the NorPD (dispensed prescriptions from pharmacies) and from the NPR (hospital-administered bDMARDs based on the Norwegian classification of medical procedures) (Supplementary Data S1, available at Rheumatology online). For glucocorticoids, only oral forms were included.
Statistical analysis
Mortality rates were analysed for RA, PsA and axSpA patients and the Norwegian population ≥18 years from January 2010 until December 2017. The analysis was performed using the survival package in R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Overall survival was estimated using the Kaplan–Meier estimator. The analysis was adjusted for age as a continuous variable through using it as the time scale. In this approach we assume that patients of the same age face the same health risks regardless of the year of diagnosis. This assumption might be violated in a rapidly developing medical field. Individuals were left-censored before their age of entry and right-censored after their age of exit. Cause-specific mortality rates for the three most common causes of death were estimated with the cumulative incidence competing risks method [11].
Individuals older than 95 on 1 January, 2010 were removed from the dataset. All-cause and cause-specific hazard ratios (HRs) for deaths from CVD, respiratory diseases and malignancies were estimated using the Cox proportional hazards regression model, with comparison to the total Norwegian adult population, using age as the time scale. All HRs were further adjusted by stratification for sex, education level, health region and age group (three groups based on their age on 1 January, 2010 [<55, 55–74, 75–95]). Combining the adjustment variables, there were a total of 72 strata. Health region was missing for 1013 (0.02%) individuals, and was imputed by its most common value stratified on sex and work status. Education level was missing for 201 661 (4.45%) individuals, and was imputed by its most common value stratified on sex, work status and county of residence. A sensitivity analysis performed by imputing the missing data with chained equations [12] yielded very similar results (data not shown). Strata-specific HRs for cause-specific and all-cause mortality were also estimated for age and sex groups, as well as groups of ever vs never use of bDMARDs (≥1 dispensed prescription or infusion in hospital) and/or oral glucocorticoids (≥1 dispensed prescription).
Ethics approval
The study was approved by the Norwegian General Data Protection Regulation (16/00482-11/CDG), the South East Health Authority Ethical Committee (2016/588) and the Data Protection Officers at Oslo University Hospital (2016/924) and Diakonhjemmet Hospital (7/12-2019). The Norwegian Cardio-Rheuma Register comprises routinely recorded administrative data, and no written consent from study subjects was required.
Results
We identified 36 095 RA patients (24 276 [67%] seropositive), 18 700 PsA patients and 16 524 axSpA patients. The patients’ baseline characteristics and medications are shown in Table 1.
Table 1.
Cohort characteristics
| Characteristic | RA (n = 36 095) | PsA (n = 18 700) | axSpA (n = 16 524) |
|---|---|---|---|
| Women, n (%) | 25 174 (69.7) | 9991 (53.4) | 7441 (45.0) |
| Age, mean (s.d.), years | 59.08 (15.79) | 49.98 (13.96) | 43.85 (15.26) |
| Education level, n (%) | |||
| Below upper secondary | 11 447 (31.7) | 5097 (27.3) | 3996 (24.2) |
| Upper secondary or post-secondary non-tertiary | 17 068 (47.2) | 9201 (49.2) | 7704 (46.6) |
| Higher | 7580 (21.0) | 4402 (23.5) | 4825 (29.2) |
| Baseline comorbidities, n (%) | |||
| Hypertension or antihypertensive medication | 15 843 (43.9) | 6613 (35.4) | 4214 (25.5) |
| Diabetes or antidiabetic medication | 3081 (8.5) | 1560 (8.3) | 748 (4.5) |
| Coronary disease | 2050 (5.7) | 719 (3.8) | 539 (3.3) |
| Heart failure | 654 (1.8) | 106 (0.6) | 150 (0.9) |
| Atrial fibrillation | 780 (2.2) | 227 (1.2) | 197 (1.2) |
| Stroke | 1036 (2.9) | 298 (1.6) | 234 (1.4) |
| Malignancy | 2570 (7.1) | 713 (3.8) | 607 (3.7) |
| Chronic kidney disease | 405 (1.1) | 115 (0.6) | 150 (0.9) |
| Chronic respiratory disease | 2517 (7.0) | 841 (4.5) | 654 (4.0) |
| Use of DMARDs during follow-up, n (%) | |||
| Any DMARD | 28 792 (79.8) | 13 686 (73.2) | 8716 (52.7) |
| Any conventional DMARD | 27 266 (75.5) | 12 494 (66.8) | 3768 (22.8) |
| Any biologic DMARD | 11 362 (31.5) | 5782 (30.9) | 7337 (44.4) |
| Methotrexate | 24 237 (67.1) | 11 216 (60.0) | 2461 (14.9) |
| Oral glucocorticoids | 25 466 (70.6) | 8510 (45.5) | 5624 (34.0) |
Age, sex, education level, comorbidities and use of DMARDs among the RA, PsA and axSpA cohorts. axSpA: axial spondyloarthritis.
All-cause mortality
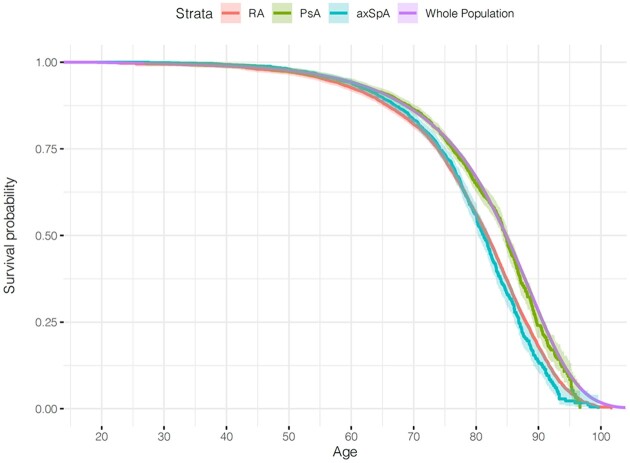
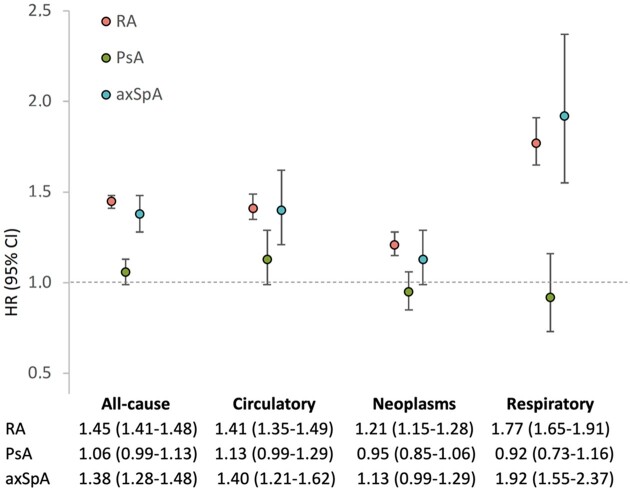
A total of 5657 deaths was recorded among patients with RA during 205 787 person-years, 911 deaths among patients with PsA during 107 445 person-years, 732 deaths among patients with axSpA during 90 331 person-years and 310 699 deaths in the general population during 31 228 501 person-years. Compared with the general population, RA and axSpA patients, but not PsA patients, had decreased survival (Fig. 1). Sex-, education level-, health region- and age group-adjusted HR for all-cause mortality in RA was 1.45 (95% CI: 1.41, 1.48), in PsA 1.06 (95% CI: 0.99, 1.13) and in axSpA 1.38 (95% CI: 1.28, 1.48). Excess mortality was more pronounced among seropositive (adjusted HR 1.52, 95% CI: 1.47, 1.57) compared with seronegative RA patients (HR 1.30, 95% CI: 1.24, 1.36).
Fig. 1.
Kaplan–Meier survival curves for overall survival
Kaplan–Meier survival curves showing overall survival rate among RA, PsA and axSpA cohorts as well as the general population, with age at inclusion to the study (in years) as the time scale. axSpA: axial spondyloarthritis.
Cause-specific mortality
Table 2 presents data on the frequency of the causes of death for patients with RA, PsA and axSpA as well as for the general population. The three leading causes of death among patients with IJDs as well as the general population were diseases of the circulatory system, malignancies and diseases of the respiratory system. These three causes of death were explored further. The cumulative incidences of cause-specific death are shown in Table 3. Sex-, education level-, health region- and age group-adjusted HRs for cause-specific mortality are shown in Fig. 2. In RA, we found excess mortality from CVD, neoplasms and respiratory diseases. The HR point estimates for mortality from these diseases were slightly higher for seropositive compared with seronegative RA patients (Supplementary Table S2, available at Rheumatology online). PsA patients did not have excess mortality from CVD, neoplasms or respiratory diseases. AxSpA patients had increased risk of death from CVD and respiratory diseases.
Table 2.
Causes of death
|
n (% of all deaths) |
||||
|---|---|---|---|---|
| Cause of death (ICD-10 code) | RA (n = 5657) | PsA (n = 911) | axSpA (n = 732) | General population (n = 310 699) |
| Circulatory system (I00–I99) | 1594 (28.2) | 233 (25.6) | 177 (24.2) | 91 311 (29.4) |
| Ischaemic heart diseases (I20–I25) | 584 (10.3) | 85 (9.3) | 61 (8.3) | 33 511 (10.8) |
| Cerebrovascular diseases (I60–I69) | 307 (5.4) | 36 (4.0) | 40 (5.5) | 21 402 (6.9) |
| Neoplasms (C00–D48) | 1403 (24.8) | 306 (33.6) | 222 (30.3) | 85 467 (27.5) |
| Malignant neoplasm of larynx and trachea/bronchus/lung (C32–C34) | 368 (6.5) | 76 (8.3) | 45 (6.1) | 17 085 (5.5) |
| Malignant neoplasm of colon (C18) | 135 (2.4) | 26 (2.9) | 16 (2.2) | 9122 (2.9) |
| Malignant neoplasm of prostate (C61) | 60 (1.1) | 14 (1.5) | 17 (2.3) | 7932 (2.6) |
| Malignant neoplasm of lymphatic/haematopoietic tissue (C81–C96) | 149 (2.6) | 25 (2.7) | 20 (2.7) | 6848 (2.2) |
| Malignant neoplasm of pancreas (C25) | 91 (1.6) | 33 (3.6) | 16 (2.2) | 5485 (1.8) |
| Malignant neoplasm of breast (C50) | 80 (1.4) | 8 (0.9) | 8 (1.1) | 4868 (1.6) |
| Respiratory system (J00–J99) | 745 (13.2) | 73 (8.0) | 87 (11.9) | 31 298 (10.1) |
| Chronic lower respiratory diseases (J40–J47) | 358 (6.3) | 42 (4.6) | 54 (7.4) | 15 867 (5.1) |
| Pneumonia (J12–J18) | 235 (4.2) | 16 (1.8) | 17 (2.3) | 11 136 (3.6) |
| External causes of injury and poisoning (V01–Y89) | 189 (3.3) | 61 (6.7) | 49 (6.7) | 18 990 (6.1) |
| Mental and behavioural disorders (F00–F99) | 200 (3.5) | 31 (3.4) | 14 (1.9) | 18 686 (6.0) |
| Nervous system and sense organs (G00–H95) | 165 (2.9) | 28 (3.1) | 23 (3.1) | 14 405 (4.6) |
| Digestive system (K00–K93) | 246 (4.3) | 41 (4.5) | 42 (5.7) | 9569 (3.1) |
| Endocrine, nutritional and metabolic diseases (E00–E90) | 148 (2.6) | 23 (2.5) | 18 (2.5) | 7646 (2.5) |
| Infectious and parasitic diseases (A00–B99) | 224 (4.0) | 27 (3.0) | 19 (2.6) | 7004 (2.3) |
| Genitourinary system (N00–N99) | 115 (2.0) | 17 (1.9) | 13 (1.8) | 6398 (2.1) |
| Musculoskeletal system/connective tissue (M00–M99) | 351 (6.2) | 17 (1.9) | 25 (3.4) | 1697 (0.5) |
| Other | 275 (4.9) | 54 (5.9) | 41 (5.6) | 17 934 (5.8) |
| Missing | 2 (0.0) | 0 (0.0) | 2 (0.3) | 294 (0.1) |
Causes of death during the follow-up among patients with RA, PsA and axial spondyloarthritis (axSpA) as well as the general population.
Table 3.
Cumulative incidence (%) of cause-specific death among patients with inflammatory joint diseases and the general population
| Age | n eventsa | n at riskb | Circulatory system, % | Neoplasms, % | Respiratory system, % | Other, % | Survived, % |
|---|---|---|---|---|---|---|---|
| RA | |||||||
| 55 | 113 | 3776 | 1.1 | 1.3 | 0.1 | 2.1 | 95.4 |
| 65 | 383 | 5777 | 2.4 | 4.4 | 0.7 | 4.1 | 88.4 |
| 75 | 1079 | 4518 | 5.7 | 10.6 | 2.7 | 8.7 | 72.2 |
| 85 | 2248 | 2359 | 15.7 | 19.3 | 7.7 | 20.4 | 36.9 |
| 95 | 1735 | 148 | 27.1 | 23.3 | 12.1 | 32.5 | 5.0 |
| PsA | |||||||
| 55 | 83 | 2880 | 0.9 | 1.1 | 0.0 | 1.8 | 96.2 |
| 65 | 156 | 2803 | 2.1 | 2.9 | 0.4 | 3.5 | 91.2 |
| 75 | 286 | 1084 | 5.0 | 8.2 | 1.6 | 7.2 | 78.0 |
| 85 | 250 | 247 | 12.9 | 17.5 | 3.8 | 17.7 | 48.1 |
| 95 | 128 | 10 | 25.6 | 25.3 | 6.5 | 33.3 | 9.3 |
| axSpA | |||||||
| 55 | 81 | 2018 | 0.6 | 1.0 | 0.0 | 2.2 | 96.2 |
| 65 | 124 | 1565 | 2.0 | 3.1 | 0.8 | 4.4 | 89.7 |
| 75 | 204 | 582 | 6.1 | 8.6 | 2.3 | 9.5 | 73.5 |
| 85 | 232 | 136 | 15.9 | 20.5 | 9.1 | 21.4 | 33.1 |
| 95 | 87 | 4 | 25.6 | 26.3 | 13.7 | 32.2 | 2.3 |
| General population | |||||||
| 55 | 20 383 | 507 188 | 0.5 | 1.1 | 0.1 | 2.0 | 96.3 |
| 65 | 26 709 | 451 083 | 1.5 | 3.5 | 0.4 | 3.6 | 91.0 |
| 75 | 50 740 | 249 441 | 4.2 | 9.1 | 1.7 | 6.5 | 78.5 |
| 85 | 86 041 | 146 128 | 12.7 | 18.0 | 5.1 | 15.0 | 49.3 |
| 95 | 115 353 | 26 402 | 27.2 | 24.2 | 9.5 | 29.1 | 10.0 |
Number of events is the number of deaths that occurred between two consecutive ages (i.e. 18–54, 55–64, 65–74, 75–84, and 85–94).
Number of people alive and included in the dataset at a specific age (i.e. 55, 65, 75, 85, 95). axSpA: axial spondyloarthritis.
Fig. 2.
Hazard ratios from Cox proportional hazard regression models for all-cause and cause-specific mortality
Sex-, education level-, health region- and age-group-adjusted HRs with 95% CI from stratified Cox proportional hazard regression models (with age as the time scale) for all-cause and cause-specific mortality among RA, PsA and axSpA patients compared to the general population. axSpA: axial spondyloarthritis; HR: hazard ratio.
Mortality by sex, age and use of bDMARDs/glucocorticoids
HRs for all-cause and cause-specific mortality in RA, PsA and axSpA compared with the general population by sex and age groups are shown in Table 4. For RA, we found no significant sex differences in the relative risk of all-cause or cause-specific death. We found a small but significant increase in all-cause and cardiovascular mortality among women with PsA but not men with PsA. For axSpA, the estimated HR for all-cause mortality was higher among men compared with women, but the 95% CIs overlapped slightly. In addition, we observed slightly increased mortality from neoplasms among men with axSpA. In RA and PsA, HRs for all-cause mortality were similar across age groups. However, in axSpA, the HR increased in older age groups, with no excess mortality among axSpA patients <55 years. Among RA patients, the HR for CVD death was highest for those aged <55 and lowest for those aged ≥75, and a similar trend was apparent among PsA patients.
Table 4.
Sex-, age-group- and medication-group-specific relative risk of all-cause and cause-specific mortality
| HR (95% CI) |
|||
|---|---|---|---|
| Subgroup | RA | PsA | axSpA |
| All causes of death | |||
| Men | 1.38 (1.32, 1.45) | 1.02 (0.93, 1.11) | 1.47 (1.35, 1.60) |
| Women | 1.48 (1.43, 1.53) | 1.10 (1.00, 1.21) | 1.19 (1.05, 1.36) |
| <55 years | 1.43 (1.26, 1.64) | 1.01 (0.85, 1.19) | 1.05 (0.88, 1.26) |
| 55–74 years | 1.51 (1.45, 1.58) | 1.02 (0.94, 1.12) | 1.38 (1.25, 1.52) |
| ≥75 years | 1.40 (1.36, 1.45) | 1.15 (1.02, 1.30) | 1.64 (1.44, 1.87) |
| Ever bDMARDs, ever GCs | 1.17 (1.08, 1.27) | 1.09 (0.91, 1.30) | 1.25 (1.03, 1.52) |
| Ever bDMARDs, never GCs | 0.71 (0.55, 0.91) | 0.64 (0.46, 0.90) | 0.74 (0.54, 1.01) |
| Never bDMARDs, ever GCs | 1.56 (1.52, 1.61) | 1.22 (1.12, 1.32) | 1.72 (1.55, 1.91) |
| Never bDMARDs, never GCs | 1.26 (1.17, 1.35) | 0.85 (0.74, 0.97) | 1.23 (1.08, 1.39) |
| Diseases of the circulatory system | |||
| Men | 1.33 (1.22, 1.45) | 1.05 (0.88, 1.26) | 1.47 (1.24, 1.75) |
| Women | 1.46 (1.37, 1.55) | 1.23 (1.02, 1.48) | 1.24 (0.93, 1.64) |
| <55 years | 1.97 (1.46, 2.65) | 1.55 (1.11, 2.16) | 1.16 (0.76, 1.78) |
| 55–74 years | 1.59 (1.46, 1.74) | 1.08 (0.90, 1.30) | 1.46 (1.19, 1.78) |
| ≥75 years | 1.33 (1.25, 1.41) | 1.07 (0.87, 1.33) | 1.41 (1.10, 1.81) |
| Ever bDMARDs, ever GCs | 1.10 (0.93, 1.30) | 1.29 (0.90, 1.84) | 1.78 (1.24, 2.56) |
| Ever bDMARDs, never GCs | 0.95 (0.60, 1.50) | 0.93 (0.50, 1.72) | 0.75 (0.37, 1.50) |
| Never bDMARDs, ever GCs | 1.48 (1.40, 1.57) | 1.13 (0.94, 1.34) | 1.31 (1.03, 1.66) |
| Never bDMARDs, never GCs | 1.38 (1.22, 1.57) | 1.11 (0.90, 1.42) | 1.51 (1.20, 1.91) |
| Neoplasms | |||
| Men | 1.29 (1.18, 1.40) | 0.95 (0.81, 1.11) | 1.23 (1.05, 1.44) |
| Women | 1.16 (1.09, 1.25) | 0.95 (0.81, 1.11) | 0.97 (0.77, 1.22) |
| <55 years | 1.20 (0.96, 1.49) | 0.80 (0.60, 1.08) | 0.91 (0.65, 1.26) |
| 55–74 years | 1.25 (1.17, 1.34) | 0.92 (0.80, 1.06) | 1.06 (0.89, 1.26) |
| ≥75 years | 1.16 (1.06, 1.26) | 1.21 (0.95, 1.55) | 1.69 (1.30, 2.20) |
| Ever bDMARDs, ever GCs | 0.89 (0.77, 1.03) | 0.90 (0.67, 1.22) | 0.90 (0.63, 1.30) |
| Ever bDMARDs, never GCs | 0.36 (0.21, 0.64) | 0.37 (0.19, 0.75) | 0.15 (0.05, 0.45) |
| Never bDMARDs, ever GCs | 1.43 (1.35, 1.52) | 1.30 (1.13, 1.49) | 1.85 (1.57, 2.19) |
| Never bDMARDs, never GCs | 0.77 (0.65, 0.91) | 0.53 (0.40, 0.70) | 0.75 (0.56, 0.98) |
| Diseases of the respiratory system | |||
| Men | 1.82 (1.61, 2.05) | 0.83 (0.58, 1.18) | 1.85 (1.41, 2.43) |
| Women | 1.75 (1.60, 1.92) | 1.01 (0.75, 1.37) | 2.03 (1.46, 2.83) |
| <55 years | 1.81 (1.02, 3.21) | 0.19 (0.03, 1.34) | 1.40 (0.58, 3.37) |
| 55–74 years | 1.82 (1.62, 2.05) | 1.05 (0.79, 1.38) | 1.81 (1.36, 2.40) |
| ≥75 years | 1.74 (1.58, 1.91) | 0.85 (0.56, 1.29) | 2.23 (1.60, 3.13) |
| Ever bDMARDs, ever GCs | 1.62 (1.31, 2.00) | 1.00 (0.52, 1.92) | 1.46 (0.73, 2.91) |
| Ever bDMARDs, never GCs | 0.51 (0.19, 1.36) | NE | 0.34 (0.05, 2.45) |
| Never bDMARDs, ever GCs | 1.99 (1.83, 2.16) | 1.13 (0.86, 1.50) | 3.19 (2.49, 4.07) |
| Never bDMARDs, never GCs | 1.00 (0.78, 1.29) | 0.63 (0.37, 1.06) | 0.83 (0.49, 1.40) |
Adjusted HRs with 95% CI from Cox proportional hazard regression models among patients with RA, PsA and axSpA compared with the general population. Sex-specific HRs were adjusted for education level, health region and age group, and age-group-specific HRs were adjusted for sex, education level and health region. Medication-group-specific HRs were adjusted for sex, age group, education level and health region. axSpA: axial spondyloarthritis; bDMARDs: biologic DMARDs; HR: hazard ratio.; GCs: glucocorticoids; NE: no events.
Stratified analyses by ever-use vs never-use of bDMARDs and/or glucocorticoids revealed an increased risk of all-cause and cardiovascular death among RA and axSpA patients without bDMARDs regardless of their glucocorticoid use. Among RA and axSpA patients with bDMARD use, increased mortality was restricted to patients with glucocorticoid use. In PsA, mortality was increased among patients who had used glucocorticoids but not bDMARDs. Across all three IJDs, patients who had used bDMARDs but not glucocorticoids had lowest adjusted HRs for all-cause mortality (HRs and 95% CIs ≤1.01), and patients who had used glucocorticoids but not bDMARDs had highest HRs for all-cause mortality.
Discussion
In this Norwegian nationwide registry study, we showed that RA and axSpA patients had decreased survival compared with the general population even in the 2010s in a country with good access to modern but expensive treatments for IJDs [13]. We observed no increased mortality among the PsA cohort as a whole. However, women (but not men) with PsA had a slightly increased mortality rate. As expected, the three leading causes of death for all the IJD cohorts as well as the general population were CVDs, malignancies and respiratory diseases. RA and axSpA patients had a 1.4-fold greater risk of CVD death compared with the general population, and an almost doubled risk of dying from respiratory diseases compared with the general population.
In RA, all-cause mortality is increased by a factor of 1.5 according to a meta-analysis of observational longitudinal studies of early RA cohorts [2]. A few recent population-based studies from Finland, Norway, the UK and Canada have suggested that the mortality gap between inception RA cohorts and the general population may have been closing or at least narrowing during the 2000s [14–17]. Of special interest is the Norwegian study conducted within the Oslo RA register by Provan et al. in which 10-year all-cause and cardiovascular mortality did not increase among an RA inception cohort diagnosed between 2004 and 2008, although survival was impaired among earlier RA inception cohorts [16]. These results indicate that modern treatment strategies may have had a positive impact on survival in RA. However, these data were from a single centre, Diakonhjemmet Hospital in Oslo, with a longstanding focus on CVD prevention among IJD patients [18]. These results may, therefore, highlight the potential results that can be achieved in an optimal setting and might not be generalizable to the rest of Norway or Europe. Another recent study from central Norway (the HUNT study) indicated that mortality among RA patients is increased (HR 1.24, 95% CI: 1.03, 1.44) [19]. Like the HUNT study, our study was not an inception cohort study, and this may be another reason for the different results reported by Provan et al. Furthermore, recent Swedish nationwide and UK-based inception cohort studies showed that despite decreasing mortality rates over time, RA continues to be associated with increased mortality [20, 21], especially after a disease duration of ≥10 years [20].
Contemporary information on mortality in axSpA is limited. A meta-analysis including observational studies on mostly AS but also PsA and undifferentiated SpA did not confirm increased mortality rates in SpA compared with the general population (risk ratio 1.23, 95% CI: 0.96, 1.57) [22]. Despite this, many studies have identified reduced survival among AS patients [7, 9]. A study that included 677 AS patients from northern Norway who were followed up until 2009 reported a standardized mortality ratio of 1.61 (95% CI: 1.29, 1.93). This increase in mortality was confined to male AS patients [9]. A 2016 Swedish register-based study that included >8000 AS patients reported an HR of 1.60 for all-cause mortality, with similar estimates for both men and women [7]. In our study, both men and women with axSpA had excess mortality, although the HR point estimate was slightly higher among men. Factors generally associated with increased mortality in both RA and axSpA include the overall comorbidity burden and disease activity [9, 23, 24].
In contrast to RA and axSpA, all-cause mortality was not greater among the PsA cohort as a whole and only slightly greater among women and those aged ≥75 years. Results from previous mortality studies among PsA cohorts have been inconsistent, mainly supporting either no or a slightly increased mortality risk among PsA patients [25–27]. A Danish nationwide cohort study conducted between 1998 and 2014 revealed that survival was impaired in severe psoriasis but not in PsA [26]. A study using the British Society for Rheumatology Biologics Register showed that, among patients with severe PsA requiring bDMARD treatment, mortality may have increased by 50%, in part driven by deaths from coronary heart disease [28]. A population-based study using the Health Improvement Network data in the UK concluded that, unlike patients with RA, patients with PsA were not at increased risk for all-cause or cause-specific mortality, with the exception of suicide deaths [5].
Patients with RA are at increased risk of various types of CVD, which results from the combined and complexly intertwined effects of traditional CVD risk factors and systemic inflammation [29]. Similarly, CVD risk is increased in both PsA and axSpA [30, 31]. Among RA patients, cardiovascular mortality rates are ∼60% higher compared with the general population, according to a meta-analysis of 17 observational studies [32]. In AS, similar estimates prevail, with a HR for CVD death of 1.36 (95% CI: 1.13, 1.65), according to a population-based retrospective cohort study from Ontario, Canada [6]. Our findings of an ∼40% increase in the risk of CVD death among RA and axSpA patients are well-aligned with these previous estimates. Similar to all-cause mortality, some studies have found a reduction in the relative risk of CVD death with no excess risk among most recent RA inception cohorts [16, 33, 34]. These findings, however, would need confirmation by larger RA cohorts and/or with a longer follow-up period of a minimum of 10 years.
For PsA, results regarding the risk of CVD mortality have been more conflicting [4, 5, 26] despite multiple reports identifying an increased prevalence of CVD and its risk factors among PsA patients [35]. We were not able to show a statistically significant increase in the risk of CVD death among our entire PsA cohort (HR 1.13, 95% CI: 0.99, 1.29). However, CVD mortality was higher among women (HR 1.23, 95% CI: 1.02, 1.48). While the reason for this sex difference is not clarified by the current study, CVD risk factors such as obesity, hypertension, smoking and diabetes may be over-represented among women with PsA [36].
There were some noteworthy differences in the relative risk of CVD mortality between age groups. In the PsA cohort, the youngest age group (<55 years) had a 1.6-fold risk of CVD death compared with the general population. Also, among RA patients, the relative risk for CVD death was highest among those <55 years. Similar findings of a higher relative risk of CVD events and mortality among younger patients have been made previously in RA and PsA cohorts [25, 37, 38]. These findings underscore the role of IJD-related inflammation as a non-traditional CVD risk factor especially in young adults, and that patients with IJDs require active CVD risk factor screening and management already early in their life.
The risk of death from neoplasms increased 1.2-fold among our RA cohort compared with the general population. A meta-analysis of nine observational studies concluded that the overall incidence of malignancies is increased among RA patients (pooled standardized incidence ratio 1.09, 95% CI: 1.06, 1.13), with a more clearly increased risk of lymphoma and lung cancer [39]. In axSpA and PsA, we did not detect a statistically significant increase in malignancy-related mortality, with the exception of a slightly increased risk of malignancy-related death among men with axSpA. An Australian study of >2100 AS patients and 1:5 matched controls showed that not only was the crude risk of cancer among AS patients increased by 15%, but AS patients had an increased risk of 5-year mortality following a cancer diagnosis [40]. Among PsA patients, the incidence of malignancies seems to be similar to that of the general population, with the exception of an increased risk of non-melanoma skin cancer [28].
The risk of death from respiratory diseases, mainly represented by chronic lower respiratory diseases and pneumonias, was greater among both RA and axSpA patients but not PsA patients. Factors that may contribute to increased mortality from respiratory diseases among RA patients include smoking, which is an important risk factor for seropositive RA, and perhaps interstitial lung disease, which is an extra-articular manifestation of RA that is linked to a worse prognosis [41]. Unfortunately, we did not have data on smoking status and could not estimate its confounding effect, which is a limitation. Nevertheless, our findings of increased risk from both CVD and respiratory diseases highlight the importance of support for smoking cessation among IJD patients.
Long-term use of glucocorticoids, especially at medium or high dosages, is associated with a dose-dependent increase in mortality, and increases the risk of cardiovascular, infectious, gastrointestinal and metabolic diseases [42]. In line with previous studies [24, 42], glucocorticoid use in our IJD cohorts was linked to reduced survival, whereas bDMARD users without glucocorticoid use did not have excess mortality compared with the general population. In fact, these patients were characterized by a relative ‘mortality deficit’ compared with the general population. Our results, however, are likely to be influenced by channelling bias: it may be less likely that bDMARDs are prescribed to patients with severe comorbidities that reduce survival such as malignancies and severe heart disease, whereas glucocorticoids may be preferred when comorbidities prevent the use of DMARDs. Furthermore, we were not able to distinguish between indications for glucocorticoids, and their use may be related to some other significant comorbidity than IJD. Glucocorticoid and bDMARD use may also be associated with high disease activity, which is associated with decreased survival [4, 8, 9]. Unfortunately, we lack data on disease activity and could not adjust for this in our analyses.
It is important to note that our IJD cohorts were not inception cohorts; we included all patients with ≥2 records of RA, PsA and axSpA in specialized care during a 10-year period. Some patients are likely to have had their IJD diagnosis decades earlier. We addressed this by using age as the time scale in our survival analyses. Our results may not be generalizable to patients with a very mild IJD that does not require follow-up in specialized care, nor to most recent IJD cohorts or patients with a short IJD duration. We lack data on disease duration and the fulfilment of classification criteria for IJDs, and our case identification method has not been validated in Norway.
In conclusion, in the era of modern treatments for IJDs in a country with good access to bDMARDs, patients with RA and axSpA still have shortened life expectancy. Mortality rates among PsA patients were not similarly increased, although women with PsA had a slightly reduced survival. This may be related to an overall milder disease course and may not be generalizable to severe PsA. Strategies to improve survival among IJD patients are needed across all ages, including effective suppression of disease activity, support for smoking cessation and further attention to the prevention of the most prevalent comorbidities, especially CVD.
Supplementary Material
Acknowledgements
We sincerely thank Cynthia Crowson from the Department of Quantitative Health Sciences at the Mayo Clinic, Rochester, MN, USA, for her help in conducting statistical analyses, as well as health project advisor Merete Tholin and interest policy advisor Ann Kristin Bakke, both representing the Norwegian Rheumatology Association, and patient representative Thalita Blanck from the user board (REVMA Pasientråd) at the National Advisory Unit On Rehabilitation in Rheumatology at Diakonhjemmet Hospital for their advice on dissemination and communication of our results to patients and their spouses.
All listed authors made substantial contributions to the study’s conception and design as well as the analysis and interpretation of the data. A.M.K. and A.K. drafted the article, and S.R., S.L., J.S., G.W., E.H., T.K.K. and A.G.S. critically revised the manuscript. All listed authors gave their final approval of the version to be published.
Funding: This work was supported by a research grant to A.K. from the FOREUM Foundation for Research in Rheumatology and by grants from the South Eastern Regional Health Authorities of Norway (2016063 for S.R. and 2013064 for A.G.S.). The funding sources had no role in the study design; the collection, analysis, and interpretation of the data; the writing of the report; or the decision to submit the article for publication.
Disclosure statement: A.M.K. has received speaker fees from Boehringer-Ingelheim and Sanofi; has participated in the advisory boards of Pfizer, Gilead and Boehringer-Ingelheim; and has received congress sponsorship from Pfizer, Celgene, UCB Pharma, Mylan and Roche. S.R. has received speaker honoraria from AbbVie, UCB and Pfizer.JS: None. GW: None. E.H. has received consulting fees from Abbvie, Novartis, Pfizer, Gilead, Eli Lilly, Galapagos and Janssen as well as speaker fees from Pfizer and Abbvie. T.K.K. has received speaker fees from Amgen, Celltrion, Egis, Evapharma, Ewopharma, Hikma, Oktal, Sandoz and Sanofi; fees for consulting from AbbVie, Amgen, Biogen, Celltrion, Eli Lilly, Gilead, Mylan, Novartis, Pfizer, Sandoz and Sanofi; and research funding for Diakonhjemmet Hospital from AbbVie, Amgen, BMS, MSD, Novartis, Pfizer and UCB. A.G.S. has received speaker honoraria and/or consulting fees from AbbVie, Bayer, Eli Lilly, Novartis and Sanofi as well as a collaborative agreement for independent research from Eli Lilly, who had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. A.K., S.L., J.S. and G.W. have no disclosures.
Contributor Information
Anne M Kerola, Preventive Cardio-Rheuma clinic, Division of Rheumatology and Research, Diakonhjemmet Hospital, Oslo, Norway; Department of Internal Medicine, Päijät-Häme Joint Authority for Health and Wellbeing, Lahti; Faculty of Medicine, University of Helsinki, Helsinki, Finland.
Amirhossein Kazemi, Division of Rheumatology and Research, Diakonhjemmet Hospital.
Silvia Rollefstad, Preventive Cardio-Rheuma clinic, Division of Rheumatology and Research, Diakonhjemmet Hospital, Oslo, Norway.
Siri Lillegraven, Division of Rheumatology and Research, Diakonhjemmet Hospital.
Joseph Sexton, Division of Rheumatology and Research, Diakonhjemmet Hospital.
Grunde Wibetoe, Preventive Cardio-Rheuma clinic, Division of Rheumatology and Research, Diakonhjemmet Hospital, Oslo, Norway.
Espen A Haavardsholm, Division of Rheumatology and Research, Diakonhjemmet Hospital; Faculty of Medicine, University of Oslo, Oslo, Norway.
Tore K Kvien, Division of Rheumatology and Research, Diakonhjemmet Hospital.
Anne Grete Semb, Preventive Cardio-Rheuma clinic, Division of Rheumatology and Research, Diakonhjemmet Hospital, Oslo, Norway.
Data availability statement
The data underlying this article were provided by the register keepers (i.e. the Norwegian Institute of Public Health, Statistics Norway, and the Norwegian Tax Administration) by permission of Norwegian Data Protection Authority. It cannot be shared publicly due to patient privacy.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Kerola AM, Rollefstad S, Kazemi A. et al. Psoriatic arthritis, axial spondyloarthritis and rheumatoid arthritis in Norway: nationwide prevalence and use of biologic agents. Scand J Rheumatol (in press), doi: 10.1080/03009742.2021.1997436. [DOI] [PubMed] [Google Scholar]
- 2. Dadoun S, Zeboulon-Ktorza N, Combescure C. et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine 2013;80:29–33. [DOI] [PubMed] [Google Scholar]
- 3. Sokka T, Abelson B, Pincus T.. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol 2008;26:S35–61. [PubMed] [Google Scholar]
- 4. Juneblad K, Rantapää-Dahlqvist S, Alenius GM.. Disease activity and increased risk of cardiovascular death among patients with psoriatic arthritis. J Rheumatol 2016;43:2155–61. [DOI] [PubMed] [Google Scholar]
- 5. Ogdie A, Maliha S, Shin D. et al. Cause-specific mortality in patients with psoriatic arthritis and rheumatoid arthritis. Rheumatology (Oxford) 2017;56:907–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haroon NN, Paterson JM, Li P, Inman RD, Haroon N.. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med 2015;163:409–16. [DOI] [PubMed] [Google Scholar]
- 7. Exarchou S, Lie E, Lindström U. et al. Mortality in ankylosing spondylitis: results from a nationwide population-based study. Ann Rheum Dis 2016;75:1466–72. [DOI] [PubMed] [Google Scholar]
- 8. Book C, Saxne T, Jacobsson LTH.. Prediction of mortality in rheumatoid arthritis based on disease activity markers. J Rheumatol 2005;32:430–4. [PubMed] [Google Scholar]
- 9. Bakland G, Gran JT, Nossent JC.. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis 2011;70:1921–5. [DOI] [PubMed] [Google Scholar]
- 10. Bakken IJ, Ariansen AMS, Knudsen GP, Johansen KI, Vollset SE.. The Norwegian patient registry and the Norwegian Registry for Primary Health Care: research potential of two nationwide health-care registries. Scand J Public Health 2020;48:49–55. [DOI] [PubMed] [Google Scholar]
- 11. Therneau T. survival: Survival Analysis. R package version 3.2-12. Chapter 2.3: Competing risks. 2021. https://CRAN.R-project.org/package=survival (18 August 2021, date last accessed).
- 12. van Buuren S, Groothuis-Oudshoorn K.. mice: Multivariate Imputation by Chained Equations in R. J Stat Soft 2011;45:1–67. [Google Scholar]
- 13. Putrik P, Ramiro S, Kvien TK. et al. ; Working Group ‘Equity in access to treatment of rheumatoid arthritis in Europe’. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis 2014;73:198–206. [DOI] [PubMed] [Google Scholar]
- 14. Lacaille D, Avina-Zubieta JA, Sayre EC, Abrahamowicz M.. Improvement in 5-year mortality in incident rheumatoid arthritis compared with the general population—closing the mortality gap. Ann Rheum Dis 2017;76:1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abhishek A, Nakafero G, Kuo CF. et al. Rheumatoid arthritis and excess mortality: down but not out. A primary care cohort study using data from Clinical Practice Research Datalink. Rheumatology (Oxford) 2018;57:977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Provan SA, Lillegraven S, Sexton J. et al. Trends in all-cause and cardiovascular mortality in patients with incident rheumatoid arthritis: a 20-year follow-up matched case-cohort study. Rheumatology (Oxford) 2020;59:505–12. [DOI] [PubMed] [Google Scholar]
- 17. Puolakka K, Kautiainen H, Pohjolainen T, Virta L.. No increased mortality in incident cases of rheumatoid arthritis during the new millennium. Ann Rheum Dis 2010;69:2057–8. [DOI] [PubMed] [Google Scholar]
- 18. Rollefstad S, Kvien TK, Holme I. et al. Treatment to lipid targets in patients with inflammatory joint diseases in a preventive cardio-rheuma clinic. Ann Rheum Dis 2013;72:1968–74. [DOI] [PubMed] [Google Scholar]
- 19. Houge IS, Hoff M, Thomas R, Videm V.. Mortality is increased in patients with rheumatoid arthritis or diabetes compared to the general population – the Nord-Trøndelag Health Study. Sci Rep 2020;10:3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holmqvist M, Ljung L, Askling J.. Mortality following new-onset rheumatoid arthritis: has modern rheumatology had an impact? Ann Rheum Dis 2018;77:85–91. [DOI] [PubMed] [Google Scholar]
- 21. Humphreys JH, Warner A, Chipping J. et al. Mortality trends in patients with early rheumatoid arthritis over 20 years: results from the Norfolk Arthritis Register. Arthritis Care Res 2014;66:1296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JH, Choi IA.. Cardiovascular morbidity and mortality in patients with spondyloarthritis: a meta-analysis. Int J Rheum Dis 2021;24:477–86. [DOI] [PubMed] [Google Scholar]
- 23. Zhao SS, Robertson S, Reich T. et al. Prevalence and impact of comorbidities in axial spondyloarthritis: systematic review and meta-analysis. Rheumatology (Oxford) 2020;59:iv47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Listing J, Kekow J, Manger B. et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFα inhibitors and rituximab. Ann Rheum Dis 2015;74:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elalouf O, Muntyanu A, Polachek A. et al. Mortality in psoriatic arthritis: risk, causes of death, predictors for death. Semin Arthritis Rheum 2020;50:571–5. [DOI] [PubMed] [Google Scholar]
- 26. Skov L, Thomsen SF, Kristensen LE. et al. Cause-specific mortality in patients with psoriasis and psoriatic arthritis. Br J Dermatol 2019;180:100–7. [DOI] [PubMed] [Google Scholar]
- 27. Buckley CE, Cavill CR, Taylor GJ. et al. Mortality in psoriatic arthritis – a single-center study from the UK. J Rheumatol 2010;37:2141–4. [DOI] [PubMed] [Google Scholar]
- 28. Fagerli KM, Kearsley-Fleet L, Mercer LK. et al. Malignancy and mortality rates in patients with severe psoriatic arthritis requiring tumour-necrosis factor alpha inhibition: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2019;58:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerola AM, Rollefstad S, Semb AG.. Atherosclerotic cardiovascular disease in rheumatoid arthritis: impact of inflammation and antirheumatic treatment. Eur Cardiol 2021;16:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eriksson JK, Jacobsson L, Bengtsson K, Askling J.. Is ankylosing spondylitis a risk factor for cardiovascular disease, and how do these risks compare with those in rheumatoid arthritis? Ann Rheum Dis 2017;76:364–70. [DOI] [PubMed] [Google Scholar]
- 31. Ogdie A, Yu YD, Haynes K. et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis 2015;74:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meune C, Touzé E, Trinquart L, Allanore Y.. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2009;48:1309–13. [DOI] [PubMed] [Google Scholar]
- 33. Kerola AM, Nieminen TVM, Virta LJ. et al. No increased cardiovascular mortality among early rheumatoid arthritis patients: a nationwide register study in 2000-2008. Clin Exp Rheumatol 2015;33:391–8. [PubMed] [Google Scholar]
- 34. Myasoedova E, Gabriel SE, Matteson EL. et al. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: dawn of a new era in cardiovascular disease in RA? J Rheumatol 2017;44:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jamnitski A, Symmons D, Peters MJL. et al. Cardiovascular comorbidities in patients with psoriatic arthritis: a systematic review. Ann Rheum Dis 2013;72:211–6. [DOI] [PubMed] [Google Scholar]
- 36. Landgren AJ, Bilberg A, Eliasson B. et al. Cardiovascular risk factors are highly overrepresented in Swedish patients with psoriatic arthritis compared with the general population. Scand J Rheumatol 2020;49:195–9. [DOI] [PubMed] [Google Scholar]
- 37. Lassere MN, Rappo J, Portek IJ, Sturgess A, Edmonds JP.. How many life years are lost in patients with rheumatoid arthritis? Secular cause-specific and all-cause mortality in rheumatoid arthritis, and their predictors in a long-term Australian cohort study. Intern Med J 2013;43:66–72. [DOI] [PubMed] [Google Scholar]
- 38. Widdifield J, Paterson JM, Huang A, Bernatsky S.. Causes of death in rheumatoid arthritis: how do they compare to the general population? Arthritis Care Res 2018;70:1748–55. [DOI] [PubMed] [Google Scholar]
- 39. Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S.. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelty E, Raymond W, Inderjeeth C. et al. Cancer diagnosis and mortality in patients with ankylosing spondylitis: a Western Australian retrospective cohort study. Int J Rheum Dis 2021;24:216–22. [DOI] [PubMed] [Google Scholar]
- 41. Kelly CA, Nisar M, Arthanari S. et al. Rheumatoid arthritis related interstitial lung disease – improving outcomes over 25 years: a large multicentre UK study. Rheumatology (Oxford) 2021;60:1882–90. [DOI] [PubMed] [Google Scholar]
- 42. Bijlsma JWJ, Buttgereit F.. Adverse events of glucocorticoids during treatment of rheumatoid arthritis: lessons from cohort and registry studies. Rheumatology (Oxford) 2016;55:ii3–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the register keepers (i.e. the Norwegian Institute of Public Health, Statistics Norway, and the Norwegian Tax Administration) by permission of Norwegian Data Protection Authority. It cannot be shared publicly due to patient privacy.