Abstract
Over the past decades, substantial advances in neonatal medical care have increased the survival of extremely premature infants. However, there continues to be significant morbidity associated with preterm birth with common complications including bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), neuronal injury such as intraventricular hemorrhage (IVH) or hypoxic ischemic encephalopathy (HIE), as well as retinopathy of prematurity (ROP). Common developmental immune and inflammatory pathways underlie the pathophysiology of such complications providing the opportunity for multisystem therapeutic approaches. To date, no single therapy has proven to be effective enough to prevent or treat the sequelae of prematurity. In the past decade mesenchymal stem/stromal cell (MSC)—based therapeutic approaches have shown promising results in numerous experimental models of neonatal diseases. It is now accepted that the therapeutic potential of MSCs is comprised of their secretome, and several studies have recognized the small extracellular vesicles (sEVs) as the paracrine vector. Herein, we review the current literature on the MSC-EVs as potential therapeutic agents in neonatal diseases and comment on the progress and challenges of their translation to the clinical setting.
Keywords: mesenchymal stem/stromal cells, extracellular vesicles, MSC-EVs, neonatal, prematurity, bronchopulmonary dysplasia
Graphical Abstract
Graphical Abstract.
Significance Statement.
Mesenchymal stromal/stem cell extracellular vesicles (MSC-EVs) have been reported to exert considerable therapeutic potential in a multitude of preclinical disease models. Prematurity and perinatal stressors disturb the physiologic equilibrium by inducing inflammation, growth arrest, and loss of vascular support, thus potentially reprogramming normal development. MSC-EV therapy has the potential to modulate these common pathophysiologic pathways and has emerged as a promising treatment option for many perinatal diseases. This review provides a detailed summary of the current literature on the therapeutic potential of MSC-EVs in neonatal diseases, as well as highlights future perspectives of the field.
Introduction
Preterm birth remains one of the world’s most significant public health problems, accounting for 10.6% of live births worldwide and approximately 15 million premature infants each year.1 Current technological and medical advancements have improved the survival of preterm infants; however, despite optimal medical support, they are at increased risk of suffering from several complications of prematurity contributing disproportionately to neonatal morbidity and mortality.2 In fact, according to WHO, preterm birth complications are among the leading causes of pediatric mortality under 5 years of age, causing approximately 1 million deaths in 2015.3
The sequelae of prematurity are mostly affecting vital and developing organs, such as the lungs, the brain, and the gastrointestinal tract. Consequently, some severe complications of preterm birth are bronchopulmonary dysplasia (BPD), periventricular leukomalacia (PVL), hypoxic/ischemic encephalopathy (HIE), necrotizing enterocolitis (NEC), and retinopathy of prematurity (ROP). Recent studies support that these sequelae, occurring at such a critical developmental time point, may follow premature babies to infancy, childhood, and even in adulthood, very often leading to lifelong morbidities.4-7 Even though the use of glucocorticoids, surfactant replacement, and supportive care have ameliorated the severity and changed the phenotype of these diseases, none of the existing treatment methods is curative. Hydrocortisone treatment starting from postnatal day 14 to 28 was recently reported by the NICHD Neonatal Research Network to be ineffective in decreasing moderate or severe BPD compared to placebo.8 Thus, with no single effective treatment, it is a necessity to explore new therapeutic options able to prevent and cure the complications of prematurity.
In the last decade, mesenchymal stem/stromal cells (MSCs) and their extracellular vesicles have emerged as a promising therapeutic agent for several diseases and a number of preclinical studies have successfully shown encouraging results in various pre-clinical models of neonatal disease.9,10 This review summarizes the current literature on the potential therapeutic applications of MSC-EVs in neonatal diseases, including BPD, HIE, NEC, and others, as well as comments on the progress and challenges of their translation to the clinic.
From MSCs to the Extracellular Vesicles
MSCs are somatic stem cells of mesodermal origin capable of differentiating into a variety of mesoderm-derived cells, such as adipocytes, osteocytes, chondrocytes, fibroblasts, and skeletal muscle cells. They can be isolated from a range of tissues, including bone marrow, adipose tissue, amniotic fluid, umbilical cord Wharton’s jelly (WJ), umbilical cord blood, and placenta.11,12 Lately, there has been a great diversification of the tissue source of MSCs for clinical use, from predominantly bone marrow until 2008, to almost equal use of adipose tissue, bone marrow and perinatal derived MSC sources.13 Several preclinical studies have highlighted the therapeutic potential of MSCs in a multitude of diseases as potent immunomodulatory, neuroprotective, angiogenic, and regenerative mediators.9,10,14 While initially MSCs were thought to home into the damaged tissue and differentiate into resident cells, no long-term engraftment or differentiation in significant numbers has been observed. In contrast, subsequent studies highlighted a paracrine mechanism responsible for their beneficial activities and EVs have been identified as one of the key mediators of this effect.15-20
EVs are a heterogeneous class of spherical lipid bilayer microparticles released from virtually every cell type. They were originally perceived as a “garbage disposal” cell mechanism, to eliminate undesirable cellular components, and indeed this arguably remains their function in most cell types. Nevertheless, evolution apparently co-opted the EV biogenetic pathways in certain cell types to generate EV subpopulations (signalosomes) designed to transfer intercellular signals and affect the proximal microenvironment in a paracrine manner.21-23
While the full definition of EV sub-types and their biogenesis will not be actively explored herein, in general, EVs are sub-categorized into three major classes: (1) the small EVs (sEVs) (~30–150 nm in diameter), which include the exosomes and are generated through the endosomal pathway as intraluminal vesicles (ILVs) inside the multivesicular bodies (MVBs), released as sEVs upon merging of MVBs with the plasma membrane; (2) the microvesicles (~100 nm-1 μm in diameter), which are formed through budding of the plasma membrane; (3) and the apoptotic bodies (>1 μm in diameter), which are released by apoptotic cells.21,23 The molecular composition and the bioactive cargo of each EV subpopulation are tightly dependent on the type and state of parent cells, as well as on their biogenesis pathway, and is comprised of a variable combination of lipids, proteins, and diverse types of nucleic acid (DNA, mRNA, lnc-RNA, micro-RNA (miRs)). The profound heterogeneity in EV biogenesis, biophysical properties (size, density, and predominant protein markers), as well as the variety of EV isolation techniques has highlighted the need for a universal consensus regarding EV characterization methodologies. To that end, the pioneering work of the International Society of Extracellular Vesicles has led the efforts to establish standardized nomenclature, definitions, and methodological practices in the EV field.22,24,25
BPD and MSC Derived EVs
Bronchopulmonary dysplasia is a chronic lung disease with multifactorial pathophysiology. It primarily occurs in premature infants requiring respiratory support with mechanical ventilation and supplemental oxygen. It was first described in 1967 by Northway et al., as a sequela of respiratory distress syndrome (RDS), characterized by emphysematous alveoli, prominent fibrosis, airway muscle hypertrophy, pulmonary arteriole lesions, and pulmonary hypertension leading to cor pulmonale.26 Since then, surfactant supplementation, antenatal steroids, and advancements in respiratory support have significantly altered the disease phenotype. Nowadays, the severe histopathologic features of the “old BPD” are limited to alveolar simplification and dysmorphic capillary morphology in the “new BPD”.27 Interestingly, despite the milder phenotype, BPD remains a leading cause of significant morbidity and mortality in premature infants, with potentially long-term respiratory and neurodevelopmental complications, oftentimes lasting beyond childhood.2,5-7,28 Therefore, more effective therapeutic approaches continue to be a necessity.
On this note, MSCs have shown promising results in numerous preclinical models of BPD.9,28-30 However, several animal studies supported that MSC conditioned media (CM) might be more effective in preserving the alveolar and vascular integrity than the parental cells. This finding was accompanied by minimal MSC engraftment in the injured lung indicating a paracrine mechanism of MSC protective action.15-17,31,32 Notably, MSC-CM dosing, as well as the identification and the actual concentration of their crucial bioactive factors, are important parameters to be determined to enable further research and potential clinical translation. Detailed analyses of the MSC-CM highlighted the sEVs as the primary mediator of this paracrine effect. Our group first reported this association in a model of hypoxia-induced pulmonary hypertension, a complication of severe BPD. After fractionating the MSC-CM, it was observed that MSC-EVs were able to suppress pulmonary macrophage influx, inhibit vascular remodeling, and ameliorate pulmonary hypertension, while exosome-depleted CM or fibroblast-CM had no such effect.17
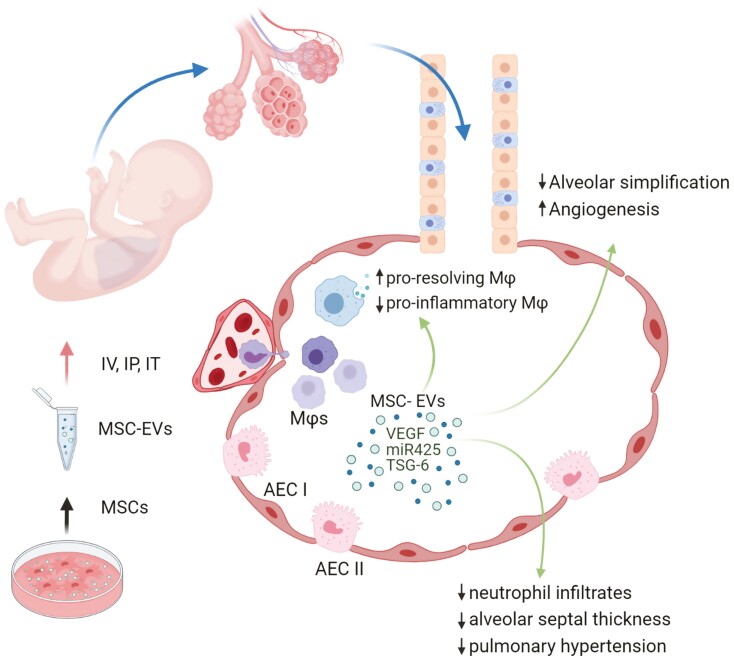
We subsequently demonstrated that a single dose of MSC-EVs was sufficient to significantly improve lung morphology in a murine model of BPD. MSV-EV treatment was able to decrease lung fibrosis, ameliorate pulmonary vascular remodeling, improve pulmonary function test results, and alleviate associated pulmonary hypertension.18 Interestingly, this study highlighted the immunomodulatory capacity of MSC-EVs as a potential mechanism of their protective action. In fact, MSC-EV treatment was able to modulate the macrophage phenotype both in vitro and in vivo, by suppressing the proinflammatory “M1-like” state, while enhancing a pro-resolving “M2-like” state (Table 1, Fig. 1).
Table 1.
Synopsis of technical details and main results of studies on MSC-EVs in BPD.
| Reference | MSC source | MSC product | Isolation | Disease model | Route | Dose/ frequency | Main result/ action↑↓ | Pathway/active factor |
|---|---|---|---|---|---|---|---|---|
| Lee et al. (2012) | BM-MSCs (mouse) and WJMSCs (human) | CM and Exosomes | Ultrafiltration, PEG, size exclusion chromatography, UC | In vivo: HPH |
IV | 1 Dose of CM: 50 μL of 5 μg protein 1 or 2 doses of exosomes: 0.1 μg or 10 μg protein |
Inhibited vascular remodeling and HPH ↓ Influx of macrophages ↓ Pro-inflammatory and pro-proliferative mediators |
↓ STAT3activation and ↑ miR-17 superfamily and lung levels of miR-204. |
| Willis et al. (2018) | BM-MSCs (human) and WJMSCs (human) | EVs angiogenesis | Differential centrifugation, TFF, OptiPrepTM cushion density flotation | In vivo: BPD (hyperoxia 75% O2) In vitro: mouse BMDM Or alveolar macrophages |
IV | 1 dose 50 μL of EVs ≈ 0.5 × 106 cell equivalents |
Prevented lung fibrosis ↑ Alveolarization, ↑ Pulmonary function and modulated macrophage phenotype |
Modulate macrophage Phenotype: ↓ pro-inflammatory and ↑ Anti-inflammatory state |
| Ahn et al. (2018) | hUC MSCs | Cells and EVs | UC | In vivo: BPD hyperoxia (90%O2) in vitro: rat lung epithelial cell line challenged with H2O2 |
IT | 1 Dose onPN5 50 µL of: MSCs: 1 × 105 cells OR EVs: 20 µg of protein |
MSCs and EVs equally: ↑alveolarization,↑vascularization and ↓inflammatory response |
Transfer of VEGF |
| Chaubey et al. (2018) | GA hUC-MSCs | CM and Exosomes | 10-Fold concentrated CM &UC | In vivo: BPD hyperoxia (95% O2) in vitro: lung epithelial cell line challenged with H2O2 |
IP | 2 Doses PN2 & PN4 CM:10 μg protein. Exosomes: 0.7 × 106 cell equivalents |
↓ Pulmonary inflammation ↓ Alveolar-capillary leakage ↓alveolar simplification, ↓PH and RVH ↓ Cell death in brain and ↑Myelination |
TSG-6 |
| Braun et al. (2018) | BM-MSCs (Rat) | Exosomes | UC | In vivo: BPD hyperoxia (85%O2) in vitro: HUVEC tube formation assay |
IP | Daily injection of 15 mg protein ≈ 3.4 × 109 exosomes | In vivo: ↓alveolar simplification, ↑Small vessel number and inhibited RVH In vitro: ↑ tube-like formation by HUVEC |
VEGF mediated mechanism |
| Porzionato et al. (2018) | hUC MSCs | Cells and EVs | TFF | In vivo: BPD hyperoxia (60%O2) |
IT | 3 Doses (PN3, PN7, PN10) MSCs: 6 × 106 cells EVs: 0.64 × 1010 particles/ml |
Both EVs and MSCs: ↓hyperoxia-induced damage. EVs: better alveolarization and vascularization |
|
| Porzionato et al. (2020) | hUC MSCs | EVs | TFF | In vivo: BPD hyperoxia (60%O2) |
IT | 4 Times (PN3, PN7, PN10, and PN21) EVs: 0.64 x1010 particles/ml |
↑ Alveolarization ↓ PA remodeling MSC-EV preserved: –The interstitial, alveolar and perivascular CD163+ macrophages –↑ Cell proliferation |
M2 macrophage polarization could play a role through anti-inflammatory and proliferative mechanisms. |
| Willis et al. (2020) | WJMSCs (human) | EVs | Differential centrifugation, TFF, OptiPrepTM cushion density flotation | in vivo: BPD (hyperoxia 75% O2) In vitro: mouse BMDM |
IV | For early intervention: at PN4 1 dose of 0.5 × 106 cell equivalents For late intervention: at PN18 1 dose of 1 × 106 cell equivalents For serial late intervention: 4 doses (PN18-PN39) of 1 × 106 cell equivalents |
Early intervention: See previous publication Late intervention: –1 dose:partially restores alveolar simplification. –serial doses: ↑ alveolarization,↓ fibrosis, ↓ vascular muscularization and ↓microvascular loss Early and late MEx interventions ↓ RVH and ↑ functional exercise capacity |
|
| You et al. (2020) | hUC MSCs | EVs | Serial centrifugation, UC | In vivo: BPD (85% O2) In vitro: HUVEC tube formation assay and cell survival of MLE-2 under hyperoxic conditions |
IT | 1 Dose 20 mg of protein on PN7 |
In vivo: ↓ alveolar simplification and lung function,↓ PH, ↑ Ki-67+ and ↓ TUNEL+ lung cells, ↑ type II AECs, ↑ pulmonary vascular endothelial cells in vitro: ↑ tube formation of hyperoxic HUVECs ↑ proliferation and ↓apoptosis in MLE-12 |
PTEN/Akt signaling pathway: ↓ expression of PTEN & cleaved-caspase3, and ↑ expression of p-Akt and VEGF-a |
| Wu et al. (2021) | BM-MSCs (rat) | Exosomes | Exosome isolation reagent & centrifugation for 1 h at 12,000 g | In vivo: Hyperoxia lung injury (90% O2) in vitro: lung epithelial cell line (RLE-6TN) challenged with H2O2 |
IV | 1 Dose 800 μg of protein |
In vivo: ↑ alveolarization, ↓inflammatory influx in lung In vitro: ↓ oxidative damage on RLE-6TN, |
miR-425 in BMSCs-EVs activates the PI3K/AKT axis by targeting PTEN and thus inhibits hyperoxic injury |
| Willis GR et al. (2021) | WJMSCs and BM-MSCs (human) | MEx (small EVs) | Differential centrifugation, TFF, OptiPrepTM cushion density flotation | In vivo: BPD (hyperoxia 75% O2) in vitro: mouse BMDMy pretreated with MEx |
IV | 1 Dose 50 μL of EVs ≈ 0.5 × 106 cell equivalents on PN4 |
MSC-EVs: –co-localized with F4/80+, CD64+myeloid cells –preserved the lung CD45+ cells, especially AMφ and Ly6C low monocytes -↓ AMφ inflammatory activation Adoptive transfer of MSC-EV-educated-BMDMy prevented the hyperoxic injury similarly to MSC-EV treatment |
MSC-EV modulate myeloid cells into a Ly6C/G+, CX3CR1+, CCR2− phenotype, with immunosuppressive capacity, possibly through transcriptomic and epigenetic reprogramming |
| Reis M. et al. (2021) | WJMSCs and HDF (human dermal fibroblast) | MEx (small EVs) | Differential centrifugation, TFF, OptiPrepTM cushion density flotation | In vivo: BPD (hyperoxia 75% O2) in vitro: T cell autoreactivity assessment |
IV | 1 Dose 50 μL of EVs ≈ 0.5 × 106 cell equivalents on PN4 |
MSC-EVs: –prevented the development of BPD –preserved the thymic medullary architecture ↑development of regulatory T cells ↓ T cell autoreactivity ↑ genes related to maturation, antigen presentation and oxidative stress in DCs and mTECs |
Modulation of thymic antigen presenting cell populations (DCs and mTECs) |
| Fernandez-Gonzalez et al. (2021) | WJMSCs (human) & BMSCs(human) | MEx (small EVs) | Differential centrifugation, TFF, OptiPrepTM cushion density flotation | In vivo: BPD (hyperoxia 75% O2) |
IV | 1 Dose 50 μl of EVs ≈ 0.5x106 cell equivalents on PN4 |
Lung: ↑ Vascularization and ↑alveolarization Brain: ↑myelination ↓ astrogliosis ↓activation of microglial cells Retina: Preserved the retinal thickness, ↓gliosis and ↓microglial activation and invasion into the outer nuclear layer. |
Macrophage/microglia immunomodulation |
Abbreviations: AECs, alveolar epithelial cells; Amφ, alveolar macrophages; BMDM, bone marrow derived monocytes; BMDMy, bone marrow derived myeloid cells; BM-MSCs, bone marrow mesenchymal stem cells; BPD, bronchopulmonary dysplasia CM, conditioned media; DCs, dendritic cells; EVs, extracellular vesicles; GA hUC-MSCs, early gestational age mesenchymal stem cells; HDF, human dermal fibroblast; HPH, hypoxia-induced pulmonary hypertension; hUC MSCs, human umbilical cord blood mesenchymal stem cells; HUVEC, human endothelial cells; IP, intraperitoneally IT, intratracheally; IV, intravenously; MEx, mesenchymal stem cell derived small extracellular vesicles; miR, microRNA; mTEC, medullary thymic epithelial cells; PEG, polyethylene glycol; PH, pulmonary hypertension; PN, post-natal day; RVH, right ventricular hypertrophy; TFF, tangential flow filtration; TSG-6, tumor necrosis factor alpha-stimulated gene-6; UC, ultracentrifugation; VEGF, vascular endothelial growth factor; WJ-MSCs, umbilical cord Wharton’s jelly mesenchymal stem cells.
Figure 1.
MSC-EV therapeutic effects in BPD. MSC-EVs prevent the development of BPD by acting on multiple disease components. They modulate macrophage activation by enhancing a pro-resolving rather than a proinflammatory phenotype, resulting in the prevention of inflammation and reducing inflammatory cell infiltration. MSC-EVs promote lung alveolarization and vascularization, thus blocking the development of alveolar simplification and growth arrest observed in BPD. They potentially confer protection of the lung epithelial cells from oxidative injury, as well as have a systemic effect protecting other organs (brain, eye, and thymus) from oxygen toxicity (not shown).
More recently, our group documented a very interesting and unique ability of MSC-EVs, their potential of preventing, but also reverting BPD.33 Specifically, early MSC-EV administration prevented hyperoxia-induced alveolar simplification, pulmonary vascular muscularization and microvascular loss, in a murine model of hyperoxia-induced BPD. In parallel, serial MSC-EV treatment after prolonged hyperoxic exposure was able to significantly ameliorate the established injury by dramatically improving alveolarization, pulmonary fibrosis, and vascular remodeling. This was also accompanied by functional improvement as demonstrated by the exercise capacity tests serving as a surrogate for cardio-pulmonary function of the affected mice.33
In an effort to shed light on the therapeutic mechanism of MSC-EVs and their cell interactions, we employed mass cytometry analysis of the whole lung CD45+ cell populations.34 The analysis revealed three major observations: 1. the MSC-EVs co-localized with the recipient F4/80+, CD64+ myeloid cells indicating their direct interaction; 2. MSC-EV treatment was able to prevent hyperoxia-induced reduction of pulmonary CD45+ cell number and preserve alveolar macrophage (AMφ) and Ly6Clow monocyte subpopulations; 3. MSC-EV treatment blunted the hyperoxia-induced inflammatory activation of AMφs. Further analysis of MSC-EV and myeloid cell interaction indicated that MSC-EV pre-conditioning of bone marrow derived myeloid cells (BMDMy) induced a Ly6C/G+, CX3CR1+, CCR2- phenotype, with immunosuppressive capacity, and possibly promoted a CCR2low monocyte population by implementing transcriptomic and epigenetic reprogramming of BMD-monocytes. Notably, adoptive transfer of BMDMy “educated” by MSC-EVs prevented the hyperoxic injury conferring similar histological and functional results as the MSC-EVs treatment,34 replicating our previous findings on the bleomycin model of pulmonary fibrosis.35
Other groups have corroborated similar beneficial effects of MSC-EVs in preclinical models of BPD (Table 1). Porzionato et al. reported that intratracheal administration of MSCs or their EVs ameliorated hyperoxia-induced damage, with EVs being more effective regarding lung vascularization and alveolarization.36,37 They also documented that hyperoxia reduced the number of CD163+ macrophages (M2-like marker) both in interstitial, alveolar and perivascular compartments, while MSC-EV treatment preserved this population.37
Interestingly, while several groups demonstrate analogous beneficial effect of MSC-EVs in BPD preclinical studies, they identify a variety of different agents responsible for their protective action (Table 1, Fig. 1). Ahn et al. and Braun et al. report improvement of BPD features by MSC-EV treatment, while they identify vascular endothelial growth factor (VEGF) as a key player of their action.38,39 You et al. associated the amelioration in alveolarization and angiogenesis with PTEN/Akt pathway and their downstream targets, such as caspase 3 and VEGF-A.40 Wu et al. reported that MSC-EV protective effect is mediated by the delivery of miR-425 into the lung cells. Inhibition of miR-425 expression in MSCs reversed the EV protective effect against oxidative damage of a lung epithelial cell line challenged with H2O2. Supportive evidence suggests that miR-425 activates the PI3K/AKT axis by targeting PTEN and thus inhibits hyperoxic injury.41
On the other hand, Chaubey et al., detected tumor necrosis factor-stimulated gene 6 (TSG-6), an immunomodulatory glycoprotein, in MSC-EVs and pinpoint it as an important component of their activity. Knockdown of TSG-6 in MSC-EVs abrogated their therapeutic effect, while administration of TSG-6 in vivo was able to attenuate BPD-associated pathologies in lung, heart, and brain. Notably, when examining the brains in this model, they noticed that the EV- treated group had less neuronal apoptosis and restored myelination.42
More recently, our group reported the beneficial effect of MSC-EVs in organ systems other than the lung in the setting of hyperoxia-induced BPD. Fernandez-Gonzalez et al. observed significant hyperoxia-induced injury to the neonatal brain and retina occurring simultaneously to the lung disrupted vascularization and alveolarization findings of BPD. Regarding the neonatal brain, hyperoxia exposure decreased myelination, and increased astrogliosis and activation of microglial cells compared to the normoxia controls. Interestingly, a single intravenous administration of MSC-EVs was able to prevent the hyperoxic effects on myelin sheath, astroglia and microglia restoring them to normoxic controls.43 The retina observations are discussed in the ROP section of this review, as the mechanism of disease is more relevant. We also reported significant oxygen toxicity on the thymus of the newborn pups and, therefore, on the developing adaptive immune system.44 More specifically, Reis et al., showed that hyperoxia led to significant involution of the thymic medulla, which was accompanied by disrupted generation of Foxp3+ regulatory T cells at a multiorgan level, as well as increased T cell autoreactivity (Table 1). Systemic administration of MSC-EVs was able not only to prevent the development of BPD in the lung but also to preserve the thymic medullary architecture and the development of regulatory T cells. MSC-EVs had the ability to prevent oxygen-induced T cell autoreactivity to levels comparable to normoxic controls. Implementing single-cell RNA sequencing, we demonstrated as a potential mechanism of MSC-EV treatment the modulation of thymic antigen presenting cell populations, such as dendritic cells (DCs) and medullary thymic epithelial cells (mTECs). Specifically, upon MSC-EV treatment these cell populations exhibited increased expression of genes related to maturation, antigen presentation, and cellular protection against oxidative stress injury.44 A summary of the studies on BPD and their main results are depicted in Figure 1 and Table 1.
MSC-EVs for Perinatal Brain Injuries
Hypoxic ischemic encephalopathy is a serious perinatal complication occurring in 1-8 per 1000 live births in industrialized countries and approximately 26 per 1000 live births in underdeveloped countries.45 HIE refers to neurologic dysfunction resulting from inadequate brain perfusion and oxygenation.46 Common etiologies include acute blood loss secondary to placental abruption, fetal/maternal hemorrhage, or umbilical cord prolapse. Ultimately, oxygen deprivation results in cell injury, particularly in highly susceptible oligodendrocytes that structurally support brain tissue. Even though advances in obstetric and neonatal care have significantly reduced mortality, survivors still remain at risk of long-term unfavorable neurodevelopmental outcomes, such as cognitive disorders (20–50%), epilepsy,47 or cerebral palsy (5-10%).48-50 Therefore, novel neuroprotective strategies are needed to optimize outcomes and disease prognosis.
Initially, the therapeutic potential of MSCs was investigated in preclinical HIE models. MSCs demonstrated significantly enhanced neuroprotection, neuro-regeneration, and functional recovery, along with attenuated neuroinflammation.14,51-54 Interestingly, similar results were observed after MSC-CM administration in an HIE rat model.55 Although the exact therapeutic moiety was not identified, these findings were attributed to the several neurotrophic factors contained in MSC-CM, especially insulin-like growth factor-1 (IGF-1) and brain-derived neurotrophic factor (BDNF).55
Ophelders et al. were the first to recapitulate the MSC neuroprotective effects by delivering MSC-EVs in a HIE ovine model (Table 2).19,54 Initially, they showed that MSCs were able to enhance myelination, while decreasing white matter injury, oligodendrocyte loss, and microglia proliferation.54 To investigate the potential mechanism of action, they tested the efficacy of MSC secretome. Notably, MSC-EVs improved brain function, reduced the total number and duration of seizures, and histologically restored subcortical white matter myelination; but neuroinflammation was not prevented in this study.19 In later studies, EV-mediated neuroprotection was linked to the preservation of the blood–brain barrier (BBB) integrity.56 The latter functions as a highly selective filter, preventing systemically circulating substances, such as microorganisms and medications, from entering the cerebrospinal fluid and the central nervous system. During HIE, BBB disruption by free radicals permits immune cells to enter the brain and induce neuroinflammation.57,58 The same group demonstrated that MSC-EVs prevented HIE-induced BBB albumin leakage, possibly by targeting the Annexin A1/formyl peptide receptor axis.56
Table 2.
Summary of technical details and main results of studies on MSC-EVs in neonatal brain diseases.
| Reference | MSC source | MSC product | Isolation | Disease model | Route | Dose/ frequency | Main result/action↑↓ | Pathway/active factor |
|---|---|---|---|---|---|---|---|---|
| Ophelders et al. (2016) | BM-MSCs (human) | EVs | MSC-CM filtration, PEG, low-speed centrifugation | In vivo: HIE transient UCO in the preterm ovine fetus |
In utero IV | 2 Doses 2 × 107 cell equivalents 1 h following UCO and 4 days after the insult |
↓ Total number and duration of seizures Preserved baroreceptor reflex sensitivity ↓ hypomyelination |
|
| Drommelschmidt et. al. (2017) | BM-MSCs (human) | EVs | PEG, UC | In vivo: LPS induced perinatal brain injury |
IP | 2 doses 1 × 108 cell equivalents/kg 3 h prior to and 24 h after IP injection of the vehicle or LPS |
↓Neuronal degeneration ↓ microgliosis ↓ reactive astrogliosis Prevented myelination deficits and white matter microstructural abnormalities ↑ cognitive function |
|
| Joerger-Messerli et al. (2018) | WJMSC (human) | EVs | Serial centrifugation | In vitro: OGD in the mouse neuroblastoma cell line neuro2a (N2a) |
1 Dose 0.1 mg/mL or 1 mg/mL of EVs either 24 h or 1 h before, or 6 h after OGD induction |
↓ DNA fragmentation and Casp3 expression | Delivery of let-7-5p-miR targeting proapoptotic genes | |
| C. Sisa et al. (2019) | BM-MSCs (human) | EVs | UC | In vivo: HIE modified Rice–Vannucci model |
IN | 1 Dose 6 µL of EVs 1.25 × 109 particles/dose |
↓ Microglia activation ↓ apoptosis ↓ brain tissue volume loss ↑ behavioral outcomes |
|
| R. Gussenhoven et al. (2019) | BM-MSCs (human) | EVs | PEG, low-speed centrifugation and UC | In vivo: HIE model ovine fetus UCO model in vitro: OGD in primary fetal endothelial cells |
IV | 2 Doses 2 × 107 cell equivalents at 1 h and 4 days after injury |
In vivo: ↓ BBB leakage in vitro: Restored endothelial barrier integrity |
Annexin A1 (ANXA1) in MSC-EVs targets the formyl peptide receptor (FPR) activation. |
| Thomi et al. (2019a) | WJ-MSC (human) |
Exosome | Serial centrifugation and UC | In vivo: LPS induced perinatal brain injury & modified Rice–Vannucci model in vitro: LPS stimulation of BV-2 microglia and primary mixed glial cells. |
IN | 1 Dose of 50 mg/kg. | In vivo: ↓ neuroinflammation ↓ pro-inflammatory cytokine production ↓ microgliosis in vitro: ↓inflammatory gene expression |
interfered with the TLR-4 signaling pathway, ↓degradation of IkBa and ↓phosphorylation of MAP kinase family molecules. |
| G.Thomi et al. (2019b) | WJ-MSC (human) |
Exosomes | Serial centrifugation and UC | In vivo: LPS induced perinatal brain injury & modified Rice–Vannucci model |
IN | 1 Dose of 50 mg/ kg | ↑ Animal survival ↓neuronal cell death Preserved: myelination, mature oligodendroglia and neuron cell counts ↑functional recovery ↑ the learning ability of treated animals. |
|
| Xin et al. (2019) | BM-MSCs (mouse) |
EVs | Centrifugation, filtration, ultrafiltration and EVs isolation kit (qEV, iZonScience) | In vivo: HIE modified Rice–Vannucci model |
ICV | 1 Dose of 100μg/ml 24 h after HI |
Neuroprotective effect ↓neuronal apoptosis and neuroinflammation skewed microglia and brain monocyte/macrophage toward a more anti-inflammatory phenotype. |
MSC-EVs transfer of miR-21a-5q to neurons which targets Timp3 gene. |
| Kaminski et al. (2019) | BM-MSCs (human) | EVs | Sequential centrifugation, PEG and UC | In vivo: HIE modified Rice–Vannucci model |
IP | 3 Doses (day 1, 3, and 5 after injury) 1 × 105 cell equivalents/g |
↓Striatal tissue loss ↓ microglia and astroglia activation In microglia: ↓ TNFa, ↑ YM-1 and TGFb In astroglia: ↓ C3,↑neural growth factors(BDNF, VEGF, EGF). ↑neuronal and vessel density ↑ cell proliferation in the neurogenic sub-ventricular zone juxtaposed to the striatum. Improved oligodendrocyte maturation and myelination |
Immunomodulation of microglia and astroglia phenotype (M1/M2 & A1/A2) |
| Chu et al. (2020) | BM-MSCs (mouse) |
EVs and H2S-EVs | Centrifugation, filtration, ultrafiltration and EVs isolation kit (qEV, iZonScience) | In vivo: HIEmodifiedRice–Vannucci model |
ICV | 1 Dose 100 μg EVs 1.5 × 108 particles 24 h following HI insult |
↑ Cognitive function MSC-EVs were found in both microglia and neurons 2h post-administration H2S-EVs were more potent at: ↓ brain tissue loss ↑ a more anti-inflammatory brain environment ↑ long-term cognitive and memory outcomes |
EV delivery of miR-7b-5p results in microglia and monocyte immunomodulation H2S MSC pre-treatment ↑ miR-7b-5p EV content miR-7b-5p delivery into the cells induces further miR-7b-5p expression |
| Han et al. (2021) | hUC-MSCs | EVs | Serial centrifugation & UC | In vivo: HIE modified Rice–Vannucci model in vitro: OGD to primary neurons |
IP | 4 Doses (prior and after the injury) 2 × 105 cell equivalents | In vitro: ↓ neuronal apoptosis in vivo: ↓ edema formationand infarction volume Ameliorated the neurological severity score |
EV delivery of miR-410 prevents neuronal apoptosis by an HDAC1-dependent EGR2/Bcl2 axis |
| Xin et al. (2021) | BM-MSCs(mouse) | EVs | Differential centrifugation & UC | In vivo: HIE modified Rice–Vannucci model |
ICV | 1 dose of 100 μg of EVs 24 h after HI |
↓ OPN expression induced by HI insult in microglia and macrophages restored synaptic reorganization ↑ synaptic protein expression ↓ edema and infarction volume |
EVs ↓OPN expression through NF-κB involvement |
| Ahn et al. (2020) | hUC-MSCs | Cells & EVs | UC | In vivo: IVH rodent model in vitro: rat cortical neuronal cells challenged with thrombin |
ICV | 1 × 105 MSCs or 20 μg of EVs at P6. |
In vitro: ↓ thrombin-induced neuronal cell death In vivo: ↓neuronal cell death, ↓ astrogliosis ↓ inflammatory responses ↑myelin basic protein and neurogenesis ↓ progression of post hemorrhagic hydrocephalus Ameliorated behavioral tests |
BDNF transfer via EVs |
| Pathipati et al. (2021) | BM-MSCs (mouse) |
EVs | Sequential centrifugation, filtration, ExoQuick TC Ultra |
In vivo: perinatal rodent stroke model in vitro: Microglia cells of HI mice |
ICV or IN | 1 Dose 1 μg/μL or 5 μg/μL |
↓ Edema and infarction volume MSC-sEV reside in microglia/macrophages of the injury site ↓ microglial morphological transformation ↓ cytokine/chemokine concentration ↓ caspase-3-dependent apoptotic cell death |
Modulate the microglia phenotype and cytokine production |
Abbreviations: BBB, blood–brain barrier; BDNF, brain derived neurotrophic factor; BM-MSCs, bone marrow mesenchymal stem cells; CM, conditioned media; EGF, epidermal growth factor; EVs, extracellular vesicles; H2S, hydrogen sulfide; H2S-EVs, hydrogen sulfide conditioned mesenchymal stem cell derived extracellular vesicles; HIE, hypoxic ischemic encephalopathy; hUC MSCs, human umbilical cord blood mesenchymal stem cells; ICV, intracerebroventricularly; IN, intranasally; IP, intraperitoneally; IT, intratracheally; IV, intravenously; LPS, lipopolysaccharide; MEx, mesenchymal stem cell derived small extracellular vesicles; miR, microRNA; OGD, oxygen/ glucose deprivation assay; OPN, osteopontin; PEG, polyethylene glycol; PN, post-natal day; TFF, tangential flow filtration; TNF-α, tumor necrosis factor alpha; UC, ultracentrifugation; UCO, umbilical cord occlusion; VEGF, vascular endothelial growth factor; WJ-MSCs, umbilical cord Wharton’s jelly mesenchymal stem cells; YM-1: CH3L3, chitinase 3 like protein 3.
Several groups have attributed the neuroprotective effects of MSC-EVs to their immunomodulatory capacity (Table 2). Kaminski et al. using human MSC-EVs in a rodent model of HIE reported significantly reduced microglia and astroglia activation, along with alterations in their inflammatory profile. Specifically, MSC-EVs significantly decreased pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α) expression, accompanied by upregulation of the M2-like marker YM-1 (CHIL3), and the anti-inflammatory cytokine transforming growth factor beta (TGFβ) in injured cortex.59 Similarly, MSC-EVs significantly downregulated astrocytic pro-inflammatory complement marker C3, while enhancing pro-regenerative marker S110A10 and mRNA expression of important growth factors, such as BDNF, VEGF, EGF, and IGF-1. These alterations were associated with increased neuronal and vascular density and significant improvement of oligodendrocyte maturation and myelination.59 Similarly, other groups reported a reduction of HIE-induced microglia activation by MSC-EV treatment, accompanied by improved behavioral outcomes, and decreased brain tissue loss.59-62 On the same note, Xin et al. correlated the neuroprotective properties of MCV-EVs with downregulation of HIE-induced microglial/macrophage osteopontin expression, a proinflammatory mediator in the CNS, mediated potentially via inhibition of the NF-κB inflammatory cascade.62
Meanwhile, other groups have associated the beneficial effects observed with MSC-EVs in HIE preclinical models with miR activity (Table 2). In 2018, human MSC-EVs were shown to exert neuroprotective effects in a mouse neuroblastoma cell line (N2a) via EV-contained miRs of the let-7-5p family that regulated caspase 3.20 Using an analogous model of oxygen–glucose deprivation/reoxygenation in vitro, Han et al. replicated the neuroprotective effects of MSC-EVs; notably, inhibition of neuronal apoptosis was abrogated following treatment with RNase A.63 Similarly, beneficial outcomes were recorded following MSC-EV administration in vivo, and were particularly associated with miR-410 by the same group.63 In another HIE mouse model, mouse-derived BM-MSC-EVs containing miR-21a-5p achieved both anti-inflammatory and anti-apoptotic effects; the latter was abolished following pretreatment with miR-21a-5p inhibitor.61 On the other hand, MSC pretreatment with hydrogen sulfide yielded EVs with significantly enhanced protective properties and miR-7b-5p content. Again, any additional benefit compared to the morphologically similar EVs without hydrogen sulfide pretreatment was lost following miR-7b-5p knockdown.64
Notably, MSC-EVs have shown remarkable neuroprotective potential in other brain injury models besides HIE (Table 2). Thomi et al. explored the therapeutic effect of MSC-EVs in an in vivo model of combined LPS and hypoxic-ischemic perinatal brain injury. MSC-EVs improved the survival rate and rescued normal myelination, mature oligodendroglia, and neuronal cell counts. They significantly improved the learning ability and memory of treated animals 4 weeks post-injury but were unable to prevent long-term memory impairment. MSC-EVs dampened the LPS-induced neuroinflammation, both in vivo and in vitro, possibly through a TLR-4/CD14 signaling pathway preventing the degradation of IkBα and the phosphorylation of MAP kinase family molecules, such as ERK1/2, JNK, and p38.65,66 Notably, bio-distribution studies demonstrated even distribution of MSC-EVs throughout the whole brain, as well as the deep layers 3 h post-intranasal administration.65 Similarly, in a model of LPS-induced perinatal brain injury, Drommelschmidt et al. demonstrated that MSC-EVs decreased neuronal damage, microgliosis and reactive astrogliosis, as well as prevented myelination defects and white matter injury. Even though MSC-EVs did not alter activity and anxiety parameters or learning behavior of adolescent and adult rats, they improved the long-term cognitive function.67 Ahn et al. were able to show identical efficacy of umbilical cord MSC-EVs to the parent cells in a rodent model of neonatal intraventricular hemorrhage (IVH). MSC-EVs attenuated IVH induced neuro-inflammation and apoptosis, as well as prevented progression of post-hemorrhagic hydrocephalus, and improved behavioral outcomes possibly by BDNF transfer.68 Pathipati et al. were able to recapitulate the neuroprotective effects of BM-MSCs with the use of BM-MSC-EVs in an in vivo model of perinatal stroke. Mouse BM-MSC-EVs were able to significantly reduce the infarct volume and the caspase 3 dependent apoptosis by modulating microglial cytokine and chemokine profile in the injury site. Importantly, they observed similar therapeutic effects with either intranasal or intracerebroventricular EV administration, while EVs were specifically located in microglia/macrophages of the injury site.69 These findings facilitate the MSC-EVs transition from the bench to the bedside, as they indicate an effective non-invasive administration route and postulate their targeted therapeutic effects to the injury site. The studies on neonatal brain injury models and their main results are summarized in Table 2.
MSC-EVs for ROP
Another sequelae of premature birth, which can be seen alone or associated with BPD, is ROP. This is a potentially blinding vasculo-proliferative retinal disease, which remains the second leading cause of childhood blindness in the US after cortical visual impairment.70 The pathophysiology of ROP includes two phases: phase 1 involves delayed physiologic retinal vascular development, and phase 2 involves vasoproliferation. Premature delivery exposes the immature retina to higher-than-normal oxygen levels, even in ambient air. This hyperoxic status decreases hypoxia inducible factor 1α (HIF1α), leading to decreased VEGF, as well as IGF-1 levels, thus halting retinal vessel growth. Subsequently, this leads to impaired retinal oxygen supply resulting in increased angiogenic signaling, which promotes disorganized proliferation of leaky and immature retina vessels possibly leading to vitreo-retinal traction and retinal detachment.71,72 Although current treatment options, such as laser photocoagulation, target disease progression and reduce the incidence of blindness from ROP, treated patients often still have suboptimal visual acuity. Thus, less invasive alternative treatments focusing on disease prevention need to be explored.
Several groups have reported beneficial effects of intravitreal administration of MSCs and their CM for retinal vascular injury either in oxygen-induced retinopathy (OIR), a preclinical model of ROP, or in ischemia-reperfusion models. MSC treatment was able to decrease the area of neovascularization, preserve retinal thickness and prevent the loss of retinal ganglion cells.73-76 In addition, BM-MSCs and their CM were able to inhibit neovascularization and diminish initial vaso-obliteration potentially by restoring neuronal semaphorin 3E (Sema3E) levels leading to reduction of interleukin-17A (IL-17A) and other proinflammatory factors in myeloid cells.76 Accordingly, Moisseiev et al., reported preserved retinal vascular flow, attenuated neovascularization, and reduced retinal thinning following human BM-MSC-exosome treatment in an OIR model. Proteomic analysis of BM-MSC-exosomes, to assess factors mediating their protective effects, demonstrated pro-survival-associated proteins, such as cAMP response element-binding protein (CREB) pathway. Notably, BM-MSC-exosome treatment did not provoke any immunogenicity or had any adverse effects.77 More recently, Fernandez-Gonzalez et al. investigated the retina of mouse pups exposed to 7 days of hyperoxia in a rodent model of BPD treated with hWJ-MSC-EVs. Hyperoxia exposure resulted in reduction of retinal thickness, as well as induction of gliosis. In addition, hyperoxia induced microglia activation and invasion into the outer nuclear layer depicted as increased ionized calcium binding adaptor molecule (Iba-1) immunofluorescence. Interestingly, a single dose of MSC-EVs was able to preserve retinal thickness, decrease gliosis and prevent microglial activation and invasion of the outer nuclear layer.43Table 3 summarizes the main details of the studies on ROP.
Table 3.
Summary of studies on MSC-EVs in ROP, NEC, and perinatal lung growth.
| Reference | MSC source | MSC product | Isolation | Disease model | Route | Dose/ frequency | Main result/action↑↓ | Pathway/active factor |
|---|---|---|---|---|---|---|---|---|
| ROP | ||||||||
| Mathew B. et al. (2019) | BM- MSCs (human) |
EVs | Sequential centrifugation, ultrafiltration & Exo Quick-TC EV | In vivo: rat model of retinal ischemia in vitro: OGD of R28 retinal cells |
IVit | 1 Dose 4 μL of 1 × 109 particles/mL 24 h post-injury in both the ischemic and non-ischemic eyes |
↓ Cell death & ↑ cell proliferation ↑ functional recovery ↓ neuro-inflammation & apoptosis |
Delivery of pro-survival proteins from the cAMP response element-binding protein (CREB) pathway |
| Moisseiev et al. (2017) | BM-MSCs (human) cultured in 1% O2 for 48hr | Exosomes | Serial centrifugation, TFF, VivaSpin filtration column | In vivo Oxygen-induced Retinopathy (OIR) (75% O2) |
IVit | 1 Dose 1 μl ≈ 20 μg protein on PN12 |
Partially preserved Retinal vascular flow ↓ retinal thinning ↓ retinal neovascularization no immunogenicity or ocular/systemic adverse effects were observed |
|
| NEC | ||||||||
| Rager et al. (2016) | BMSCs (rat) |
Cells and Exosomes | In vivo: P100 PureExo Exosome Isolation kit in vitro: serial centrifugation & UC |
In vivo NEC rodent model in vitro: Intestinal epithelial cell wound healing assay IEC-6 cells |
IP | 1 Dose MSCs: 3 × 105 cells or Exosomes: 2.5 × 109 5h post-delivery |
In vivo: ↓ the incidence and severity of disease preserve gut barrier function in vitro: ↑wound healing of IEC-6 cells |
|
| McCulloh et al. (2018) | AF-MSC, BM-MSC, AF-NSC and E-NSC (rat) |
Exosomes | Differential UC | In vivo: NEC rodent model |
IP | 1 Dose 50 μL of MSC-Exosomes 1 h post-delivery. 1.3 × 105 EVs/50 μL, 6.4 × 105 EVs/50 μL, 3.2 × 106 EVs/50 μL, 1.6 × 107 EVs/50 μL, 8.0 × 107 EVs 50 μL, 4.0 × 108 EVs/50 μL |
↓ NEC incidence and severity Equal efficacy of EVs and parent MSCs Best results at 8 × 107 or 4.0 × 108 EVs/50 μL |
|
| LIet al. (2020) | AFSC (rat) | Cells and EVs | ExoQuick | In vivo: NEC mouse model in vitro: Intestinal epithelial cell wound healing assay IEC-18 cells |
IP | 2 Doses 2 × 106 AFSCsOr AFSC-EVs derived from 200 μL of CM of 2 × 106 AFSCs at PN 6 and 7 Or at PN3 and 4 prior to NEC injury induction |
AFSC and EV attenuate NEC intestinal injury ↑cellular proliferation, ↓inflammation regenerating a normal intestinal epithelium |
Activate the Wnt signaling pathway |
| O’Connell et al. (2021) | AFSC (human) | EVs | Sequential centrifugation, UC | In vivo: NEC mouse model | IP | 2 Doses 100 μL of 3.0 × 107 cell equivalents PN 6 and 7 |
↓ Intestinal injury ↓NEC incidence ↓intestinal inflammation (IL -6, TNF-α) ↑ Intestinal stem cell expressionand ↑cellular proliferation |
|
| Perinatal lung growth | ||||||||
| Abele et al. (2021) | BM-MSCs (human) | EVs | Differential centrifugation, TFF, OptiPrepTM cushion density flotation | In vivo: chorioamnionitis rat model In vitro: Fetal Lung Explants |
IA | 1 Dose 0.25 × 106 cell equivalents ≈ to 4.25 × 108 particles |
↓Placental inflammatory cytokines normalized spiral artery architecture preserved distal lung growth and mechanics In vitro: ↑ distal lung branching ↑ VEGF & SPC gene expression |
|
| Taglauer et al. (2021) | WJMSCs (human) | EVs | Differential centrifugation, TFF, OptiPrepTM cushion density flotation | In vivo: lung injury in experimental preeclampsia, Hmox1-null model in vitro: Fetal lung explants |
IV | 3 Doses (weeks 1, 2, and 3 of pregnancy) ≈ 5 × 106 cell equivalents |
Normalization of lung developmental genes ↑ pup birth weight ↓ alveolar simplification and lung developmental arrest altered the amniotic fluid proteomic profile AF of MEx treated pregnancies: ↑ distal lung branching ↑lung developmental genes |
Normalization of fetal lung development by altering the AF proteomic profile |
Abbreviations: AF, amniotic fluid; AF-MSCs, amniotic fluid-derived mesenchymal stem cells; AF-NSCs, amniotic fluid-derived neural stem cells; E-NSCs, neonatal enteric neuronal stem cells; BM-MSCs, bone marrow mesenchymal stem cells; CM, conditioned media; EVs, extracellular vesicles; hUC MSCs, human umbilical cord blood mesenchymal stem cells; IA, intra-amniotic; IL-6, interleukin 6; IP, intraperitoneally; IT, intratracheally; IV, intravenously; IVit, intravitreally; LPS, lipopolysaccharide; MEx, mesenchymal stem cell derived small extracellular vesicles; miR, microRNA; NEC, necrotizing enterocolitis; OGD, oxygen/glucose deprivation assay; PEG, polyethylene glycol; PN, post-natal day; ROP, retinopathy of prematurity; SPC, surfactant protein-C; TFF, tangential flow filtration; TNF-α, tumor necrosis factor alpha; UC, ultracentrifugation; VEGF, vascular endothelial growth factor; WJ-MSCs, umbilical cord Wharton’s jelly mesenchymal stem cells.
MSC-EVs for NEC
Necrotizing enterocolitis is a devastating gastrointestinal disease of prematurity, primarily affecting preterm infants with a birth weight of less than 1500 g. It is estimated to affect approximately 1-3 infants per 1000 live births in the US, with 20%-40% requiring surgical intervention.2,78,79 NEC has a multifactorial etiology, with several contributing factors, such as prematurity, formula feeding, and bacterial contamination. The immature gastrointestinal mucosa and the naïve immune system facilitate the invasion of gas-forming bacteria into intestinal epithelium, leading to extensive intestinal inflammation, full-thickness necrosis, and perforation. This devastating injury often results in systemic inflammation, short bowel syndrome, prolonged neonatal hospitalization, impaired growth, and poor long-term neurodevelopment.80,81 Even though, early recognition and aggressive treatment have significantly improved the clinical outcomes, NEC still accounts for substantial morbidity, mortality, and high costs for families and society. Therefore, the exploration of alternative treatment strategies is essential.
Several preclinical studies have demonstrated the protective effects of MSCs in NEC models. MSCs from variable sources improve survival rate, weight gain and significantly attenuated mucosal damage following intraperitoneal or intravenous delivery.10,82 More recently, McCulloh et al., compared the therapeutic efficacy of MSCs from different sources (amniotic fluid (AF)-MSCs, BM-MSCs, amniotic fluid-neuronal stem cells (AF-NSCs), and neonatal enteric neural stem cells (E-NSCs)) and observed similar therapeutic effects on reducing the incidence and severity of experimental NEC, as well as preserving the intestinal permeability.83,84
Rager et al. from the same group were the first to report the equivalent protective effects of MSC-EVs in a neonatal rat model of NEC (Table 3). A single intraperitoneal injection of MSC-EVs was equally potent to the parent cell in reducing NEC incidence and severity, as well as, preserving the integrity of the gut barrier.85 Later the same group compared the efficacy of EVs derived from AF-MSCs, BM-MSCs, AF-NSCs, and E-NSCs reporting similar efficacy between the different EVs, equivalent to the respective parent cell treatment.86 More recently, Li et al. demonstrated that AF-MSCs and their EVs reduced intestinal injury by activating the Wnt signaling pathway. Both treatments increased cellular proliferation, reduced intestinal inflammation (Interleukin-6, TNF-α), and ultimately regenerated a normal intestinal epithelium. The latter was mediated through increased intestinal stem cells and epithelial proliferation via Wnt signaling. Interestingly, the timing of EV administration was instrumental for their therapeutic effect, as delivery prior to NEC induction failed to prevent injury.87 Later, the same group reported similar protective effects on intestinal inflammation and regeneration with the use of human AF-MSC CM and EVs. Functional proteomic analysis identified several protein clusters associated with immune and cell cycle regulation possibly responsible for their effects.88,89 Taken together, these studies highlight a promising regenerative potential of MSC EV-based therapies for the treatment of NEC, which call for further exploration. A summary of the studies on NEC is presented in Table 3.
The Antenatal Effect of MCS-EVs
Preterm birth is inevitably associated with maternal and placental health, as preeclampsia and intrauterine growth restriction (IUGR) are common reasons for indicated preterm delivery.90 Accumulating evidence highlight the potent effects of antenatal adverse factors on postnatal health, especially on respiratory outcomes.91,92 Mestan et al. demonstrated that histological and cord blood biomarkers related to preeclampsia vascular hypoperfusion were predictive of BPD and pulmonary hypertension in the newborn.93,94 Preeclampsia itself, as well as IUGR status have also been significantly implicated with increased BPD risk95,96 and therefore can potentially impact the neonatal pulmonary health long-term. Similarly, placental inflammation or infection due to chorioamnionitis hinders normal lung growth97 and can lead to worse outcomes.98 The above suggests that prematurity, as well as infant postnatal health and development are significantly associated with the antenatal placental health, highlighting the uteroplacental equilibrium as a potential therapeutic target.
On that note, recently our group demonstrated the protective effect of MSC-EVs on preeclampsia, preeclampsia-associated IUGR status, and lung outcomes (Table 3).99,100 Using a preclinical model of preeclampsia—the heme oxygenase (Hmox1)-null mouse—Taglauer et al., showed that intravenous antenatal MSC-EV therapy was able to prevent core preeclamptic features, as well as significantly improve fetal loss and intrauterine growth restriction.99 Newborn pups of preeclamptic mothers demonstrated significant alveolar simplification altered bronchial epithelial morphology and alterations in lung developmental genes, further confirming the adverse effect of prenatal conditions on the developing lung.100 Interestingly, weekly systemic administration of MSC-EVs to the pregnant preeclamptic mothers was able to prevent the aforementioned deleterious effects on the neonatal lung. Possibly, MSC-EVs confer their therapeutic effect indirectly, as the direct MSC-EV application on lung explants had no effect. MSC-EV therapy significantly altered the cytokine and proteomic profiles of the preeclamptic amniotic fluid (AF), which evidently was the mediator of MSC-EV therapeutic effect on lung development. These alterations are possibly associated with immunomodulation of uteroplacental leukocytes, as mass cytometry analysis showed that a single MSC-EV injection altered the abundance, surface marker repertoire, and cytokine profile of multiple immune cell populations of the uteroplacental environment.99,100
On the same note, using a rat model of endotoxin (ETX) induced-chorioamnionitis, Abele et al. evaluated the effect of intrauterine MSC-EV treatment on the placenta and the neonatal lung. The placentas of the ETX group demonstrated increased inflammatory markers (NLRP-3, IL-1ß) and altered spiral artery morphology. Analysis of ETX group neonatal lungs showed decreased alveolarization and pulmonary vessel density, increased right ventricular hypertrophy, and worse lung mechanics compared to healthy controls; further supporting the impact of antenatal environment on postnatal lung health. Interestingly, intrauterine MSC-EV therapy reduced placental inflammatory cytokines and normalized spiral artery architecture. Additionally, the pups of the MSC-EV group had preserved distal lung growth and mechanics. Finally, MSC-EV treatment on fetal lung explants in vitro conferred enhanced distal lung branching and increased VEGF and surfactant protein C gene expression compared to ETX exposure.101
Consequently, the above studies (Table 3) highlight the detrimental role of the dysregulated intrauterine environment on postnatal lung development, and the tremendous potential of MSC-EVs to modulate both the uteroplacental equilibrium, as well as restore neonatal lung development even in the antenatal setting.
MSC-EV Clinical Translation
Despite the rapidly growing interest and research on MSC-EVs, the field is still in its infancy and there are several challenges to be addressed to achieve an optimal transition to the clinic. One of the major hurdles is the heterogeneity of the MSC-EV preparations brought by the absence of standardized and consolidated criteria for EV production. To this end members of 4 academic societies (SOCRATES, ISCT, ISEV, and ISBT) have proposed specific harmonization criteria for MCS-EV isolation, purification, and characterization, with the hope to help achieve the homogeneity required for the clinic.25 Another important challenge is the need of a thorough evaluation of MSC-EV potency and purity prior to use in the clinic with a reliable functionality assay, as it has been shown that EV preparations might differ in the particle number, potency, and purity resulting in ambiguous functionality.102,103 This important quality control step will certainly facilitate the optimal transition to the bedside.
Notably, variables such as the dosage, the appropriate frequency, and the optimal timing of administration remain debatable. Single or multiple, as well as early versus late administration of the MSC-EVs, still need to be determined and might vary depending on the disease of interest. On that note, there are several preclinical studies reporting beneficial results with a single dose of MSC-EVs, when delivered early in the disease process,18,33,34,44,100 while at the same time some studies have shown reversal or amelioration of the disease features with multiple dosages in later time points.33 In addition, potential safety concerns need to be addressed and monitored, as the exact contents, purity, and heterogeneity of each EV preparation vary, as well as their potential hemocompatibility.104 So far, the results from the preclinical studies examining the therapeutic potential of MSC-EVs, such as those cited in this review, have not reported any major side effects. Lastly, EV preservation and storage are important as certain storage conditions might affect the EV potency.105 The former obstacle of scalability of MCS-EV production for the use in clinical trials is being resolved by the rapid increase in companies stepping into the field of EV production. Evidently, more work is required to better standardize the EV production, isolation, and characterization, as well as to decipher their molecular and cellular mechanism of action. Some important steps to this end are already being done by academic experts in the field with efforts toward standardization and harmonization, such as the MISEV2018 and publications regarding the minimal experimental requirements for EVs.22,25,103,106,107
The promising therapeutic effects of MSC-EVs in several preclinical studies have increased the excitement for their translation to the clinic. Even though to date there are some clinical trials exploring the safety and efficacy of MSCs in BPD, IVH, HIE, and a case study for NEC108-113 (NCT04873752, NCT03635450) to the best of our knowledge there are only 2 on the MSC secretome for neonatal diseases. The first one is a phase I study exploring the safety of MSC-EV therapy for the prevention of BPD (NCT03857841), which was discontinued due to business decisions by the sponsor company. The second one is a study exploring the safety and efficacy of MSC paracrine factors on HIE (NCT02854579) whose status is unknown.
Arguably, the neonatal preterm population is most vulnerable and poses both technical and ethical considerations that may complicate enrollment in clinical trials. The appropriate dosing, as well as the most suitable route of administration (systemic or intratracheal) are factors that need to be considered. Additionally, the timing of intervention is of great importance in terms of disease stage and severity of illness. Based on animal work, early treatment is most effective, but it may lead to babies receiving treatment who otherwise would not have developed the disease. Conversely, treating the most severely ill patients in the setting of what may be an advanced diseases with scarring and fibrosis may not provide meaningful results. Nonetheless, the incidence of BPD is rising, and given the lack of effective therapy to date, there is a great need for well-designed clinical trials to evaluate novel therapies such as MSC-EVs.
When looking at MSC-EV therapy in adult patients there are some clinical trials demonstrating safety and indication of therapeutic efficacy.106,114,115 At the same time, there are clinical trials listed on Clinicaltials.gov across the spectrum of diseases (ARDS, COVID-19, Type I diabetes, epidermolysis bulosa, Crohn’s disease, burns) preparing to start.
Conclusion: Final Remarks
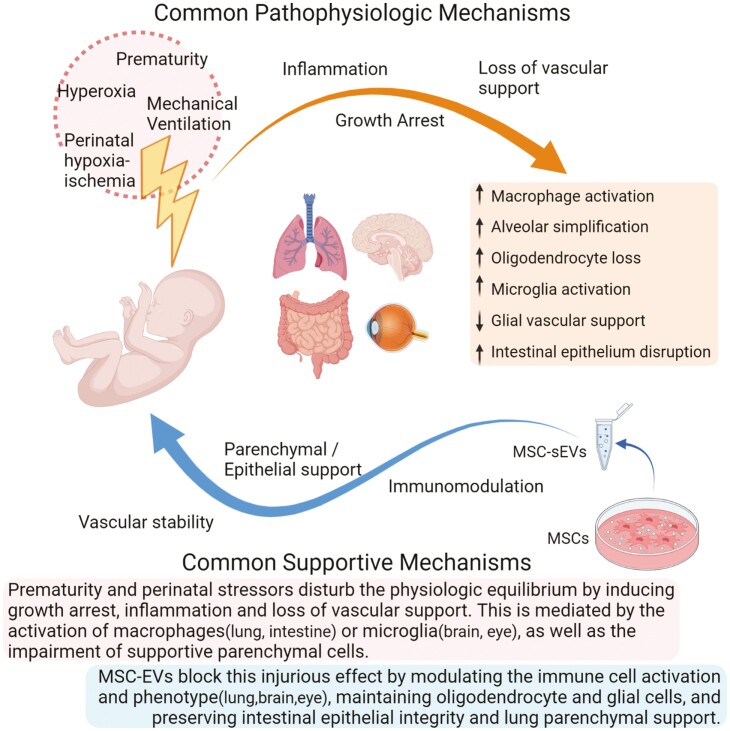
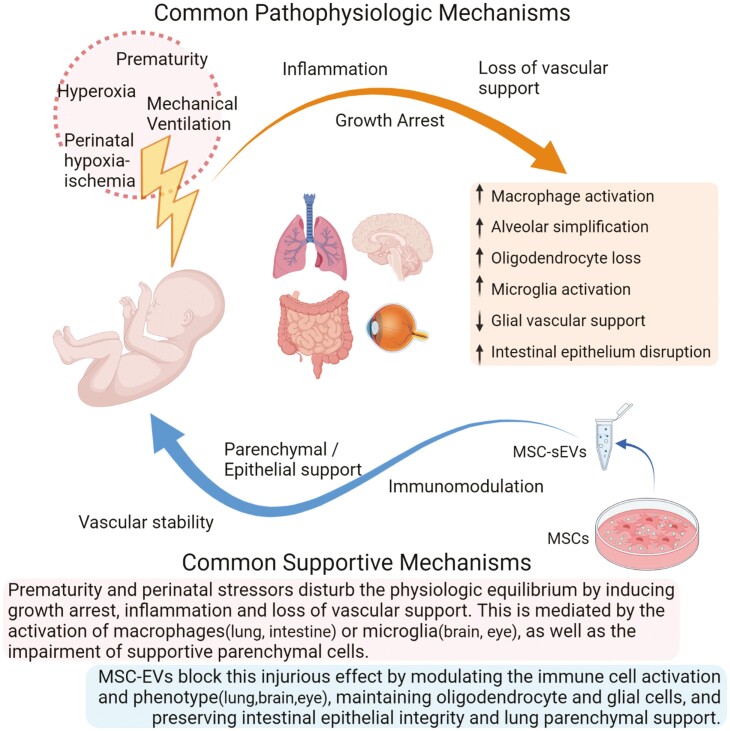
Despite the significant advances in neonatal care, there is still a need for novel therapeutic approaches for the prevention and treatment of neonatal diseases. Premature birth sequelae share some common pathophysiologic mechanisms, such as tissue immaturity, oxygen toxicity, or low oxygenation, as well as the activation of immune cells that are critical for maintaining the vascular and tissue homeostasis. Such disruption in local homeostasis can result in the developmental arrest of the implicated tissues, potentially with long-term consequences. MSC-based therapies have shown promising therapeutic potential for such complex diseases with multifactorial etiologies. It is now widely accepted that MSC therapeutic capacity is comprised in their secretome, with the major therapeutic vector being the MSC-secreted EVs. Several preclinical studies have demonstrated beneficial results of MSC-EV treatment in the full spectrum of neonatal diseases (Tables 1–3). As summarized in these Tables, the MSC source of EVs may vary from BM to amnion and umbilical cord, but the beneficial results observed, and the proposed mechanism of action is comparable. Even though the detailed molecular mechanisms of MSC-EV action remain the focus of intensive and thorough research, their beneficial effects on perinatal pathologies seem to principally rely on immunomodulation. The immunomodulatory reprogramming of the tissue resident, as well as the circulating immune cells, in a pro-homeostatic phenotype is probably favoring the parenchymal support and vascular stability resulting in tissue repair and homeostasis (Fig. 2).
Figure 2.
Schematic illustration of the common pathophysiologic mechanisms shared by neonatal diseases, as well as the common supportive effects of MSC-EV treatment. Prematurity and perinatal stressors disturb the physiologic equilibrium by inducing growth arrest, inflammation and loss of vascular support. This is mediated by the activation of macrophages (lung, intestine) or microglia (bran, eye), as well as the impairment of supportive parenchymal cells. MSC-EVs block this injurious effect by modulating the immune cell activation and phenotype (lung, brain, and eye), maintaining oligodendrocyte and glial cells, and preserving intestinal epithelial integrity and lung parenchymal support.
Indeed, EV-based therapeutics may represent the next-generation drug delivery system, providing an impressive efficacy for the treatment of numerous diseases of complex pathophysiology. However, their clinical application and development remain in their infancy hampered by technical, mechanistic and standardization issues. The need for standardized MSC-sEV production that follows good manufacturing practices, as well as the minimal criteria required for EV characterization as suggested by the ISEV and other academic societies, are crucial for the optimal transition to the clinic. Additionally, quality control of the final EV product regarding purity and potency is very important, as EV preparations might differ in particle number, potency, and purity resulting in ambiguous functionality.102,103 The next important variables are the dosing, the appropriate frequency as well as the optimal timing of administration and storage. Importantly, the quest for the active component of MSC-EVs remains long and complex, despite the rigorous research. Even though several studies have identified different miRs or single proteins as the effector molecule of EV function, their vastly diverse cargo (combination of DNA, RNA, proteins, and lipids) renders improbable a single moiety to be responsible for their action. Instead, it is more likely that an “orchestra” of active elements or enzymatic components exerts the MSC-EV beneficial effect. One study proposed that based on biochemical and biologically relevant concentrations, protein rather than RNA transfer may be the more likely mechanism of MSC-EV action.116 Clearly, more work is required to better standardize the EV production, isolation, and characterization, as well as to decipher their molecular, cellular, and epigenetic mechanism of action that results in long-lasting effects.
Contributor Information
Eleni Delavogia, Division of Newborn Medicine, Department of Pediatrics, Boston Children’s Hospital, Boston, MA, USA; Department of Pediatrics, Harvard Medical School, Boston, MA, USA.
Dimitrios P Ntentakis, Retina Service, Angiogenesis Laboratory, Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, MA, USA.
John A Cortinas, Division of Newborn Medicine, Department of Pediatrics, Boston Children’s Hospital, Boston, MA, USA; Department of Pediatrics, Harvard Medical School, Boston, MA, USA.
Angeles Fernandez-Gonzalez, Division of Newborn Medicine, Department of Pediatrics, Boston Children’s Hospital, Boston, MA, USA; Department of Pediatrics, Harvard Medical School, Boston, MA, USA.
S Alex Mitsialis, Division of Newborn Medicine, Department of Pediatrics, Boston Children’s Hospital, Boston, MA, USA; Department of Pediatrics, Harvard Medical School, Boston, MA, USA.
Stella Kourembanas, Division of Newborn Medicine, Department of Pediatrics, Boston Children’s Hospital, Boston, MA, USA; Department of Pediatrics, Harvard Medical School, Boston, MA, USA.
Funding
This work was supported by NIH R01 HL146128 (SK), R21 AI134025 (SK), Hood Foundation Major Grants Initiative to Advance Child Health (SK), and United Therapeutics Research Grant (SK & SAM). ED was supported by George and Marie Vergottis Postdoctoral Fellowship Award, Harvard Medical School.
Conflict of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
E.D.: conception and design, collection and/or assembly of data, manuscript writing, figure artwork and illustrations, final approval of manuscript. D.P.N.: manuscript review, manuscript writing, manuscript editing. J.A.C.: manuscript review, editing. A.FG.: manuscript review, editing, final approval of manuscript. S.A.M., S.K.: conception and design, financial support, manuscript editing, final approval of manuscript.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37-e46. 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stoll BJ, Hansen N, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388(10063):3027-3035. 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serenius F, Ewald U, Farooqi A, et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatr. 2016;170(10):954-963. 10.1001/jamapediatrics.2016.1210. [DOI] [PubMed] [Google Scholar]
- 5. Stocks J, Hislop A, Sonnappa S.. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1(9):728-742. 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 6. Yang J, Kingsford RA, Horwood J, et al. Lung function of adults born at very low birth weight. Pediatrics. 2020;145(2):e20292359. 10.1542/peds.2019-2359. [DOI] [PubMed] [Google Scholar]
- 7. Collaco JM, McGrath-Morrow SA.. Bronchopulmonary dysplasia as a determinant of respiratory outcomes in adult life. Pediatr Pulmonol. 2021;56(11):3464-3471. 10.1002/ppul.25301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watterberg KL, Walsh MC, Lei L, et al. Hydrocortisone to improve survival without bronchopulmonary dysplasia. N Engl J Med. 2022;386(12):1121-1131. 10.1056/NEJMoa2114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitsialis SA, Kourembanas S.. Stem cell-based therapies for the newborn lung and brain: possibilities and challenges. Semin Perinatol. 2016;40(3):138-151. 10.1053/j.semperi.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tayman C, Duygu U, Emine K, et al. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr Res. 2011;70(5):489-494. 10.1203/PDR.0b013e31822d7ef2. [DOI] [PubMed] [Google Scholar]
- 11. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 12. Jin HJ, Rajasingh J, Pisano C, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986-18001. 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moll G, Ankrum JA, Kamhieh-Milz J, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149-163. 10.1016/j.molmed.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 14. Hegyi B, Kornyei Z, Ferenczi S, et al. Regulation of mouse microglia activation and effector functions by bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2014;23(21):2600-2612. 10.1089/scd.2014.0088. [DOI] [PubMed] [Google Scholar]
- 15. Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180(11):1122-1130. 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansmann G, Fernandez-Gonzalez A, Aslam M, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2(2):170-181. 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126(22):2601-2611. 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willis GR, Fernandez-Gonzalez A, Anastas J, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197(1):104-116. 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ophelders DR, Wolfs TG, Jellema RK, et al. Mesenchymal stromal cell-derived extracellular vesicles protect the fetal brain after hypoxia-ischemia. Stem Cells Transl Med. 2016;5(6):754-763. 10.5966/sctm.2015-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joerger-Messerli MS, Oppliger B, Spinelli M, et al. Extracellular vesicles derived from Wharton’s jelly mesenchymal stem cells prevent and resolve programmed cell death mediated by perinatal hypoxia-ischemia in neuronal cells. Cell Transplant. 2018;27(1):168-180. 10.1177/0963689717738256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeung V, Willis GR, Taglauer E, et al. Paving the road for mesenchymal stem cell-derived exosome therapy in bronchopulmonary dysplasia and pulmonary hypertension. Stem Cell-Based Therapy for Lung Dis. 2019:131-152. 10.1007/978-3-030-29403-8_8. [DOI] [Google Scholar]
- 22. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): a position statement of the International Society for Extracellular Vesicles and update of the Misev2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raposo G, Stoorvogel W.. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383. 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials - an isev position paper. J Extracell Vesicles. 2015;4:30087. 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Witwer KW, Van-Balkom BW, Bruno S, et al. Defining mesenchymal stromal cell (Msc)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8(1):1609206. 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Northway WH, Jr., Rosan RC, Porter DY.. Pulmonary disease following respirator therapy of hyaline-membrane disease. bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357-368. 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 27. Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8(1):73-81. 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 28. Thébaud B. Mesenchymal stromal cell therapy for respiratory complications of extreme prematurity. Am J Perinatol. 2018;35(6):566-569. 10.1055/s-0038-1639371. [DOI] [PubMed] [Google Scholar]
- 29. Nitkin CR, Rajasingh J, Pisano C, et al. Stem cell therapy for preventing neonatal diseases in the 21st century: current understanding and challenges. Pediatr Res. 2020;87(2):265-276. 10.1038/s41390-019-0425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Augustine S, Cheng W, Avey MT, et al. Are all stem cells equal? systematic review, evidence map, and meta-analyses of preclinical stem cell-based therapies for bronchopulmonary dysplasia. Stem Cells Transl Med. 2020;9(2):158-168. 10.1002/sctm.19-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180(11):1131-1142. 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ionescu L, Byrne RN, Van-Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303(11):L967-L977. 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Willis GR, Fernandez-Gonzalez A, Reis M, et al. Mesenchymal stromal cell-derived small extracellular vesicles restore lung architecture and improve exercise capacity in a model of neonatal hyperoxia-induced lung injury. J Extracell Vesicles. 2020;9(1):1790874. 10.1080/20013078.2020.1790874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Willis GR, Reis M, Gheinani AH, et al. Extracellular vesicles protect the neonatal lung from hyperoxic injury through the epigenetic and transcriptomic reprogramming of myeloid cells. Am J Respir Crit Care Med. 2021;204(12):1418-1432. 10.1164/rccm.202102-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mansouri N, Willis GR, Fernandez-Gonzalez A, et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019;4(21):e128060. 10.1172/jci.insight.128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Porzionato A, Zaramella P, Dedja A, et al. Intratracheal administration of clinical-grade mesenchymal stem cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2019;316(1):L6-L19. 10.1152/ajplung.00109.2018. [DOI] [PubMed] [Google Scholar]
- 37. Porzionato A, Zaramella P, Dedja A, et al. Intratracheal administration of mesenchymal stem cell-derived extracellular vesicles reduces lung injuries in a chronic rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2021;320(5):L688-L704. 10.1152/ajplung.00148.2020. [DOI] [PubMed] [Google Scholar]
- 38. Ahn SY, Park WS, Kim YE, et al. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med. 2018;50(4):1-12. 10.1038/s12276-018-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Braun RK, Chetty C, Balasubramaniam V, et al. Intraperitoneal injection of msc-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem Biophys Res Commun. 2018;503(4):2653-2658. 10.1016/j.bbrc.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. You J, Zhou O, Liu J, et al. Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles alleviate lung injury in rat model of bronchopulmonary dysplasia by affecting cell survival and angiogenesis. Stem Cells Dev. 2020;29(23):1520-1532. 10.1089/scd.2020.0156. [DOI] [PubMed] [Google Scholar]
- 41. Wu Y, Li J, Yuan R, et al. Bone marrow mesenchymal stem cell-derived exosomes alleviate hyperoxia-induced lung injury via the manipulation of microrna-425. Arch Biochem Biophys. 2021;697:108712. 10.1016/j.abb.2020.108712. [DOI] [PubMed] [Google Scholar]
- 42. Chaubey S, Thueson S, Ponnalagu D, et al. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor tsg-6. Stem Cell Res Ther. 2018;9(1):173. 10.1186/s13287-018-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fernandez-Gonzalez A, Willis GR, Yeung V, et al. Therapeutic effects of mesenchymal stromal cell-derived small extracellular vesicles in oxygen-induced multi-organ disease: a developmental perspective. Front Cell Dev Biol. 2021;9:647025. 10.3389/fcell.2021.647025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reis M, Willis GR, Fernandez-Gonzalez A, et al. Mesenchymal stromal cell-derived extracellular vesicles restore thymic architecture and T cell function disrupted by neonatal hyperoxia. Front Immunol. 2021;12:640595. 10.3389/fimmu.2021.640595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Douglas-Escobar M, Weiss MD.. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015;169(4):397-403. 10.1001/jamapediatrics.2014.3269. [DOI] [PubMed] [Google Scholar]
- 46. Kurinczuk JJ, White-Koning M, Badawi N.. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):329-338. 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 47. Pierrat V, Haouari N, Liska A, et al. Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Arch Dis Child Fetal Neonatal Ed. 2005;90(3):F257-F261. 10.1136/adc.2003.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee AC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74(Suppl 1):50-72. 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Robertson C, Finer N.. Term infants with hypoxic-ischemic encephalopathy: outcome at 3.5 years. Dev Med Child Neurol. 1985;27(4):473-484. 10.1111/j.1469-8749.1985.tb04571.x. [DOI] [PubMed] [Google Scholar]
- 50. Ravichandran L, Allen VM, Allen AC, et al. Incidence, intrapartum risk factors, and prognosis of neonatal hypoxic-ischemic encephalopathy among infants born at 35 weeks gestation or more. J Obstet Gynaecol Can. 2020;42(12):1489-1497. 10.1016/j.jogc.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 51. van Velthoven CT, Kavelaars A, van Bel F, et al. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun. 2010;24(3):387-393. 10.1016/j.bbi.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 52. Xia G, Hong X, Chen X, et al. Intracerebral transplantation of mesenchymal stem cells derived from human umbilical cord blood alleviates hypoxic ischemic brain injury in rat neonates. J Perinat Med. 2010;38(2):215-221. 10.1515/jpm.2010.021. [DOI] [PubMed] [Google Scholar]
- 53. Kim ES, Ahn SY, Im GH, et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr Res. 2012;72(3):277-284. 10.1038/pr.2012.71. [DOI] [PubMed] [Google Scholar]
- 54. Jellema RK, Wolfs TG, Passos VL, et al. Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS One. 2013;8(8):e73031. 10.1371/journal.pone.0073031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei X, Du Z, Zhao L, et al. Ifats collection: the conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells. 2009;27(2):478-488. 10.1634/stemcells.2008-0333. [DOI] [PubMed] [Google Scholar]
- 56. Gussenhoven R, Klein L, Ophelders DR, et al. Annexin A1 as neuroprotective determinant for blood-brain barrier integrity in neonatal hypoxic-ischemic encephalopathy. J Clin Med. 2019;8(2):137. 10.3390/jcm8020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kumar A, Mittal R, Khanna HD, et al. Free radical injury and blood-brain barrier permeability in hypoxic-ischemic encephalopathy. Pediatrics. 2008;122(3):e722-e727. 10.1542/peds.2008-0269. [DOI] [PubMed] [Google Scholar]
- 58. Moretti R, Pansiot J, Bettati D, et al. Blood-brain barrier dysfunction in disorders of the developing brain. Front Neurosci. 2015;9:40. 10.3389/fnins.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kaminski N, Koster C, Mouloud Y, et al. Mesenchymal stromal cell-derived extracellular vesicles reduce neuroinflammation, promote neural cell proliferation and improve oligodendrocyte maturation in neonatal hypoxic-ischemic brain injury. Front Cell Neurosci. 2020;14:601176. 10.3389/fncel.2020.601176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sisa C, Kholia S, Naylor J, et al. Mesenchymal stromal cell derived extracellular vesicles reduce hypoxia-ischaemia induced perinatal brain injury. Front Physiol. 2019;10:282. 10.3389/fphys.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xin D, Li T, Chu X, et al. Mesenchymal stromal cell-derived extracellular vesicles modulate microglia/macrophage polarization and protect the brain against hypoxia-ischemic injury in neonatal mice by targeting delivery of Mir-21a-5p. Acta Biomater. 2020;113:597-613. 10.1016/j.actbio.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 62. Xin D, Li T, Chu X, et al. Mscs-Extracellular vesicles attenuated neuroinflammation, synapse damage and microglial phagocytosis after hypoxia-ischemia injury by preventing osteopontin expression. Pharmacol Res. 2021;164:105322. 10.1016/j.phrs.2020.105322. [DOI] [PubMed] [Google Scholar]
- 63. Han J, Yang S, Hao X, et al. Extracellular vesicle-derived microrna-410 from mesenchymal stem cells protects against neonatal hypoxia-ischemia brain damage through an Hdac1-dependent Egr2/Bcl2 Axis. Front Cell Dev Biol. 2020;8:579236. 10.3389/fcell.2020.579236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chu X, Liu D, Li T, et al. Hydrogen sulfide-modified extracellular vesicles from mesenchymal stem cells for treatment of hypoxic-ischemic brain injury. J Control Release. 2020;328:13-27. 10.1016/j.jconrel.2020.08.037. [DOI] [PubMed] [Google Scholar]
- 65. Thomi G, Joerger-Messerli M, Haesler V, et al. Intranasally administered exosomes from umbilical cord stem cells have preventive neuroprotective effects and contribute to functional recovery after perinatal brain injury. Cells. 2019;8(8):855. 10.3390/cells8080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thomi G, Surbek D, Haesler V, et al. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther. 2019;10(1):105. 10.1186/s13287-019-1207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Drommelschmidt K, Serdar M, Bendix I, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. 2017;60:220-232. 10.1016/j.bbi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 68. Ahn SY, Sung DK, Kim YE, et al. Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cell-derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats. Stem Cells Transl Med. 2021;10(3):374-384. 10.1002/sctm.20-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pathipati P, Lecuyer M, Faustino J, et al. Mesenchymal Stem Cell (Msc)-derived extracellular vesicles protect from neonatal stroke by interacting with microglial cells. Neurotherapeutics. 2021;18(3):1939-1952. 10.1007/s13311-021-01076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bashinsky AL. Retinopathy of prematurity. N C Med J. 2017;78(2):124-128. 10.18043/ncm.78.2.124. [DOI] [PubMed] [Google Scholar]
- 71. Cavallaro G, Filippi L, Bagnoli P, et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol. 2014;92(1):2-20. 10.1111/aos.12049. [DOI] [PubMed] [Google Scholar]
- 72. Hartnett ME, Penn JS.. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367(26):2515-2526. 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang JD, An Y, Zhang J-S, et al. Human bone marrow mesenchymal stem cells for retinal vascular injury. Acta Ophthalmol. 2017;95(6):e453-e461. 10.1111/aos.13154. [DOI] [PubMed] [Google Scholar]
- 74. Li N, Li XR, Yuan JQ.. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. 2009;247(4):503-514. 10.1007/s00417-008-1009-y. [DOI] [PubMed] [Google Scholar]
- 75. Kim KS, Park J-M, Kong T, et al. Retinal angiogenesis effects of Tgf-Β1 and paracrine factors secreted from human placental stem cells in response to a pathological environment. Cell Transplant. 2016;25(6):1145-1157. 10.3727/096368915X688263. [DOI] [PubMed] [Google Scholar]
- 76. Noueihed B, Rivera JC, Dabouz R, et al. Mesenchymal stromal cells promote retinal vascular repair by modulating Sema3e and Il-17a in a model of ischemic retinopathy. Front Cell Dev Biol. 2021;9:630645. 10.3389/fcell.2021.630645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moisseiev E, Anderson JD, Oltjen S, et al. Protective effect of intravitreal administration of exosomes derived from mesenchymal stem cells on retinal ischemia. Curr Eye Res. 2017;42(10):1358-1367. 10.1080/02713683.2017.1319491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Han SM, Hong CR, Knell J, et al. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: a multicenter cohort analysis. J Pediatr Surg. 2020;55(6):998-1001. 10.1016/j.jpedsurg.2020.02.046. [DOI] [PubMed] [Google Scholar]
- 79. Neu J, Walker WA.. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255-264. 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mutanen A, Pierro A, Zani A.. Perioperative complications following surgery for necrotizing enterocolitis. Eur J Pediatr Surg. 2018;28(2):148-151. 10.1055/s-0038-1636943. [DOI] [PubMed] [Google Scholar]
- 81. Biouss G, Antounians L, Li B, et al. Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain. J Neuroinflammation. 2019;16(1):97. 10.1186/s12974-019-1481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang J, Watkins D, Chen C-L, et al. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg. 2012;215(4):534-545. 10.1016/j.jamcollsurg.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. McCulloh CJ, Olson JK, Wang Y, et al. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J Surg Res. 2017;214:278-285. 10.1016/j.jss.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McCulloh CJ, Olson JK, Zhou Y, et al. Stem cells and necrotizing enterocolitis: a direct comparison of the efficacy of multiple types of stem cells. J Pediatr Surg. 2017;52(6):999-1005. 10.1016/j.jpedsurg.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rager TM, Olson JK, Zhou Y, et al. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. 2016;51(6):942-947. 10.1016/j.jpedsurg.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McCulloh CJ, Olson JK, Wang Y, et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg. 2018;53(6):1215-1220. 10.1016/j.jpedsurg.2018.02.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li B, Lee C, O’Connell JS, et al. Activation of wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis. 2020;11(9):750. 10.1038/s41419-020-02964-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. O’Connell JS, Li B, Zito A, et al. Treatment of necrotizing enterocolitis by conditioned medium derived from human amniotic fluid stem cells. PLoS One. 2021;16(12):e0260522. 10.1371/journal.pone.0260522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. O’Connell JS, Lee C, Farhat N, et al. Administration of extracellular vesicles derived from human amniotic fluid stem cells: a new treatment for necrotizing enterocolitis. Pediatr Surg Int. 2021;37(3):301-309. 10.1007/s00383-020-04826-6. [DOI] [PubMed] [Google Scholar]
- 90. Goldenberg RL, Culhane JF, Iam JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75-84. 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Manuck TA, Levy PT, Gyamfi-Bannerman C, et al. Prenatal and perinatal determinants of lung health and disease in early life: a National Heart, Lung, and Blood Institute workshop report. JAMA Pediatr. 2016;170(5):e154577. 10.1001/jamapediatrics.2015.4577. [DOI] [PubMed] [Google Scholar]
- 92. Morrow LA, Wagner BD, Ingram DA, et al. Antenatal determinants of bronchopulmonary dysplasia and late respiratory disease in preterm infants. Am J Respir Crit Care Med. 2017;196(3):364-374. 10.1164/rccm.201612-2414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mestan KK, Check J, Minturn L, et al. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35(8):570-574. 10.1016/j.placenta.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mestan KK, Gotteiner N, Porta N, et al. Cord blood biomarkers of placental maternal vascular underperfusion predict bronchopulmonary dysplasia-associated pulmonary hypertension. J Pediatr. 2017;185:33-41. 10.1016/j.jpeds.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Soudée S, Vuillemin L, Alberti C, et al. Fetal growth restriction is worse than extreme prematurity for the developing lung. Neonatology. 2014;106(4):304-310. 10.1159/000360842. [DOI] [PubMed] [Google Scholar]
- 96. Tagliaferro T, Jain D, Vanbuskirk S, et al. Maternal preeclampsia and respiratory outcomes in extremely premature infants. Pediatr Res. 2019;85(5):693-696. 10.1038/s41390-019-0336-5. [DOI] [PubMed] [Google Scholar]
- 97. Hirsch K, Taglauer E, Seedorf G, et al. Perinatal hypoxia-inducible factor stabilization preserves lung alveolar and vascular growth in experimental bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2020;202(8):1146-1158. 10.1164/rccm.202003-0601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hartling L, Liang Y, Lacaze-Masmonteil T.. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2012;97(1):F8-F17. 10.1136/adc.2010.210187. [DOI] [PubMed] [Google Scholar]
- 99. Taglauer ES, Fernandez-Gonzalez A, Willis GR, et al. Mesenchymal stromal cell-derived extracellular vesicle therapy prevents preeclamptic physiology through intrauterine immunomodulation†. Biol Reprod. 2021;104(2):457-467. 10.1093/biolre/ioaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Taglauer ES, Fernandez-Gonzalez A, Willis GR, et al. Antenatal mesenchymal stromal cell extracellular vesicle therapy prevents preeclamptic lung injury in mice. Am J Respir Cell Mol Biol. 2022;66(1):86-95. 10.1165/rcmb.2021-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Abele AN, Taglauer E, Almeda M, et al. Antenatal mesenchymal stromal cell extracellular vesicle treatment preserves lung development in a model of bronchopulmonary dysplasia due to chorioamnionitis. Am J Physiol Lung Cell Mol Physiol. 2022;322(2):L179-L190. 10.1152/ajplung.00329.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Antounians L, Tzanetakis A, Pellerito O, et al. The regenerative potential of amniotic fluid stem cell extracellular vesicles: lessons learned by comparing different isolation techniques. Sci Rep. 2019;9(1):1837. 10.1038/s41598-018-38320-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mitsialis SA. The unsettling ambiguity of therapeutic extracellular vesicles from mesenchymal stromal cells. Am J Respir Cell Mol Biol. 2020;62(5):539-540. 10.1165/rcmb.2019-0382ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Moll G, Ankrum JA, Olson SD, et al. Improved MSC minimal criteria to maximize patient safety: a call to embrace tissue factor and hemocompatibility assessment of MSC products. Stem Cells Transl Med. 2022;11(1):2-13. 10.1093/stcltm/szab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gelibter S, Marostica G, Mandelli A, et al. The impact of storage on extracellular vesicles: a systematic study. J Extracell Vesicles. 2022;11(2):e12162. 10.1002/jev2.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Willis GR, Kourembanas S, Mitsialis SA.. Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front Cardiovasc Med. 2017;4:63. 10.3389/fcvm.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Poupardin R, Wolf M, Strunk D.. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv Drug Deliv Rev. 2021;176:113872. 10.1016/j.addr.2021.113872. [DOI] [PubMed] [Google Scholar]
- 108. Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 2014;164(5):966-972.e6. 10.1016/j.jpeds.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 109. Ahn SY, Chang YS, Kim JY, et al. Two-year follow-up outcomes of premature infants enrolled in the phase I trial of mesenchymal stem cells transplantation for bronchopulmonary dysplasia. J Pediatr. 2017;185:49-54.e2. 10.1016/j.jpeds.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 110. Ahn SY, Chang YS, Lee MH, et al. Stem cells for bronchopulmonary dysplasia in preterm infants: a randomized controlled phase II trial. Stem Cells Transl Med. 2021;10(8):1129-1137. 10.1002/sctm.20-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Powell SB, Silvestri JM.. Safety of intratracheal administration of human umbilical cord blood derived mesenchymal stromal cells in extremely low birth weight preterm infants. J Pediatr. 2019;210:209-213.e2. 10.1016/j.jpeds.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 112. Akduman H, Dilli D, Ergun E, et al. Successful mesenchymal stem cell application in supraventricular tachycardia-related necrotizing enterocolitis: a case report. Fetal Pediatr Pathol. 2021;40(3):250-255. 10.1080/15513815.2019.1693672. [DOI] [PubMed] [Google Scholar]
- 113. Ahn SY, Chang YS, Sung SI, et al. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: phase I dose-escalation clinical trial. Stem Cells Transl Med. 2018;7(12):847-856. 10.1002/sctm.17-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kordelas L, Rebmann V, Ludwig A-K, et al. Msc-Derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970-973. 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 115. Nassar W, El-Ansary M, Sabry D, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21. 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Toh WS, Lai RC, Zhang B, et al. Msc exosome works through a protein-based mechanism of action. Biochem Soc Trans. 2018;46(4):843-853. 10.1042/BST20180079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.